Photodegradation Assessment of Calcipotriol in the Presence of UV Absorbers by UHPLC/MSE
Abstract
1. Introduction
2. Experimental
2.1. Materials and Methods
Chemicals and Reagents
2.2. Standard Solution
2.3. Photodegradation Studies of Calcipotriol
2.4. UHPLC/MSE Analysis
2.5. Method Validation
2.5.1. Specificity
2.5.2. Linearity
2.5.3. Limit of Detection (LOD) and Limit of Quantification (LOQ)
2.5.4. Precision
2.5.5. Accuracy
2.5.6. Robustness
2.6. In Silico Toxicity Prediction
2.7. Molecular Modeling
2.8. Statistical Analysis
3. Results and Discussion
3.1. Method Validation
3.2. UV Irradiation of Calcipotriol
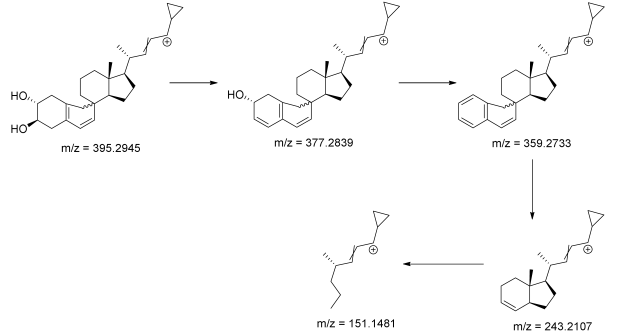
3.3. Identification of Degradation Photoproducts
3.4. In Silico Toxicity Predictions
3.5. Molecular Modeling
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rendon, A.; Schäkel, K. Psoriasis Pathogenesis and Treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef]
- Gisondi, P.; Gracia-Cazaña, T.; Kurzen, H.; Galván, J. Calcipotriol/Betamethasone Dipropionate for the Treatment of Psoriasis: Mechanism of Action and Evidence of Efficacy and Safety versus Topical Corticosteroids. J. Clin. Med. 2024, 13, 4484. [Google Scholar] [CrossRef]
- Fabbrocini, G.; De Simone, C.; Dapavo, P.; Malagoli, P.; Martella, A.; Calzavara-Pinton, P. Long-term maintenance treatment of psoriasis: The role of calcipotriol/betamethasone dipropionate aerosol foam in clinical practice. J. Dermatol. Treat. 2022, 33, 2425–2432. [Google Scholar] [CrossRef]
- Adamczyk, M.; Bartosinska, J.; Krasowska, D. Efficacy and safety of agents containing betamethasone dipropionate and calcipotriol in topical therapy of psoriasis. Przegl. Dermatol. 2018, 105, 434–450. [Google Scholar]
- Qasem, S.F.; Ashkanani, H.; Ali, A. Therapeutic Advancements in the Management of Psoriasis: A Clinical Overview and Update. Cureus 2025, 17, e79097. [Google Scholar] [CrossRef]
- Yan, B.X.; Chen, X.Y.; Ye, L.R.; Chen, J.Q.; Zheng, M.; Man, X.Y. Cutaneous and Systemic Psoriasis: Classifications and Classification for the Distinction. Front. Med. 2021, 8, 649408. [Google Scholar] [CrossRef]
- Uzelac, M. Psoriasis is More Than Skin Disease. AIDASCO Rev. 2024, 2, 2–3. [Google Scholar] [CrossRef]
- Douglas, W.S.; Poulin, Y.; Decroix, J.; Ortonne, J.P.; Mrowietz, U.; Gulliver, W.; Krogstad, A.L.; Larsen, F.G.; Iglesias, L.; Buckley, C.; et al. A New Calcipotriol/Betamethasone Formulation with Rapid Onset of Action was Superior to Monotherapy with Betamethasone Dipropionate or Calcipotriol in Psoriasis Vulgaris. Acta Derm. Venereol. 2002, 82, 131–135. [Google Scholar] [CrossRef]
- Norsgaard, H.; Kurdykowski, S.; Descargues, P.; Gonzalez, T.; Marstrand, T.; Dünstl, G.; Røpke, M. Calcipotriol counteracts betamethasone-induced decrease in extracellular matrix components related to skin atrophy. Arch. Dermatol. Res. 2014, 306, 719–729. [Google Scholar] [CrossRef]
- Lind, M.; Nielsen, K.T.; Schefe, L.H.; Nørremark, K.; Norsgaard, H.; Pedersen, B.T.; Petersson, K. Supersaturation of Calcipotriene and Betamethasone Dipropionate in a Novel Aerosol Foam Formulation for Topical Treatment of Psoriasis Provides Enhanced Bioavailability of the Active Ingredients. Dermatol. Ther. Heidelb. 2016, 6, 413–425. [Google Scholar] [CrossRef]
- Koo, J.; Tyring, S.; Werschler, W.P.; Bruce, S.; Olesen, M.; Villumsen, J.; Bagel, J. Superior efficacy of calcipotriene and betamethasone dipropionate aerosol foam versus ointment in patients with psoriasis vulgaris—A randomized phase II study. J. Dermatol. Treat. 2016, 27, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Kryczyk-Poprawa, A.; Kwiecień, A.; Opoka, W. Photostability of Topical Agents Applied to the Skin: A Review. Pharmaceutics 2019, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Baertschi, S.W.; Clapham, D.; Foti, C.; Kleinman, M.H.; Kristensen, S.; Reed, R.A.; Templeton, A.C.; Tønnesen, H.H. Implications of In-Use Photostability: Proposed Guidance for Photostability Testing and Labeling to Support the Administration of Photosensitive Pharmaceutical Products, Part 2: Topical Drug Product. J. Pharm. Sci. 2015, 104, 2688–2701. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, M.; Hecker, D.; Martinez, J.; Sapadin, A.; Patel, B. Interactions between calcipotriene and ultraviolet light. J. Am. Acad. Dermatol. 1997, 37, 93–95. [Google Scholar] [CrossRef]
- Jahani, M.; Akaberi, M.; Heidari, T.; Kamali, H.; Nejabat, M.; Rajabi, O.; Hadizadeh, F. Simultaneous determination of mometasone furoate and calcipotriol in a binary mixture by validated HPLC and chemometric-assisted UV spectrophotometric methods and identification of degradation products by LC-MS. Iran. J. Basic Med. Sci. 2023, 26, 37–47. [Google Scholar] [CrossRef]
- Bhogadi, R.K.; Satyanarayana, A.; Rao, N.S.; Arutla, S.; Reddy, A.M. Stability Indicating RP-HPLC Method for Estimation of Impurities of Vitamin D3 Analogue and Corticosteroid Used as Antipsoriatic Drugs. An Attempt to Characterize Pre-Calcipotriene. Am. J. Analyt. Chem. 2015, 6, 1050–1058. [Google Scholar] [CrossRef]
- Milczarek, M.; Filip-Psurska, B.; Martowicz, A.; Krupa, M.; Krajewski, K.; Kutner, A.; Wietrzyk, J. Synthesis and Biological Activity of Diastereomeric and Geometric Analogs of Calcipotriol, PRI-2202 and PRI-2205, Against Human HL-60 Leukemia and MCF-7 Breast Cancer Cells. Cancers 2013, 5, 1355–1378. [Google Scholar] [CrossRef]
- Kutner, A.; Chodyński, M.; Ryznar, T.; Fitak, H.; Winiarski, J.; Górecki, B.; Burzyńska, A.; Szelejewski, W. Process for Preparation of Pharmaceutically Pure Anhydrous Calcipotriol. U.S. Patent US7700580B2, 20 April 2010. [Google Scholar]
- Grodner, B.; Żołek, T.; Kutner, A. Nonaqueous Capillary Electrophoretic Separation of Analogs of (24R)-1,24-Dihydroxyvitamin D3 Derivative as Predicted by Quantum Chemical Calculations. Molecules 2023, 28, 5055. [Google Scholar] [CrossRef]
- SynZeal Research. Calcipotriol Beta Isomer. Available online: https://www.synzeal.com/en/calcipotriol-beta-isomer (accessed on 27 May 2025).
- Kryczyk-Poprawa, A.; Zupkó, I.; Bérdi, P.; Żmudzki, P.; Piotrowska, J.; Pękala, E.; Berdys, A.; Muszyńska, B.; Opok, W. Photodegradation of Bexarotene and Its Implication for Cytotoxicity. Pharmaceutics 2021, 13, 1220. [Google Scholar] [CrossRef]
- Kryczyk-Poprawa, A.; Zupkó, I.; Bérdi, P.; Żmudzki, P.; Popiół, J.; Muszyńska, B.; Opoka, W. Photostability Testing of a Third-Generation Retinoid-Tazarotene in the Presence of UV Absorbers. Pharmaceutics 2020, 12, 899. [Google Scholar] [CrossRef]
- Kryczyk-Poprawa, A.; Żmudzki, P.; Koczurkiewicz, P.; Pękala, E.; Hubicka, U. Photostability of Terbinafine Under UVA Irradiation: The Effect of UV Absorbers. Photochem. Photobiol. 2019, 95, 911–923. [Google Scholar] [CrossRef]
- Kryczyk, A.; Żmudzki, P.; Hubicka, U. Determination of bifonazole and identification of its photocatalytic degradation products using UPLC-MS/MS. Biomed. Chromatogr. 2017, 31, 3955. [Google Scholar] [CrossRef]
- Nitulescu, G.; Lupuliasa, D.; Adam-Dima, I.; Nitulescu, G.M. Ultraviolet Filters for Cosmetic Applications. Cosmetics 2023, 10, 101. [Google Scholar] [CrossRef]
- Jesus, A.; Sousa, E.; Cruz, M.T.; Cidade, H.; Lobo, J.M.S.; Almeida, I.F. UV Filters: Challenges and Prospects. Pharmaceuticals 2022, 15, 263. [Google Scholar] [CrossRef] [PubMed]
- Manasfi, T.; Coulomb, B.; Ravier, S.; Boudenne, J.L. Degradation of Organic UV filters in Chlorinated Seawater Swimming Pools: Transformation Pathways and Bromoform Formation. Environ. Sci. Technol. 2017, 51, 13580–13591. [Google Scholar] [CrossRef] [PubMed]
- ICH-Q2 (R2): Guideline on validation of analytical procedures. In Proceedings of the International Conference on Harmonization, Geneva, Switzerland, 14 June 2024; Available online: https://www.ema.europa.eu/en/ich-q2r2-validation-analytical-procedures-scientific-guideline (accessed on 25 April 2025).
- Sander, T. Osiris Property Explorer. Available online: https://www.organic-chemistry.org/prog/peo/ (accessed on 30 May 2025).
- Tocchini-Valentini, G.; Rochel, N.; Wurtz, J.M.; Moras, D. Crystal Structures of the Vitamin D Nuclear Receptor Liganded with the Vitamin D Side Chain Analogues Calcipotriol and Seocalcitol, Receptor Agonists of Clinical Importance. Insights into a Structural Basis for the Switching of Calcipotriol to a Receptor Antagonist by Further Side Chain Modification. J. Med. Chem. 2004, 47, 1956–1961. [Google Scholar] [CrossRef]
- ICH: Q 1 B: Photostability Testing of New Active Substances and Medicinal Products. Available online: https://www.ema.europa.eu/en/ich-q1b-photostability-testing-new-active-substances-medicinal-products-scientific-guideline (accessed on 13 April 2025).
- Venkatraman, R.K.; Orr-Ewing, A.J. Photochemistry of Benzophenone in Solution: A Tale of Two Different Solvent Environments. J. Am. Chem. Soc. 2019, 141, 15222–15229. [Google Scholar] [CrossRef]
- Walling, C.; Gibian, M.J. Hydrogen Abstraction Reactions by the Triplet States of Ketones. J. Am. Chem. Soc. 1965, 87, 3413–3417. [Google Scholar] [CrossRef]
- Singh, A.K.; Palit, D.K.; Mukherjee, T. Triplet excited states and radical intermediates formed in electron pulse radiolysis of amino and dimethylamino derivatives of benzophenone. J. Phys. Chem. A 2002, 106, 6084–6093. [Google Scholar] [CrossRef]
- Cirunay, J.J.; Vander Heyden, Y.; Plaizier-Vercammen, J. LC separation of calcipotriol from its photodegradation products and protection possibilities using adjuvants. J. Pharm. Biomed. Anal. 2001, 26, 31–41. [Google Scholar] [CrossRef]
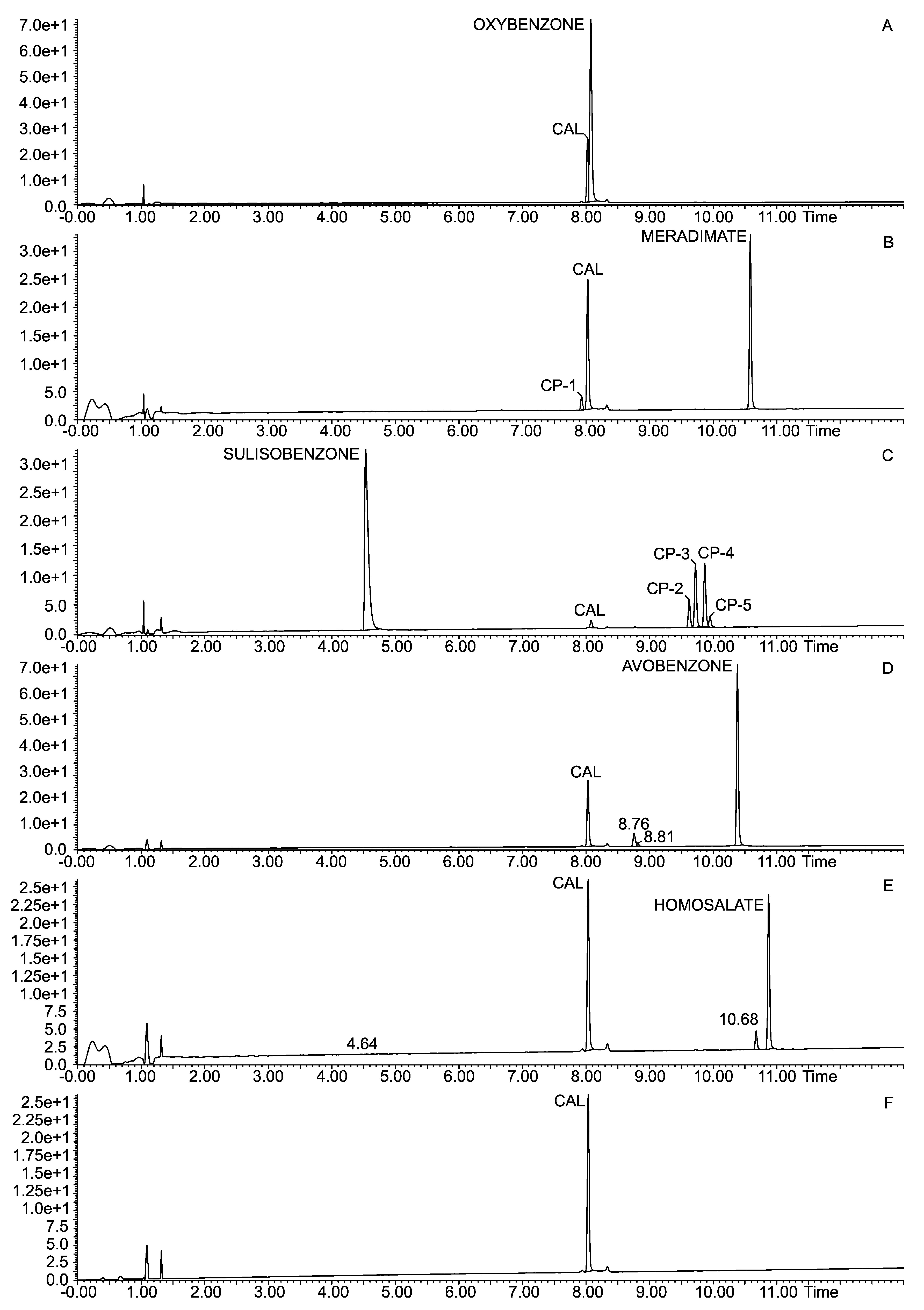

| Parameter | Calcipotriol |
|---|---|
| Retention time (min) a | 8.04 |
| Limit of detection (μg mL−1) | 7.39 |
| Limit of quantitation (μg mL−1) | 50 |
| Linear range (μg mL−1) | 15–150 |
| Regression equation (y): b | |
| Slope (a ± Sa) | 83,451 ± 955 |
| Intercept (b ± Sb) | −86,683 ± 79,389 |
| t = b/Sb c | 1.09188 < tα,f statistically insignificant |
| Normality of residuals d (Shapiro–Wilk test) b | 0.92444 (p = 0.1549) |
| Correlation coefficient | 0.999 |
| R2 value | 0.9979 |
| Recovery level 75% | 99.17 |
| Recovery level 100% | 101.55 |
| Recovery level 150% | 99.82 |
| Precision (CV) | 2.41 |
| Compound | RT [min] | [M+H]+ | Fragmentation Ions | Structure |
|---|---|---|---|---|
| calcipotriol | 7.93 | 395.2945 * | 151.1481, 243.2107, 359.2733, 377.2839 | 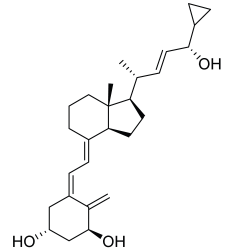 |
| CP-1 | 8.08 | 395.2945 * | 151.1481, 243.2107, 359.2733, 377.2839 |  |
| CP-2 | 9.62 | 395.2945 * | 151.1481, 243.2107, 359.2733, 377.2839 | 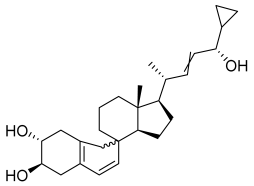 |
| CP-3 | 9.72 | |||
| CP-4 | 9.87 | |||
| CP-5 | 9.95 | |||
| Calcipotriol |
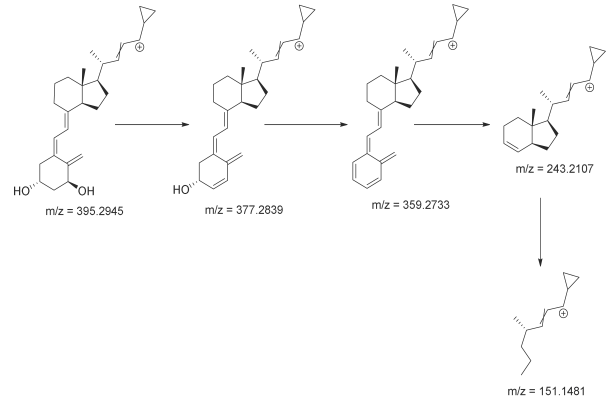 |
| CP-2—CP-5 |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Król, M.; Żmudzki, P.; Bucki, A.; Kryczyk-Poprawa, A. Photodegradation Assessment of Calcipotriol in the Presence of UV Absorbers by UHPLC/MSE. Appl. Sci. 2025, 15, 8124. https://doi.org/10.3390/app15158124
Król M, Żmudzki P, Bucki A, Kryczyk-Poprawa A. Photodegradation Assessment of Calcipotriol in the Presence of UV Absorbers by UHPLC/MSE. Applied Sciences. 2025; 15(15):8124. https://doi.org/10.3390/app15158124
Chicago/Turabian StyleKról, Małgorzata, Paweł Żmudzki, Adam Bucki, and Agata Kryczyk-Poprawa. 2025. "Photodegradation Assessment of Calcipotriol in the Presence of UV Absorbers by UHPLC/MSE" Applied Sciences 15, no. 15: 8124. https://doi.org/10.3390/app15158124
APA StyleKról, M., Żmudzki, P., Bucki, A., & Kryczyk-Poprawa, A. (2025). Photodegradation Assessment of Calcipotriol in the Presence of UV Absorbers by UHPLC/MSE. Applied Sciences, 15(15), 8124. https://doi.org/10.3390/app15158124






