The Effect of Cs-Controlled Triple-Cation Perovskite on Improving the Sensing Performance of Deep-Ultraviolet Photodetectors
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
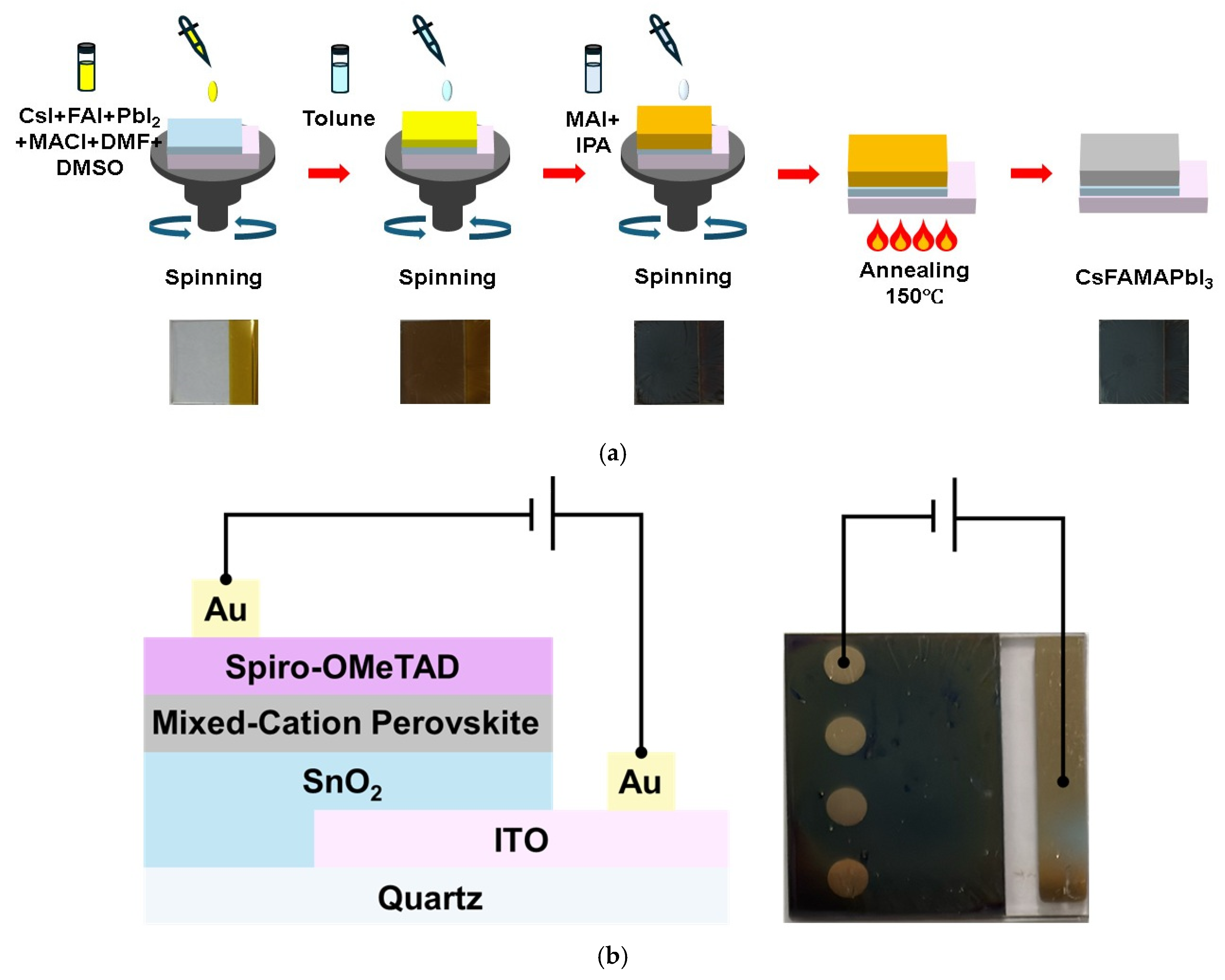
2.2. Sample Fabrication Process
2.2.1. Substrate Preparation
2.2.2. SnO2 Deposition
2.2.3. Perovskite Film Deposition
2.2.4. Hole Transport Layer and Electrode Deposition
2.3. Sample Measurements
2.3.1. Structural and Morphological Analysis
2.3.2. Optical Characterization+
2.3.3. Electrical and PD Performance
3. Results and Discussion
3.1. Characterization of Triple-Cation Perovskite Thin Films
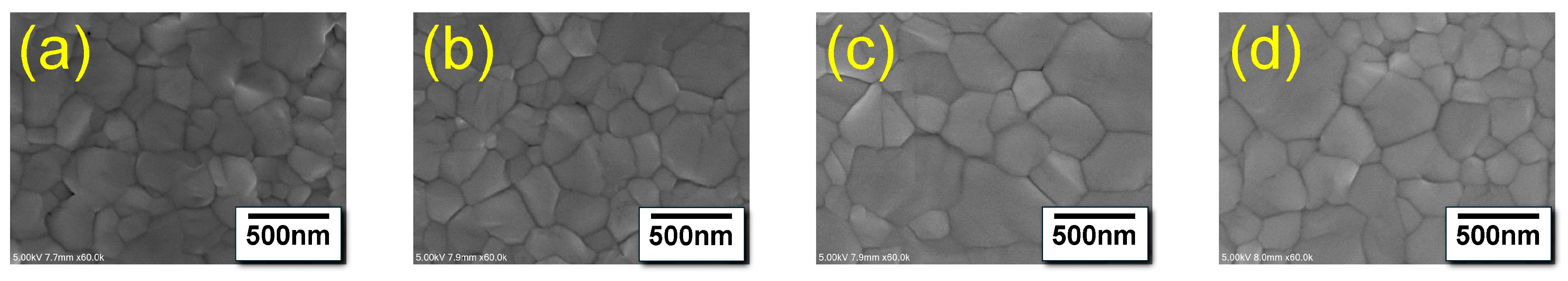
3.1.1. Structural Analysis of Triple-Cation Perovskite Thin Films
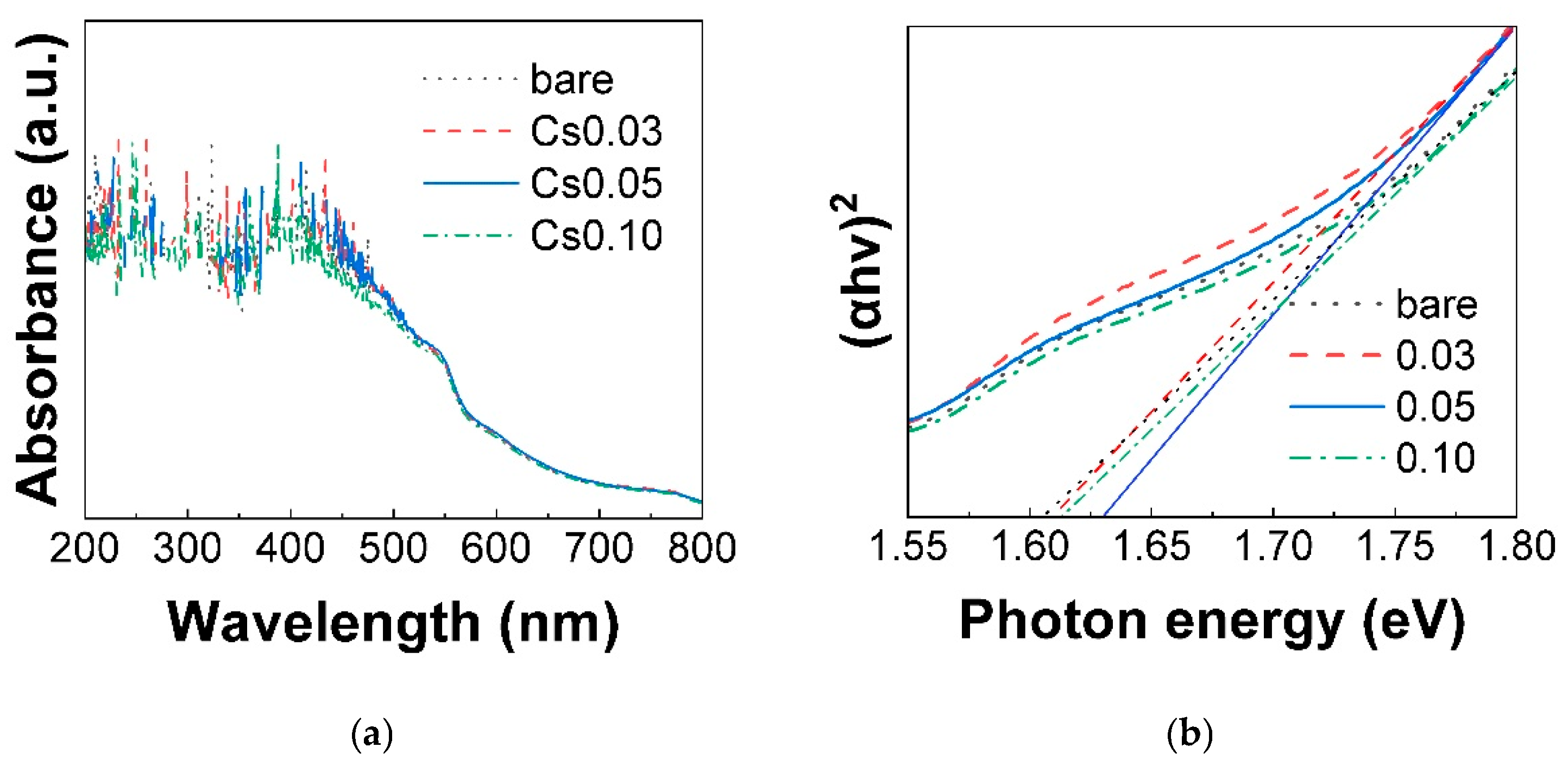
3.1.2. Optical Properties of Mixed-Cation Perovskite Thin Films
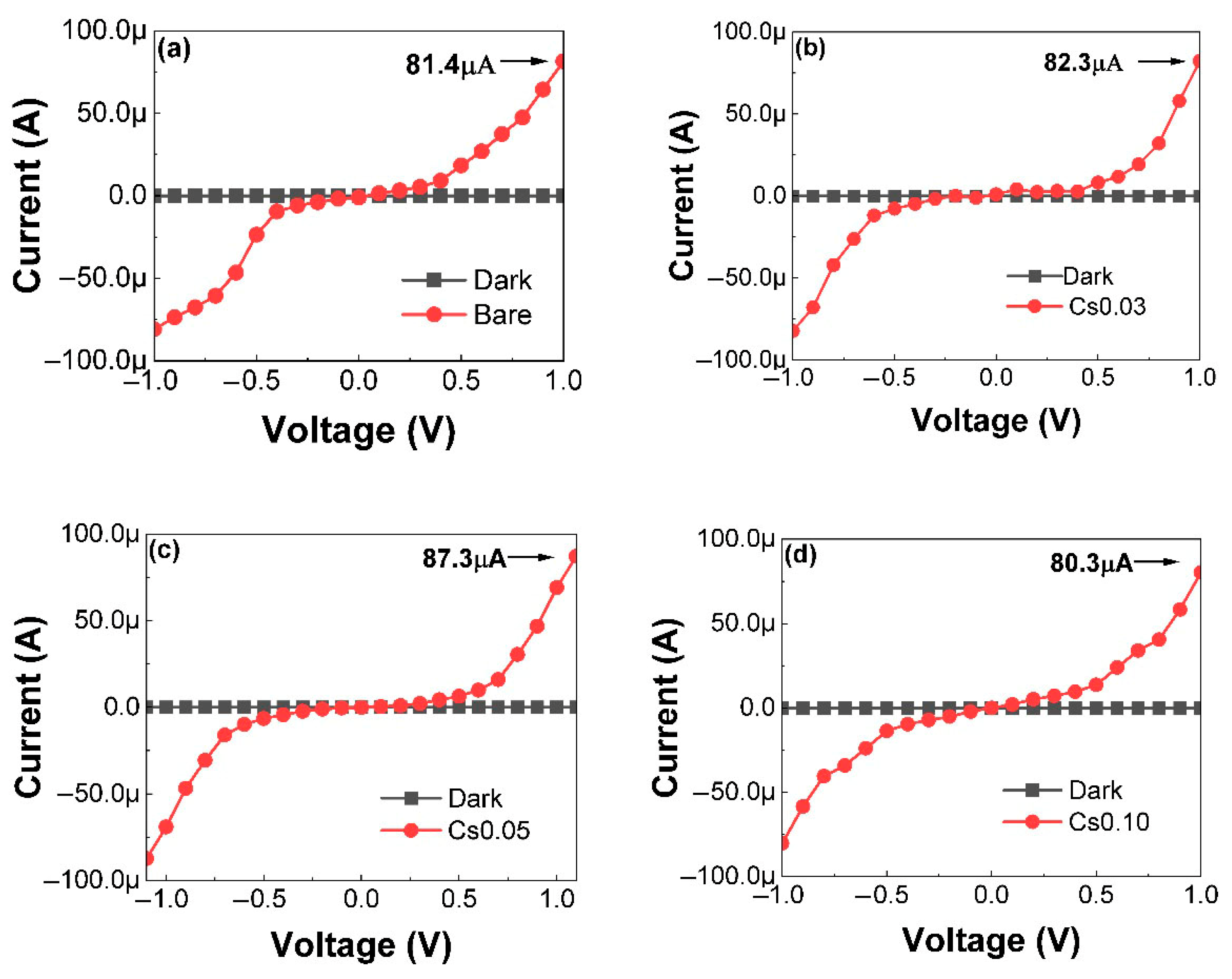
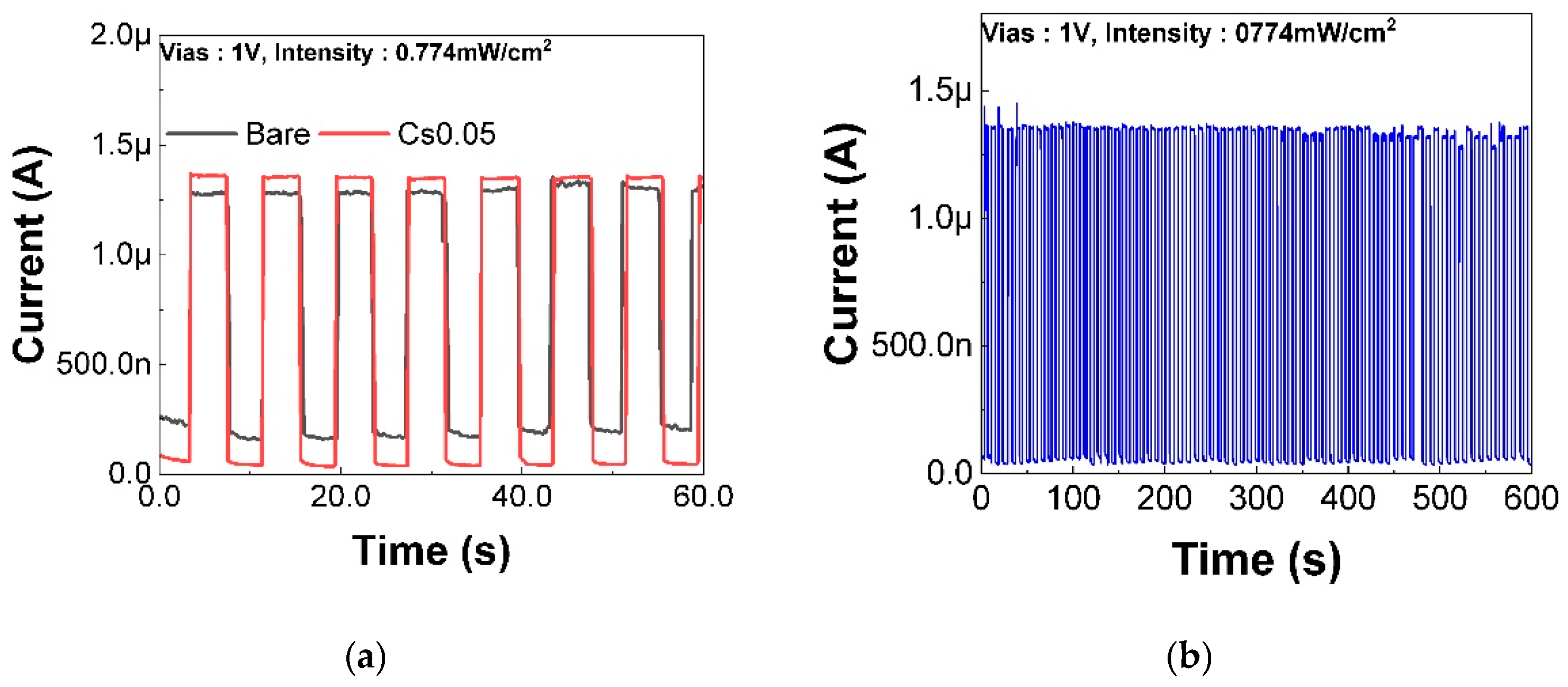
3.1.3. Performance Evaluation of Sensors with Triple-Cation Perovskite Structure
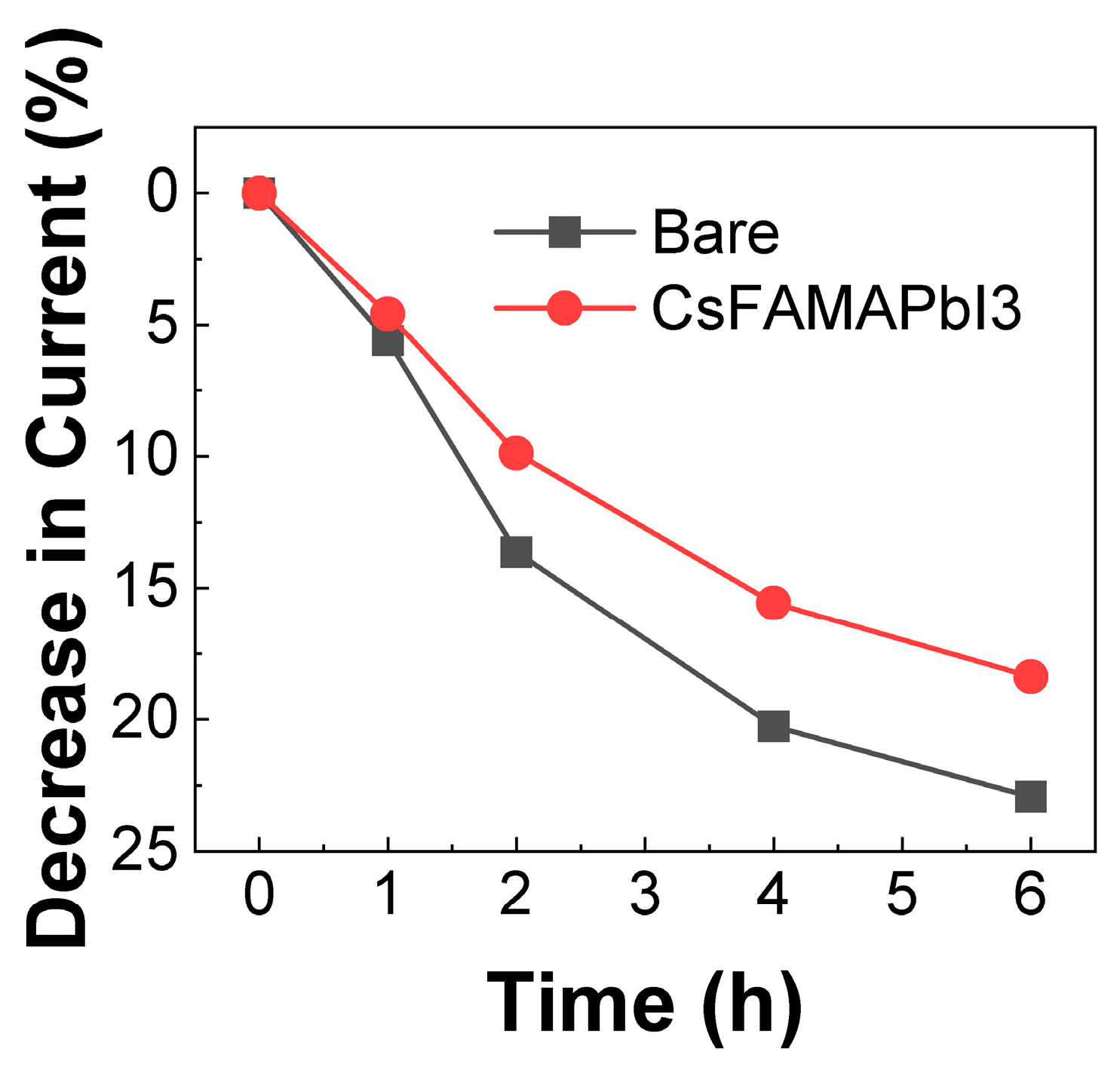
3.2. Stability of Sensors with Triple-Structure Perovskite
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qadir, A.; Shafique, S.; Iqbal, T.; Ali, H.; Xin, L.; Ruibing, S.; Shi, T.; Xu, H.; Wang, Y.; Hu, Z. Recent advancements in polymer-based photodetector: A comprehensive review. Sens. Actuators A Phys. 2024, 370, 115267. [Google Scholar] [CrossRef]
- Miao, J.; Zhang, F. Recent progress on highly sensitive perovskite photodetectors. J. Mater. Chem. C 2019, 7, 1741–1791. [Google Scholar] [CrossRef]
- Rehman, W.; McMeekin, D.P.; Patel, J.B.; Milot, R.L.; Johnston, M.B.; Snaith, H.J.; Herz, L.M. Photovoltaic mixed-cation lead mixed-halide perovskites: Links between crystallinity, photo-stability and electronic properties. Energy Environ. Sci. 2017, 10, 361–369. [Google Scholar] [CrossRef]
- Razeghi, M.; Rogalski, A. Semiconductor ultraviolet detectors. J. Appl. Phys. 1996, 79, 7433–7473. [Google Scholar] [CrossRef]
- Jansen-van Vuuren, R.D.; Armin, A.; Pandey, A.K.; Burn, P.L.; Meredith, P. Organic photodiodes: The future of full color detection and image sensing. Adv. Mater. 2016, 28, 4766–4802. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Zhou, H.; Li, L. Hybrid organic–inorganic perovskite photodetectors. Small 2017, 13, 1702107. [Google Scholar] [CrossRef]
- Sang, L.; Liao, M.; Sumiya, M. A comprehensive review of semiconductor ultraviolet photodetectors: From thin film to one-dimensional nanostructures. Sensors 2013, 13, 10482–10518. [Google Scholar] [CrossRef]
- Zhao, Z.; Xu, C.; Niu, L.; Zhang, X.; Zhang, F. Recent progress on broadband organic photodetectors and their applications. Laser Photonics Rev. 2020, 14, 2000262. [Google Scholar] [CrossRef]
- Žigulić, N.; Glučina, M.; Lorencin, I.; Matika, D. Military decision-making process enhanced by image detection. Information 2023, 15, 11. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A.; Singh, R.P.; Rab, S.; Suman, R. Significance of sensors for industry 4.0: Roles, capabilities, and applications. Sens. Int. 2021, 2, 100110. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, J.; Feng, B.; Song, H.; Shen, L. Recent Progress in Organic Photodetectors and their Applications. Adv. Sci. 2020, 7, 1902585. [Google Scholar] [CrossRef]
- Wang, H.; Sun, Y.; Chen, J.; Wang, F.; Han, R.; Zhang, C.; Kong, J.; Li, L.; Yang, J. A review of perovskite-based photodetectors and their applications. Nanomaterials 2022, 12, 4390. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Seol, D.J.; Cho, A.N.; Park, N.G. High-efficiency perovskite solar cells based on the black polymorph of HC (NH2)2PbI3. Adv. Mater. 2014, 26, 4991–4998. [Google Scholar] [CrossRef] [PubMed]
- Stoumpos, C.C.; Malliakas, C.D.; Kanatzidis, M.G. Semiconducting tin and lead iodide perovskites with organic cations: Phase transitions, high mobilities, and near-infrared photoluminescent properties. Inorg. Chem. 2013, 52, 9019–9038. [Google Scholar] [CrossRef] [PubMed]
- Jeon, N.J.; Noh, J.H.; Yang, W.S.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. Compositional engineering of perovskite materials for high-performance solar cells. Nature 2015, 517, 476–480. [Google Scholar] [CrossRef]
- Pellet, N.; Gao, P.; Gregori, G.; Yang, T.Y.; Nazeeruddin, M.K.; Maier, J.; Grätzel, M. Mixed-organic-cation Perovskite photovoltaics for enhanced solar-light harvesting. Angew. Chem. Int. Ed. 2014, 53, 3151–3157. [Google Scholar] [CrossRef] [PubMed]
- Saliba, M.; Matsui, T.; Domanski, K.; Seo, J.Y.; Ummadisingu, A.; Zakeeruddin, S.M.; Correa-Baena, J.-P.; Tress, W.R.; Abate, A.; Hagfeldt, A.; et al. Incorporation of rubidium cations into perovskite solar cells improves photovoltaic performance. Science 2016, 354, 206–209. [Google Scholar] [CrossRef]
- Chen, H. Two-Step Sequential Deposition of Organometal Halide Perovskite for Photovoltaic Application. Adv. Funct. Mater. 2017, 27, 1605654. [Google Scholar] [CrossRef]
- Kang, D.H.; Kim, S.Y.; Lee, J.W.; Park, N.G. Efficient surface passivation of perovskite films by a post-treatment method with a minimal dose. J. Mater. Chem. A 2021, 9, 3441–3450. [Google Scholar] [CrossRef]
- Kong, T.; Kim, Y.; Cho, J.; Choi, H.; Zhang, Y.; Ahn, H.; Woo, J.; Kim, D.; Lee, J.; Sirringhaus, H.; et al. Thermal Annealing-Induced Phase Conversion in N-type Triple-Cation Lead-Based Perovskite Field Effect Transistors. ACS Appl. Mater. Interfaces 2025, 17, 8501–8512. [Google Scholar] [CrossRef]
- Saliba, M.; Matsui, T.; Seo, J.Y.; Domanski, K.; Correa-Baena, J.P.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Tress, W.; Abate, A.; Hagfeldt, A.; et al. Cesium-containing triple cation perovskite solar cells: Improved stability, reproducibility and high efficiency. Energy Environ. Sci. 2016, 9, 1989–1997. [Google Scholar] [CrossRef]
- Ašmontas, S.; Čerškus, A.; Gradauskas, J.; Grigucevičienė, A.; Leinartas, K.; Lučun, A.; Juškėnas, R. Cesium-containing triple cation perovskite solar cells. Coatings 2021, 11, 279. [Google Scholar] [CrossRef]
- Ašmontas, S.; Čerškus, A.; Gradauskas, J.; Grigucevičienė, A.; Juškėnas, R.; Leinartas, K.; Širmulis, E. Impact of cesium concentration on optoelectronic properties of metal halide perovskites. Materials 2022, 15, 1936. [Google Scholar] [CrossRef] [PubMed]
- Bartel, C.J.; Sutton, C.; Goldsmith, B.R.; Ouyang, R.; Musgrave, C.B.; Ghiringhelli, L.M.; Scheffler, M. New tolerance factor to predict the stability of perovskite oxides and halides. Sci. Adv. 2019, 5, eaav0693. [Google Scholar] [CrossRef]
- Liu, X.; Chen, M.; Zhang, Y.; Xia, J.; Yin, J.; Li, M.; Brooks, K.G.; Hu, R.; Gao, X.; Kim, Y.-H.; et al. High-efficiency perovskite photovoltaic modules achieved via cesium doping. Chem. Eng. J. 2022, 431, 133713. [Google Scholar] [CrossRef]
- Niemann, R.G.; Gouda, L.; Hu, J.; Tirosh, S.; Gottesman, R.; Cameron, P.J.; Zaban, A. Cs+ incorporation into CH3NH3PbI3 perovskite: Substitution limit and stability enhancement. J. Mater. Chem. A 2016, 4, 17819–17827. [Google Scholar] [CrossRef]
- Wang, S.; Li, M.H.; Zhang, Y.; Jiang, Y.; Xu, L.; Wang, F.; Hu, J.S. Surface n-type band bending for stable inverted CsPbI3 perovskite solar cells with over 20% efficiency. Energy Environ. Sci. 2023, 16, 2572–2578. [Google Scholar] [CrossRef]
- McMeekin, D.P.; Sadoughi, G.; Rehman, W.; Eperon, G.E.; Saliba, M.; Hörantner, M.T.; Haghighirad, A.A.; Sakai, N.; Korte, L.; Rech, B.; et al. A mixed-cation lead mixed-halide perovskite absorber for tandem solar cells. Science 2016, 351, 151–155. [Google Scholar] [CrossRef]
- Nguyen, T.M.H.; Lee, S.K.; Kim, S.; Bark, C.W. Practical demonstration of deep-ultraviolet detection with wearable and self-powered halide perovskite-based photodetector. ACS Appl. Mater. Interfaces 2021, 13, 57609–57618. [Google Scholar] [CrossRef]
- Nguyen, T.M.H.; Kim, S.; Bark, C.W. Solution-processed and self-powered photodetector in vertical architecture using mixed-halide perovskite for highly sensitive UVC detection. J. Mater. Chem. A 2021, 9, 1269–1276. [Google Scholar] [CrossRef]
- Nguyen, T.M.H.; Tran, M.H.; Bark, C.W. Deep-ultraviolet transparent electrode design for high-performance and self-powered perovskite photodetector. Nanomaterials 2023, 13, 2979. [Google Scholar] [CrossRef]
- Hong, S.B.; Choi, H.W. A Study on UVC Photodetector Using Mixed-Cation Perovskite with High Detection Rate as Light-Absorption Layer. Nanomaterials 2022, 12, 1185. [Google Scholar] [CrossRef]
- Wang, Y.C.; Chiang, C.H.; Chang, C.M.; Maeda, H.; Fukui, N.; Wang, I.T.; Nishihara, H. Two-Dimensional Bis(dithiolene) iron(II) self-powered UV photodetectors with ultrahigh air stability. Adv. Sci. 2021, 8, 2100564. [Google Scholar] [CrossRef]
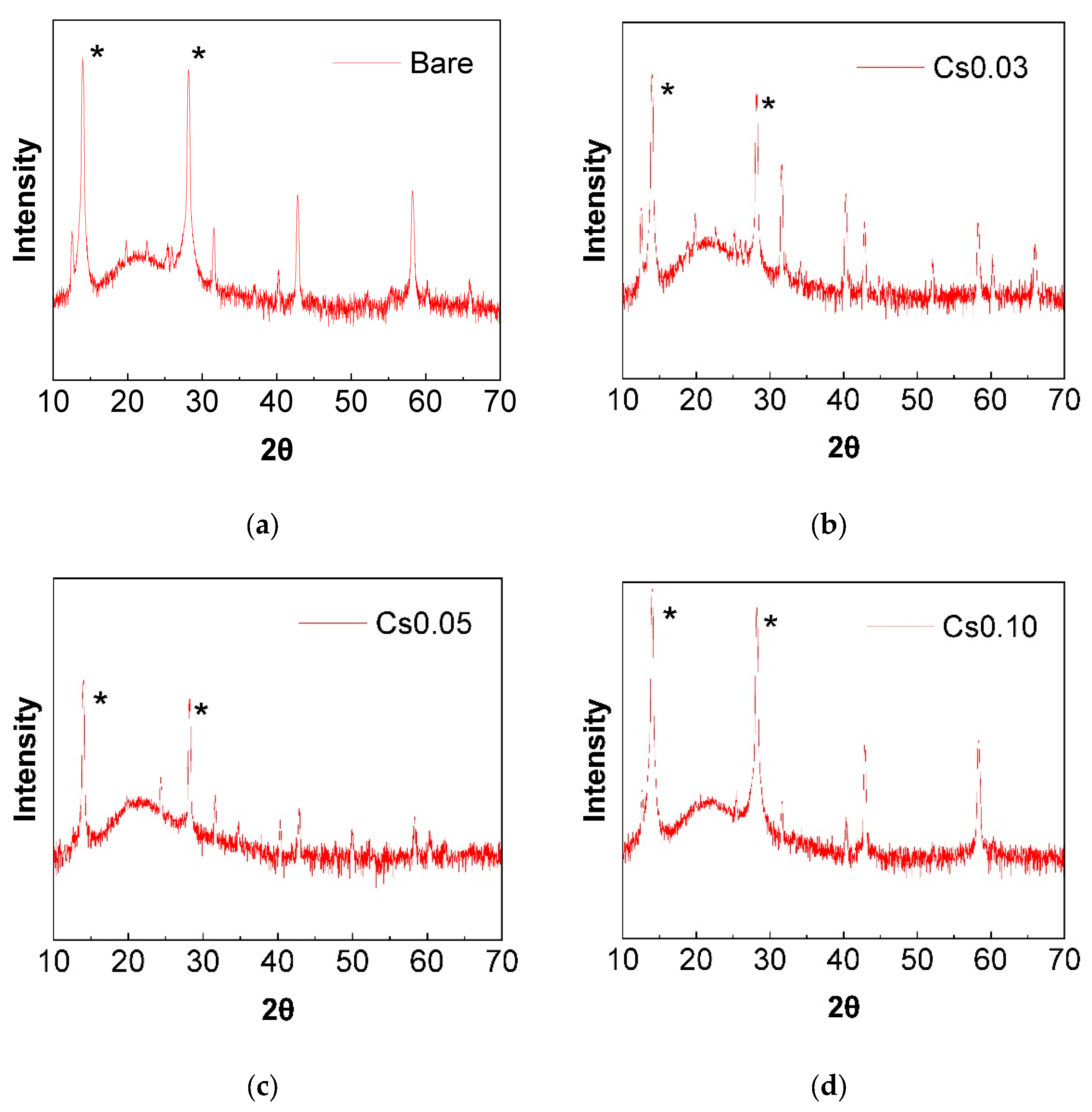

| Cs Content | Tolerance Factor (t) | Octahedral Factor (μ) |
|---|---|---|
| Bare (FAMAPbI3) | 0.98 | 0.54 |
| 0.03 | 0.97 | 0.54 |
| 0.05 | 0.97 | 0.54 |
| 0.10 | 0.96 | 0.54 |
| Photoactive Material | Detectable Wavelength (nm) | On/Off Ratio | Detectivity (Jones) | Structure | Refs |
|---|---|---|---|---|---|
| Cs0.05FA0.9MA0.1PbI3 | 254 | 23.94 | 3.52 × 1014 | NIP | this study |
| (FAPbI3)1-X(MAPbBr3)X | 254 | 337.14 | 7.57 × 1010 | NIP | [29] |
| (FAPbI3)0.97(MAPbBr3)0.03 | 254 | 103 | 4.65 × 1011 | NIP | [30] |
| FAMAPb(IxClyBr1-x-y) | 254 | 1.05 × 1012 | 4.71 × 1012 | NIP | [31] |
| (FA)x(MA)1−xPbI3 | 254 | - | 4.47 × 1013 | NIP | [32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.S.; Kim, S.; Choi, H.W. The Effect of Cs-Controlled Triple-Cation Perovskite on Improving the Sensing Performance of Deep-Ultraviolet Photodetectors. Appl. Sci. 2025, 15, 7982. https://doi.org/10.3390/app15147982
Kim JS, Kim S, Choi HW. The Effect of Cs-Controlled Triple-Cation Perovskite on Improving the Sensing Performance of Deep-Ultraviolet Photodetectors. Applied Sciences. 2025; 15(14):7982. https://doi.org/10.3390/app15147982
Chicago/Turabian StyleKim, Jun Seo, Sangmo Kim, and Hyung Wook Choi. 2025. "The Effect of Cs-Controlled Triple-Cation Perovskite on Improving the Sensing Performance of Deep-Ultraviolet Photodetectors" Applied Sciences 15, no. 14: 7982. https://doi.org/10.3390/app15147982
APA StyleKim, J. S., Kim, S., & Choi, H. W. (2025). The Effect of Cs-Controlled Triple-Cation Perovskite on Improving the Sensing Performance of Deep-Ultraviolet Photodetectors. Applied Sciences, 15(14), 7982. https://doi.org/10.3390/app15147982







