Hydrogen–Rock Interactions in Carbonate and Siliceous Reservoirs: A Petrophysical Perspective
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. X-Ray Diffraction (XRD) Analysis
3.2. Porosity, Permeability, and Textural Characteristics
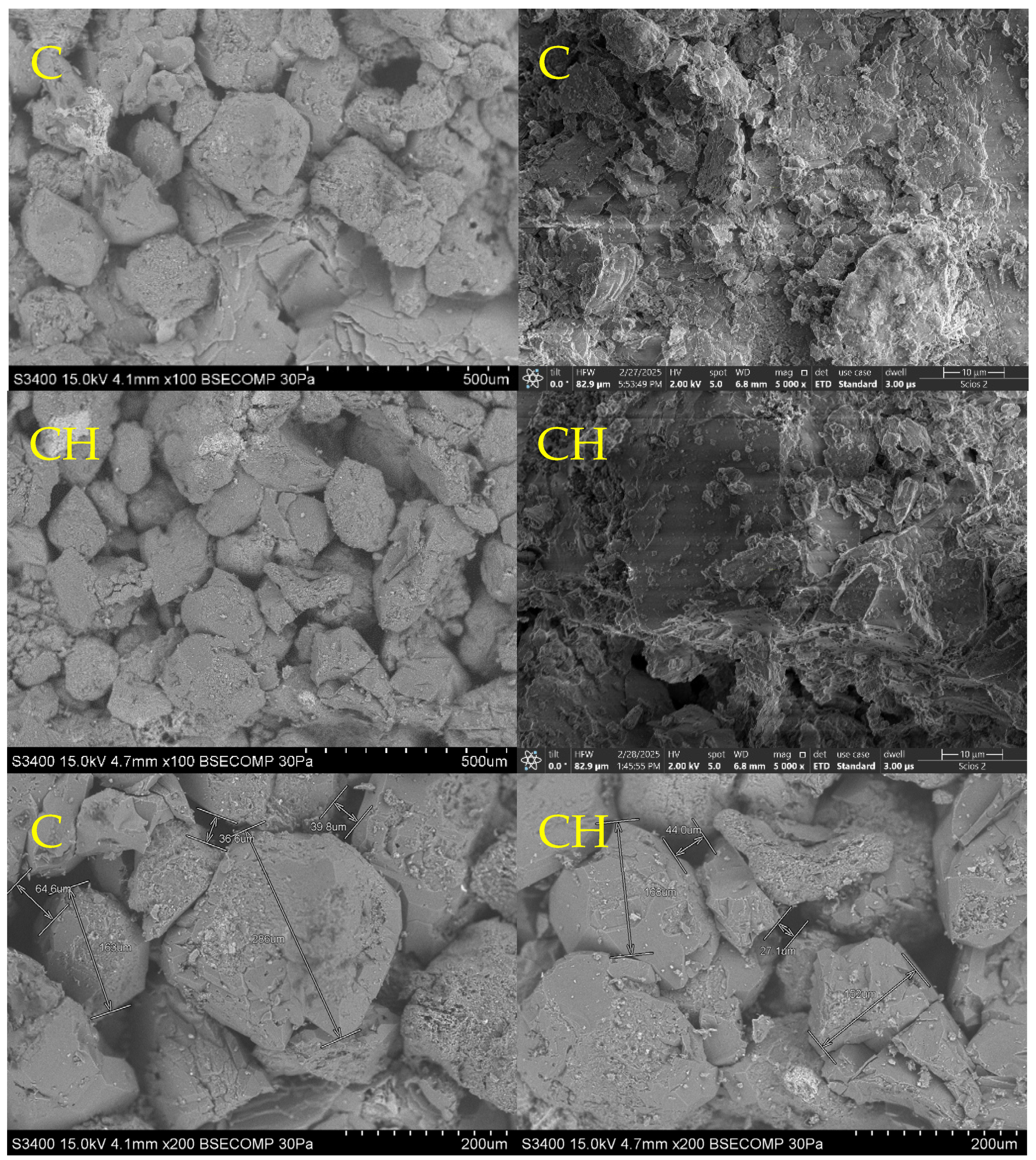
3.3. SEM-EDS Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| bar | Unit of pressure |
| BET | Brunauer–Emmett–Teller (method for measuring specific surface area) |
| BJH | Barrett–Joyner–Halenda (method for pore size distribution analysis) |
| CaCO3 | Calcite |
| CaMg(CO3)2 | Dolomite |
| cm3/g | Cubic centimeters per gram (pore volume unit) |
| CO2 | Carbon dioxide |
| Cu-Kα | Copper K-alpha radiation (X-ray source used in XRD) |
| Dv(d) | Mean pore diameter (physical parameter in pore size analysis) |
| EDS | Energy-dispersive X-ray spectroscopy |
| GASPERM | Brand/model of permeameter used for permeability measurements |
| H2, H3, H4 | Types of hysteresis loops in nitrogen adsorption isotherms according to IUPAC classification |
| He-Porosimeter | Helium porosimeter used for porosity measurements |
| IUPAC | International Union of Pure and Applied Chemistry |
| m2/g | Square meters per gram (surface area unit) |
| mD | Millidarcy (unit of permeability) |
| nm | Nanometer (length unit) |
| NOVA 2200e | Model of gas adsorption analyzer used for BET/BJH measurements |
| p/p0 | Relative pressure (used in adsorption isotherms) |
| SEM | Scanning electron microscopy |
| UHS | Underground hydrogen storage |
| XRD | X-ray diffraction |
| θ-θ | Theta–theta geometry (XRD measurement configuration) |
References
- IEA. Global Hydrogen Review 2023; International Energy Agency: Paris, France, 2023; p. 68.
- Caglayan, D.G.; Weber, N.; Heinrichs, H.U.; Linßen, J.; Robinius, M.; Kukla, P.A.; Stolten, D. Technical potential of salt caverns for hydrogen storage in Europe. Int. J. Hydrogen Energy 2020, 45, 6793–6805. [Google Scholar] [CrossRef]
- Li, P.; Shi, X.; Li, Y.; Zhu, S.; Liu, W.; Ma, H.; Yang, C. Optimizing geological hydrogen storage in bedded salt formations: Assessing permeation and leakage dynamics. J. Energy Storage 2025, 122, 116709. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Hu, S. Underground hydrogen storage in depleted gas fields: Progress, challenges, and outlook. J. Energy Storage 2024, 102, 113842. [Google Scholar] [CrossRef]
- Luboń, K.; Tarkowski, R. Hydrogen Storage in Deep Saline Aquifers: Non-Recoverable Cushion Gas after Storage. Energies 2024, 17, 1493. [Google Scholar] [CrossRef]
- Bade, S.O.; Taiwo, K.; Ndulue, U.F.; Tomomewo, O.S.; Aisosa Oni, B. A review of underground hydrogen storage systems: Current status, modeling approaches, challenges, and future prospective. Int. J. Hydrogen Energy 2024, 80, 449–474. [Google Scholar] [CrossRef]
- Ali, M.; Isah, A.; Yekeen, N.; Hassanpouryouzband, A.; Sarmadivaleh, M.; Okoroafor, E.R.; Al Kobaisi, M.; Mahmoud, M.; Vahrenkamp, V.; Hoteit, H. Recent progress in underground hydrogen storage. Energy Environ. Sci. 2025, 18, 5740–5810. [Google Scholar] [CrossRef]
- Shojaee, A.; Ghanbari, S.; Wang, G.; Mackay, E. Interplay between microbial activity and geochemical reactions during underground hydrogen storage in a seawater-rich formation. Int. J. Hydrogen Energy 2024, 50, 1529–1541. [Google Scholar] [CrossRef]
- Al-Yaseri, A.; Amao, A.; Fatah, A. Experimental investigation of shale/hydrogen geochemical interactions. Fuel 2023, 346, 128272. [Google Scholar] [CrossRef]
- Al-Yaseri, A.; Yekeen, N.; Al-Mukainah, H.; Hassanpouryouzband, A. Geochemical interactions in geological hydrogen Storage: The role of sandstone clay content. Fuel 2024, 361, 130728. [Google Scholar] [CrossRef]
- Hassanpouryouzband, A.; Adie, K.; Cowen, T.; Thaysen, E.M.; Heinemann, N.; Butler, I.B.; Wilkinson, M.; Edlmann, K. Geological Hydrogen Storage: Geochemical Reactivity of Hydrogen with Sandstone Reservoirs. ACS Energy Lett. 2022, 7, 2203–2210. [Google Scholar] [CrossRef] [PubMed]
- Driba, D.L.; Beckingham, L.E. Unraveling hydrogen induced geochemical reaction mechanisms through coupled geochemical modeling and machine learning. Appl. Geochem. 2025, 183, 106330. [Google Scholar] [CrossRef]
- Thiyagarajan, S.R.; Emadi, H.; Hussain, A.; Patange, P.; Watson, M. A comprehensive review of the mechanisms and efficiency of underground hydrogen storage. J. Energy Storage 2022, 51, 104490. [Google Scholar] [CrossRef]
- Zeng, L.; Keshavarz, A.; Xie, Q.; Iglauer, S. Hydrogen storage in Majiagou carbonate reservoir in China: Geochemical modelling on carbonate dissolution and hydrogen loss. Int. J. Hydrogen Energy 2022, 47, 24861–24870. [Google Scholar] [CrossRef]
- Al-Yaseri, A.; Al-Mukainah, H.; Yekeen, N. Experimental insights into limestone-hydrogen interactions and the resultant effects on underground hydrogen storage. Fuel 2023, 344, 128000. [Google Scholar] [CrossRef]
- Aslannezhad, M.; Ali, M.; Kalantariasl, A.; Sayyafzadeh, M.; You, Z.; Iglauer, S.; Keshavarz, A. A review of hydrogen/rock/brine interaction: Implications for Hydrogen Geo-storage. Prog. Energy Combust. Sci. 2023, 95, 101066. [Google Scholar] [CrossRef]
- Al-Yaseri, A.; Al-Mukainah, H.; Yekeen, N.; Al-Qasim, A.S. Experimental investigation of hydrogen-carbonate reactions via computerized tomography: Implications for underground hydrogen storage. Int. J. Hydrogen Energy 2023, 48, 3583–3592. [Google Scholar] [CrossRef]
- Ugarte, E.R.; Tetteh, D.; Salehi, S. Experimental studies of well integrity in cementing during underground hydrogen storage. Int. J. Hydrogen Energy 2024, 51, 473–488. [Google Scholar] [CrossRef]
- Mwakipunda, G.C.; Franck Kouassi, A.K.; Ayimadu, E.T.; Komba, N.A.; Nadege, M.N.; Mgimba, M.M.; Ngata, M.R.; Yu, L. Underground hydrogen storage in geological formations: A review. J. Rock Mech. Geotech. Eng. 2025; in press. [Google Scholar] [CrossRef]
- Bo, Z.; Zeng, L.; Chen, Y.; Xie, Q. Geochemical reactions-induced hydrogen loss during underground hydrogen storage in sandstone reservoirs. Int. J. Hydrogen Energy 2021, 46, 19998–20009. [Google Scholar] [CrossRef]
- Cheng, C.; Busch, B.; Kontny, A.; Hilgers, C. Underground hydrogen storage in sandstone reservoirs: Effects of geochemical reactivity of hydrogen on reservoir performance. Int. J. Hydrogen Energy 2025, 105, 492–504. [Google Scholar] [CrossRef]
- Flesch, S.; Pudlo, D.; Albrecht, D.; Jacob, A.; Enzmann, F. Hydrogen underground storage—Petrographic and petrophysical variations in reservoir sandstones from laboratory experiments under simulated reservoir conditions. Int. J. Hydrogen Energy 2018, 43, 20822–20835. [Google Scholar] [CrossRef]
- Al-Yaseri, A.; Fatah, A.; Adebayo, A.R.; Al-Qasim, A.S.; Patil, P.D. Pore structure analysis of storage rocks during geological hydrogen storage: Investigation of geochemical interactions. Fuel 2024, 361, 130683. [Google Scholar] [CrossRef]
- Kaczmarek, S.E.; Gregg, J.M.; Bish, D.L.; Machel, H.G.; Fouke, B.W. Dolomite, Very High-Magnesium Calcite, and Microbes—Implications for the Microbial Model of Dolomitization. In Characterization and Modeling of Carbonates–Mountjoy Symposium 1; SEPM (Society for Sedimentary Geology): Tulsa, OK, USA, 2017; Volume 109. [Google Scholar] [CrossRef]
- Medina-Carrasco, S.; Valverde, J.M. In situ XRD analysis of dolomite calcination under CO2 in a humid environment. CrystEngComm 2020, 22, 6502–6516. [Google Scholar] [CrossRef]
- Al-Jaroudi, S.S.; Ul-Hamid, A.; Mohammed, A.-R.I.; Saner, S. Use of X-ray powder diffraction for quantitative analysis of carbonate rock reservoir samples. Powder Technol. 2007, 175, 115–121. [Google Scholar] [CrossRef]
- Zhao, T.; Xu, C.; Ma, Y.; Zeng, Y.; Wang, N. Study on preparation and structure of chrysanthemum-shaped micron calcium carbonate based on inverse microemulsion. Micro Nano Lett. 2020, 15, 1151–1155. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, Z.; Hu, Y.; He, J.; Tian, M.; Zhou, J.; Zhou, Q.; Chen, S.; Chen, D.; Chen, P.; et al. Novel Insights into the Hydroxylation Behaviors of α-Quartz (101) Surface and its Effects on the Adsorption of Sodium Oleate. Minerals 2019, 9, 450. [Google Scholar] [CrossRef]
- Liu, R.; Mei, X.; Zhang, J.; Zhao, D.-B. Characteristics of clay minerals in sediments of Hemudu area, Zhejiang, China in Holocene and their environmental significance. China Geol. 2019, 2, 8–15. [Google Scholar] [CrossRef]
- Braid, H.; Taylor, K.; Hough, E.; Rochelle, C.; Niasar, V.; Ma, L. Hydrogen-induced mineral alteration: A review in the context of underground hydrogen storage (UHS) in saline aquifers. Earth-Sci. Rev. 2024, 259, 104975. [Google Scholar] [CrossRef]
- Davies, K.; Esteban, L.; Sarout, J.; Keshavarz, A.; Iglauer, S. Hydrogen-Induced Transformations in Dolomite: Unlocking Natural Hydrogen Exploration and Subsurface Storage in Carbonates. Energy Fuels 2025, 39, 6644–6653. [Google Scholar] [CrossRef]
- Opoku Duartey, K.; Ampomah, W.; Rahnema, H.; Mehana, M. Underground Hydrogen Storage: Transforming Subsurface Science into Sustainable Energy Solutions. Energies 2025, 18, 748. [Google Scholar] [CrossRef]
- Kharya, A.; Sachan, H.K.; Tiwari, S.K.; Singhal, S.; Singh, P.C.; Rai, S.; Kumar, S.; Mehta, M.; Gautam, P.K.R. New occurrence of albitite from Nubra valley, Ladakh: Characterization from mineralogy and whole rock geochemistry. Curr. Sci. 2016, 111, 1531–1535. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, J.; Xu, L.; Li, P.; Liu, Y.; Dang, W. Pore Structure and Fractal Characteristics of Deep Shale: A Case Study from Permian Shanxi Formation Shale, from the Ordos Basin. ACS Omega 2022, 7, 9229–9243. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhang, T.; Chen, X.; He, Y.; Liang, X. Model construction of micro-pores in shale: A case study of Silurian Longmaxi Formation shale in Dianqianbei area, SW China. Pet. Explor. Dev. 2018, 45, 412–421. [Google Scholar] [CrossRef]
- Truche, L.; Jodin-Caumon, M.-C.; Lerouge, C.; Berger, G.; Mosser-Ruck, R.; Giffaut, E.; Michau, N. Sulphide mineral reactions in clay-rich rock induced by high hydrogen pressure. Application to disturbed or natural settings up to 250 °C and 30 bar. Chem. Geol. 2013, 351, 217–228. [Google Scholar] [CrossRef]
- Gelencsér, O.; Árvai, C.; Mika, L.T.; Breitner, D.; LeClair, D.; Szabó, C.; Falus, G.; Szabó-Krausz, Z. Effect of hydrogen on calcite reactivity in sandstone reservoirs: Experimental results compared to geochemical modeling predictions. J. Energy Storage 2023, 61, 106737. [Google Scholar] [CrossRef]
- Yekta, A.E.; Pichavant, M.; Audigane, P. Evaluation of geochemical reactivity of hydrogen in sandstone: Application to geological storage. Appl. Geochem. 2018, 95, 182–194. [Google Scholar] [CrossRef]
- Liu, X.; Fu, M.; Gluyas, J.; Song, R.; Lan, H.; Fan, Y.; Wu, D. Dolomitization Facilitated by Clay Minerals on Mixed Siliciclastic-Carbonate Shoals of Carboniferous Age in the Tarim Basin, China: Constraints on Element Mobility and Isotope Geochemistry. Minerals 2025, 15, 419. [Google Scholar] [CrossRef]









| Mineral Phase | Crystal Plane—Miller Index (hkl) | (°) | Content % in Core A | Content % in Core AH |
|---|---|---|---|---|
| Chlorite (Ch) | 001 | 6.2 | 5.62 | - |
| Illite (I) | 001 | 8.9 | 2.84 | 2.41 |
| Quartz (Q) | 100 | 20.8 | 2.49 | 2.10 |
| Dolomite (D) | 012 | 24.1 | 1.92 | - |
| Quartz (Q) | 101 | 26.6 | 4.13 | 3.46 |
| Albite (A) | 112 | 27.92 | 2.03 | - |
| Calcite (C) | 104 | 29.4 | 1.32 | - |
| Dolomite (D) | 104 | 30.88 | 12.20 | 10.53 |
| Dolomite (D) | 110 | 37.25 | 1.31 | - |
| Dolomite (D) | 113 | 41.05 | 3.17 | 3.13 |
| Dolomite (D) | 202 | 44.85 | 2.04 | - |
| Dolomite (D) | 018 | 50.4 | 2.19 | 2.21 |
| Dolomite (D) | 116 | 50.9 | 2.68 | 2.84 |
| Mineral Phase | Crystal Plane—Miller Index (hkl) | (°) | Content % in Core B | Content % in Core BH |
|---|---|---|---|---|
| Vaterite (V) | 004 | 20.9 | 2.61 | - |
| Vaterite (V) | 110 | 24.8 | 3.87 | 3.43 |
| Vaterite (V) | 112 | 27.1 | 6.05 | 4.82 |
| Calcite (C) | 104 | 29.3 | 6.42 | 6.37 |
| Calcite (C) | 006 | 31.2 | 2.35 | - |
| Vaterite (V) | 114 | 32.7 | 5.13 | 5.44 |
| Calcite (C) | 113 | 39.38 | 1.66 | - |
| Calcite (C) | 202 | 43.2 | 1.11 | - |
| Vaterite (C) | 300 | 43.8 | 2.98 | 3.50 |
| Calcite (C) | 018 | 47.4 | 1.75 | 1.75 |
| Calcite (C) | 116 | 48.5 | 2.68 | 2.94 |
| Vaterite (V) | 224 | 55.7 | 1.27 | 1.64 |
| Calcite (C) | 122 | 57.3 | 0.52 | - |
| Mineral Phase | Crystal Plane—Miller Index (hkl) | (°) | Content % in Core B | Content % in Core BH |
|---|---|---|---|---|
| Chlorite (Ch) | 001 | 6.2 | 6.87 | 5.94 |
| Illite (I) | 001 | 8.9 | 2.62 | - |
| Chlorite (Ch) | 002 | 12.5 | 2.19 | 3.22 |
| Quartz (Q) | 100 | 20.8 | 3.57 | 3.78 |
| Quartz (Q) | 101 | 26.7 | 14.64 | 13.87 |
| Albite (A) | 112 | 27.92 | 2.58 | - |
| Calcite (C) | 104 | 29.4 | 3.90 | - |
| Quartz (Q) | 110 | 36.45 | 1.52 | 1.77 |
| Quartz (Q) | 102 | 39.5 | 1.92 | 1.68 |
| Quartz (Q) | 200 | 42.5 | 1.16 | 1.39 |
| Quartz (Q) | 201 | 45.9 | 1.30 | 1.52 |
| Quartz (Q) | 112 | 50.2 | 2.02 | 2.88 |
| Quartz (Q) | 211 | 60.2 | 1.50 | 1.64 |
| Core | Porosity (%) | Permeability (mD) | BET Surface Area (m2/g) | Total Pore Volume (cm3/g) | Mean Pore Diameter (nm) | Adsorbed N2 Volume (cm3/g) |
|---|---|---|---|---|---|---|
| A | 23.95 | 574.84 | 3.270 | 0.0214 | 3.3 | 14.376 |
| AH | 23.87 | 592.44 | 4.023 | 0.245 | 5.09 | 23.35 |
| B | 19.89 | 132.16 | 6.93 | 0.0175 | 3.023 | 26.11 |
| BH | 16.33 | 107.43 | 5.80 | 0.0143 | 2.853 | 21.17 |
| C | 24.50 | 581.96 | 19.048 | 0.0291 | 3.107 | 27.81 |
| CH | 24.13 | 567.43 | 18.87 | 0.0284 | 3.305 | 27.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doukeh, R.; Ghețiu, I.V.; Chiș, T.V.; Stoica, D.B.; Brănoiu, G.; Ramadan, I.N.; Gavrilă, Ș.A.; Petrescu, M.G.; Harkouss, R. Hydrogen–Rock Interactions in Carbonate and Siliceous Reservoirs: A Petrophysical Perspective. Appl. Sci. 2025, 15, 7957. https://doi.org/10.3390/app15147957
Doukeh R, Ghețiu IV, Chiș TV, Stoica DB, Brănoiu G, Ramadan IN, Gavrilă ȘA, Petrescu MG, Harkouss R. Hydrogen–Rock Interactions in Carbonate and Siliceous Reservoirs: A Petrophysical Perspective. Applied Sciences. 2025; 15(14):7957. https://doi.org/10.3390/app15147957
Chicago/Turabian StyleDoukeh, Rami, Iuliana Veronica Ghețiu, Timur Vasile Chiș, Doru Bogdan Stoica, Gheorghe Brănoiu, Ibrahim Naim Ramadan, Ștefan Alexandru Gavrilă, Marius Gabriel Petrescu, and Rami Harkouss. 2025. "Hydrogen–Rock Interactions in Carbonate and Siliceous Reservoirs: A Petrophysical Perspective" Applied Sciences 15, no. 14: 7957. https://doi.org/10.3390/app15147957
APA StyleDoukeh, R., Ghețiu, I. V., Chiș, T. V., Stoica, D. B., Brănoiu, G., Ramadan, I. N., Gavrilă, Ș. A., Petrescu, M. G., & Harkouss, R. (2025). Hydrogen–Rock Interactions in Carbonate and Siliceous Reservoirs: A Petrophysical Perspective. Applied Sciences, 15(14), 7957. https://doi.org/10.3390/app15147957








