How Does Left Ventricular Ejection Fraction Affect the Multimodal Assessment of Congestion in Patients with Acute Heart Failure? Results from a Prospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Data Collection and Variables
2.4. Echographic Evaluation
- In the hepatic veins, waveforms were classified as normal, mildly, or severely altered based on the S/D wave ratio and flow direction.
- Portal vein pulsatility was categorized as <30%, 30–50%, or >50%, with higher pulsatility indicating greater congestion.
- Intrarenal venous flow was categorized as continuous, biphasic, or monophasic, with monophasic indicating severe congestion.
2.5. Statistical Analysis
2.6. Ethical Considerations
3. Results
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HF | Heart failure |
| LVEF | Left ventricular ejection fraction |
| VExUS | Venous Excess Ultrasound |
| HFpEF | Heart failure with preserved ejection fraction |
| HFrEF | Heart failure with reduced ejection fraction |
| CA125 | Carbohydrate antigen 125 |
| NTproBNP | N-terminal pro-B-type natriuretic peptide |
| POCUS | Point-of-care ultrasonography |
| LUS | Lung ultrasound |
| IVC | Inferior vena cava |
| AHF | Acute heart failure |
| CKD | Chronic kidney disease |
| NYHA | New York Heart Association |
References
- Riccardi, M.; Sammartino, A.M.; Piepoli, M.; Adamo, M.; Pagnesi, M.; Rosano, G.; Metra, M.; von Haehling, S.; Tomasoni, D. Heart failure: An update from the last years and a look at the near future. ESC Heart Fail. 2022, 9, 3667–3693. [Google Scholar] [CrossRef] [PubMed]
- Husain-Syed, F.; McCullough, P.A.; Birk, H.W.; Renker, M.; Brocca, A.; Seeger, W.; Ronco, C. Cardio-Pulmonary-Renal Interactions: A Multidisciplinary Approach. J. Am. Coll. Cardiol. 2015, 65, 2433–2448. [Google Scholar] [CrossRef] [PubMed]
- Sicras-Mainar, A.; Sicras-Navarro, A.; Palacios, B.; Varela, L.; Delgado, J.F. Epidemiology and treatment of heart failure in Spain: The HF-PATHWAYS study. Rev. Esp. Cardiol. 2022, 75, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Kazory, A.; Elkayam, U. Cardiorenal interactions in acute decompensated heart failure: Contemporary concepts facing emerging controversies. J. Card. Fail. 2014, 20, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Marteles, M.; Garcés-Horna, V.; Morales-Rull, J.L.; Casado, J.; Carrera-Izquierdo, M.; Conde-Martel, A.; Dávila-Ramos, M.F.; Llácer, P.; Salamanca-Bautista, P.; Ruiz, R.; et al. Combining loop and thiazide diuretics across the left ventricular ejection fraction spectrum. JACC Heart Fail. 2024, 12, 1719–1730. [Google Scholar] [CrossRef] [PubMed]
- Koratala, A.; Kazory, A. Point of Care Ultrasonography for Objective Assessment of Heart Failure: Integration of Cardiac, Vascular, and Extravascular Determinants of Volume Status. Cardiorenal Med. 2021, 11, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Gracia, J.; Demissei, B.G.; ter Maaten, J.M.; Cleland, J.G.; O’Connor, C.M.; Metra, M.; Ponikowski, P.; Teerlink, J.R.; Cotter, G.; Davison, B.A.; et al. Prevalence, predictors and clinical outcome of residual congestion in acute decompensated heart failure. Int. J. Cardiol. 2018, 258, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Morrow, D.A.; Velazquez, E.J.; Devore, A.D.; Desai, A.S.; Duffy, C.I.; Ambrosy, A.P.; Gurmu, Y.; Mccague, K.; Rocha, R.; Braunwald, E. Clinical Outcomes in Patients with Acute Decompensated Heart Failure Randomly Assigned to Sacubitril/Valsartan or Enalapril in the PIONEER-HF Trial. Circulation 2019, 139, 2285–2288. [Google Scholar] [CrossRef] [PubMed]
- Long, B.; Koyfman, A.; Gottlieb, M. Diagnosis of acute heart failure in the emergency department: An evidence-based review. West. J. Emerg. Med. 2019, 20, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Maisel, A.; Mueller, C.; Adams, K., Jr.; Anker, S.D.; Aspromonte, N.; Cleland, J.G.; Cohen-Solal, A.; Dahlstrom, U.; DeMaria, A.; Di Somma, S.; et al. State of the art: Using natriuretic peptide levels in clinical practice. Eur. J. Heart Fail. 2008, 10, 824–839. [Google Scholar] [CrossRef] [PubMed]
- Koratala, A.; Reisinger, N. POCUS for nephrologists: Basic principles and a general approach. Kidney360 2021, 2, 1660–1668. [Google Scholar] [CrossRef] [PubMed]
- Jobs, A.; Vonthein, R.; König, I.R.; Schäfer, J.; Nauck, M.; Haag, S.; Fichera, C.F.; Stiermaier, T.; Ledwoch, J.; Schneider, A.; et al. Inferior vena cava ultrasound in acute decompensated heart failure: Design rationale of the CAVA-ADHF-DZHK10 trial. ESC Heart Fail. 2020, 7, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Rola, P.; Miralles-Aguiar, F.; Argaiz, E.; Beaubien-Souligny, W.; Haycock, K.; Karimov, T.; Dinh, V.A.; Spiegel, R. Clinical applications of the venous excess ultrasound (VExUS) score: Conceptual review and case series. Ultrasound J. 2021, 13, 32. [Google Scholar] [CrossRef] [PubMed]
- Pastore, M.C.; Ilardi, F.; Stefanini, A.; Mandoli, G.E.; Palermi, S.; Bandera, F.; Benfari, G.; Esposito, R.; Lisi, M.; Pasquini, A.; et al. Bedside ultrasound for hemodynamic monitoring in cardiac intensive care unit. J. Clin. Med. 2022, 11, 7538. [Google Scholar] [CrossRef] [PubMed]
- Narula, J.; Chandrashekhar, Y.; Braunwald, E. Time to Add a Fifth Pillar to Bedside Physical Examination: Inspection, Palpation, Percussion, Auscultation, and Insonation. JAMA Cardiol. 2018, 3, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Llàcer, P.; Romero, G.; Trullàs, J.C.; de la Espriella, R.; Cobo, M.; Quiroga, B.; Casado, J.; Slon-Roblero, M.F.; Morales-Rull, J.L.; Morgado, J.I.; et al. Consensus on the approach to hydrosaline overload in acute heart failure. SEMI/SEC/S.E.N. recommendations. Rev. Esp. Cardiol. 2024, 77, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Beaubien-Souligny, W.; Rola, P.; Haycock, K.; Bouchard, J.; Lamarche, Y.; Spiegel, R.; Denault, A.Y. Quantifying systemic congestion with Point-Of-Care ultrasound: Development of the venous excess ultrasound grading system. Ultrasound J. 2020, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Assavapokee, T.; Rola, P.; Assavapokee, N.; Koratala, A. Decoding VExUS: A practical guide for excelling in point-of-care ultrasound assessment of venous congestion. Ultrasound J. 2024, 16, 48. [Google Scholar] [CrossRef] [PubMed]
- Landi, I.; Guerritore, L.; Iannaccone, A.; Ricotti, A.; Rola, P.; Garrone, M. Assessment of venous congestion with venous excess ultrasound score in the prognosis of acute heart failure in the emergency department: A prospective study. Eur. Heart J. Open. 2024, 4, oeae050. [Google Scholar] [CrossRef] [PubMed]
- Gamarra, Á.; Salamanca, J.; Díez-Villanueva, P.; Cuenca, S.; Vázquez, J.; Aguilar, R.J.; Diego, G.; Rodríguez, A.P.; Alfonso, F. Ultrasound imaging of congestion in heart failure: A narrative review. Cardiovasc Diagn Ther. 2025, 15, 233–250. [Google Scholar] [CrossRef] [PubMed]
- Rubio Gracia, J.; Sánchez Marteles, M.; Pérez Calvo, J.I. Involvement of systemic venous congestion in heart failure. Rev. Clin. Esp. 2017, 217, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Di Fiore, V.; Del Punta, L.; De Biase, N.; Pellicori, P.; Gargani, L.; Dini, F.L.; Armenia, S.; Vigni, M.L.; Maremmani, D.; Masi, S. Integrative assessment of congestion in heart failure using ultrasound imaging. Int. Emerg. Med. 2025, 20, 11–22. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.V.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin–Neprilysin Inhibition versus Enalapril in Heart Failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Omote, K.; Verbrugge, F.H.; Borlaug, B.A. Heart failure with preserved ejection fraction: Mechanisms and treatment strategies. Annu. Rev. Med. 2022, 73, 321–337. [Google Scholar] [CrossRef] [PubMed]
- Borlaug, B.A.; Sharma, K.; Shah, S.J.; Ho, J.E. Heart failure with preserved ejection fraction: JACC Scientific Statement. J. Am. Coll. Cardiol. 2023, 81, 1810–1834. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Dewan, P.; Anand, I.S.; Desai, A.S.; Packer, M.; Ziler, M.R.; Pfeffer, M.A.; Solomon, S.D.; Abraham, W.T.; Shah, S.J.; et al. Clinical characteristics and outcomes in patients with heart failure: Are there thresholds justifying a clínical classification? Circulation 2023, 148, 732–749. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Rev. Esp. Cardiol. 2022, 75, 523. [Google Scholar] [PubMed]
- Espriella, R.; Santas, E.; Zegri Reiriz, I.; Górriz, J.L.; Cobo Marcos, M.; Nuñez, J. Quantification and treatment of congestion in heart failure: A clinical and pathophysiological overview. Nefrología 2022, 42, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Van Aelst, L.N.L.; Arrigo, M.; Placido, R.; Akiyama, E.; Girerd, N.; Zannad, F.; Manivet, P.; Rossignol, P.; Badoz, M.; Sadoune, M.; et al. Acutely decompensated heart failure with preserved and reduced ejection fraction present with comparable haemodynamic congestion. Eur. J. Heart Fail. 2018, 20, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Menghoum, N.; Badii, M.C.; Deltombe, M.; Lejeune, S.; Roy, C.; Vancraeynest, D.; Pasquet, A.; Gerber, B.L.; Horman, S.; Gruson, D.; et al. Carbohydrate antigen 125: A useful marker of congestion, fibrosis, and prognosis in heart failure with preserved ejection fraction. ESC Heart Fail. 2024, 11, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Tuersun, R.; Abudouwayiti, A.; Li, Y.; Pan, Y.; Aimaier, S.; Wen, Z.Y.; Gao, W.T.; Ma, L.J.; Mahemuti, A.; Zheng, Y.Y. Serum CA125: A prognostic biomarker for mortality in chronic heart failure. BMC Cardiovasc. Disord. 2025, 25, 227. [Google Scholar] [CrossRef] [PubMed]
- Palazzuoli, A.; Ruocco, G.; Pellicori, P.; Gargani, L.; Coiro, S.; Lamiral, Z.; Ambrosio, G.; Rastogi, T.; Girerd, N. Multi-modality assessment of congestion in acute heart failure: Associations with left ventricular ejection fraction and prognosis. Curr. Probl. Cardiol. 2024, 49, 102374. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, V.; Vikneswaran, G.; Rola, P.; Raju, S.; Bhat, R.S.; Jayakumar, A.; Alva, A. Combination of Inferior Vena Cava Diameter, Hepatic Venous Flow, and Portal Vein Pulsatility Index: Venous Excess Ultrasound Score (VEXUS Score) in Predicting Acute Kidney Injury in Patients with Cardiorenal Syndrome: A Prospective Cohort Study. Indian J. Crit. Care Med. 2020, 9, 7839. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Ramos, J.A.; Aguirre-Acevedo, D.C.; Pana-Toloza, M.C. Point of care ultrasound impact in acute heart failure hospitalization: A retrospective cohort study. Am. J. Emerg. Med. 2023, 66, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Trullàs, J.C.; Moreno-García, M.C.; Mittelbrunn-Alquézar, V.; Conde-Martel, A.; Soler-Rangel, L.; Montero-Pérez-Barquero, M.; Casado, J.; Sánchez-Marteles, M.; Arévalo-Lorido, J.C.; Pérez-Silvestre, J.; et al. The RICA-2 registry: Design and baseline characteristics of the first 1000 patients. Rev. Clin. Esp. 2024, 224, 522–533. [Google Scholar] [CrossRef] [PubMed]

| Variables | HFpEF (>50%) | HFrEF (<50%) | p-Value |
|---|---|---|---|
| Hypertension | 52 (87) | 26 (87) | 1.000 |
| Diabetes | 20 (33) | 11 (37) | 0.754 |
| Flutter/Atrial fibrillation | 52 (87) | 16 (53) | <0.001 * |
| Dyslipidemia | 33 (55) | 19 (63) | 0.451 |
| CKD | 17 (28) | 15 (50) | 0.043 * |
| Congestion score (points) | 4 ± 2 | 5 ± 2 | 0.119 |
| Pleural effusion | 31 (52) | 23 (77) | 0.022 * |
| Ascites | 3 (5) | 6 (20) | 0.020 * |
| Inferior vena cava distension (mm) | 22.4 ± 4.3 | 22.8 ± 4.8 | 0.693 |
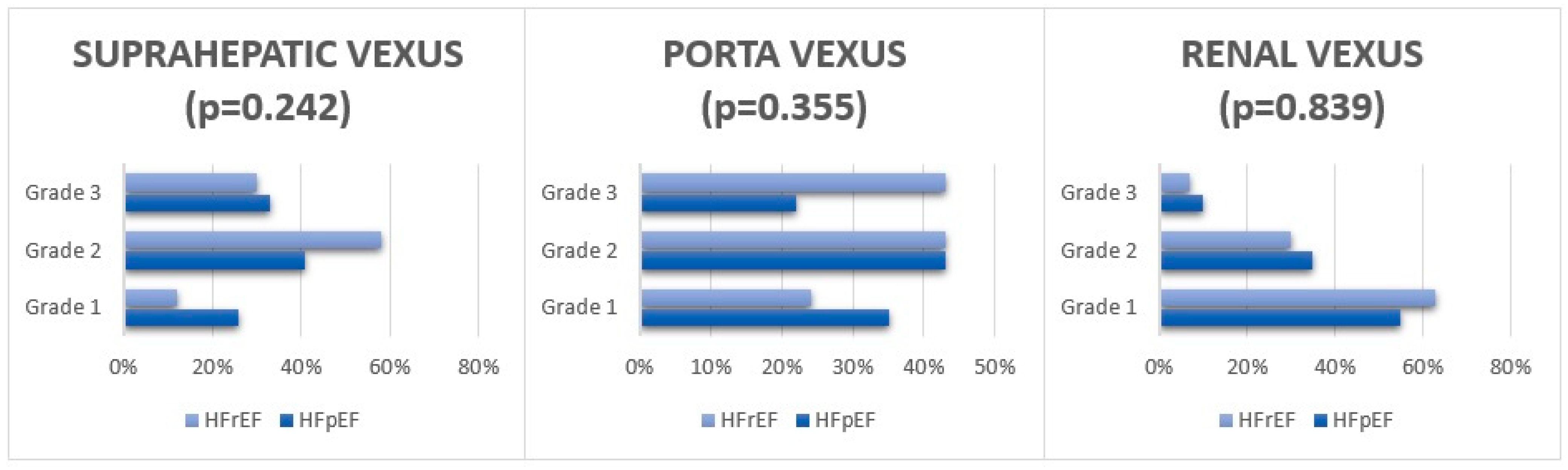
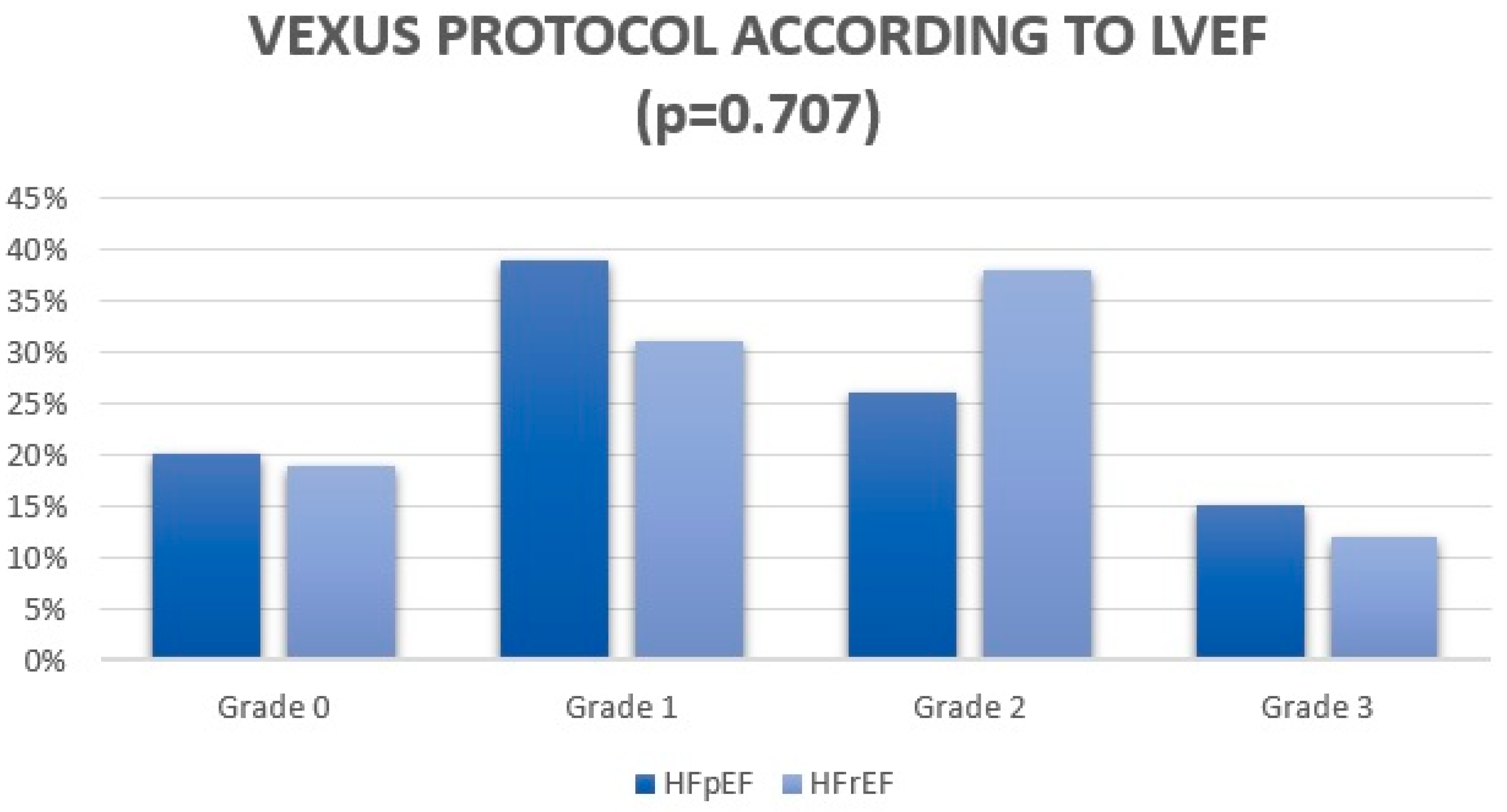
| VExUS grade 0–1 | 31 (59) | 14 (50) | 0.526 |
| VExUS grade 2–3 | 21 (41) | 14 (50) | 0.526 |
| Uric acid (mg/dL) | 7.0 ± 3.8 | 7.2 ± 3.5 | 0.466 |
| Urea (mg/dL) | 66.7 ± 34 | 75.3 ± 23 | 0.223 |
| Creatinine (mg/dL) | 1.3 ± 0.5 | 1.5 ± 0.5 | 0.077 |
| eGFR (mL/min/1.73 m2) | 52 ± 29 | 44 ± 24 | 0.270 |
| Sodium (mmol/L) | 141 ± 3.5 | 140 ± 5.1 | 0.097 |
| Potassium (mmol/L) | 4.02 ± 0.4 | 4.31 ± 0.9 | 0.056 |
| Chloride (mmol/L) | 100 ± 5 | 101 ± 6 | 0.820 |
| Creatinine in urine (mg/dL) | 37.5 ± 28.5 | 33.7 ± 21.3 | 0.564 |
| Chloride in urine (mg/dL) | 86 ± 59 | 87 ± 63 | 0.960 |
| Sodium in urine (mg/dL) | 93.5 ± 48.5 | 88 ± 61 | 0.438 |
| Potassium in urine (mg/dL) | 25.1 ± 10.1 | 27.4 ± 11.3 | 0.354 |
| Urea in urine (mg/dL) | 522 ± 412 | 536 ± 470 | 0.883 |
| ACR in urine (mg/g) | 92 (RIC: 237) | 121 (RIC: 236) | 0.406 |
| NTproBNP (ng/dL) | 4665 (RIC: 5662) | 11,278 (RIC: 20,179) | <0.001 * |
| Ca 125 (U/mL) | 37.3 (RIC: 51.6) | 70.6 (RIC: 120) | 0.032 * |
| Hemoglobin (g/dL) | 12.4 ± 2 | 12 ± 2.4 | 0.368 |
| Hematocrit (%) | 38.2 ± 6.1 | 36.3 ± 7.5 | 0.215 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esterellas-Sánchez, L.K.; Campos-Sáenz de Santamaría, A.; Albines Fiestas, Z.S.; Crespo-Aznarez, S.; Sánchez-Marteles, M.; Garcés-Horna, V.; Alcaine-Otín, A.; Gimenez-Lopez, I.; Rubio-Gracia, J. How Does Left Ventricular Ejection Fraction Affect the Multimodal Assessment of Congestion in Patients with Acute Heart Failure? Results from a Prospective Study. Appl. Sci. 2025, 15, 8157. https://doi.org/10.3390/app15158157
Esterellas-Sánchez LK, Campos-Sáenz de Santamaría A, Albines Fiestas ZS, Crespo-Aznarez S, Sánchez-Marteles M, Garcés-Horna V, Alcaine-Otín A, Gimenez-Lopez I, Rubio-Gracia J. How Does Left Ventricular Ejection Fraction Affect the Multimodal Assessment of Congestion in Patients with Acute Heart Failure? Results from a Prospective Study. Applied Sciences. 2025; 15(15):8157. https://doi.org/10.3390/app15158157
Chicago/Turabian StyleEsterellas-Sánchez, Laura Karla, Amelia Campos-Sáenz de Santamaría, Zoila Stany Albines Fiestas, Silvia Crespo-Aznarez, Marta Sánchez-Marteles, Vanesa Garcés-Horna, Alejandro Alcaine-Otín, Ignacio Gimenez-Lopez, and Jorge Rubio-Gracia. 2025. "How Does Left Ventricular Ejection Fraction Affect the Multimodal Assessment of Congestion in Patients with Acute Heart Failure? Results from a Prospective Study" Applied Sciences 15, no. 15: 8157. https://doi.org/10.3390/app15158157
APA StyleEsterellas-Sánchez, L. K., Campos-Sáenz de Santamaría, A., Albines Fiestas, Z. S., Crespo-Aznarez, S., Sánchez-Marteles, M., Garcés-Horna, V., Alcaine-Otín, A., Gimenez-Lopez, I., & Rubio-Gracia, J. (2025). How Does Left Ventricular Ejection Fraction Affect the Multimodal Assessment of Congestion in Patients with Acute Heart Failure? Results from a Prospective Study. Applied Sciences, 15(15), 8157. https://doi.org/10.3390/app15158157






