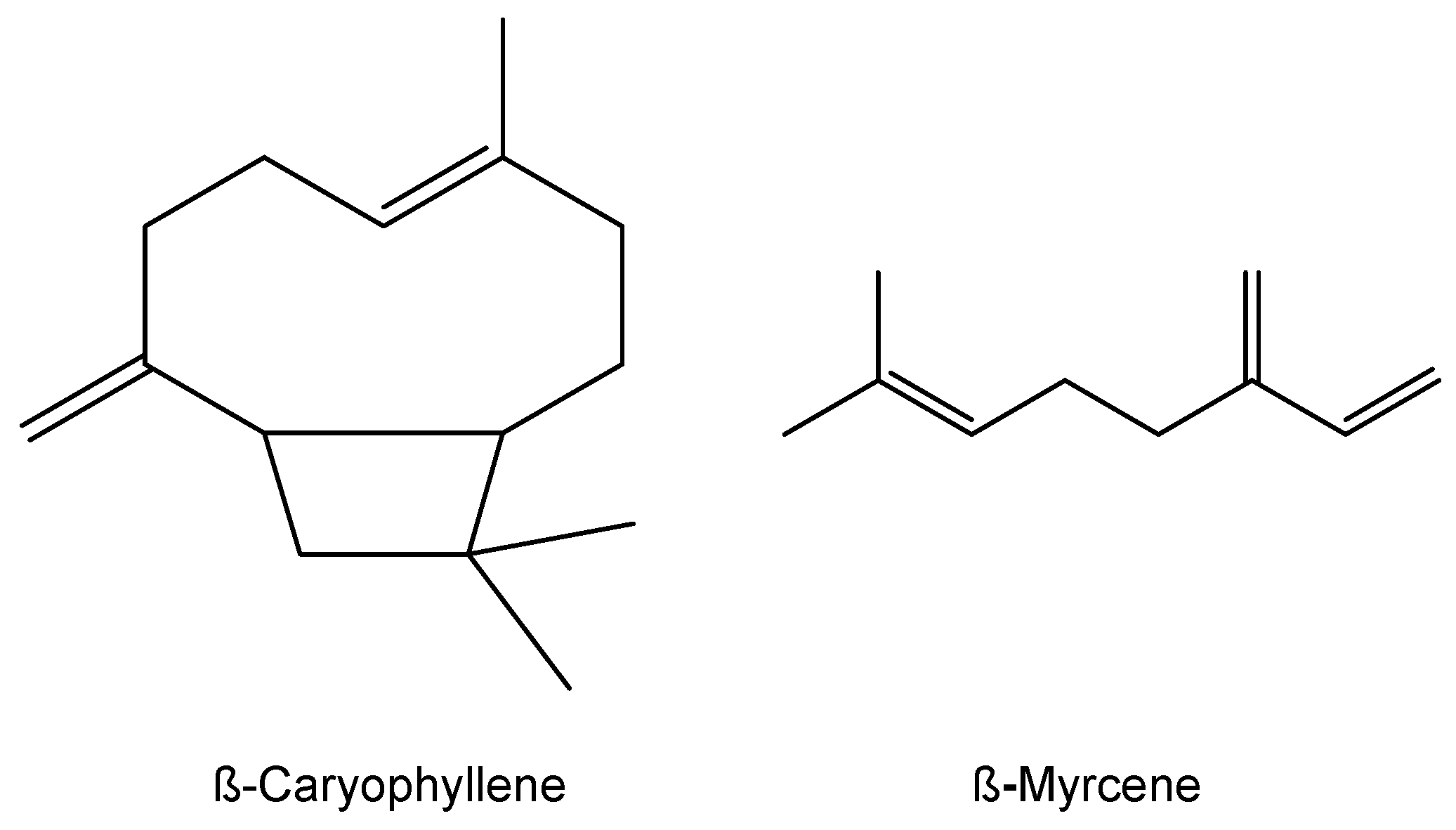
Polyoxometalates Surrounded by Organic Cations or Immobilized on Functionalized Merrifield Resin as Catalysts for Oxidation of β-Myrcene and β-Caryophyllene
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Synthesis of Objects
2.3.1. Heteropolyacid (HPA) Synthesis [51]
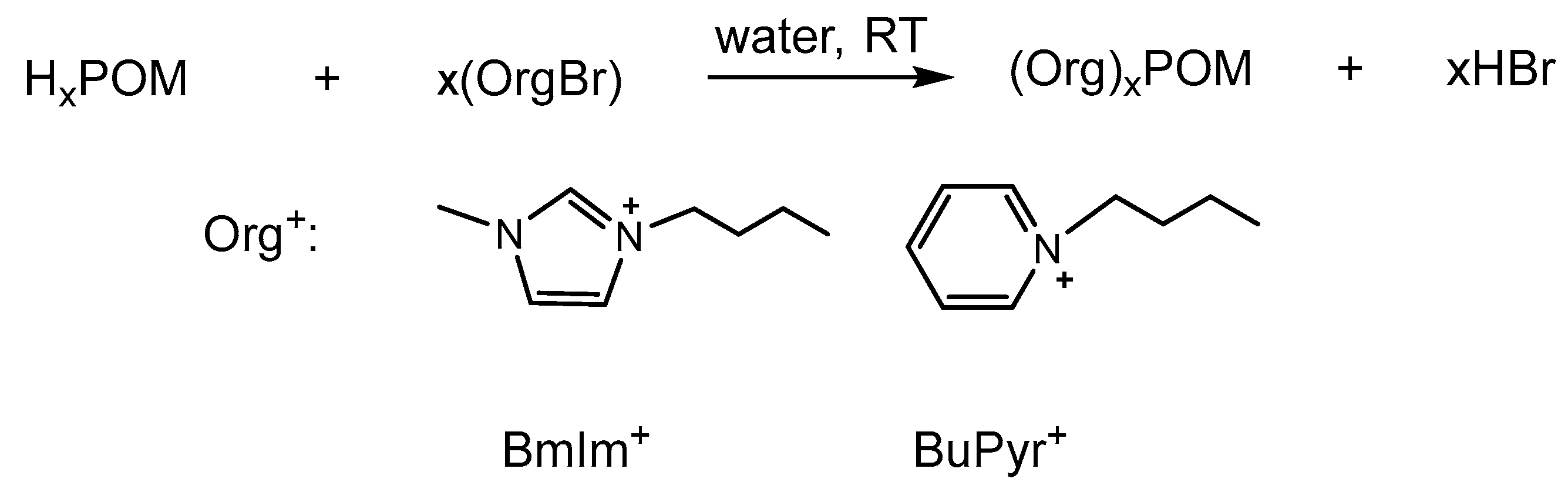
2.3.2. General Synthesis of Organic Salts of POMs
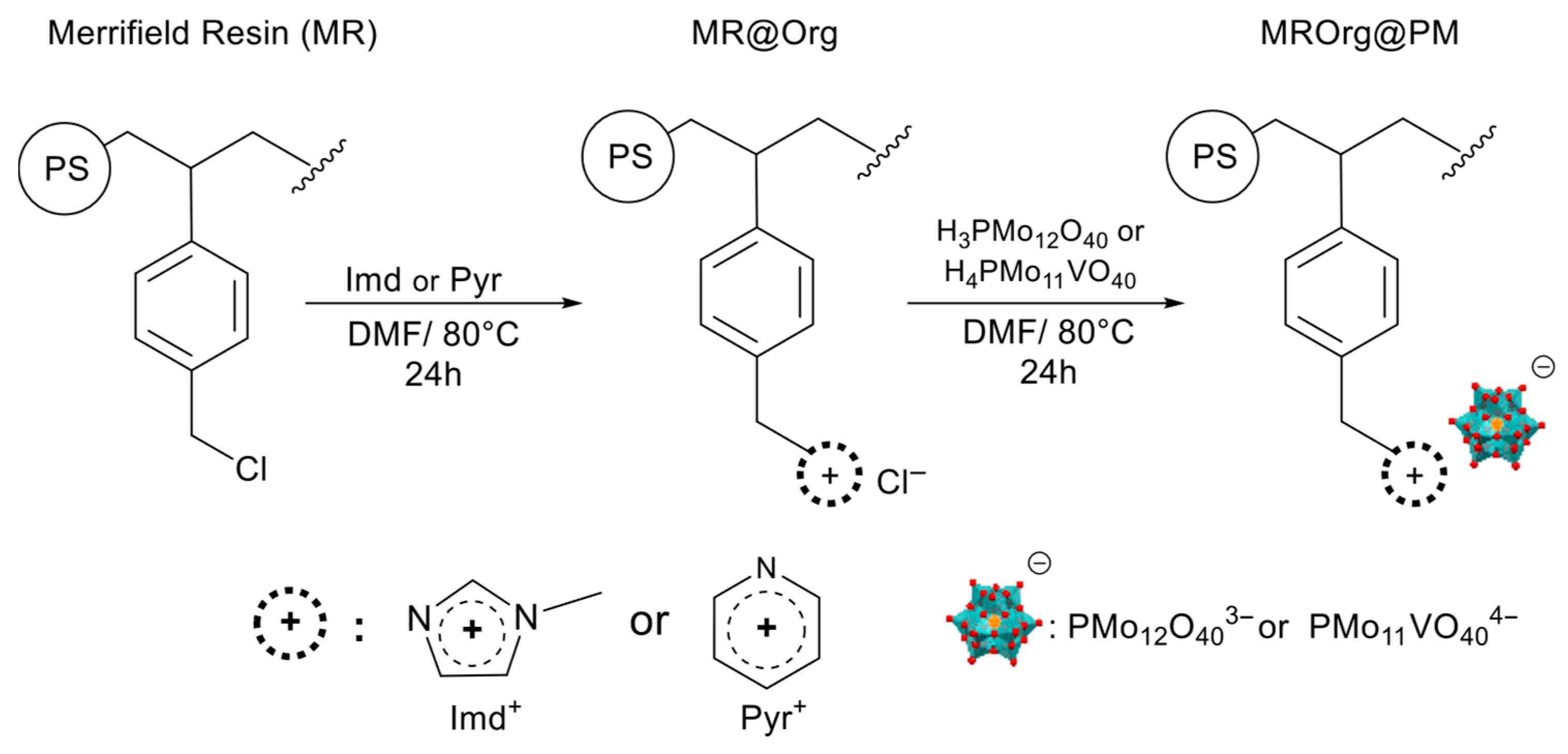
2.3.3. MR@Org and MROrg@POMs Synthesis [52]
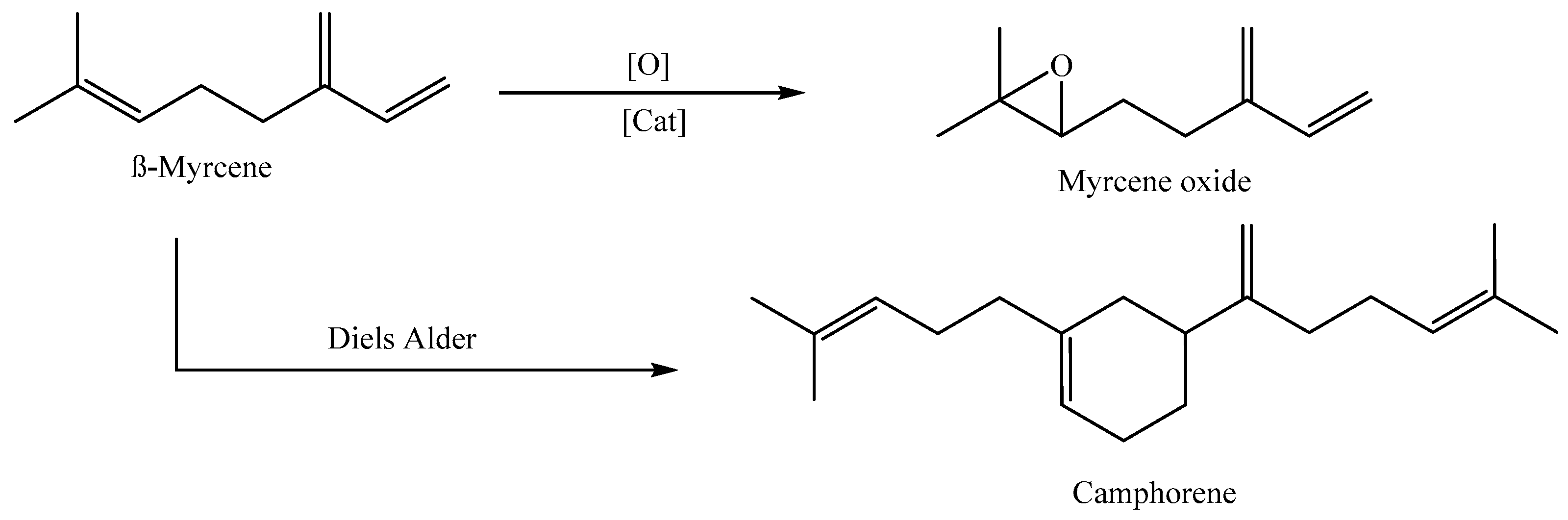
2.3.4. Synthesis of β-Myrcene Oxide (7-8-Epoxy β-Myrcene) [53]
2.4. Catalytic Oxidation Procedure
3. Results and Discussion
3.1. Synthetic Pathways of the Catalysts
3.1.1. Molecular POMs with Organic Cations
3.1.2. Immobilized POMs on Functionalized MRs
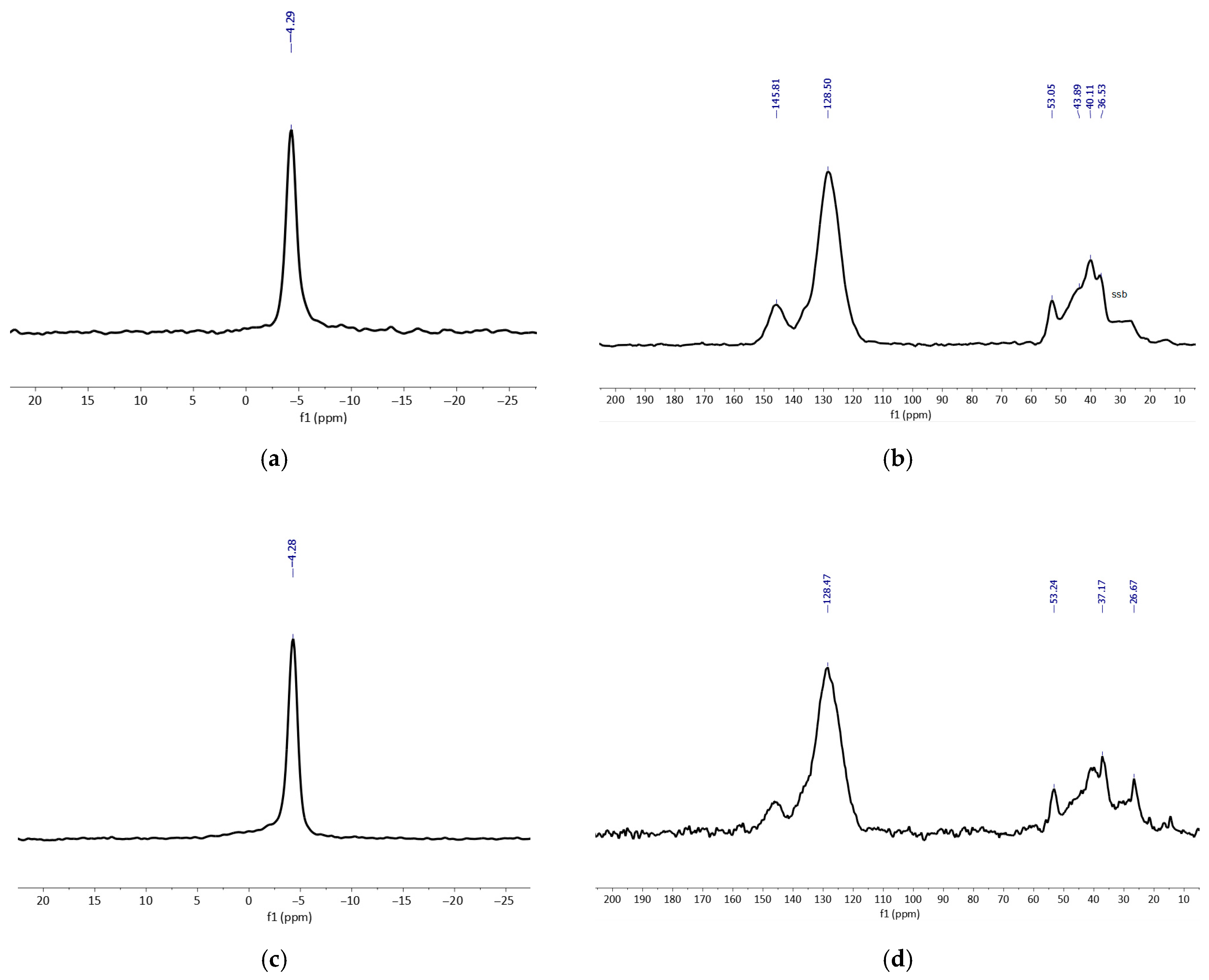
3.2. Characterization of the Catalysts
3.2.1. Molecular POMs with Organic Cations
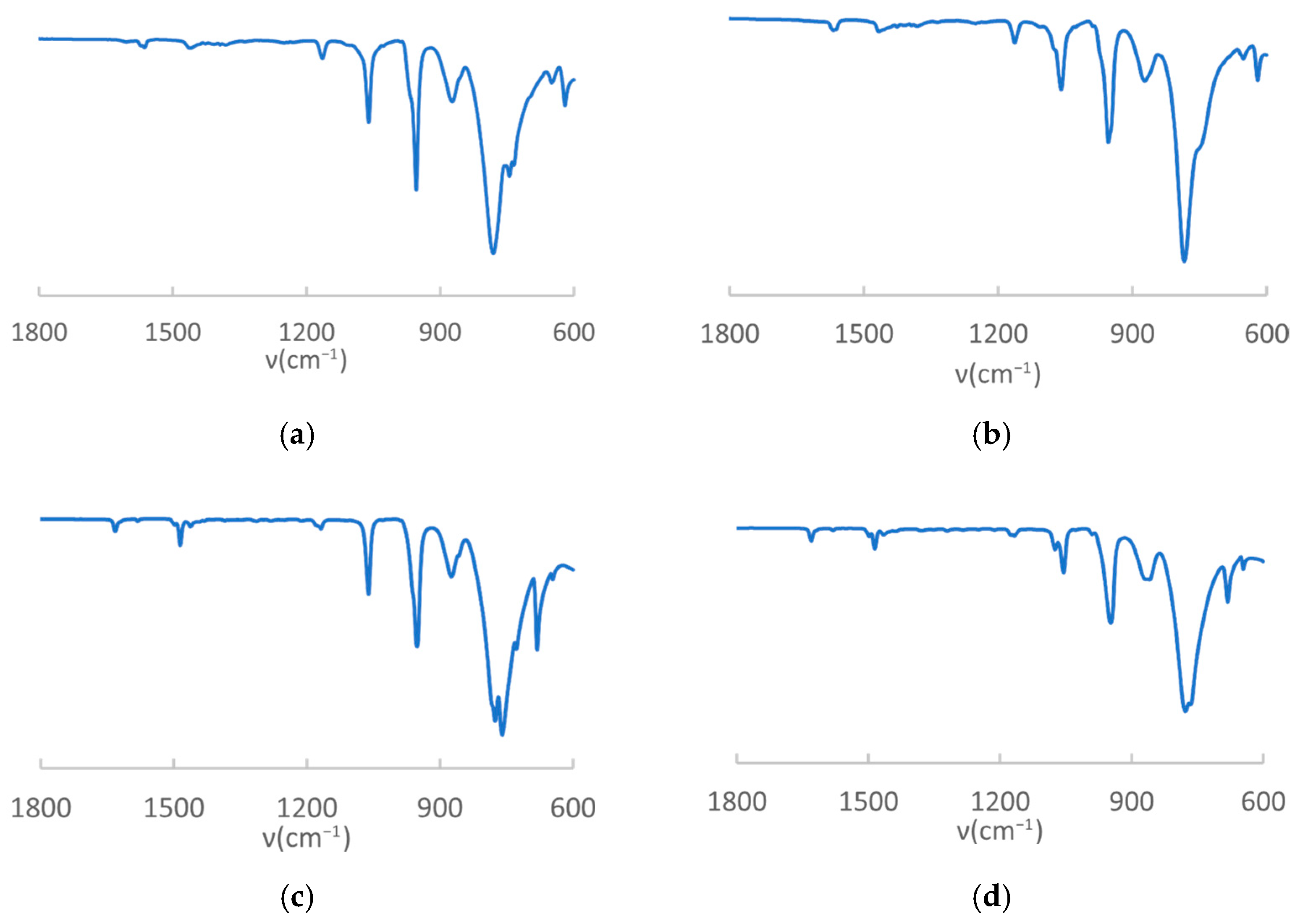
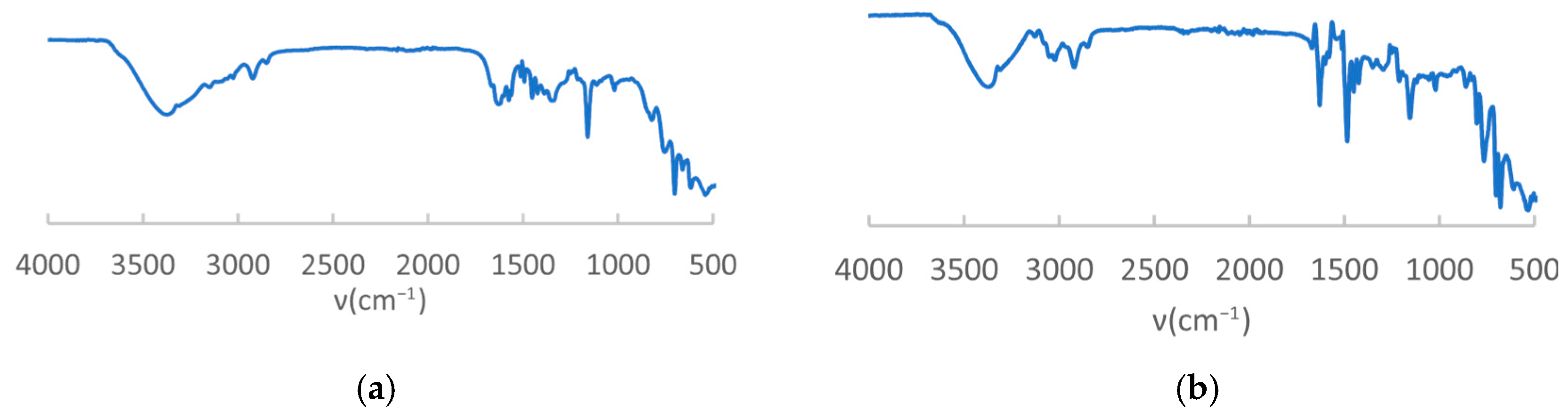
- IR characterization
3.2.2. Immobilized POMs on Functionalized MRs
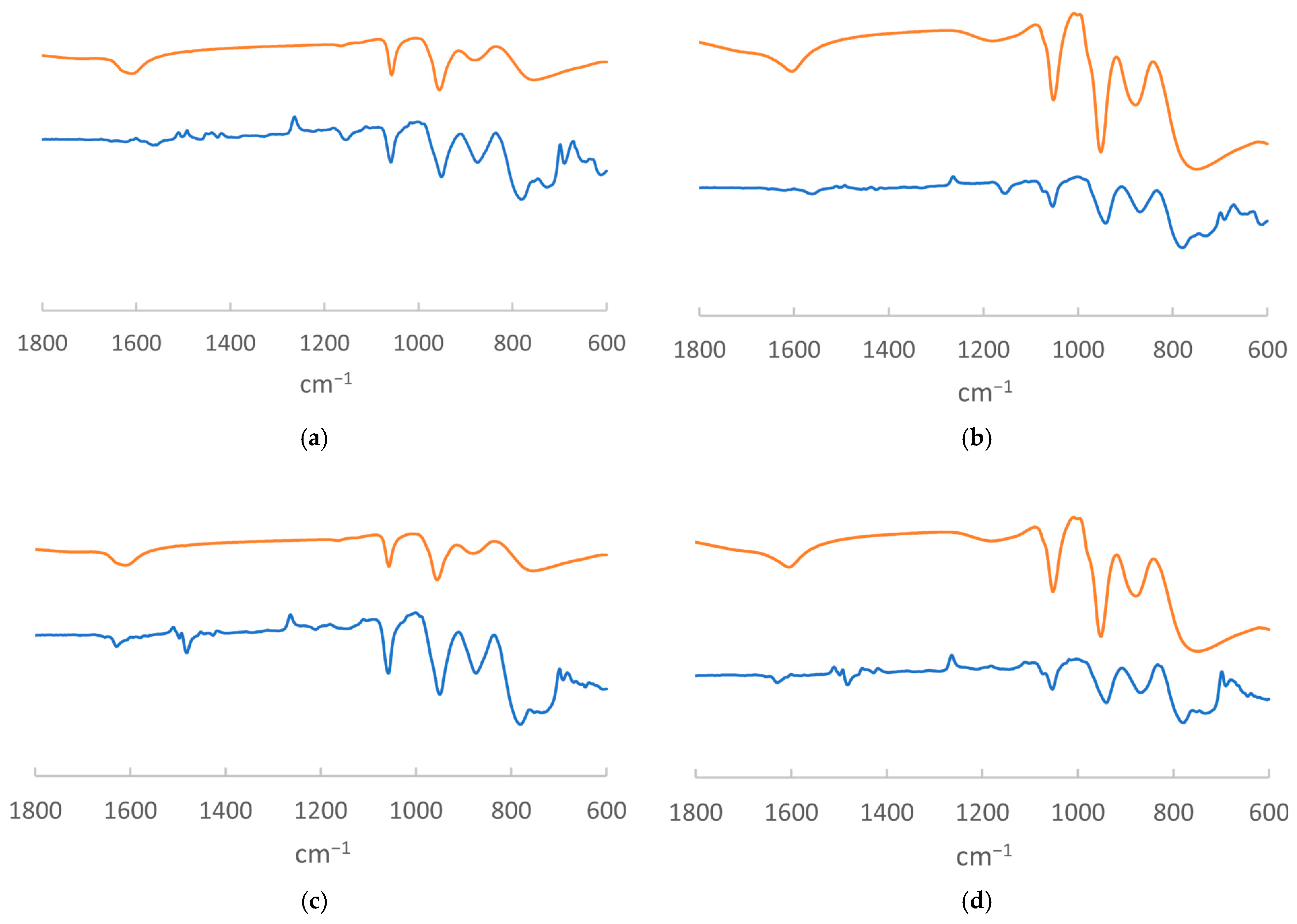
- IR Characterization of MR@Org
- Quantification of the functional groups on MR@Org
- Quantification of POM loading on MROrg@POM
- IR Analysis of MROrg@POM
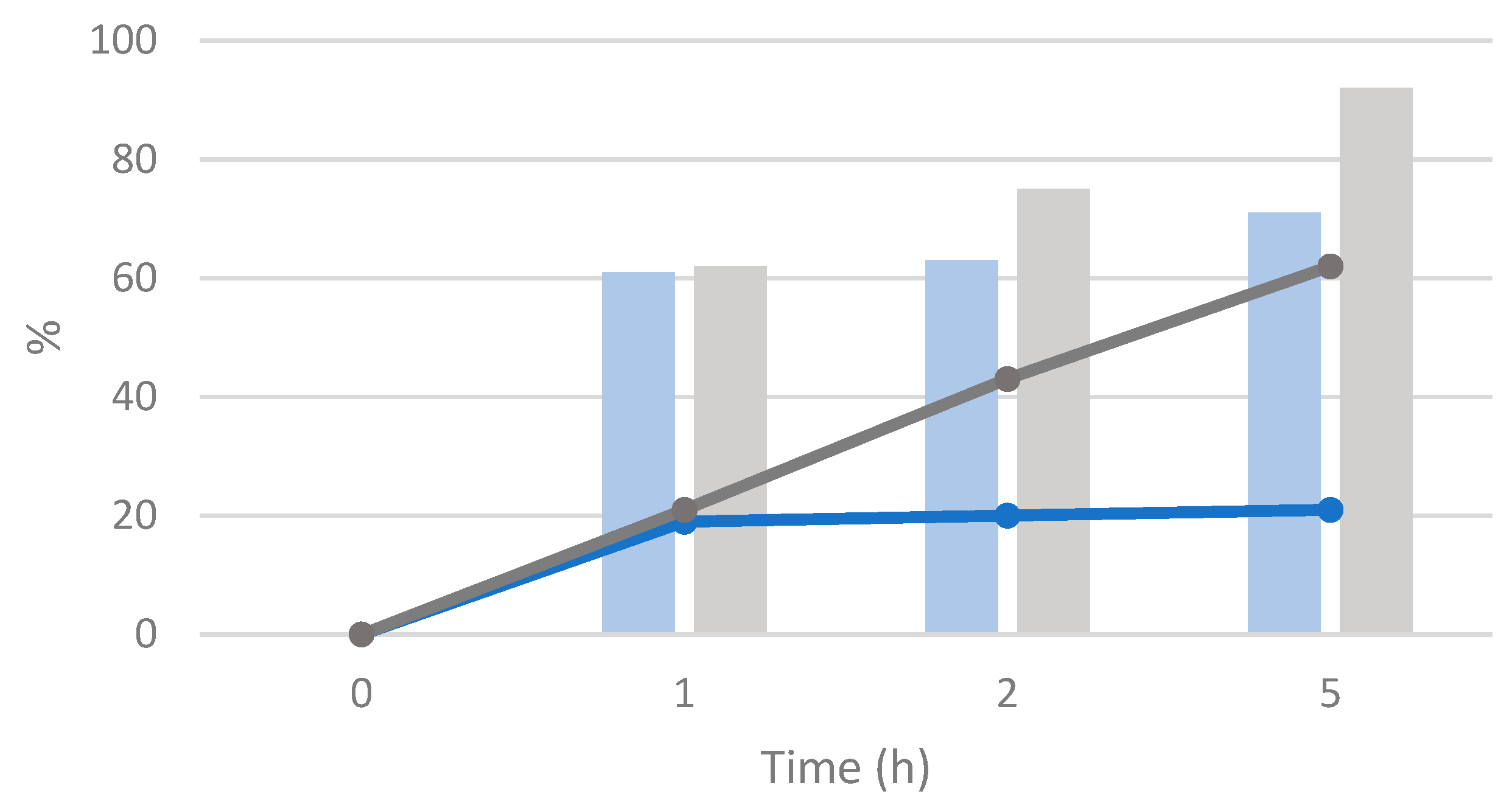
3.3. Swelling Test of Merrifield Resin with β-Myrcene
3.4. Catalyzed Oxidation of β-Myrcene
3.4.1. General Experimental Considerations
3.4.2. Effect of H2O2 as Oxidant with Organic Salts of POMs
3.4.3. Effect of TBHP as Oxidant with Organic Salts of POMs
3.4.4. Effect of H2O2 as Oxidant with Immobilized POMs
3.4.5. Effect of TBHP as Oxidant with Immobilized POMs
3.4.6. Diels–Alder Investigation
3.5. β-Caryophyllene Oxidation
3.5.1. General Experimental Considerations
3.5.2. Effect of H2O2 as Oxidant with Organic Salts of POMs
3.5.3. Effect of TBHP as Oxidant with Organic Salts of POMs
3.5.4. Solvent Influence
3.5.5. Effect of H2O2 as Oxidant with Immobilized POMs
3.5.6. Effect of TBHP as Oxidant with Immobilized POMs
3.5.7. Leaching Experiment with MRImd@PMo11VO40
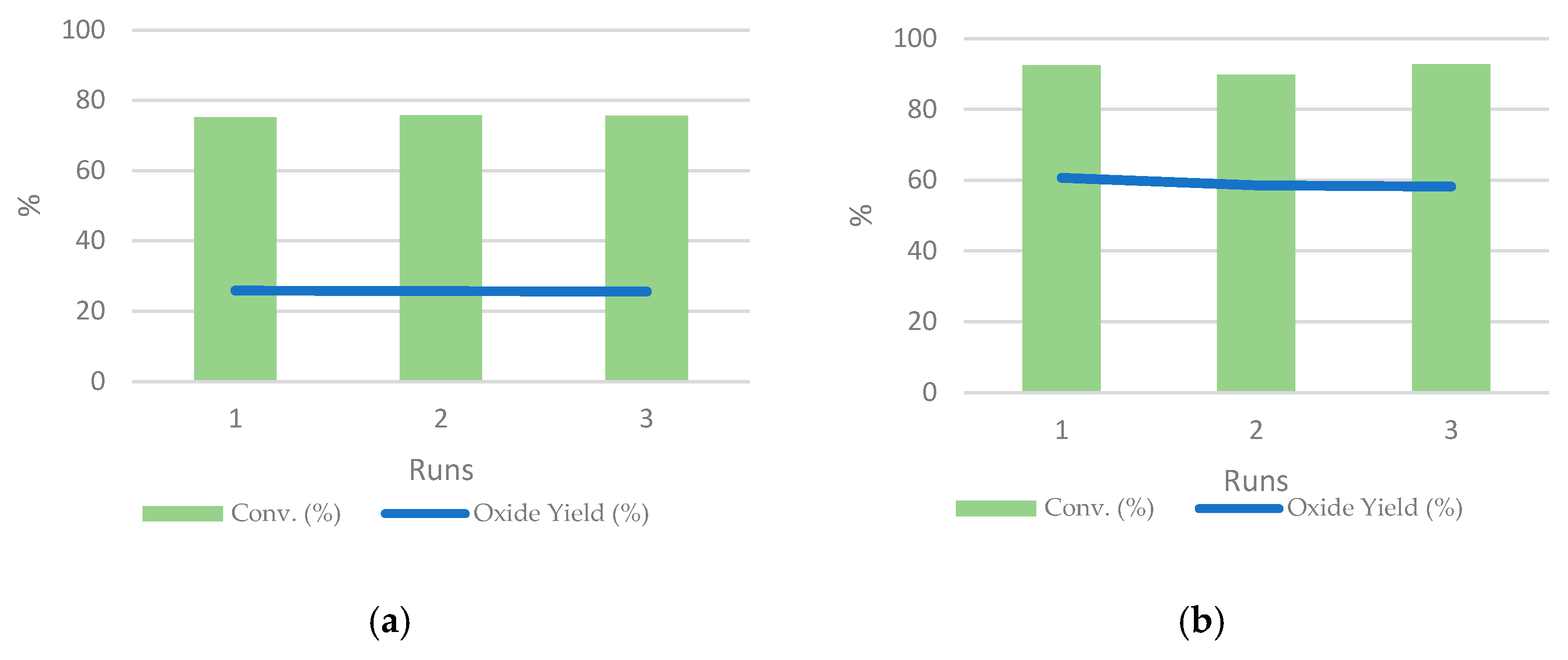
3.6. Recyclability of the Catalysts with Both Substrates and MRImd@PMo11VO40
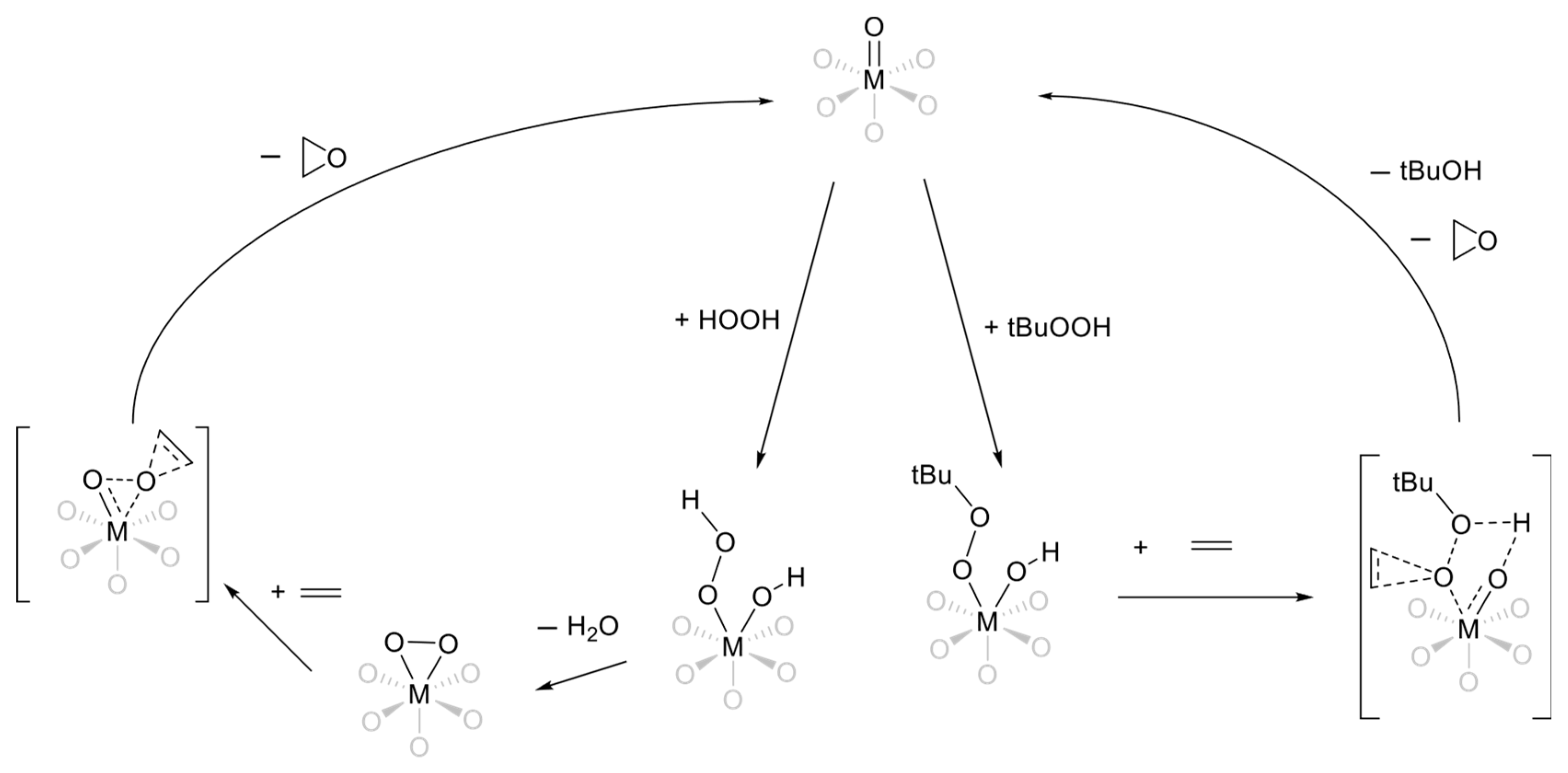
3.7. Effect of Tert-Butanol on Oxidation—A Putative Explanation
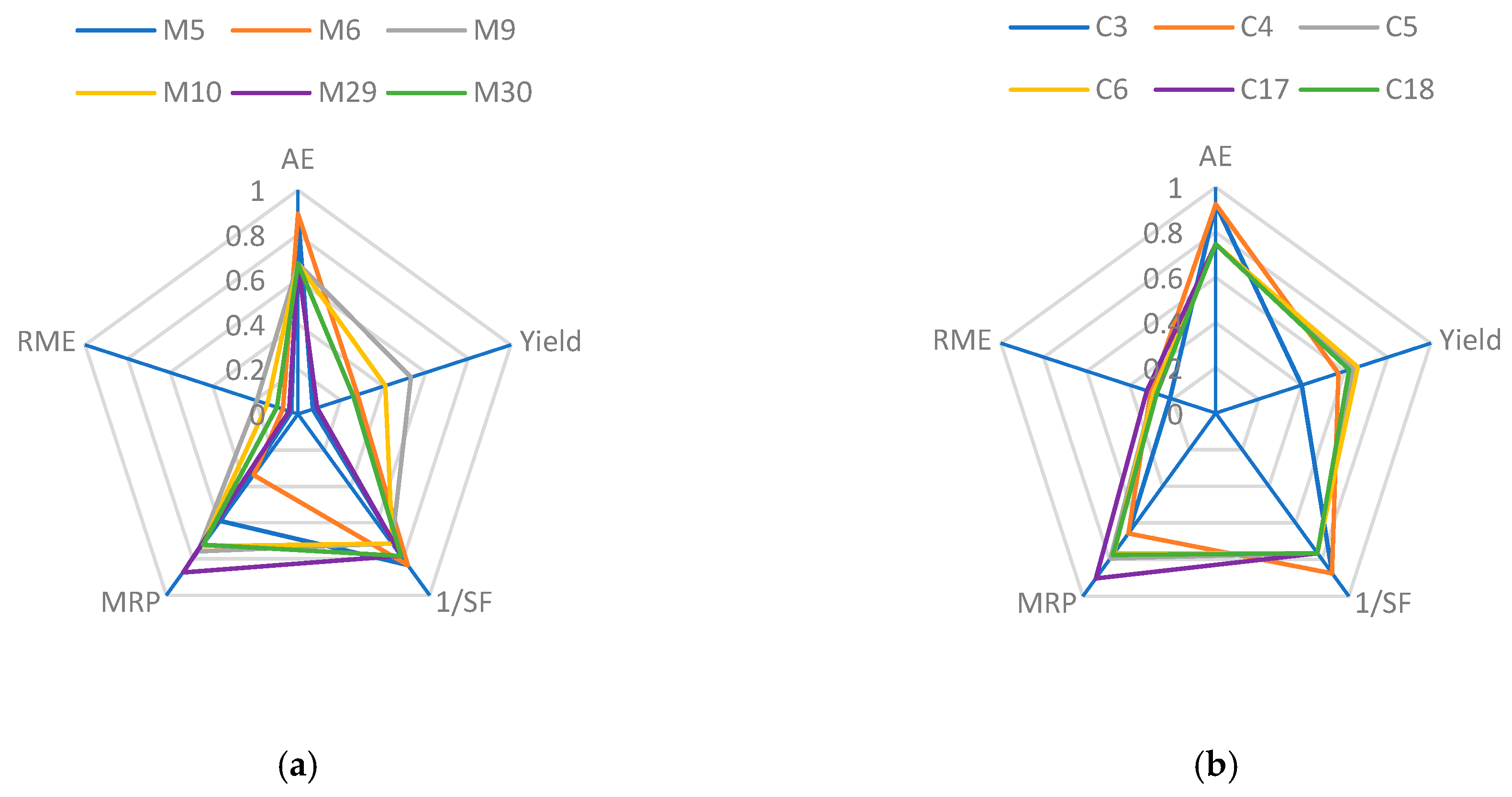
3.8. Green Metric Considerations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| aq | Aqueous |
| dec | Decane |
| TBHP | tert-butyl hydroperoxide |
| POM | Polyoxometalate |
| HPA | Heteropolyacid |
| BmIm | Butylmethyl imidazolium |
| BuPyr | Butyl pyridinium |
| Imd | Methyl imidazolium |
| Pyr | Pyridinium |
| MR | Merrifield resin |
References
- Faaij, A. Modern Biomass Conversion Technologies. Mitig. Adapt. Strateg. Glob. Change 2006, 11, 343–375. [Google Scholar] [CrossRef]
- Ahorsu, R.; Medina, F.; Constantí, M. Significance and Challenges of Biomass as a Suitable Feedstock for Bioenergy and Biochemical Production: A Review. Energies 2018, 11, 3366. [Google Scholar] [CrossRef]
- Dodds, D.R.; Gross, R.A. Chemicals from Biomass. Science 2007, 318, 1250–1251. [Google Scholar] [CrossRef] [PubMed]
- Haveren, J.V.; Scott, E.L.; Sanders, J. Bulk Chemicals from Biomass. Biofuels Bioprod. Biorefin. 2008, 2, 41–57. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 2000; ISBN 978-0-19-850698-0. [Google Scholar]
- De, S.; Burange, A.S.; Luque, R. Conversion of Biomass-Derived Feedstocks into Value-Added Chemicals over Single-Atom Catalysts. Green Chem. 2022, 24, 2267–2286. [Google Scholar] [CrossRef]
- Corma, A.; Iborra, S.; Velty, A. Chemical Routes for the Transformation of Biomass into Chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Harman-Ware, A.E. Conversion of Terpenes to Chemicals and Related Products. In Chemical Catalysts for Biomass Upgrading; Crocker, M., Santillan-Jimenez, E., Eds.; Wiley: Hoboken, NJ, USA, 2020; pp. 529–568. ISBN 978-3-527-34466-6. [Google Scholar]
- Geron, C.; Rasmussen, R.; Arnts, R.R.; Guenther, A. A Review and Synthesis of Monoterpene Speciation from Forests in the United States. Atmos. Environ. 2000, 34, 1761–1781. [Google Scholar] [CrossRef]
- Behr, A.; Johnen, L. Myrcene as a Natural Base Chemical in Sustainable Chemistry: A Critical Review. ChemSusChem 2009, 2, 1072–1095. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.J.; Linforth, R.S.T. (Eds.) Food Flavour Technology, 1st ed.; Wiley: Hoboken, NJ, USA, 2010; ISBN 978-1-4051-8543-1. [Google Scholar]
- Behr, A.; Johnen, L.; Neubert, P. A Sustainable Route from the Renewable Myrcene to Methyl Ethersvia Direct Hydroalkoxylation. Catal. Sci. Technol. 2012, 2, 88–92. [Google Scholar] [CrossRef]
- Fahlbusch, K.; Hammerschmidt, F.; Panten, J.; Pickenhagen, W.; Schatkowski, D.; Bauer, K.; Garbe, D.; Surburg, H. Flavors and Fragrances. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH, Ed.; Wiley: Hoboken, NJ, USA, 2003; ISBN 978-3-527-30385-4. [Google Scholar]
- Anastasiou, D.E. Investigating the Epoxidation of Poly-β-Myrcene: Optimization, Kinetics, and Thermodynamics. J. Polym. Environ. 2023, 31, 4044–4051. [Google Scholar] [CrossRef]
- Asempour, F.; Yang, R.; Maric, M. Thermadapt Shape Memory Vitrimeric Polymyrcene Elastomer. React. Funct. Polym. 2024, 201, 105941. [Google Scholar] [CrossRef]
- Ricci, G.; Boccia, A.C.; Palucci, B.; Sommazzi, A.; Masi, F.; Scoti, M.; De Stefano, F.; De Rosa, C. Synthesis of Stereoregular Polymyrcenes Using Neodymium-, Iron- and Copper-Based Catalysts. Polym. Chem. 2024, 15, 1367–1376. [Google Scholar] [CrossRef]
- Li, T.; Zhang, M.; He, J.; Ni, P. Synthesis and Characterization of Graft Copolymers with Poly(ε-Caprolactone) Side Chain Using Hydroxylated Poly(β-Myrcene-Co-α-Methyl Styrene). Molecules 2024, 29, 2363. [Google Scholar] [CrossRef] [PubMed]
- Ling, F.-W.; Luo, M.-C.; Chen, M.-K.; Zeng, J.; Li, S.-Q.; Yin, H.-B.; Wu, J.-R.; Xu, Y.-X.; Huang, G. Terminally and Randomly Functionalized Polyisoprene Lead to Distinct Aggregation Behaviors of Polar Groups. Polymer 2019, 178, 121629. [Google Scholar] [CrossRef]
- Hahn, C.; Wagner, M.; Müller, A.H.E.; Frey, H. MyrDOL, a Protected Dihydroxyfunctional Diene Monomer Derived from β-Myrcene: Functional Polydienes from Renewable Resources via Anionic Polymerization. Macromolecules 2022, 55, 4046–4055. [Google Scholar] [CrossRef]
- Sell, C.S. Terpenoids. In Kirk-Othmer Encyclopedia of Chemical Technology; Kirk-Othmer, Ed.; Wiley: Hoboken, NJ, USA, 2006; ISBN 978-0-471-48494-3. [Google Scholar]
- Ma, S.-J.; Yu, J.; Yan, D.-W.; Wang, D.-C.; Gao, J.-M.; Zhang, Q. Meroterpene-like Compounds Derived from β-Caryophyllene as Potent α-Glucosidase Inhibitors. Org. Biomol. Chem. 2018, 16, 9454–9460. [Google Scholar] [CrossRef] [PubMed]
- Machado, K.D.C.; Islam, M.T.; Ali, E.S.; Rouf, R.; Uddin, S.J.; Dev, S.; Shilpi, J.A.; Shill, M.C.; Reza, H.M.; Das, A.K.; et al. A Systematic Review on the Neuroprotective Perspectives of Beta-caryophyllene. Phytother. Res. 2018, 32, 2376–2388. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.F.; Su, J.-H.; Shiue, R.-T.; Pan, X.-J.; Dai, C.-F.; Kuo, Y.-H.; Sheu, J.-H. New β-Caryophyllene-Derived Terpenoids from the Soft Coral Sinularia n Anolobata. J. Nat. Prod. 2004, 67, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Giersch, W.K.; Boschung, A.F.; Snowden, R.L.; Schulte-Elte, K.H. Thermoconversion of Caryophyllene- to Farnesene-Type Sesquiterpenes. Short Access to the Enantiomers of (6RS,7RS)- and (6RS,7SR)-6,7-epoxy-6,7-dihydro-β-farnesenes. Helv. Chim. Acta 1994, 77, 36–40. [Google Scholar] [CrossRef]
- Cheng, X.; Harzdorf, N.; Khaing, Z.; Kang, D.; Camelio, A.M.; Shaw, T.; Schmidt, C.E.; Siegel, D. Neuronal Growth Promoting Sesquiterpene-Neolignans; Syntheses and Biological Studies. Org. Biomol. Chem. 2012, 10, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.S.; Oliveira, T.C.; Júnior, L.M.R.; Figueiras, A.; Nunes, L.C.C. β-Caryophyllene Delivery Systems: Enhancing the Oral Pharmacokinetic and Stability. Curr. Pharm. Des. 2018, 24, 3440–3453. [Google Scholar] [CrossRef] [PubMed]
- Sköld, M.; Karlberg, A.-T.; Matura, M.; Börje, A. The Fragrance Chemical β-Caryophyllene—Air Oxidation and Skin Sensitization. Food Chem. Toxicol. 2006, 44, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Alberti, T.; Barbosa, W.; Vieira, J.; Raposo, N.; Dutra, R. (−)-β-Caryophyllene, a CB2 Receptor-Selective Phytocannabinoid, Suppresses Motor Paralysis and Neuroinflammation in a Murine Model of Multiple Sclerosis. Int. J. Mol. Sci. 2017, 18, 691. [Google Scholar] [CrossRef] [PubMed]
- Mitzner, B.M.; Theimer, E.T.; Steinbach, L.; Wolt, J. α-Myrcene (2-Methyl-6-Methylene-1,7-Octadiene). J. Org. Chem. 1965, 30, 646–648. [Google Scholar] [CrossRef]
- Gagan, S.; Sarang, K.; Rudzinski, K.J.; Liu, R.; Szmigielski, R.; Zhang, Y. Synthetic Strategies for Oxidation Products from Biogenic Volatile Organic Compounds in the Atmosphere: A Review. Atmos. Environ. 2023, 312, 120017. [Google Scholar] [CrossRef]
- Barrero, A.F.; Molina, J.; Enrique Oltra, J.; Altarejos, J.; Barragán, A.; Lara, A.; Segura, M. Stereochemistry of 14-Hydroxy-β-Caryophyllene and Related Compounds. Tetrahedron 1995, 51, 3813–3822. [Google Scholar] [CrossRef]
- Mahamat Ahmat, Y.; Kamkui, H.M.; Kaliaguine, S. Scaled up Epoxidation of Terpenes in Microemulsion. Catal. Today 2025, 449, 115202. [Google Scholar] [CrossRef]
- Imperio, D.; Casali, E.; Del Grosso, E.; Caprioglio, D.; Minassi, A.; Panza, L. Trifluoromethyl Ketone Galactose Catalyst for Asymmetric Epoxidation: Experimental and Theoretical Model. Eur. J. Org. Chem. 2024, 27, e202301163. [Google Scholar] [CrossRef]
- Cheng, X.; Harzdorf, N.L.; Shaw, T.; Siegel, D. Biomimetic Syntheses of the Neurotrophic Natural Products Caryolanemagnolol and Clovanemagnolol. Org. Lett. 2010, 12, 1304–1307. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Mao, W.; Zhang, L.; Zhao, Y.; Huang, H.; Xiao, Y.; Mao, L.; Fu, Z.; Yu, N.; Yin, D. An Ultrathin Amino-Acid Based Copper(II) Coordination Polymer Nanosheet for Efficient Epoxidation of β-Caryophyllene. Mol. Catal. 2021, 511, 111754. [Google Scholar] [CrossRef]
- Li, S.; Shi, L.; Zhang, L.; Huang, H.; Xiao, Y.; Mao, L.; Tan, R.; Fu, Z.; Yu, N.; Yin, D. Ionic Liquid-Mediated Catalytic Oxidation of β-Caryophyllene by Ultrathin 2D Metal-Organic Framework Nanosheets under 1 Atm O2. Mol. Catal. 2020, 496, 111196. [Google Scholar] [CrossRef]
- Dileep, R.; Rudresha, B.J. An Ionic Liquid Immobilized Copper Complex for Catalytic Epoxidation. RSC Adv. 2015, 5, 65870–65873. [Google Scholar] [CrossRef]
- Yamazaki, S. An Effective Procedure for the Synthesis of Acid-Sensitive Epoxides: Use of 1-Methylimidazole as the Additive on Methyltrioxorhenium-Catalyzed Epoxidation of Alkenes with Hydrogen Peroxide. Org. Biomol. Chem. 2010, 8, 2377. [Google Scholar] [CrossRef] [PubMed]
- Salles, L.; Brégeault, J.-M.; Thouvenot, R. Comparison of Oxoperoxophosphatotungstate Phase Transfer Catalysis with Methyltrioxorhenium Two-Phase Catalysis for Epoxidation by Hydrogen Peroxide. C. R. Acad. Sci. Ser. IIC Chem. 2000, 3, 183–187. [Google Scholar] [CrossRef]
- Mahha, Y.; Salles, L.; Piquemal, J.-Y.; Briot, E.; Atlamsani, A.; Brégeault, J.-M. Environmentally Friendly Epoxidation of Olefins under Phase-Transfer Catalysis Conditions with Hydrogen Peroxide. J. Catal. 2007, 249, 338–348. [Google Scholar] [CrossRef]
- Tibbetts, J.D.; Cunningham, W.B.; Vezzoli, M.; Plucinski, P.; Bull, S.D. Sustainable Catalytic Epoxidation of Biorenewable Terpene Feedstocks Using H2O2 as an Oxidant in Flow Microreactors. Green Chem. 2021, 23, 5449–5455. [Google Scholar] [CrossRef]
- Cunningham, W.B.; Tibbetts, J.D.; Hutchby, M.; Maltby, K.A.; Davidson, M.G.; Hintermair, U.; Plucinski, P.; Bull, S.D. Sustainable Catalytic Protocols for the Solvent Free Epoxidation and Anti-Dihydroxylation of the Alkene Bonds of Biorenewable Terpene Feedstocks Using H2O2 as Oxidant. Green Chem. 2020, 22, 513–524. [Google Scholar] [CrossRef]
- Escande, V.; Petit, E.; Garoux, L.; Boulanger, C.; Grison, C. Switchable Alkene Epoxidation/Oxidative Cleavage with H2O2/NaHCO3: Efficient Heterogeneous Catalysis Derived from Biosourced Eco-Mn. ACS Sustain. Chem. Eng. 2015, 3, 2704–2715. [Google Scholar] [CrossRef]
- Evtushok, V.Y.; Ivanchikova, I.D.; Lopatkin, V.A.; Maksimchuk, N.V.; Podyacheva, O.Y.; Suboch, A.N.; Stonkus, O.A.; Kholdeeva, O.A. Heterolytic Alkene Oxidation with H2O2 Catalyzed by Nb-Substituted Lindqvist Tungstates Immobilized on Carbon Nanotubes. Catal. Sci. Technol. 2021, 11, 3198–3207. [Google Scholar] [CrossRef]
- Shen, Y.; Jiang, P.; Wai, P.; Gu, Q.; Zhang, W. Recent Progress in Application of Molybdenum-Based Catalysts for Epoxidation of Alkenes. Catalysts 2019, 9, 31. [Google Scholar] [CrossRef]
- Cherevan, A.S.; Nandan, S.P.; Roger, I.; Liu, R.; Streb, C.; Eder, D. Polyoxometalates on Functional Substrates: Concepts, Synergies, and Future Perspectives. Adv. Sci. 2020, 7, 1903511. [Google Scholar] [CrossRef] [PubMed]
- Tarlani, A.; Abedini, M.; Nemati, A.; Khabaz, M.; Amini, M.M. Immobilization of Keggin and Preyssler Tungsten Heteropolyacids on Various Functionalized Silica. J. Colloid Interface Sci. 2006, 303, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gayet, F.; Guillo, P.; Agustin, D. Organic Solvent-Free Olefins and Alcohols (Ep) Oxidation Using Recoverable Catalysts Based on [PM12O40]3− (M = Mo or W) Ionically Grafted on Amino Functionalized Silica Nanobeads. Materials 2019, 12, 3278. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.C.; Kumar Sutar, A.; Lin, C.-C. Polymer-Supported Schiff Base Complexes in Oxidation Reactions. Coord. Chem. Rev. 2009, 253, 1926–1946. [Google Scholar] [CrossRef]
- Pisk, J.; Agustin, D. Molybdenum, Vanadium, and Tungsten-Based Catalysts for Sustainable (Ep) Oxidation. Molecules 2022, 27, 6011. [Google Scholar] [CrossRef] [PubMed]
- Tsigdinos, G.A.; Hallada, C.J. Molybdovanadophosphoric Acids and Their Salts. I. Investigation of Methods of Preparation and Characterization. Inorg. Chem. 1968, 7, 437–441. [Google Scholar] [CrossRef]
- Pisk, J.; Agustin, D.; Poli, R. Organic Salts and Merrifield Resin Supported [PM12O40]3− (M = Mo or W) as Catalysts for Adipic Acid Synthesis. Molecules 2019, 24, 783. [Google Scholar] [CrossRef] [PubMed]
- Hioki, H.; Ooi, H.; Hamano, M.; Mimura, Y.; Yoshio, S.; Kodama, M.; Ohta, S.; Yanai, M.; Ikegami, S. Enantioselective Total Synthesis and Absolute Stereostructure of Hippospongic Acid A. Tetrahedron 2001, 57, 1235–1246. [Google Scholar] [CrossRef]
- Noorhisham, N.A.; Amri, D.; Mohamed, A.H.; Yahaya, N.; Ahmad, N.M.; Mohamad, S.; Kamaruzaman, S.; Osman, H. Characterisation Techniques for Analysis of Imidazolium-Based Ionic Liquids and Application in Polymer Preparation: A Review. J. Mol. Liq. 2021, 326, 115340. [Google Scholar] [CrossRef]
- Lawrenson, S.; North, M.; Peigneguy, F.; Routledge, A. Greener Solvents for Solid-Phase Synthesis. Green Chem. 2017, 19, 952–962. [Google Scholar] [CrossRef]
- Mitsuaki, K.; Hiroyuki, M.; Yukiko, M.; Yoshiyasu, F. Convenient Synthesis of Chiral Epoxyisoprenoids by Yeast Reduction. Tetrahedron Lett. 1990, 31, 4025–4026. [Google Scholar] [CrossRef]
- Runckel, W.J.; Goldblatt, L.A. Inhibition of Myrcene Polymerization during Storage. Ind. Eng. Chem. 1946, 38, 749–751. [Google Scholar] [CrossRef]
- Keller, C.L.; Walkling, C.J.; Zhang, D.D.; Baldwin, L.C.; Austin, J.S.; Harvey, B.G. Designer Biosynthetic Jet Fuels Derived from Isoprene and α-Olefins. ACS Sustain. Chem. Eng. 2023, 11, 4030–4039. [Google Scholar] [CrossRef]
- Kafuku, K.; Oyamada, T.; Nishi, M. A NEW DITERPENE—γ-CAMPHORENE. Bull. Chem. Soc. Jpn. 1933, 8, 144–148. [Google Scholar] [CrossRef]
- Francomano, F.; Caruso, A.; Barbarossa, A.; Fazio, A.; La Torre, C.; Ceramella, J.; Mallamaci, R.; Saturnino, C.; Iacopetta, D.; Sinicropi, M.S. β-Caryophyllene: A Sesquiterpene with Countless Biological Properties. Appl. Sci. 2019, 9, 5420. [Google Scholar] [CrossRef]
- Gyrdymova, Y.V.; Rubtsova, S.A. Caryophyllene and Caryophyllene Oxide: A Variety of Chemical Transformations and Biological Activities. Chem. Pap. 2022, 76, 1–39. [Google Scholar] [CrossRef]
- Cederbaum, A.I.; Qureshi, A.; Cohen, G. Production of Formaldehyde and Acetone by Hydroxyl-Radical Generating Systems during the Metabolism of Tertiary Butyl Alcohol. Biochem. Pharmacol. 1983, 32, 3517–3524. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Smith, F.R.; Ichikawa, N.; Baba, T.; Itow, M. Decomposition of Hydrogen Peroxide in Aqueous Solutions at Elevated Temperatures. Int. J. Chem. Kinet. 1991, 23, 971–987. [Google Scholar] [CrossRef]
- Haidar, A.A.H.; Agustin, D. Role of Organic Solvent and Influence of Oxidant in the Oxidation of Linalool Catalyzed by Molybdenum and Vanadium Complexes. Tetrahedron Green Chem. 2023, 2, 100029. [Google Scholar] [CrossRef]

| Imd or Pyr Content (mmol/g MR) 1 | POM Loading (mmol/g of MR@POM) 2 | ||
|---|---|---|---|
| MR@Imd | 1.5 | MRIm@PMo12O40 | 0.444 |
| MRIm@PMo11VO40 | 0.402 | ||
| MR@Pyr | 1.6 | MRPyr@PMo12O40 | 0.426 |
| MRPyr@PMo11VO40 | 0.43 |
| Catalysts | Entry | T (°C) | Oxidant | β-Myrcene Conv (%) | Myrcene Oxide Yield (%) |
|---|---|---|---|---|---|
| None | M1 | 80 | None | 47 2 | DA * |
| (BmIm)3PMo12O40 | M2 | 70 | H2O2aq | 75 | 1 |
| M3 | 70 | H2O2aq + Toluene | 82 | 29 | |
| M4 | 80 | TBHPaq | 40 | 7 | |
| M5 | 80 | TBHPdec | 89 | 39 | |
| (BuPyr)3PMo12O40 | M6 | 70 | H2O2aq | 79 | 2 |
| M7 | 70 | H2O2aq + Toluene | 29 | 2 | |
| M8 | 80 | TBHPaq | 60 | 10 | |
| M9 | 80 | TBHPdec | 80 | 15 | |
| (BmIm)4PMo11VO40 | M10 | 70 | H2O2aq | 87 | 7 |
| M11 | 70 | H2O2aq + Toluene | 87 | 28 | |
| M12 | 70 | H2O2aq + EtOAc | 95 | 2 | |
| M13 | 70 | H2O2aq + EtOAc + CH3CN 1 | 94 | 2 | |
| M14 | 80 | TBHPaq | 81 | 53 | |
| M15 | 80 | TBHPdec | 88 | 41 | |
| (BmIm)4PMo11VO40 | M16 | 80 | NNone | 37 2 | |
| M17 | 40 | H2O2aq | 20 | 5 | |
| M18 | 40 | TBHPaq | 24 | 4 | |
| (BuPyr)4PMo11VO40 | M19 | 70 | H2O2aq | 75 | 1 |
| M20 | 70 | H2O2aq + Toluene | 26 | 2 | |
| M21 | 80 | TBHPaq | 70 | 41 | |
| M22 | 80 | TBHPdec | 85 | 9 | |
| Naked MR | M23 | 70 | H2O2aq | 47 | |
| M24 | 80 | TBHPaq | 42 | ||
| M25 | 80 | TBHPdec | 39 | ||
| MRImd@PMo12O40 | M26 | 70 | H2O2aq | 94 | 6 |
| M27 | 80 | TBHPaq | 64 | 6 | |
| MRPyr@PMo12O40 | M28 | 70 | H2O2aq | 94 | 5 |
| M29 | 80 | TBHPaq | 95 | 5 | |
| MRImd@PMo11VO40 | M30 | 70 | H2O2aq | 98 | 9 |
| M31 | 80 | TBHPaq | 88 | 9 | |
| M32 | 80 | TBHPdec | 75 | 26 | |
| MRPyr@PMo11VO40 | M33 | 70 | H2O2aq | 95 | 5 |
| M34 | 80 | TBHPaq | 85 | 5 |
| Catalysts | Entry | T (°C) | Conditions | β-Caryo 1 Conv (%) | Oxide Yield (%) |
|---|---|---|---|---|---|
| (BmIm)3PMo12O40 | C1 | 70 | H2O2aq | 98 | 26 |
| C2 | 80 | TBHPaq | 98 | 45 | |
| (BuPyr)3PMo12O40 | C3 | 70 | H2O2aq | 98 | 14 |
| C4 | 80 | TBHPaq | 98 | 24 | |
| (BmIm)4PMo11VO40 | C5 | 70 | H2O2aq | 99 | 40 |
| C6 | 80 | H2O2aq + EtOAc | 99 | 57 | |
| C7 | 80 | TBHPaq | 98 | 64 | |
| C8 | 70 | TBHPdec | 98 | 66 | |
| (BuPyr)4PMo11VO40 | C9 | 70 | H2O2aq | 99 | 15 |
| C10 | 70 | H2O2aq + EtOAc | 92 | 42 | |
| C11 | 80 | TBHPaq | 99 | 31 | |
| C12 | 80 | TBHPdec | 99 | 36 | |
| Naked MR | C13 | 70 | H2O2aq | 5 | - |
| C14 | 80 | TBHPaq | 5 | - | |
| C15 | 80 | TBHPdec | 11 | - | |
| MRImd@PMo11VO40 | C16 | 70 | H2O2aq | 94 | 36 |
| C17 | 80 | TBHPaq | 92 | 62 | |
| C18 | 80 | TBHPdec | 94 | 62 | |
| MRImd@PMo11VO40 | C19 | 80 | TBHPaq | 62 | 20 |
| Catalyst removal after 1 h * | C20 | 71 | 21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haidar, A.A.H.; Guillo, P.; Agustin, D. Polyoxometalates Surrounded by Organic Cations or Immobilized on Functionalized Merrifield Resin as Catalysts for Oxidation of β-Myrcene and β-Caryophyllene. Appl. Sci. 2025, 15, 7981. https://doi.org/10.3390/app15147981
Haidar AAH, Guillo P, Agustin D. Polyoxometalates Surrounded by Organic Cations or Immobilized on Functionalized Merrifield Resin as Catalysts for Oxidation of β-Myrcene and β-Caryophyllene. Applied Sciences. 2025; 15(14):7981. https://doi.org/10.3390/app15147981
Chicago/Turabian StyleHaidar, Ali Al Hadi, Pascal Guillo, and Dominique Agustin. 2025. "Polyoxometalates Surrounded by Organic Cations or Immobilized on Functionalized Merrifield Resin as Catalysts for Oxidation of β-Myrcene and β-Caryophyllene" Applied Sciences 15, no. 14: 7981. https://doi.org/10.3390/app15147981
APA StyleHaidar, A. A. H., Guillo, P., & Agustin, D. (2025). Polyoxometalates Surrounded by Organic Cations or Immobilized on Functionalized Merrifield Resin as Catalysts for Oxidation of β-Myrcene and β-Caryophyllene. Applied Sciences, 15(14), 7981. https://doi.org/10.3390/app15147981








