Correlations Between the Opioid System, Imidazoline Receptors, and EEG: An Investigation of Acquired Drug-Seeking Behaviors in Different Environments
Abstract
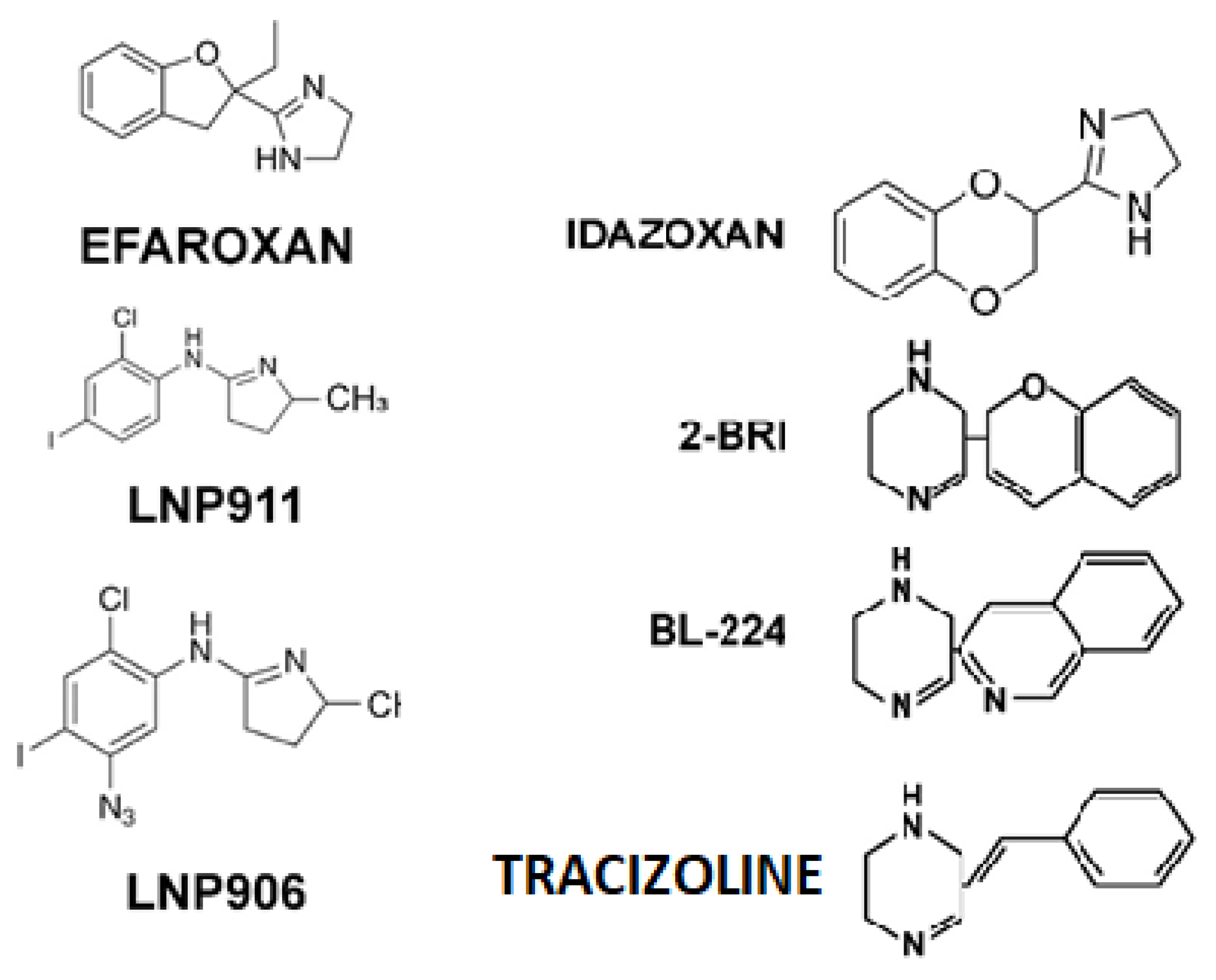
1. Introduction
2. Materials and Methods
2.1. EEG Procedure
2.2. CPP Procedure
2.3. The Preconditioning Phase
2.4. The Conditioning Phase
- − Group 1: saline solution (NaCl 0.9%), 1 mL/kg bw ip;
- − Group 2: efaroxan (1 mg/kg bw ip);
- − Group 3: idazoxan (0.3 mg/kg bw ip);
- − Group 4: tramadol (40 mg/kg bw ip);
- − Group 5: tramadol (40 mg/kg bw ip) and efaroxan (1 mg/kg bw ip);
- − Group 6: tramadol (40 mg/kg bw ip) and idazoxan (0.3 mg/kg bw ip).
2.5. The Post-Conditioning Phase
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IR | Imidazoline receptors |
| CNS | Central nervous system |
| EEG | Electroencephalography |
| CPP | Conditioned place preference |
| GPCRs | G protein-coupled receptors |
| MOR | μ-opioid receptors |
| MRI | Magnetic resonance imaging |
| fMRI | Functional MRI |
| PET | Positron emission tomography |
References
- Bousquet, P.; Hudson, A.; García-Sevilla, J.A.; Li, J.X. Imidazoline Receptor System: The Past, the Present, and the Future. Pharmacol. Rev. 2020, 72, 50–79. [Google Scholar] [CrossRef]
- Mirzaei, N.; Mota, B.C.; Birch, A.M.; Davis, N.; Romero-Molina, C.; Katsouri, L.; Palmer, E.O.C.; Golbano, A.; Riggall, L.J.; Nagy, I.; et al. Imidazoline ligand BU224 reverses cognitive deficits, reduces microgliosis and enhances synaptic connectivity in a mouse model of Alzheimer’s disease. Br. J. Pharmacol. 2021, 178, 654–671. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Su, R.B.; Li, J. Agmatine and imidazoline receptors: Their role in opioid analgesia, tolerance and dependence. Cell Mol. Neurobiol. 2008, 28, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Qin, W.; Chen, F.; Xia, Z. Long-Term Stability of Tramadol and Ketamine Solutions for Patient-Controlled Analgesia Delivery. Med. Sci. Monit. 2015, 26, 2528–2534. [Google Scholar] [CrossRef] [PubMed]
- Raffa, R.B.; Friderichs, E.; Reimann, W.; Shank, R.P.; Codd, E.E.; Vaught, J.L. Opioid and nonopioid components independently contribute to the mechanism of action of tramadol, an ‘atypical’ opioid analgesic. J. Pharmacol. Exp. Ther. 1992, 260, 275–285. [Google Scholar] [CrossRef]
- Subedi, M.; Bajaj, S.; Kumar, M.S.; Yc, M. An overview of tramadol and its usage in pain management and future perspective. Biomed. Pharmacother. 2019, 111, 443–451. [Google Scholar] [CrossRef]
- Dayer, P.; Desmeules, J.; Collart, L. Pharmacology of tramadol. Drugs 1997, 53, 18–24. [Google Scholar] [CrossRef]
- Farquhar-Smith, P.; Gubbay, A. Tramadol and acetaminophen combination for chronic non-cancer pain. Expert Opin. Pharmacother. 2013, 14, 2297–2304. [Google Scholar] [CrossRef]
- Miranda, H.F.; Sierralta, F.; Aranda, N.; Poblete, P.; Noriega, V.; Prieto, J.C. Synergism between gabapentin-tramadol in experimental diabetic neuropathic pain. Fundam. Clin. Pharmacol. 2018, 32, 581–588. [Google Scholar] [CrossRef]
- Singh, V.P.; Patil, C.S.; Kulkarni, S.K. Analysis of interaction between etoricoxib and tramadol against mechanical hyperalgesia of spinal cord injury in rats. Life Sci. 2006, 78, 1168–1174. [Google Scholar] [CrossRef]
- Yang, C.; Li, W.Y.; Yu, H.Y.; Gao, Z.Q.; Liu, X.L.; Zhou, Z.Q.; Yang, J.J. Tramadol pretreatment enhances ketamine-induced antidepressant effects and increases mammalian target of rapamycin in rat hippocampus and prefrontal cortex. J. Biomed. Biotechnol. 2012, 2012, 175619. [Google Scholar] [CrossRef]
- Ide, S.; Minami, M.; Ishihara, K.; Uhl, G.R.; Sora, I.; Ikeda, K. μ-Opioid receptor-dependent and independent components in effects of tramadol. Neuropharmacology 2006, 51, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Ide, S.; Satoyoshi, H.; Minami, M.; Satoh, M. Amelioration of the reduced antinociceptive effect of morphine in the unpredictable chronic mild stress model mice by noradrenalin but not serotonin reuptake inhibitors. Mol. Pain 2015, 11, 47. [Google Scholar] [CrossRef] [PubMed]
- Grace, A.A.; Floresco, S.B.; Goto, Y.; Lodge, D.J. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends. Neurosci. 2007, 30, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Haber, S.N.; Knutson, B. The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology 2010, 35, 4–26. [Google Scholar] [CrossRef]
- Miotto, K.; Cho, A.K.; Khalil, A.M.; Blanco, K.; Sasaki, J.D.; Rawson, R. Trends in Tramadol: Pharmacology, Metabolism, and Misuse. Anesth. Analg. 2017, 124, 44–51. [Google Scholar] [CrossRef]
- Raffa, R.B.; Buschmann, H.; Christoph, T.; Eichenbaum, G.; Englberger, W.; Flores, C.M.; Hertrampf, T.; Kögel, B.; Schiene, K.; Straßburger, W.; et al. Mechanistic and functional differentiation of tapentadol and tramadol. Expert Opin. Pharmacother. 2012, 13, 1437–1449. [Google Scholar] [CrossRef]
- Grond, S.; Sablotzki, A. Clinical pharmacology of tramadol. Clin. Pharmacokinet. 2004, 43, 879–923. [Google Scholar] [CrossRef]
- Siepsiak-Połom, M.; Szałek, E.; Porażka, J.; Karbownik, A.; Grabowski, T.; Mziray, M.; Adrych, K.; Grześkowiak, E. Ketoprofen and tramadol pharmacokinetics in patients with chronic pancreatitis. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 4044–4051. [Google Scholar] [CrossRef]
- Asari, Y.; Ikeda, Y.; Tateno, A.; Okubo, Y.; Iijima, T.; Suzuki, H. Acute tramadol enhances brain activity associated with reward anticipation in the nucleus accumbens. Psychopharmacology 2018, 235, 2631–2642. [Google Scholar] [CrossRef]
- Cha, H.J.; Song, M.J.; Lee, K.W.; Kim, E.J.; Kim, Y.H.; Lee, Y.; Seong, W.K.; Hong, S.I.; Jang, C.G.; Yoo, H.S.; et al. Dependence potential of tramadol: Behavioral pharmacology in rodents. Biomol. Ther. 2014, 22, 558–562. [Google Scholar] [CrossRef]
- Zhang, M.; Jing, L.; Liu, Q.; Wen, R.T.; Li, J.X.; Li, Y.L.; Gong, Q.; Liang, J.H. Tramadol induces conditioned place preference in rats: Interactions with morphine and buprenorphine. Neurosci. Lett. 2012, 520, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Tzschentke, T. Measuring reward with the conditioned place preference (CPP) paradigm: Update of the last decade. Addict. Biol. 2007, 12, 227–462. [Google Scholar] [CrossRef] [PubMed]
- Sprague, J.E.; Leifheit, M.; Selken, J.; Milks, M.M.; Kinder, D.H.; Nichols, D.E. In vivo microdialysis and conditioned place preference studies in rats are consistent with abuse potential of tramadol. Synapse 2002, 43, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Tzschentke, T.M.; Bruckmann, W.; Friderichs, E. Lack of sensitization during place conditioning in rats is consistent with the low abuse potential of tramadol. Neurosci. Lett. 2002, 329, 25–28. [Google Scholar] [CrossRef]
- Sutter, R.; Kaplan, P.W.; Cervenka, M.C.; Thakur, K.T.; Asemota, A.O.; Venkatesan, A.; Geocadin, R.G. Electroencephalography for diagnosis and prognosis of acute encephalitis. Clin. Neurophysiol. 2015, 126, 1524–1531. [Google Scholar] [CrossRef]
- Wrzosek, M.; Ives, J.R.; Karczewski, M.; Dziadkowiak, E.; Gruszka, E. The relationship between epileptiform discharges and background activity in the visual analysis of electroencephalographic examinations in dogs with seizures of different etiologies. Vet. J. 2017, 222, 41–51. [Google Scholar] [CrossRef]
- Cicero, T.J.; Ennis, T.; Ogden, J.; Meyer, E.R. Gender Differences in the Reinforcing Properties of Morphine. Pharmacol. Biochem. Behav. 2000, 65, 91–96. [Google Scholar] [CrossRef]
- Sarton, E.; Olofsen, E.; Romberg, R.; Den Hartigh, J.; Kest, B.; Nieuwenhuijs, D.; Burm, A.; Teppema, L.; Dahan, A. Sex Differences in Morphine Analgesia: An Experimental Study in Healthy Volunteers. Anesthesiology 2000, 93, 1245–1254. [Google Scholar] [CrossRef]
- Benelli, A.; Arletti, R.; Basaglia, R.; Bertolini, A. Male sexual behaviour: Further studies on the role of alpha 2-adrenoceptors. Pharmacol. Res. 1993, 28, 35–45. [Google Scholar] [CrossRef]
- Allan, D.R.; Penner, S.B.; Smyth, D.D. Antagonism by idazoxan at low dose but not high dose, of the natriuretic action of moxonidine. Br. J. Pharmacol. 1996, 117, 29–34. [Google Scholar] [CrossRef]
- Hosseini-Sharifabad, A.; Rabbani, M.; Sharifzadeh, M.; Bagheri, N. Acute and chronic tramadol administration impair spatial memory in rat. Res. Pharm. Sci. 2016, 11, 49–57. [Google Scholar]
- Attoh-Mensah, E.; Léger, M.; Loggia, G.; Fréret, T.; Chavoix, C.; Schumann-Bard, P. Effects of chronic tramadol administration on cognitive flexibility in mice. Psychopharmacology 2021, 238, 2883–2893. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Obata, H.; Saito, S. Antihypersensitivity effects of tramadol hydrochloride in a rat model of postoperative pain. Anesth. Analg. 2012, 115, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, F.S.; Alghamdi, B.S.; Hakami, A.Y.; Alshehri, A.A.; Althobaiti, Y.S. Melatonin attenuates morphine-induced conditioned place preference in Wistar rats. Brain Behav. 2021, 11, e2397. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; He, Y.; Zhang, J.; Li, H.; Wan, X.; Li, M.; Wang, Y.; Xu, R.; Zhang, H.; Dai, Y.; et al. Simvastatin Blocks Reinstatement of Cocaine-induced Conditioned Place Preference in Male Mice with Brain Lipidome Remodeling. Neurosci. Bull. 2021, 37, 1683–1702. [Google Scholar] [CrossRef]
- Beakley, B.D.; Kaye, A.M.; Kaye, A.D. Tramadol, Pharmacology, Side Effects, and Serotonin Syndrome: A Review. Pain Physician 2015, 18, 395–400. [Google Scholar] [CrossRef]
- Minami, K.; Ogata, J.; Uezono, Y. What is the main mechanism of tramadol? Naunyn Schmiedebergs Arch. Pharmacol. 2015, 388, 999–1007. [Google Scholar] [CrossRef]
- Gatch, M.B.; Negus, S.S.; Mello, N.K. Antinociceptive effects of monoamine reuptake inhibitors administered alone or in combination with μ opioid agonists in rhesus monkeys. Psychopharmacology 1998, 135, 99–106. [Google Scholar] [CrossRef]
- Roussin, A.; Doazan-d’Ouince, O.; Géniaux, H.; Halberer, C. French Network of Centre for Evaluation and Information on Pharmacodependence (Addictovigilance Centres) Evaluation of abuse and dependence in addiction monitoring systems: Tramadol as an example. Therapie 2015, 70, 203–221. [Google Scholar] [CrossRef]
- Daveluy, A.; Miremont-Salamé, G.; Kostrzewa, A.; Couret, A.; Lacoin, L.; Lecomte, C.; Moore, N.; Gilleron, V.; Haramburu, F. Identification of abuse and dependence cases through a hospital database. Pharmacoepidemiol. Drug Saf. 2012, 21, 1344–1349. [Google Scholar] [CrossRef]
- Abdel-Ghany, R.; Nabil, M.; Abdel-Aal, M.; Barakat, W. Nalbuphine could decrease the rewarding effect induced by tramadol in mice while enhancing its antinociceptive activity. Eur. J Pharmacol. 2015, 758, 11. [Google Scholar] [CrossRef] [PubMed]
- Baik, J.H. Dopamine signaling in reward-related behaviors. Front. Neural Circuits 2013, 7, 152. [Google Scholar] [CrossRef]
- Gao, C.; Wolf, M.E. Dopamine receptors regulate NMDA receptor surface expression in prefrontal cortex neurons. J. Neurochem. 2008, 106, 2489–2501. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Jing, X.H.; Cui, C.L.; Xing, G.G.; Zhu, B. NMDA receptors in the midbrain play a critical role in dopamine-mediated hippocampal synaptic potentiation caused by morphine. Addict. Biol. 2014, 19, 380–391. [Google Scholar] [CrossRef] [PubMed]
- Carmack, S.A.; Kim, J.S.; Sage, J.R.; Thomas, A.W.; Skillicorn, K.N.; Anagnostaras, S.G. The competitive NMDA receptor antagonist CPP disrupts cocaine-induced conditioned place preference but spares behavioral sensitization. Behav. Brain Res. 2013, 239, 155–163. [Google Scholar] [CrossRef]
- Li, S.; Zhang, X.Q.; Liu, C.C.; Wang, Z.Y.; Lu, G.Y.; Shen, H.W.; Wu, N.; Li, J.; Li, F. IRAS/Nischarin modulates morphine reward by glutamate receptor activation in the nucleus accumbens of mouse brain. Biomed. Pharmacother. 2022, 153, 113346. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, N.; Bai, F.; Li, C.; Liu, C.; Wei, J.; Zong, W.; Yang, L.; Ryabinin, A.E.; Ma, Y.; et al. Morphine-induced conditioned place preference in rhesus monkeys: Resistance to inactivation of insula and extinction. Neurobiol. Learn Mem. 2016, 131, 192–200. [Google Scholar] [CrossRef]
- Jesse, C.; Nogueira, C. Evidence for the involvement of glutamatergic and neuro-kinin 1 receptors in the antinociception elicited by tramadol in mice. Pharmacology 2009, 85, 36–40. [Google Scholar] [CrossRef]
- Olson, K.; Duron, D.; Womer, D.; Fell, R.; Streicher, J. Comprehensive molecular pharmacology screening reveals potential new receptor interactions for clinically relevant opioids. PLoS ONE 2019, 14, e0217371. [Google Scholar] [CrossRef]
- Xia, W.; Liu, G.; Shao, Z.; Xu, E.; Yuan, H.; Liu, J.; Gao, L. Toxicology of tramadol following chronic exposure based on metabolomics of the cerebrum in mice. Sci. Rep. 2020, 10, 11130. [Google Scholar] [CrossRef] [PubMed]
- Rehni, A.K.; Singh, I.; Kumar, M. Tramadol-induced seizurogenic effect: A possible role of opioid-dependent gamma-aminobutyric acid inhibitory pathway. Basic Clin. Pharmacol. Toxicol. 2008, 103, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Potschka, H.; Friderichs, E.; Löscher, W. Anticonvulsant and proconvulsant effects of tramadol, its enantiomers and its m1 metabolite in the rat kindling model of epilepsy. Br. J. Pharmacol. 2000, 131, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Talih, F.; Ghossoub, E. Moxonidine for tramadol withdrawal symptoms during detoxification. BMJ Case Rep. 2015, 2015, bcr2015210444. [Google Scholar] [CrossRef]
- Edwards, L.; Fishman, D.; Horowitz, P.; Bourbon, N.; Kester, M.; Ernsberger, P. The i1-imidazoline receptor in pc12 pheochromocytoma cells activates protein kinases c, extracellular signal-regulated kinase (erk) and c-jun n-terminal kinase (jnk). J. Neurochem. 2001, 79, 931–940. [Google Scholar] [CrossRef]
- Tanabe, M.; Kino, Y.; Honda, M.; Ono, H. Presynaptic i1-imidazoline receptors reduce gabaergic synaptic transmission in striatal medium spiny neurons. J. Neurosci. 2006, 26, 1795–1802. [Google Scholar] [CrossRef]
- Dahmani, S.; Paris, A.; Jannier, V.; Hein, L.; Rouelle, D.; Scholz, J.; Gressens, P.; Mantz, J. Dexmedetomidine increases hippocampal phosphorylated extracellular signal-regulated protein kinase 1 and 2 content by an alpha 2-adrenoceptor-independent mechanism: Evidence for the involvement of imidazoline I1 receptors. Anesthesiology 2008, 108, 457–466. [Google Scholar] [CrossRef]
- Taksande, B.; Khade, S.; Aglawe, M.; Gujar, S.; Chopde, C.; Kotagale, N. Agmatine inhibits behavioral sensitization to ethanol through imidazoline receptors. Alcohol. Clin. Exp. Res. 2019, 43, 747–757. [Google Scholar] [CrossRef]
- Shani, W.; Chris, B.; Marija, S.K.; Stephanie, K.P.; David, M.; Henchcliffe, C.; Yeona, K.; Hesterman, J.; Mangoubi, A.V. Neurophysiological Biomarkers of Parkinson’s Disease. J. Parkinsons. Dis. 2020, 10, 471–480. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, D.; Li, M.; Xuan, X.; Li, H. Brain-Computer Interface and Electrochemical Sensor Based on Boron-Nitrogen Co-Doped Graphene-Diamond Microelectrode for EEG and Dopamine Detection. ACS Sens. 2025, 10, 868–880. [Google Scholar] [CrossRef]
- Krieglstein, J.; Rieger, H.; Schütz, H. Effects of chlorpromazine and some of its metabolites on the EEG and on dopamine metabolism of the isolated perfused rat brain. Eur. J. Pharmacol. 1979, 56, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Tófoli, L.F.; de Araujo, D.B. Treating Addiction: Perspectives from EEG and Imaging Studies on Psychedelics. Int. Rev. Neurobiol. 2016, 129, 157–185. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.; Ebrahimi, M.N.; Banazadeh, M.; Shirvani, A.; Kamalahmadi, N.; Amiri, H.; Talaei, A. A systematic review on the role of EEG and fMRI-Neurofeedback training in the treatment of substance use disorders and behavioral addiction. Psychiatry Res. 2025, 349, 116474. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, J.; Leal, S.; Pereira, F.; Dinis-Oliveira, R.; Faria, J. Tramadol and tapentadol induce conditioned place preference with a differential impact on rewarding memory and incubation of craving. Pharmaceuticals 2023, 16, 86. [Google Scholar] [CrossRef]
- Bektaş, N.; Samur, D.; Arslan, R. The imidazoline receptors and ligands in pain modulation. Indian J. Pharmacol. 2015, 47, 472. [Google Scholar] [CrossRef]
- Lagard, C.; Vodovar, D.; Chevillard, L.; Callebert, J.; Caillé, F.; Pottier, G.; Liang, H.; Risède, P.; Tournier, N.; Mégarbane, B. Investigation of the Mechanisms of Tramadol-Induced Seizures in Overdose in the Rat. Pharmaceuticals 2022, 15, 1254. [Google Scholar] [CrossRef]
- Larsen, I.; Okdahl, T.; Mark, E.; Frøkjær, J.; Drewes, A. The influence of tramadol on bowel function: A randomised, placebo-controlled trial. Basic Clin. Pharmacol. Toxicol. 2024, 135, 475–490. [Google Scholar] [CrossRef]
- Osman, M.; Mustafa, M. Tramadol-induced mood elevation in a patient with no previous psychiatric history. Case Rep. Psychiatry 2018, 2018, 9574395. [Google Scholar] [CrossRef]





| Parameters | A (CNS-C3CZ) | B (CNS-C4CZ) | C (Tramadol-C4CZ) |
|---|---|---|---|
| Total N | 1827 | 2244 | 2491 |
| Mann–Whitney U | 425,567.000 | 445,894.500 | 857,224.000 |
| Wilcoxon W | 877,292.000 | 865,880.500 | 1457,284.000 |
| Test Statistic | 425,567.000 | 445,894.500 | 857,224.000 |
| Standard Error | 11,265.270 | 15,085.179 | 17,816.429 |
| Standardized Test Statistic | 0.798 | −10.761 | 5.215 |
| Asymptotic Sig. (2-sided test) | 0.425 | <0.001 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusu-Zota, G.; Trofin, D.; Gales, C.; Porumb-Andrese, E. Correlations Between the Opioid System, Imidazoline Receptors, and EEG: An Investigation of Acquired Drug-Seeking Behaviors in Different Environments. Appl. Sci. 2025, 15, 8437. https://doi.org/10.3390/app15158437
Rusu-Zota G, Trofin D, Gales C, Porumb-Andrese E. Correlations Between the Opioid System, Imidazoline Receptors, and EEG: An Investigation of Acquired Drug-Seeking Behaviors in Different Environments. Applied Sciences. 2025; 15(15):8437. https://doi.org/10.3390/app15158437
Chicago/Turabian StyleRusu-Zota, Gabriela, Dan Trofin, Cristina Gales, and Elena Porumb-Andrese. 2025. "Correlations Between the Opioid System, Imidazoline Receptors, and EEG: An Investigation of Acquired Drug-Seeking Behaviors in Different Environments" Applied Sciences 15, no. 15: 8437. https://doi.org/10.3390/app15158437
APA StyleRusu-Zota, G., Trofin, D., Gales, C., & Porumb-Andrese, E. (2025). Correlations Between the Opioid System, Imidazoline Receptors, and EEG: An Investigation of Acquired Drug-Seeking Behaviors in Different Environments. Applied Sciences, 15(15), 8437. https://doi.org/10.3390/app15158437






