A Hierarchically Structured Ni-NOF@ZIF-L Heterojunction Using Van Der Waals Interactions for Electrocatalytic Reduction of CO2 to HCOOH
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Samples
2.2. Materials Characterization
2.3. Electrochemical Measurements
2.4. Product Analysis
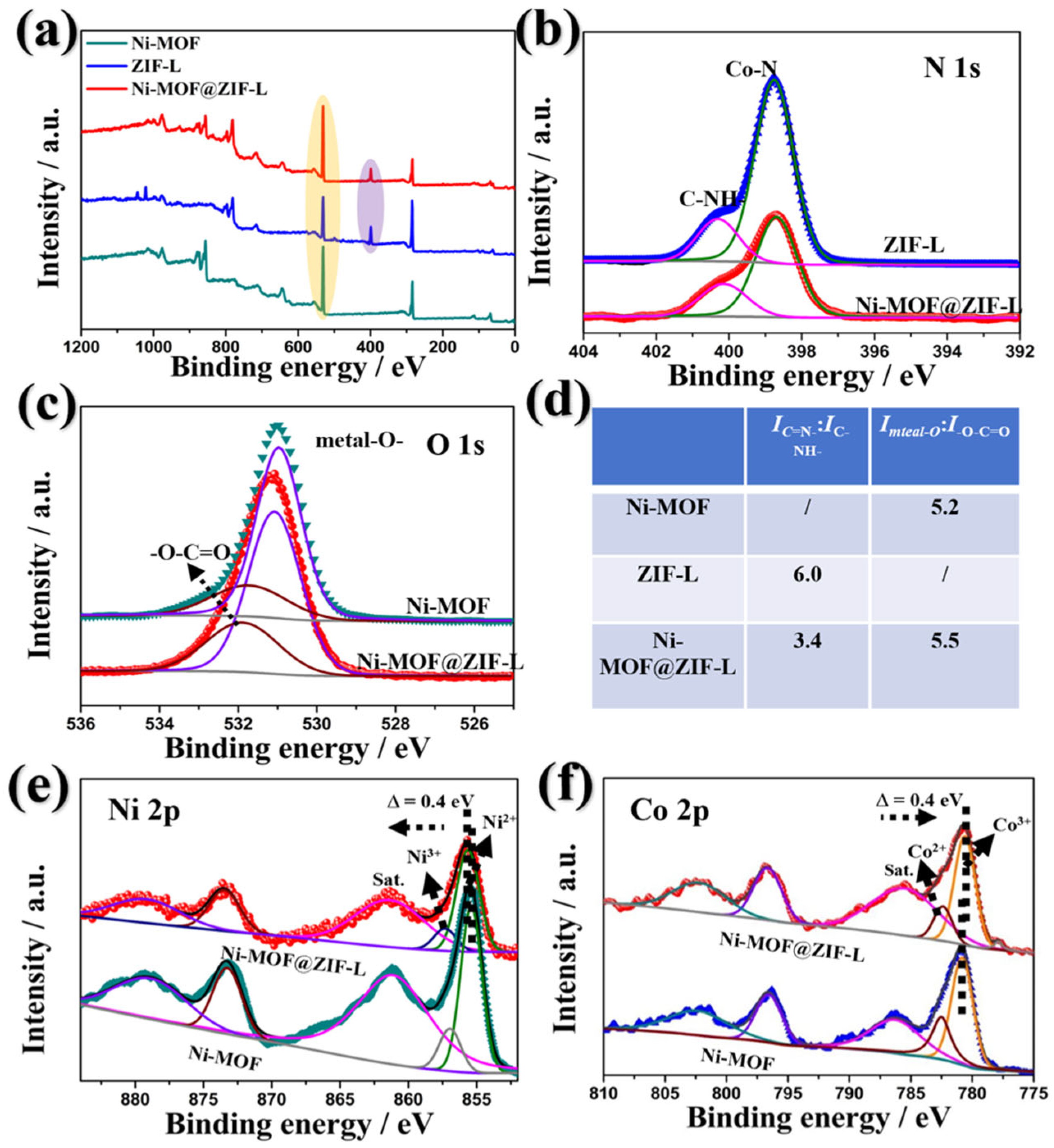
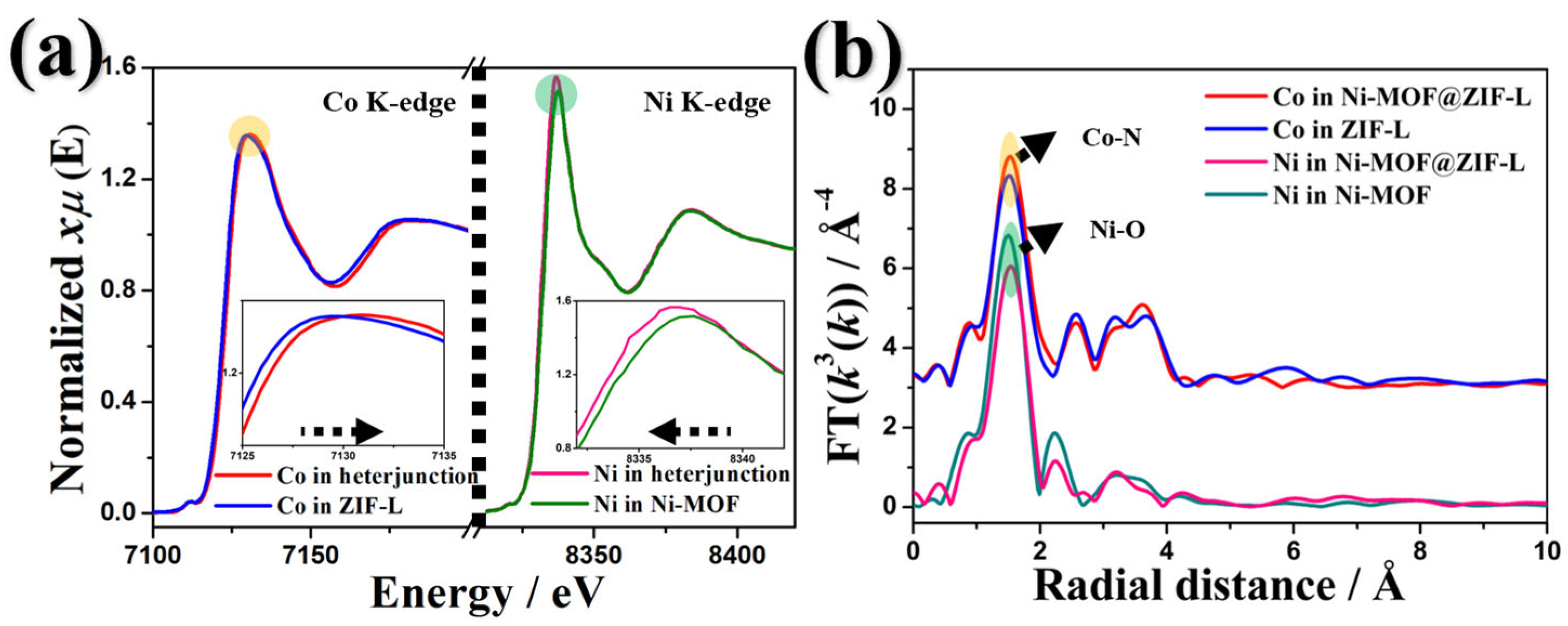
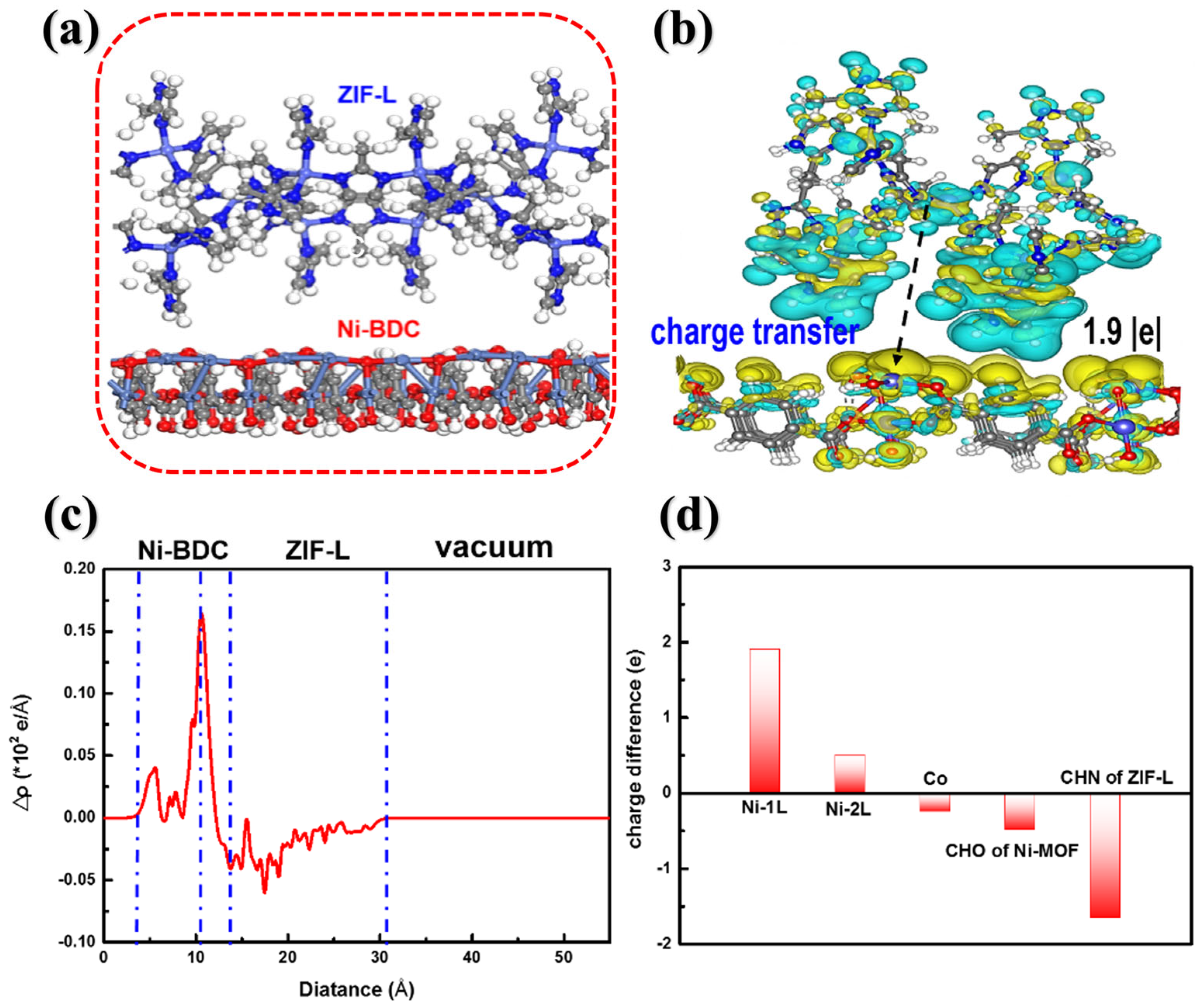
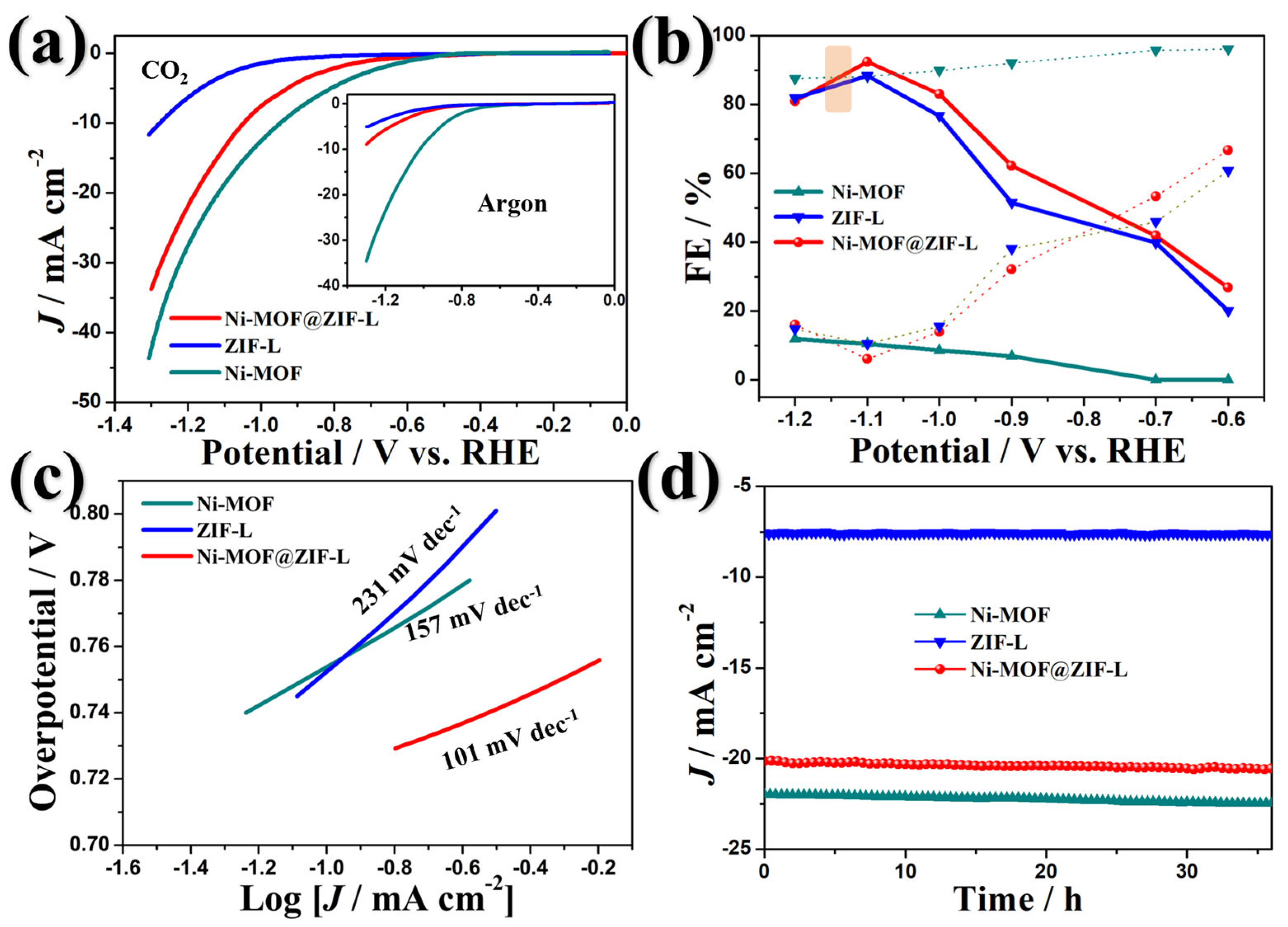
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Keith, D.W.; Holmes, G.; St Angelo, D.; Heidel, K. Carbon capture and storage: A review. Environ. Sci. Technol. 2021, 55, 7660–7670. [Google Scholar]
- Friedlingstein, P.; Jones, M.W.; O’Sullivan, M.; Andrew, R.M.; Bakker, D.C.E.; Hauck, J.; Le Quéré, C.; Peters, G.P.; Peters, W.; Pongratz, J.; et al. Global carbon budget 2021. Nat. Rev. Earth Environ. 2022, 14, 1917–2005. [Google Scholar] [CrossRef]
- Santos, A.; Delgado, J.D.; Figueroa, J.D.; Pérez, A. CO2 reduction using Ni-MOF: A comparative study. Chem. Eng. J. 2023, 435, 1397–1412. [Google Scholar]
- Kortlever, R.; Shen, J.; Schouten, K.J.P.; Calle-Vallejo, F.; Koper, M.T.M. Electrocatalytic CO2 reduction at metal surfaces. J. Phys. Chem. Lett. 2015, 6, 4073–4082. [Google Scholar] [CrossRef]
- Cui, Y.; Cheng, Y.; Yang, C.; Su, Y.; Yao, D.; Liufu, B.; Li, J.; Fang, Y.; Liu, S.; Zhong, Z.; et al. High-performance electrocatalytic CO2 reduction. ACS Sustainable Chem. Eng. 2023, 11, 11229–11238. [Google Scholar] [CrossRef]
- Hod, I.; Sampson, M.D.; Deria, P.; Kubiak, C.P.; Farha, O.K.; Hupp, J.T. CO2 electroreduction in MOF-based electrocatalysts. ACS Catal. 2015, 5, 6302–6309. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, Z.J.; Gong, J. CO2 electroreduction: Mechanisms and materials. Angew. Chem. Int. Ed. 2017, 56, 11326–11353. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, Z.J.; Chen, S.; Liu, F.; Zhuang, T.T.; Sun, Q.; Zhang, J.; Chen, W.; Huang, Y.; Li, Y.; et al. Recent advances in CO2 electroreduction. Chem. Soc. Rev. 2021, 50, 2614–2631. [Google Scholar]
- Zhang, L.; Wang, J.; Xu, Z.; Liu, H.; Li, Y.; Zhang, J. Recent progress in CO2 reduction electrocatalysts. ACS Energy Lett. 2022, 7, 3225–3236. [Google Scholar]
- Wang, Y.; Zhao, L.; Wu, X.; Zhang, X.; Wei, W.; Sun, W. High-performance CO2 reduction catalysts. Catal. Sci. Technol. 2022, 12, 1234–1245. [Google Scholar]
- Yang, H.; Wang, X.; Hu, Q.; Chai, X.; Ren, X.; Zhang, Q.; Li, Y.; Liu, Z. New insights into CO2 electroreduction. Small Methods 2020, 4, 1900826. [Google Scholar]
- Xu, Z.; Li, H.; Zhang, X.; Liu, Z.; Chen, W. Gas diffusion in catalyst layer for CO2 electroreduction. Nano Res. 2023, 16, 3029–3038. [Google Scholar]
- Zhu, H.J.; Lu, M.; Wang, Y.R.; Yao, S.J.; Zhang, M.; Kan, Y.H.; Wang, X.F.; Yan, L.K.; Liu, Q.Y.; Li, S.L.; et al. CO2 electroreduction over metal-based catalysts. Nat. Commun. 2020, 11, 497. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chu, J.; Yin, Z.; Cai, X.; Zhuang, L.; Deng, H. Advances in CO2 reduction catalysts. Chem 2018, 4, 1696–1709. [Google Scholar] [CrossRef]
- Melchionna, P.; Fornasiero, P.; Finocchio, L.; Maggio, F.; Carboni, M.G.; Bolognese, R.B. CO2 reduction on metal catalysts. ACS Catal. 2022, 12, 5678–5686. [Google Scholar]
- Liu, M.; Pang, Y.; Zhang, B.; Luna, P.D.; Voznyy, O.; Xu, J.; Li, Z.H.; Nam, D.; Kim, Y.S.; Xu, T.; et al. CO2 electroreduction on Cu-based catalysts. Nature 2016, 537, 382–388. [Google Scholar] [CrossRef]
- Zhang, E.; Wang, T.; Yu, K.; Liu, J.; Chen, W.; Li, A.; Li, J.; Zhuang, X.; Chen, J.G.; Huang, Y.; et al. CO2 electrolysis over Cu-based catalysts. J. Am. Chem. Soc. 2019, 141, 16569–16573. [Google Scholar] [CrossRef]
- Feng, D.; Wang, K.; Zhang, Y.; Yang, Y.; Li, Y. Review of CO2 electroreduction: Challenges and opportunities. Chem. Soc. Rev. 2021, 50, 2614–2631. [Google Scholar]
- Zhao, Z.J.; Liu, Z.; Zhang, Y.; Ma, S.; Zhang, J. CO2 electroreduction: Catalysis and reaction pathways. Chem. Rev. 2022, 122, 11721–11744. [Google Scholar]
- Hou, S.Z.; Zhang, X.D.; Yuan, W.W.; Li, Y.X.; Gu, Z.Y. Indium-based metal–organic framework for high-performance electroreduction of CO2 to formate. Inorg. Chem. 2020, 59, 11298–11304. [Google Scholar] [CrossRef]
- Yang, R.; Huang, Q.; Sha, X.; Gao, B.; Peng, J. Regulation of Bimetallic Coordination Centers in MOF Catalyst for Electrochemical CO2 Reduction to Formate. Int. J. Mol. Sci. 2023, 24, 13838. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shen, D.; Zhang, Q.; Lin, Y.; Peng, F. Electrochemical CO2 reduction using Ni-based catalysts. Appl. Catal. B: Environ. 2021, 283, 119630. [Google Scholar]
- Xing, Z.; Hu, L.; Ripatti, D.S.; Hu, X.; Feng, X. Bimetallic catalysts for CO2 reduction. Nat. Commun. 2021, 21, 136. [Google Scholar] [CrossRef]
- Shi, R.; Guo, J.; Zhang, X.; Waterhouse, G.I.N.; Han, Z.; Zhao, Y.; Shang, L.; Zhou, C.; Jiang, L.; Zhang, T. Efficient wettability-controlled electroreduction of CO2 to CO at Au/C interfaces. Nat. Commun. 2020, 11, 3028. [Google Scholar] [CrossRef]
- Pan, F.; Yang, Y. Electrocatalysts for CO2 reduction. Energy Environ. Sci. 2020, 13, 2275–2309. [Google Scholar] [CrossRef]
- Carton, A.; Mesbah, A.; Mazet, T.; Porcher, F.; François, M. Ab initio crystal structure of nickel(II) hydroxy-terephthalate by synchrotron powder diffraction and magnetic study. Solid State Sci. 2007, 9, 465–471. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Li, Y. Electrocatalysts for CO2 reduction: Materials and mechanisms. Chem. Commun. 2020, 49, 7379–7421. [Google Scholar]
- Wang, X.; Li, Y.; Zhang, L. CO2 electroreduction catalysts and mechanisms. J. Am. Chem. Soc. 2021, 143, 1203–1211. [Google Scholar]
- Li, Y.; Zhang, L.; Wang, X. Recent advancements in CO2 electroreduction. ACS Catal. 2022, 12, 3341–3360. [Google Scholar]
- Curcio, M.; Vittorio, O.; Cirillo, G.; De Luca, C.; Morandi, M.; Carfagna, A.L.; Curcio, M.; Puoci, F.; Rossi, F. Catalytic CO2 reduction. Molecules. 2021, 26, 4194. [Google Scholar]
- Low, J.; Teo, T.Y.K.; Ghosh, S.K.; Cai, J.; Zhang, R.; Wei, W.; Sun, Y.; Yang, Q.; Niu, Z.; Huang, Y.; et al. CO2 electroreduction catalysts. ACS Appl. Mater. Interfaces 2021, 13, 17847–17858. [Google Scholar]
- Li, H.; Zhang, Y.; Wang, X.; Zhou, X.; Jiang, X. CO2 electroreduction on Ni-based catalysts. J. Am. Chem. Soc. 2022, 144, 342–349. [Google Scholar]
- Xu, J.; Liu, T.; Zhou, Y.; Zhang, Q.; Li, Z. Advancements in CO2 electroreduction electrocatalysts. Adv. Mater. 2023, 35, 2204925. [Google Scholar]
- Taialla, O.A.; Mustapha, U.; Abdullahi, A.H.S.; Kotob, E.; Awad, M.M.; Alhassan, A.M.; Hussain, I.; Omer, K.; Ganiyu, S.A.; Alhooshani, K. Unlocking the potential of ZIF-based electrocatalysts for electrochemical reduction of CO2: Recent advances, current trends, and machine learnings. Coord. Chem. Rev. 2024, 504, 215669. [Google Scholar]
- Feng, D.; Wang, K.; Zhang, Y.; Yang, Y.; Li, Y. Systematic screening of gas diffusion layers for CO2 electroreduction. Commun. Chem. 2023, 6, 108. [Google Scholar]
- Li, J.; Wang, L.; Zhang, T.; Liu, J.; Sun, Y. Electrolyte flooding in flexible gas diffusion electrodes for CO2 electrolysis. J. Mater. Chem. A 2024, 12, 420–429. [Google Scholar]
- Zhao, Y.; Li, Z.; Liu, H.; Zhang, L.; Wang, H. Bimetallic zeolitic imidazole frameworks for CO2 electroreduction. J. Phys. Chem. C 2023, 127, 6789–6797. [Google Scholar]
- Li, F.; Thevenon, A.; Rosas-Hernández, A.; Wang, Z.; Li, Y.; Gabardo, C.M.; Ozden, A.; Dinh, C.T.; Li, J.; Wang, Y.; et al. Molecular tuning of CO2-to-ethylene conversion. Science 2021, 372, 6524. [Google Scholar] [CrossRef]
- Henkelman, G.; Arnaldsson, A.; Jónsson, H. A Fast and Robust Algorithm for Bader Decomposition of Charge Density. Comput. Mater. Sci. 2006, 36, 254–258. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Xu, Z.L.; Zhang, J.P.; Zheng, H.X. CO2 electroreduction using new catalysts. J. Am. Chem. Soc. 2017, 139, 8966–8972. [Google Scholar]
- Withers, A.J.C.; Chen, P.J.S.; Chen, L.M.M.; Liu, Y.F.Y. Nature materials for CO2 electroreduction. Nat. Mater. 2014, 13, 233–240. [Google Scholar]
- Li, Y.; Liu, Y.; Yang, X.; Wang, X.; Zhang, J.; Zhang, S.; Zhang, Q.; Gu, L.; Song, Y.; Xiao, J.; et al. Small materials for CO2 electroreduction. Small 2019, 15, 1804567. [Google Scholar]
- Motevalli, B.M.; Momeni, M.; Habibi-Yangjeh, A. CO2 electroreduction on metal catalysts. J. Phys. Chem. C 2017, 121, 25174–25181. [Google Scholar]
- Lee, S.H.; Kim, D.W.; Shin, H.C.; Joo, Y.S. Electrochemical CO2 reduction: New insights. J. Mater. Chem. A 2016, 4, 1559–1567. [Google Scholar]
- Zhang, Z.; Liu, L.; Li, Y.; Wang, X.; Zhang, S. New CO2 electroreduction catalysts. Small 2019, 15, 1900826. [Google Scholar]
- Qi, Y.; Yang, X.; Zhao, X.; Zhang, L.; Zhang, X.; Sun, X.; Sun, Y.; Xie, Y.; Xiong, Y. Electroreduction of CO2: Materials and mechanisms. Nat. Catal. 2020, 3, 383–390. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; He, X.; Zhou, J. A Hierarchically Structured Ni-NOF@ZIF-L Heterojunction Using Van Der Waals Interactions for Electrocatalytic Reduction of CO2 to HCOOH. Appl. Sci. 2025, 15, 8095. https://doi.org/10.3390/app15148095
Wu L, He X, Zhou J. A Hierarchically Structured Ni-NOF@ZIF-L Heterojunction Using Van Der Waals Interactions for Electrocatalytic Reduction of CO2 to HCOOH. Applied Sciences. 2025; 15(14):8095. https://doi.org/10.3390/app15148095
Chicago/Turabian StyleWu, Liqun, Xiaojun He, and Jian Zhou. 2025. "A Hierarchically Structured Ni-NOF@ZIF-L Heterojunction Using Van Der Waals Interactions for Electrocatalytic Reduction of CO2 to HCOOH" Applied Sciences 15, no. 14: 8095. https://doi.org/10.3390/app15148095
APA StyleWu, L., He, X., & Zhou, J. (2025). A Hierarchically Structured Ni-NOF@ZIF-L Heterojunction Using Van Der Waals Interactions for Electrocatalytic Reduction of CO2 to HCOOH. Applied Sciences, 15(14), 8095. https://doi.org/10.3390/app15148095





