Acoustic Voice Analysis as a Tool for Assessing Nasal Obstruction: A Systematic Review
Abstract
1. Introduction
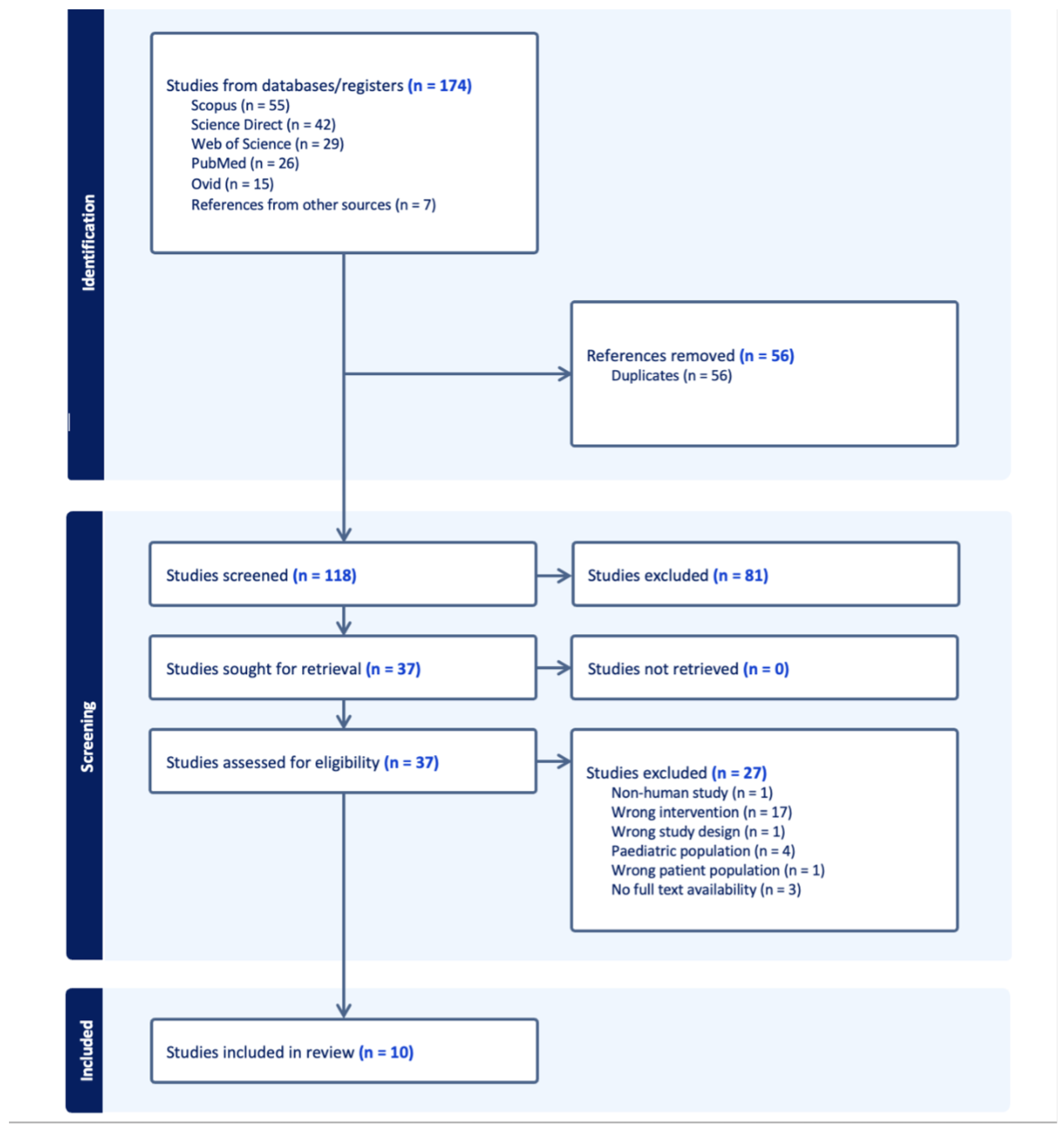
2. Materials and Methods
2.1. Search Methods
| Listing 1: Search query used in the systematic review (PubMed syntax shown) |
| ("nasal obstruction" [Title/Abstract] OR "chronic sinusitis" [Title/Abstract] OR "poliposis" [Title/Abstract] OR "nasal polyp" [Title/Abstract] OR "septal deviation" [Title/Abstract] OR "turbinate hypertrophy" [Title/Abstract] OR "choanal atresia" [Title/Abstract] OR ("swollen" [All Fields] AND "nasal tissues" [Title/Abstract]) OR "foreign body in nose" [Title/Abstract] OR "nose surgery" [Title/Abstract]) AND ("acoustic voice analysis" [Title/Abstract] OR "acoustic voice measurement" [Title/Abstract] OR "spectogram" [Title/Abstract] OR "jitter" [Title/Abstract] OR "shimmer" [Title/Abstract] OR "harmonic to noise ratio" [Title/Abstract] OR "hyponasality" [Title/Abstract] OR "acoustic analysis" [Title/Abstract] OR "nasometer" [Title/Abstract]) |
2.2. Selection Criteria
2.3. Data Extraction
2.4. Quality Assessment
3. Results
3.1. Nasal Obstruction Assessment
3.1.1. Nasometer
3.1.2. Acoustic Rhinometry
3.1.3. Anterior Rhinomanometry (ARM)
3.1.4. Nasal Obstruction Symptom Evaluation Questionnaire (NOSE)
3.1.5. Nasal Polyp Score
3.1.6. Lund–Mackay Score
3.2. Using Voice to Assess Nasal Obstruction
3.2.1. Acoustic Analysis
3.2.2. Subjective Scales: Perceptual Voice Evaluation and PROMs
3.3. Findings Summary
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jessen, M.; Malm, L. Definition, prevalence and development of nasal obstruction. Allergy 1997, 52, 3–6. [Google Scholar] [CrossRef]
- Hsu, D.W.; Suh, J.D. Anatomy and physiology of nasal obstruction. Otolaryngol. Clin. N. Am. 2018, 51, 853–865. [Google Scholar] [CrossRef] [PubMed]
- Keeler, J.; Most, S.P. Measuring nasal obstruction. Facial Plast. Surg. Clin. 2016, 24, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.G.; Witsell, D.L.; Smith, T.L.; Weaver, E.M.; Yueh, B.; Hannley, M.T. Development and validation of the Nasal Obstruction Symptom Evaluation (NOSE) scale. Otolaryngol. Neck Surg. 2004, 130, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Ciprandi, G.; Mora, F.; Cassano, M.; Gallina, A.M.; Mora, R. Visual analog scale (VAS) and nasal obstruction in persistent allergic rhinitis. Otolaryngol. Neck Surg. 2009, 141, 527–529. [Google Scholar] [CrossRef]
- Psaltis, A.J.; Li, G.; Vaezeafshar, R.; Cho, K.S.; Hwang, P.H. Modification of the Lund-Kennedy endoscopic scoring system improves its reliability and correlation with patient-reported outcome measures. Laryngoscope 2014, 124, 2216–2223. [Google Scholar] [CrossRef]
- Hilberg, O.; Jackson, A.; Swift, D.; Pedersen, O. Acoustic rhinometry: Evaluation of nasal cavity geometry by acoustic reflection. J. Appl. Physiol. 1989, 66, 295–303. [Google Scholar] [CrossRef]
- Clement, P. Committee report on standardization of rhinomanometry. Rhinology 1984, 22, 151–155. [Google Scholar]
- Blomgren, K.; Simola, M.; Hytonen, M.; Pitkaranta, A. Peak nasal inspiratory and expiratory flow measurements-practical tools in primary care? Rhinology 2003, 41, 206–210. [Google Scholar]
- Elahi, M.M.; Frenkiel, S.; Fageeh, N. Paraseptal structural changes and chronic sinus disease in relation to the deviated septum. J. Otolaryngol. 1997, 26, 236–240. [Google Scholar]
- Zehnhoff-Dinnesen, A.; Wiskirska-Woznica, B.; Neumann, K.; Nawka, T. Phoniatrics I: Fundamentals, Voice Disorders, Disorders of Language and Hearing Development; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Kummer, A.W. Cleft Palate & Craniofacial Anomalies: Effects on Speech and Resonance; Cengage Learning: Boston, MA, USA, 2013. [Google Scholar]
- Dalston, R.M.; Warren, D.W.; Dalston, E.T. Use of nasometry as a diagnostic tool for identifying patients with velopharyngeal impairment. Cleft Palate-Craniofacial J. 1991, 28, 184–189. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Maryn, Y.; Roy, N.; De Bodt, M.; Van Cauwenberge, P.; Corthals, P. Acoustic measurement of overall voice quality: A meta-analysis. J. Acoust. Soc. Am. 2009, 126, 2619–2634. [Google Scholar] [CrossRef] [PubMed]
- Yumoto, E.; Gould, W.J.; Baer, T. Harmonics-to-noise ratio as an index of the degree of hoarseness. J. Acoust. Soc. Am. 1982, 71, 1544–1550. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.S.; Yang, C.C.; Wang, C.P.; Kuo, T.B. Effect of nasal decongestion on voice spectrum of a nasal consonant-vowel. J. Voice 2005, 19, 71–77. [Google Scholar] [CrossRef]
- De Boer, G.; Bressmann, T. Application of linear discriminant analysis to the nasometric assessment of resonance disorders: A pilot study. Cleft Palate-Craniofacial J. 2015, 52, 173–182. [Google Scholar] [CrossRef]
- Naruekon, J.; Kasemsiri, P.; Thanaviratananich, S.; Prathanee, B.; Thongrong, C.; Reechaipichitkul, W. Voice quality changes after functional endoscopic sinus surgery in patients with nasal polyps. Sci. Rep. 2022, 12, 21225. [Google Scholar] [CrossRef]
- Majidi, M.R.; Asghari, M.; Abbaszadeh, E.; Demneh, A.S.; Hejrati, S. Effect of functional endoscopic sinus surgery on the voice quality among patients with rhinosinus polyposis. Iran. J. Otorhinolaryngol. 2019, 31, 197–1101. [Google Scholar]
- Mora, R.; Jankowska, B.; Dellepiane, M.; Mora, F.; Crippa, B.; Salami, A. Acoustic features of voice after septoplasty. Med. Sci. Monit. Int. Med J. Exp. Clin. Res. 2009, 15, CR269-73. [Google Scholar]
- Apaydın, E.; İkincioğulları, A.; Çolak, M.; Atan, D.; Ensari, S.; Dere, H.H. The Voice Performance After Septoplasty With Surgical Efficacy Demonstrated Through Acoustic Rhinometry and Rhinomanometry. J. Voice 2020, 34, 649.e15–649.e20. [Google Scholar] [CrossRef]
- Karakurt, S.E.; Özcan, K.M.; İkincioğullari, A.; Çetin, M.A.; Özcan, M.; Dere, H.H. Is nasal septum deviation severity a significant factor on voice in patients undergoing septoplasty? J. Coll. Physicians Surg. Pak. 2020, 30, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Altundağ, A.; Akpınar, M.E.; Koçak, I.; Tekin, M. Does nasal obstruction affect the articulation of plosive consonants? Turk. J. Ear Nose Throat 2011, 21, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Atan, D.; Özcan, K.M.; Gürbüz, A.B.T.; Dere, H. The effect of septoplasty on voice performance in patients with severe and mild nasal septal deviation. J. Craniofacial Surg. 2016, 27, 1162–1164. [Google Scholar] [CrossRef] [PubMed]
- Acar, A.; Cayonu, M.; Ozman, M.; Eryilmaz, A. Changes in Acoustic Parameters of Voice After Endoscopic Sinus Surgery in Patients with Nasal Polyposis. Indian J. Otolaryngol. Head Neck Surg. 2014, 66, 381–385. [Google Scholar] [CrossRef]
- Gulec, S.; Kulahli, I.; Sahin, M.I.; Kokoğlu, K.; Gunes, M.S.; Avci, D.; Arli, T. Effect of septoplasty on voice quality: A prospective-controlled trial. Clin. Exp. Otorhinolaryngol. 2016, 9, 238–243. [Google Scholar] [CrossRef]
- Boersma, P. Praat, a system for doing phonetics by computer. Glot. Int. 2001, 5, 341–345. [Google Scholar]
- Downs, S.H.; Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef]
- Moura, L.T.R.d.; Bedor, C.N.G.; Lopez, R.V.M.; Santana, V.S.; Rocha, T.M.B.d.S.d.; Wünsch Filho, V.; Curado, M.P. Occupational exposure to organophosphate pesticides and hematologic neoplasms: A systematic review. Rev. Bras. Epidemiol. 2020, 23, e200022. [Google Scholar] [CrossRef]
- Vieira, M.d.C.S.; Boing, L.; Machado, Z.; de Azevedo Guimarães, A.C. Sintomas do envelhecimento masculino relacionados à atividade física e qualidade de vida: Uma revisão sistemática. Rev. Bras. Ciênc. Mov. 2017, 25, 183–198. [Google Scholar] [CrossRef]
- Kennelly, J. Methodological Approach to Assessing the Evidence; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Cingi, C.; Muluk, N.B. All Around the Nose: Basic Science, Diseases and Surgical Management; Springer Nature: Cham, Switzerland, 2019. [Google Scholar]
- Clement, P.A.; Gordts, F. Consensus report on acoustic rhinometry and rhinomanometry. Rhinology 2005, 43, 169–179. [Google Scholar]
- Grymer, L.F.; Hilberg, O.; Pedersen, O.F.; Rasmussen, T.R. Acoustic rhinometry: Values from adults with subjective normal nasal patency. Rhinology 1991, 29, 35–47. [Google Scholar]
- Merkle, J.; Kohlhas, L.; Zadoyan, G.; Mösges, R.; Hellmich, M. Rhinomanometric reference intervals for normal total nasal airflow resistance. Rhinology 2014, 52, 292–299. [Google Scholar] [CrossRef]
- Thiagarajan, B. Nasal resistance its importance and measurement. ENT Scholar 2012, 1, 1–4. [Google Scholar]
- Lund, V.J.; Mackay, I.S. Staging in rhinosinusitis. Rhinology 1993, 31, 183. [Google Scholar]
- Boone, D.R.; McFarlane, S.C.; Von Berg, S.L.; Zraick, R.I. The Voice and Voice Therapy; Pearson Education: London, UK, 2013. [Google Scholar]
- Aalto, D.; Malinen, J.; Vainio, M. Formants. Oxford Research Encyclopedia of Linguistics; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Bele, I.V. The speaker’s formant. J. Voice 2006, 20, 555–578. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.F.; Freitas, D.; Junior, A.C.; Teixeira, J.P. Determination of Harmonic Parameters in Pathological Voices—Efficient Algorithm. Appl. Sci. 2023, 13, 2333. [Google Scholar] [CrossRef]
- Dhir, V.; Relekar, S.A. Normative voice data on selected parameters for young adults using VISI PITCH IV. Int. J. Sci. Eng. Res. 2020, 11, 292–304. [Google Scholar]
- KayPENTAX. SOFTWARE INSTRUCTION MANUAL Multi-Dimensional Voice Program (MDVP) Model 5105; KayPENTAX: Lincoln Park, NJ, USA, 2008. [Google Scholar]
- Tjaden, K.; Sussman, J.E.; Liu, G.; Wilding, G. Long-term average spectral (LTAS) measures of dysarthria and their relationship to perceived severity. J. Med. Speech-Lang. Pathol. 2010, 18, 125–132. [Google Scholar]
- Groll, M.D.; Hablani, S.; Stepp, C.E. The relationship between voice onset time and increase in vocal effort and fundamental frequency. J. Speech Lang. Hear. Res. 2021, 64, 1197–1209. [Google Scholar] [CrossRef]
- Dejonckere, P.H.; Remacle, M.; Fresnel-Elbaz, E.; Woisard, V.; Crevier-Buchman, L.; Millet, B. Differentiated perceptual evaluation of pathological voice quality: Reliability and correlations with acoustic measurements. Rev. Laryngol.-Otol. 1996, 117, 219–224. [Google Scholar]
- Jacobson, B.H.; Johnson, A.; Grywalski, C.; Silbergleit, A.; Jacobson, G.; Benninger, M.S.; Newman, C.W. The Voice Handicap Index (VHI): Development and Validation. Am. J. Speech-Lang. Pathol. 1997, 6, 66–70. [Google Scholar] [CrossRef]
- Fokkens, W.J.; Viskens, A.S.; Backer, V.; Conti, D.; De Corso, E.; Gevaert, P.; Scadding, G.K.; Wagemann, M.; Bernal-Sprekelsen, M.; Chaker, A. EPOS/EUFOREA update on indication and evaluation of Biologics in Chronic Rhinosinusitis with Nasal Polyps 2023. Rhinology 2023, 61, 194–202. [Google Scholar] [CrossRef]
- Dykewicz, M.S.; Wallace, D.V.; Bandi, S.; Mahdavinia, M.; Sedaghat, A.R. Patient-Reported Outcome Measures in Rhinitis and Chronic Rhinosinusitis. J. Allergy Clin. Immunol. Pract. 2024, 12, 2574–2582. [Google Scholar] [CrossRef] [PubMed]
- Worth, A.; Hammersley, V.S.; Nurmatov, U.; Sheikh, A. Systematic literature review and evaluation of patient reported outcome measures (PROMs) for asthma and related allergic diseases. Prim. Care Respir. J. 2012, 21, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Dalloul, A.H.; Miramirkhani, F.; Kouhalvandi, L. A review of recent innovations in remote health monitoring. Micromachines 2023, 14, 2157. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.R.; Awan, S.N.; Barkmeier-Kraemer, J.; Courey, M.; Deliyski, D.; Eadie, T.; Paul, D.; Švec, J.G.; Hillman, R. Recommended protocols for instrumental assessment of voice: American Speech-Language-Hearing Association expert panel to develop a protocol for instrumental assessment of vocal function. Am. J. Speech-Lang. Pathol. 2018, 27, 887–905. [Google Scholar] [CrossRef]
- Pruthi, T.; Espy-Wilson, C.Y. Acoustic parameters for automatic detection of nasal manner. Speech Commun. 2004, 43, 225–239. [Google Scholar] [CrossRef]
| Ref. | Study Design | Number of Subjects | Study/Control | Gender (m:f) |
|---|---|---|---|---|
| [18] | Prospective experimental study | 16 | 16/0 | 0:16 |
| [19] | Prospective observational study | 46 | 46/0 | 29:17 |
| [20] | Prospective observational study | 30 | 30/0 | 20:10 |
| [21] | Non randomized prospective study | 60 | 20/40 | 60:0 |
| [22] | Prospective observational study | 42 | 42 (62 initially)/0 | 25:17 |
| [23] | Prospective observational study | 69 | 69/0 | 54:15 |
| [24] | Case-control observational study | 41 | 20/21 | 21:20 |
| [25] | Prospective observational study | 43 | 43/0 | 33:10 |
| [26] | Prospective observational study | 43 | 43/0 | 31:12 |
| [27] | Prospective controlled trial | 63 | 33 (50 initially)/30 | 19:14 (study), 18:12 (control) |
| Ref. | Intervention | Control Group | Acoustic Analysis Instrument | Speech Material | Acoustic Parameters |
|---|---|---|---|---|---|
| [18] | Patient simulation | no | Praat [28] | Oral and nasal sentences | spectrogram and LTAS |
| [19] | FESS | no | Nasometer II: Model 6450, Computerized speech lab (CSL): Model 4500 | Oral, nasal and oro-nasal sentences, vowel sounds | jitter, NHR, F0, shimmer |
| [20] | FESS | no | Nasometer II: Model 6450, Computerized Speech Lab (CSL4500) | [â], [u], [i], [a], [e], [o] vowels | jitter, shimmer, HNR, F0 |
| [21] | septoplasty | yes | MDVP (Multi Dimensional Voice Program, Kay Elemetrics) | Oral, nasal and oro-nasal sentences, a, e, i vowels, | Fo, jitter, shimmer, NHR, VTI, SPI, DUV, DVB, vAm |
| [22] | septoplasty | no | Praat [28] | [a] vowel, /ma/ syllable | F0, jitter, shimmer, HNR, F1, F2, F3, F4 |
| [23] | septoplasty | no | Praat [28] | [a] vowel, /mana/ word | F0, jitter, shimmer, NHR, F1, F2, F3, F4 |
| [24] | no intervention | yes | Multi Speech Program | [pa], [ta], [ka] syllables | Voice Onset Time |
| [25] | septoplasty | no | XION Medical DIVAS 2.5 Digital Voice Analysis Software | [a] vowel | F0, jitter, shimmer |
| [26] | FESS | no | Multi-dimensional voice program | [a] vowel | F0, jitter, shimmer, NHR |
| [27] | septoplasty | yes | Multi-dimensional Voice Program | [i], [e], [a], [o], [u] vowels recorded; [n] consonant and [i] in [mini] word and [a] vowel, analysed | F0, jitter, shimmer, NHR, F1, F2, F3, F4 |
| Reference | [18] | [19] | [20] | [21] | [22] | [23] | [24] | [25] | [26] | [27] |
|---|---|---|---|---|---|---|---|---|---|---|
| Nasal Obstruction Measurements | ||||||||||
| Nasometer | ✓ | ✓ | ||||||||
| MCA | ✓ | ✓ | ||||||||
| MCV | ✓ | |||||||||
| TNV | ✓ | |||||||||
| TNR | ✓ | ✓ | ✓ | |||||||
| NOSE | ✓ | ✓ | ✓ | |||||||
| Nasal Polyp Score | § | |||||||||
| Lund–Mackay | † | † | ||||||||
| Acoustic Voice Analysis | ||||||||||
| Jitter | × | × | ✓ | × | × | × | × | × | ||
| Shimmer | × | × | ✓ | ✓ | ✓‡ | × | × | × | ||
| F0 | × | × | ✓ | × | × | ✓‡ | × | × | ||
| F1 | × | × | × | |||||||
| F2 | × | × | × | |||||||
| F3 | × | × | × | |||||||
| F4 | × | × | × | |||||||
| NHR | × | × | ✓ | × | × | × | ||||
| HNR | ✓ | |||||||||
| VTI | ✓ | |||||||||
| SPI | ✓ | |||||||||
| DUV | ✓ | |||||||||
| DVB | ✓ | |||||||||
| vAm | ✓ | |||||||||
| LTAS | ✓ | |||||||||
| VOT | × | |||||||||
| Subjective Voice Assessment | ||||||||||
| GIRBAS | × | |||||||||
| VAS | ✓ | ✓ | ||||||||
| VHI | § | ✓ | ✓‡ | ✓‡ | ||||||
| PROM for voice change | * | |||||||||
| Parameter | No. of Studies | Direction of Change | Statistical Significance (p < 0.05) | Ref. |
|---|---|---|---|---|
| Nasometry Score (Nasometer) | 2 | Significant increase ↑ in 2/2 studies (Post-op) | Yes | [19,20] |
| MCA (AR) | 2 | Significant increase ↑ in 1/2 studies [22] (Post-op), Significant decrease ↓ in 1/2 studies [24] (Obstruction vs. Control) | Yes | [22,24] |
| MCV (AR) | 1 | Significant decrease ↓ in 1/1 study (Obstruction vs. control) | Yes | [24] |
| TNV (AR) | 1 | Significant increase ↑ in 1/1 study (Post-op) | Yes | [22] |
| TNR (ARM) | 3 | Significant decrease ↓ in 3/3 studies (Post-op) | Yes | [21,22,27] |
| NOSE Score | 3 | Significant decrease ↓ in 3/3 studies (Post-op) | Yes | [22,23,25] |
| Nasal Polyp Score | 1 | Descriptive only | Not reported | [19] |
| Lund–Mackay Score | 2 | Descriptive only | Not reported | [19,20] |
| Jitter | 8 | Significant decrease ↓ in 1/8 studies [21] (Post-op) | 1 study | [19,20,21,22,23,25,26,27] |
| Shimmer | 8 | Significant decrease ↓ in 3/8 studies [21,22] (only in a subgroup [23]) (Post-op) | 3 studies | [19,20,21,22,23,25,26,27] |
| F0 | 8 | Significant increase ↑ in 1/8 studies (only in a subgroup [25])-decrease ↓ in 1/8 studies [21] (Post-op) | 2 studies | [19,20,21,22,23,25,26,27] |
| F1 | 3 | No significant change in 3/3 studies | No | [22,23,27] |
| F2 | 3 | No significant change in 3/3 studies | No | [22,23,27] |
| F3 | 3 | No significant change in 3/3 studies | No | [22,23,27] |
| F4 | 3 | No significant change in 3/3 studies | No | [22,23,27] |
| NHR | 6 | Significant decrease ↓ in 1/6 studies [21] (Post-op) | 1 study | [19,20,21,23,26,27] |
| HNR | 1 | No significant change in 1/1 study | No | [22] |
| VTI | 1 | Significant decrease ↓ in 1/1 study (Post-op) | Yes | [21] |
| SPI | 1 | Significant decrease ↓ in 1/1 study (Post-op) | Yes | [21] |
| DUV | 1 | Significant decrease ↓ in 1/1 study (Post-op) | Yes | [21] |
| DVB | 1 | Significant decrease ↓ in 1/1 study (Post-op) | Yes | [21] |
| vAm | 1 | Significant decrease ↓ in 1/1 study (Post-op) | Yes | [21] |
| LTAS | 1 | Not applicable (simulation) | Yes | [18] |
| VOT | 1 | No significant change in 1/1 study | No | [24] |
| GIRBAS | 1 | No significant change in 1/1 study | No | [19] |
| VHI | 4 | Significant decrease ↓ in 1/4 studies [22]; decrease↓ only in a subgroup in 2/4 studies [23,25]; descriptive improvement in 1/4 studies [21] (Post-op) | 3 studies | [21,22,23,25] |
| VAS | 2 | Significant increase ↑ (satisfaction) in 1/2 studies [19] (Post-op); Significant increase ↑ (symptom) in 1/2 studies (Obstruction vs. Control) [24] | Yes | [19,24] |
| PROM (Voice Change) | 1 | 36% improved | Descriptive | [27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yesilli-Puzella, G.; Degni, E.; Crescio, C.; Bracciale, L.; Loreti, P.; Rizzo, D.; Bussu, F. Acoustic Voice Analysis as a Tool for Assessing Nasal Obstruction: A Systematic Review. Appl. Sci. 2025, 15, 8423. https://doi.org/10.3390/app15158423
Yesilli-Puzella G, Degni E, Crescio C, Bracciale L, Loreti P, Rizzo D, Bussu F. Acoustic Voice Analysis as a Tool for Assessing Nasal Obstruction: A Systematic Review. Applied Sciences. 2025; 15(15):8423. https://doi.org/10.3390/app15158423
Chicago/Turabian StyleYesilli-Puzella, Gamze, Emilia Degni, Claudia Crescio, Lorenzo Bracciale, Pierpaolo Loreti, Davide Rizzo, and Francesco Bussu. 2025. "Acoustic Voice Analysis as a Tool for Assessing Nasal Obstruction: A Systematic Review" Applied Sciences 15, no. 15: 8423. https://doi.org/10.3390/app15158423
APA StyleYesilli-Puzella, G., Degni, E., Crescio, C., Bracciale, L., Loreti, P., Rizzo, D., & Bussu, F. (2025). Acoustic Voice Analysis as a Tool for Assessing Nasal Obstruction: A Systematic Review. Applied Sciences, 15(15), 8423. https://doi.org/10.3390/app15158423









