Chemometric Classification of Feta Cheese Authenticity via ATR-FTIR Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection, Preparation, and Storage
2.2. Enzyme-Linked Immunosorbent Assay (ELISA)
2.3. Instrumental Analysis
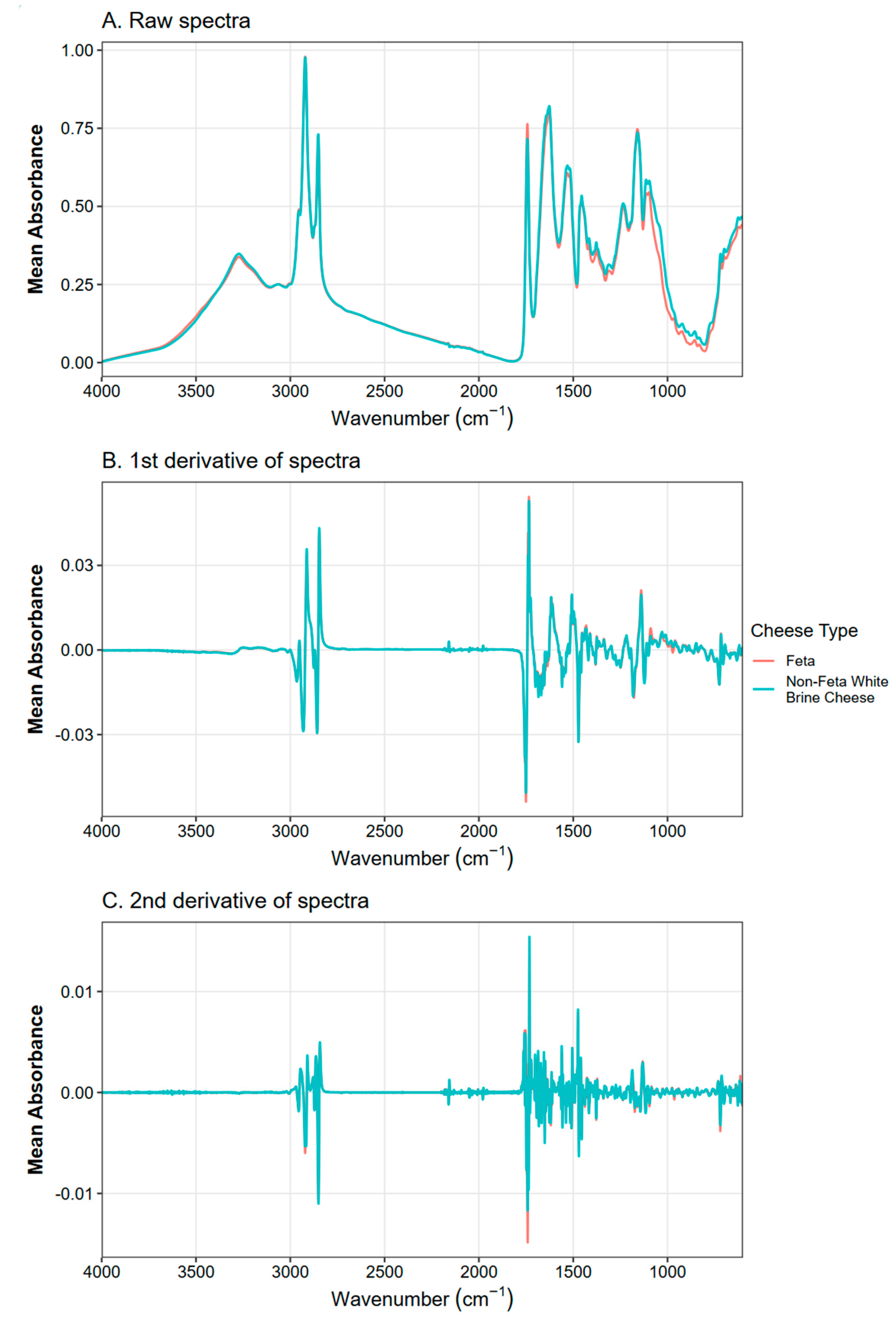
2.4. Spectral Processing
2.5. Classification Models
2.5.1. Principal Component Analysis–Linear Discriminant Analysis
2.5.2. Partial Least Squares Discriminant Analysis
3. Results
3.1. ELISA Screening for Cow’s Milk Adulteration in Feta Samples
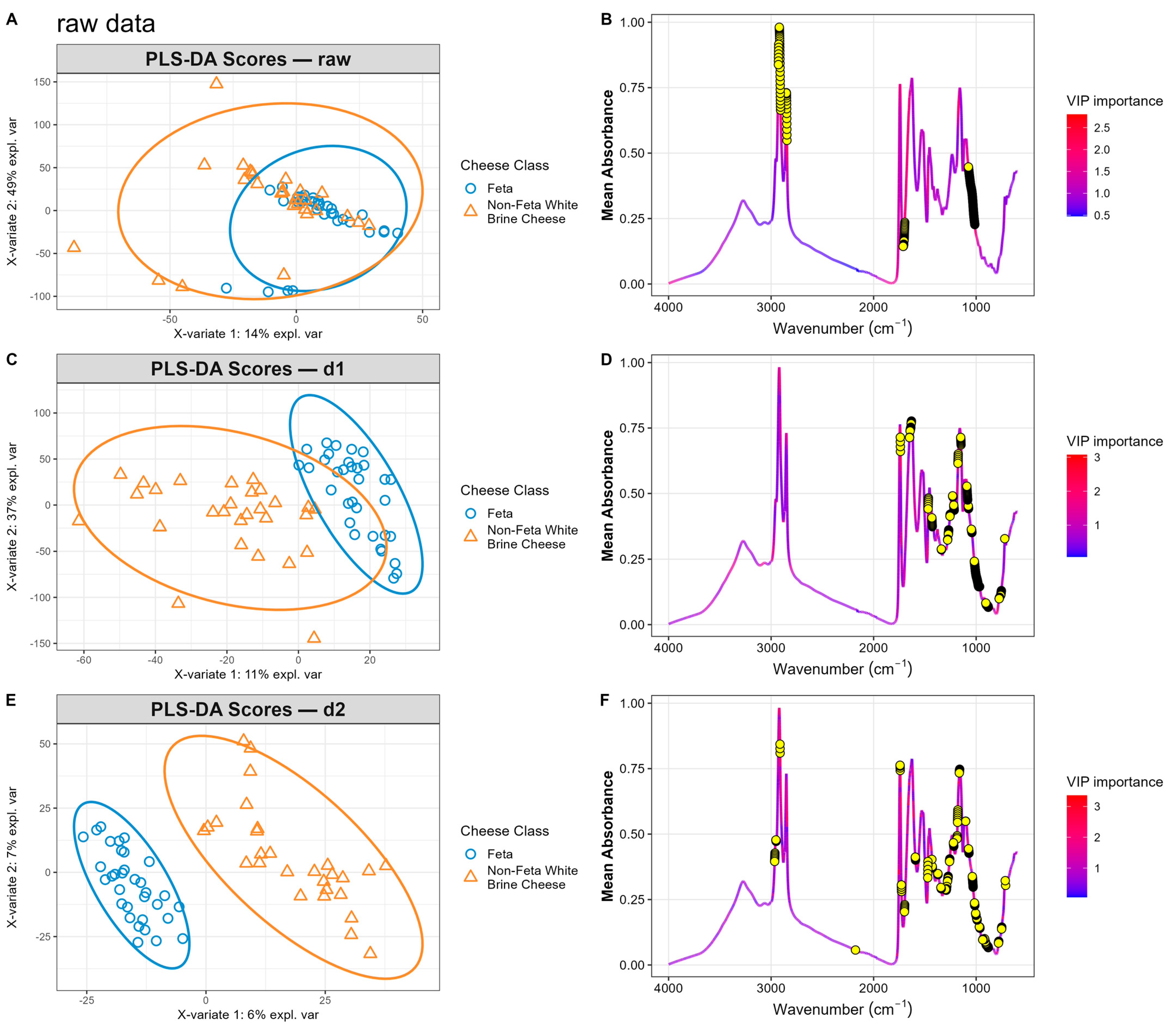
3.2. Exploratory Analysis
3.3. Classification Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATR-FTIR | Attenuated Total Reflectance–Fourier Transform Infrared Spectroscopy |
| PDO | Protected Designation of Origin |
| PCA | Principal Component Analysis |
| PLS-DA | Partial Least Squares Discriminant Analysis |
| PCA-LDA | Principal Component Analysis–Linear Discriminant Analysis |
| LOD | Limit of Detection |
| LOQ | Limit of Quantification |
| ROC | Receiver Operating Characteristic curve |
| AUC | Area Under the Curve |
| BER | Balanced Error Rate |
| LVs | Latent Variables |
| DTGS | Deuterated Triglycine Sulfate |
| VIP | Variable Importance in Projection |
| FTIR | Fourier Transform Infrared |
| OPLS-DA | Orthogonal Partial Least Squares Discriminant Analysis |
References
- Commission Regulation (EC) No 1829/2002 of 14 October 2002 Amending the Annex to Regulation (EC) No 1107/96 with Regard to the Name ‘Feta’ (Text with EEA Relevance), Vol. 277. 2002. Available online: http://data.europa.eu/eli/reg/2002/1829/oj/eng (accessed on 9 May 2025).
- Pulina, G.; Milán, M.J.; Lavín, M.P.; Theodoridis, A.; Morin, E.; Capote, J.; Thomas, D.L.; Francesconi, A.H.D.; Caja, G. Invited review: Current production trends, farm structures, and economics of the dairy sheep and goat sectors. J. Dairy Sci. 2018, 101, 6715–6729. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EC) No 1107/96 of 12 June 1996 on the Registration of Geographical Indications and Designations of Origin Under the Procedure Laid Down in Article 17 of Council Regulation (EEC) No 2081/92. Available online: https://eur-lex.europa.eu/eli/reg/1996/1107/oj/eng (accessed on 18 June 2025).
- Katsouri, E.; Magriplis, E.; Zampelas, A.; Nychas, G.J.; Drosinos, E.H. Nutritional Characteristics of Prepacked Feta PDO Cheese Products in Greece: Assessment of Dietary Intakes and Nutritional Profiles. Foods 2020, 9, 253. [Google Scholar] [CrossRef] [PubMed]
- Hammam, A.R.A.; Kapoor, R.; Salunke, P.; Metzger, L.E. Compositional and Functional Characteristics of Feta-Type Cheese Made from Micellar Casein Concentrate. Foods 2021, 11, 24. [Google Scholar] [CrossRef]
- Spognardi, S.; Passaretti, D.; Vistocco, D.; Cappelli, L.; Papetti, P. Determining the Authenticity of PDO Buffalo Mozzarella: An Approach Based on Fourier Transform Infrared (MIR-FTIR) Spectroscopy and on Chemometric Tools. Int. J. Latest Res. Sci. Technol. 2018, 7, 1–6. Available online: https://www.semanticscholar.org/paper/Determining-the-authenticity-of-PDO-buffalo-an-on-Spognardi-Passaretti/f8528e86041d8dda9f9f00ed57ec8183e2e88eac (accessed on 9 June 2025).
- Cardin, M.; Cardazzo, B.; Mounier, J.; Novelli, E.; Coton, M.; Coton, E. Authenticity and Typicity of Traditional Cheeses: A Review on Geographical Origin Authentication Methods. Foods 2022, 11, 3379. [Google Scholar] [CrossRef] [PubMed]
- Value of Production of Agricultural Products and Foodstuffs, Wines, Aromatised Wines and Spirits Protected by a Geographical Indication (GI). Available online: https://op.europa.eu/en/publication-detail/-/publication/131c84a8-3c1f-4835-9a83-fbdd0abc9b2e (accessed on 16 July 2025).
- Pidiaki, E.; Manouras, A.; Malissiova, E. Assessment of Feta Cheese Adulteration in the Region of Thessaly, Greece—Implications for Consumer Protection. In Proceedings of the IAFP’s 12th European Symposium on Food Safety, Athens, Greece, 11–13 May 2016; Available online: https://iafp.confex.com/iafp/euro16/webprogram/Paper11608.html (accessed on 9 May 2025).
- Ganopoulos, I.; Sakaridis, I.; Argiriou, A.; Madesis, P.; Tsaftaris, A. A novel closed-tube method based on high resolution melting (HRM) analysis for authenticity testing and quantitative detection in Greek PDO Feta cheese. Food Chem. 2013, 141, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Stefos, G.; Argyrokastritis, A.; Bizelis, I.; Moatsou, G.; Anifantakis, E.; Rogdakis, E. Detection of bovine mitochondrial DNA specific sequences in Feta cheese and ovine yogurt by PCR-RFLP. Milchwissenschaft 2004, 59, 509–511. [Google Scholar]
- Kritikou, A.S.; Aalizadeh, R.; Damalas, D.E.; Barla, I.V.; Baessmann, C.; Thomaidis, N.S. MALDI-TOF-MS integrated workflow for food authenticity investigations: An untargeted protein-based approach for rapid detection of PDO feta cheese adulteration. Food Chem. 2022, 370, 131057. [Google Scholar] [CrossRef] [PubMed]
- Saji, R.; Ramani, A.; Gandhi, K.; Seth, R.; Sharma, R. Application of FTIR spectroscopy in dairy products: A systematic review. Food Humanit. 2024, 2, 100239. [Google Scholar] [CrossRef]
- Mafra, I.; Honrado, M.; Amaral, J.S. Animal Species Authentication in Dairy Products. Foods. 2022, 11, 1124. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, L.; Koureas, M.; Pappas, C.; Manouras, A.; Kantas, D.; Malissiova, E. Rapid Classification of Cow, Goat, and Sheep Milk Using ATR-FTIR and Multivariate Analysis. Sci 2025, 7, 87. [Google Scholar] [CrossRef]
- Smaoui, S.; Tarapoulouzi, M.; Agriopoulou, S.; D’Amore, T.; Varzakas, T. Current State of Milk, Dairy Products, Meat and Meat Products, Eggs, Fish and Fishery Products Authentication and Chemometrics. Foods 2023, 12, 4254. [Google Scholar] [CrossRef] [PubMed]
- Ayvaz, H.; Mortas, M.; Dogan, M.A.; Atan, M.; Yildiz Tiryaki, G.; Karagul Yuceer, Y. Near- and mid-infrared determination of some quality parameters of cheese manufactured from the mixture of different milk species. J. Food Sci. Technol. 2021, 58, 3981–3992. [Google Scholar] [CrossRef] [PubMed]
- Di Donato, F.; Biancolillo, A.; Foschi, M.; D’Archivio, A.A. Application of SPORT algorithm on ATR-FTIR data: A rapid and green tool for the characterization and discrimination of three typical Italian Pecorino cheeses. J. Food Compos. Anal. 2022, 114, 104784. [Google Scholar] [CrossRef]
- Yaman, H.; Aykas, D.P.; Jiménez-Flores, R.; Rodriguez-Saona, L.E. Monitoring the ripening attributes of Turkish white cheese using miniaturized vibrational spectrometers. J. Dairy Sci. 2022, 105, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Tarapoulouzi, M.; Kokkinofta, R.; Theocharis, C.R. Chemometric analysis combined with FTIR spectroscopy of milk and Halloumi cheese samples according to species’ origin. Food Sci. Nutr. 2020, 8, 3262–3273. [Google Scholar] [CrossRef] [PubMed]
- Tzora, A.; Nelli, A.; Voidarou, C.; Fthenakis, G.; Rozos, G.; Theodorides, G.; Bonos, E.; Skoufos, I. Microbiota “Fingerprint” of Greek Feta Cheese through Ripening. Appl. Sci. 2021, 11, 5631. [Google Scholar] [CrossRef]
- Bozoudi, D.; Torriani, S.; Zdragas, A.; Litopoulou-Tzanetaki, E. Assessment of microbial diversity of the dominant microbiota in fresh and mature PDO Feta cheese made at three mountainous areas of Greece. LWT—Food Sci. Technol. 2016, 72, 525–533. [Google Scholar] [CrossRef]
- Papadakis, P.; Konteles, S.; Batrinou, A.; Ouzounis, S.; Tsironi, T.; Halvatsiotis, P.; Tsakali, E.; Van Impe, J.F.M.; Vougiouklaki, D.; Strati, I.F.; et al. Characterization of Bacterial Microbiota of P.D.O. Feta Cheese by 16S Metagenomic Analysis. Microorganisms 2021, 9, 2377. [Google Scholar] [CrossRef] [PubMed]
- Plessas, S.; Ganatsios, V.; Mantzourani, I.; Bosnea, L. White Brined Cheese Production by Incorporation of a Traditional Milk-Cereal Prebiotic Matrix with a Candidate Probiotic Bacterial Strain. Appl. Sci. 2021, 11, 6182. [Google Scholar] [CrossRef]
- Dimitrellou, D.; Kourkoutas, Y.; Banat, I.M.; Marchant, R.; Koutinas, A.A. Whey-cheese production using freeze-dried kefir culture as a starter. J. Appl. Microbiol. 2007, 103, 1170–1183. [Google Scholar] [CrossRef] [PubMed]
- Slozhenkina, M.I.; Gorlov, I.F.; Serova, O.P.; Bozhkova, S.E.; Kotova, A.S. The study of factors affecting the drying process and the quality of dry cheese. IOP Conf. Ser. Earth Environ. Sci. 2021, 640, 032018. [Google Scholar] [CrossRef]
- Cao, W.; Passot, S.; Irlinger, F.; Fonseca, F. Investigation of Freezing and Freeze-Drying for Preserving and Re-Using a Whole Microbial Cheese Community. Foods 2024, 13, 1809. [Google Scholar] [CrossRef] [PubMed]
- Rau, J.; Korte, N.; Dyk, M.; Wenninger, O.; Schreiter, P.; Hiller, E. Rapid animal species identification of feta and mozzarella cheese using MALDI-TOF mass-spectrometry. Food Control 2020, 117, 107349. [Google Scholar] [CrossRef]
- Melissari, F.; Papadakis, A.; Chatzitheodorou, D.; Tran, D.; Schouteten, J.; Athanasiou, G.; Zahariadis, T. Experiences Using Ethereum and Quorum Blockchain Smart Contracts in Dairy Production. J. Sens. Actuator Netw. 2024, 13, 6. [Google Scholar] [CrossRef]
- Tran, D.; De Steur, H.; Gellynck, X.; Papadakis, A.; Schouteten, J.J. The heterogeneity of consumer preference for blockchain-based food traceability: The role of governmental trust and information-seeking behaviour. Food Qual. Prefer. 2025, 126, 105424. [Google Scholar] [CrossRef]
- Tarapoulouzi, M.; Pashalidis, I.; Theocharis, C.R. Discrimination of Cheese Products Regarding Milk Species’ Origin Using FTIR, 1H-NMR, and Chemometrics. Appl. Sci. 2024, 14, 2584. [Google Scholar] [CrossRef]
- Foschi, M.; Biancolillo, A.; Reale, S.; Poles, F.; D’Archivio, A.A. Classification of “Ricotta” whey cheese from different milk and Designation of Origin-protected samples through infrared spectroscopy and chemometric analysis. J. Food Compos. Anal. 2025, 138, 107019. [Google Scholar] [CrossRef]
- Nicolaou, N.; Xu, Y.; Goodacre, R. Fourier transform infrared spectroscopy and multivariate analysis for the detection and quantification of different milk species. J. Dairy Sci. 2010, 93, 5651–5660. [Google Scholar] [CrossRef] [PubMed]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; Wiley: Chichester, UK, 2010. [Google Scholar]
- Skeie, S.; Feten, G.; Almøy, T.; Østlie, H.; Isaksson, T. The use of near infrared spectroscopy to predict selected free amino acids during cheese ripening. Int. Dairy J. 2006, 16, 236–242. [Google Scholar] [CrossRef]
- Balieiro, A.L.; Santos, R.A.; Pereira, M.M.; Figueiredo, R.T.; Freitas, L.S.; de Alsina, O.L.S.; Lima, A.S.; Soares, C.M.F. Adsorption Process of Molecularly Imprinted Silica for Extraction of Lactose from Milk. Braz. J. Chem. Eng. 2016, 33, 361–372. [Google Scholar] [CrossRef]
- Yaman, H.; Aykas, D.P.; Rodriguez-Saona, L.E. Monitoring Turkish white cheese ripening by portable FT-IR spectroscopy. Front. Nutr. 2023, 10, 1107491. [Google Scholar] [CrossRef] [PubMed]
- De Jesus, J.; Silva, G.; Gonçalves, B.-H.; De Souza, M.; Santos, L.; Ferrão, S. Quick Identification of the Time of Maturation of Artisanal Minas Cheese by FTIR-ATR Spectroscopy and Multivariate Techniques. J. Braz. Chem. Soc. 2020, 31, 2000–2011. [Google Scholar] [CrossRef]

| Signal Representation | Equation |
|---|---|
| Raw absorbance | - |
| First derivative | d1(ν) = (A(ν) − A(ν − Δν))/Δν |
| Second derivative | d2(ν) = (A(ν + Δν) − 2·A(ν) + A(ν − Δν))/(Δν)2 |
| Train Set | Test Set | |||||||
|---|---|---|---|---|---|---|---|---|
| Preprocessing | # PCs | AUC | Sensitivity | Specificity | AUC | Sensitivity | Specificity | Accuracy |
| Raw Spectra | 10 | 0.849 | 0.913 | 0.693 | 0.916 | 1.000 | 0.545 | 0.792 |
| First Derivative | 9 | 0.948 | 0.970 | 0.793 | 0.951 | 1.000 | 0.818 | 0.917 |
| Second Derivative | 7 | 0.980 | 0.970 | 0.827 | 0.930 | 1.000 | 0.818 | 0.917 |
| Train Set | Test Set | |||||||
|---|---|---|---|---|---|---|---|---|
| Preprocessing | # LVs | AUC | Sensitivity | Specificity | AUC | Sensitivity | Specificity | Accuracy |
| Raw Spectra | 8 | 0.992 | 0.970 | 0.929 | 0.874 | 0.846 | 0.818 | 0.833 |
| First Derivative | 3 | 1.000 | 1.000 | 0.964 | 0.923 | 1.000 | 0.727 | 0.875 |
| Second Derivative | 9 | 1.000 | 1.000 | 1.000 | 0.958 | 1.000 | 0.909 | 0.958 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimitriou, L.; Koureas, M.; Pappas, C.S.; Manouras, A.; Kantas, D.; Malissiova, E. Chemometric Classification of Feta Cheese Authenticity via ATR-FTIR Spectroscopy. Appl. Sci. 2025, 15, 8272. https://doi.org/10.3390/app15158272
Dimitriou L, Koureas M, Pappas CS, Manouras A, Kantas D, Malissiova E. Chemometric Classification of Feta Cheese Authenticity via ATR-FTIR Spectroscopy. Applied Sciences. 2025; 15(15):8272. https://doi.org/10.3390/app15158272
Chicago/Turabian StyleDimitriou, Lamprini, Michalis Koureas, Christos S. Pappas, Athanasios Manouras, Dimitrios Kantas, and Eleni Malissiova. 2025. "Chemometric Classification of Feta Cheese Authenticity via ATR-FTIR Spectroscopy" Applied Sciences 15, no. 15: 8272. https://doi.org/10.3390/app15158272
APA StyleDimitriou, L., Koureas, M., Pappas, C. S., Manouras, A., Kantas, D., & Malissiova, E. (2025). Chemometric Classification of Feta Cheese Authenticity via ATR-FTIR Spectroscopy. Applied Sciences, 15(15), 8272. https://doi.org/10.3390/app15158272







