1. Introduction
The spread of bacterial resistance to antibiotics and the associated health risks is a real and growing problem worldwide [
1,
2,
3,
4]. This is increasingly the case in a variety of environments, not just medical ones [
3,
4]. The possibility of the air-mediated transmission of agents responsible for antibiotic resistance has attracted attention in recent years. This phenomenon involves both resistance genes and resistant microorganisms, as confirmed by numerous research [
3,
5,
6]. For example, Wang et al. [
6] detected significant amounts of ampicillin-resistant bacteria (accounting for more than half of all the culturable bacteria) and vancomycin-resistant bacteria (almost one-quarter of the culturable bacteria) in bioaerosols. On the other hand, do Nascimento et al. [
7] found antibiotic resistance genes such as
mecA,
blaZ,
ermA,
ermB,
ermC in strains of
Staphylococcus bacteria isolated from air samples. Means of transport are among those places where increased exposure of people to microbial agents of air pollution can occur [
8,
9,
10,
11,
12]. A study by Jo and Lee [
13] found the presence of bacteria in a car air conditioning system in numbers as high as 2.6 × 10
3 CFU/m
3. Frąk et al. [
12] observed that the fungal concentrations in vehicle air reached 8.4 × 10
3 CFU/m
3 while bacteria concentrations accounted for about 1.7 × 10
4 CFU/m
3. It should also be noted that more than half of the bacteria and fungi isolated from the vehicle’s indoor air represent the respirable fraction, easily penetrating into the respiratory tract [
14]. This is of particular importance in view of the fact that, according to various reports, we spend an average of 45–90 min per day inside vehicles [
15,
16], and more than 4 years in a lifetime [
17].
Nowadays, the vast majority of newly manufactured cars are equipped with an air conditioning system as standard [
10,
18]. Despite the undoubted benefits of improved travel comfort, this type of installation can be a site of accumulation and secondary emission into the air of undesirable microflora, including potentially pathogenic microorganisms [
10,
18,
19,
20,
21,
22]. The phenomenon of biofilm formation inside the installation can result in an increase in the level of air pollution entering the cabin [
20]. On the other hand, the ability of microorganisms to form biofilms significantly facilitates the spread of drug resistance through gene transfer [
1].
The space inside a passenger car is a very specific indoor environment, with its relatively small area, separated from the external environment most of the time. The contaminated air conditioning system, which is a source of emissions of various substances and microorganisms, in view of the small distances inside the cabin, increases the intensity of exposure of the driver and vehicle passengers to potentially dangerous agents [
12,
18,
20,
21,
23]. The car cabin environment thus becomes a site for the potential spread of pathogenic microorganisms [
24,
25]. Contaminants of various natures can accumulate in cabin filters [
18], as well as inside ductwork in the system and in its individual components like air coolers [
26,
27]. It has been found that with prolonged use of the filter (more than two years), the microbiological quality of the air inside the vehicle drastically decreases [
12]. Studies involving analysis of the movement of PM
2.5 particles inside the vehicle have shown that the ventilation system induces themixing of air in the confined space of the cabin, resulting in similar exposure to pollutants, regardless of the place occupied in the vehicle [
28].
The indoor air quality in vehicles is an outcome of the air conditioning operational parameters [
29], intensity of use of the vehicle [
30], the frequency and timeliness of filter replacement and disinfection of the air conditioning unit [
10,
12,
19,
22,
31]. Still, virtually nothing is known about the relationship between the type of filter and the potential occurrence of antibiotic-resistant bacteria in it.
In recent years, the first research results were published confirming the possibility of the presence of drug-resistant bacteria and fungi, as well as genes, determining antibiotic resistance in air-conditioning systems [
32,
33,
34,
35]. The research concerned mainly Asian countries and very occasionally Western European countries. Unfortunately, such data are still lacking for the Central Europe area. As mentioned above, the relationship between the type of cabin filters used and the possibility of development of viable antibiotic-resistant bacteria in them has also not been recognized.
In the present study, a quantitative study of microorganisms found in the cabin filters of cars of five different brands was carried out, with a special focus on bacteria resistance to selected commonly used antibiotics: penicillin, nitrofurantoin, rifampicin, doxycycline, and gentamicin. The level of contamination with antibiotic-resistant bacteria onactivated carbon filters and filters with manufacturer-declared antibacterial properties was compared. In addition, for selected bacteria isolated from the filters, tests were performed to estimate the value of the minimum inhibitory concentration of the tested antibiotics.
2. Materials and Methods
The samples tested were cabin filters from vehicles of five different brands: Toyota, Subaru, Suzuki, Hyundai, and Volkswagen. The vehicles were mainly operated in the area of Central Europe. The filters were obtained during their periodic replacement at authorized service stations of the brands in Warsaw. Among the 20 filters tested were both filters with activated carbon and those equipped with an additional layer providing antibacterial properties.
The pieces of filter material taken from several separate places of each filter were stored in sterile containers and then transported to the laboratory where they were subjected to microbiological testing.
Sample preparation for quantitative studies consisted of placing fragments of filter material with a total weight of 2 g in 100 mL of sterile sodium pyrophosphate and then shaking for 30 min at 120 rpm to flush out the microorganisms contained in the filter. The obtained microbial suspension was inoculated in parallel onto two culture media: nutrient agar (Biocorp Ltd., Warsaw, Poland), for the determination of the total number of bacteria, and Rose Bengal Chloramphenicol medium (Biocorp Ltd., Warsaw, Poland), for the quantitative analysis of molds. Bacteria were cultured for 48 h, simultaneously at 26 °C and 37 °C, while fungi were cultured for 5 days at 26 °C. The number of microorganisms was calculated as the number of colony forming units—CFU/g of the filter.
In parallel with the determination of the total number of microorganisms, surface culture of the test suspension was performed on nutrient agar medium with the addition of individual antibiotics: penicillin, nitrofurantoin, doxycycline, rifampicin, and gentamicin, at concentrations of 100 mg/L. The cultures were incubated at 26 °C for 48 h and then the number of bacterial colonies capable of growth in the presence of antibiotics was determined. The result was reported as the number of CFU/g of the filter. The percentage of antibiotic-resistant bacteria was determined by comparing bacterial counts on individual selective media with antibiotics with the total number of bacteria in the sample.
In order to obtain pure isolates of antibiotic-resistant bacteria, the colonies of bacteria were selected from among those obtained on media with the addition of individual antibiotics, and purified by passaging using a streak plate method.
Identification of the isolates was carried out using API (Bio-Merieux Poland, Ltd., Warsaw, Poland) biochemical tests and sequencing methods. Samples for sequencing were prepared at A&A Biotechnology, Gdansk, Poland. Genomic DNA was isolated by a method based on the Genomic Mini AX Bacteria+ kit (A&A Biotechnology) with additional mechanical lysis of the sample in a FastPrep-24-type device using zirconia beads. The PCR reaction mixture contained 25 μL of PCR Mix Plus (A&A Biotechnology), 0.2 μL each of FOR and REV primers and 1 μL of test DNA. Pre-denaturation was carried out at 94 °C for 120 s, followed by 30 cycles of denaturation, primer connection, and extension, at 94 °C, 58 °C, and 72 °C, respectively, and a final extension phase for 300 s. PCR products were then purified using Gel-Out Kit (A&A Biotechnology), resuspended in 10 mM Tris-HCl buffer pH 8.0, and sent for sequencing to Macrogen (Amsterdam, The Netherlands). The resulting sequences posted were analyzed in CLC Main Workbench 22 and in BLAST software (
http://blast.ncbi.nlm.nih.gov: rRNA/ITS databases, accessed on 17 June 2025).
Determination of inhibitory concentrations of individual antibiotics against selected bacterial isolates was carried out by the dilution method. A series of solutions of individual antibiotics in twice-diluted nutrient broth (Biocorp Ltd., Warsaw, Poland) were prepared to obtain test media for microbial culture. Two test series were carried out. In the first series, antibiotics were used at concentrations of: 250 mg/L, 125 mg/L, 62.5 mg/L, 31.25 mg/L, and 15.63 mg/L. In the second series of tests, a range of concentrations was selected depending on the results obtained in the first series, in order to refine the value of the inhibitory concentration, in the range of 5 mg/L to 250 mg/L; for concentrations below 100 mg/L, the difference between successive dilutions was assumed to be 2.5 mg/L, while above 100 mg/L, the difference was 25 mg/L. The concentration ranges used in the dilution tests corresponded to the values given as the dosages used to define breakpoints according to EUCAST Clinical breakpoints tables, version 15.0 (for example: 100 mg for doxycycline, 50–100 mg for nitrofurantoin). Bacterial cultures in such prepared media were carried out for 48 h at 26 °C. Microbial growth was assessed by the spectrophotometric measurement of the optical density of the cultures at 600 nm.
3. Results and Discussion
Automotive cabin filters represent very good material for the study of microbial air pollution, because of the possibility of quantitative extraction of bioaerosol components from the filtration material [
36]. This approach allows for the analysis of air conditioning systems working under actual operating conditions, with different strategies of system operation and under varying environmental parameters, thus positively influencing the reliability of the research results and the completeness of knowledge about the inhabiting microflora [
22].
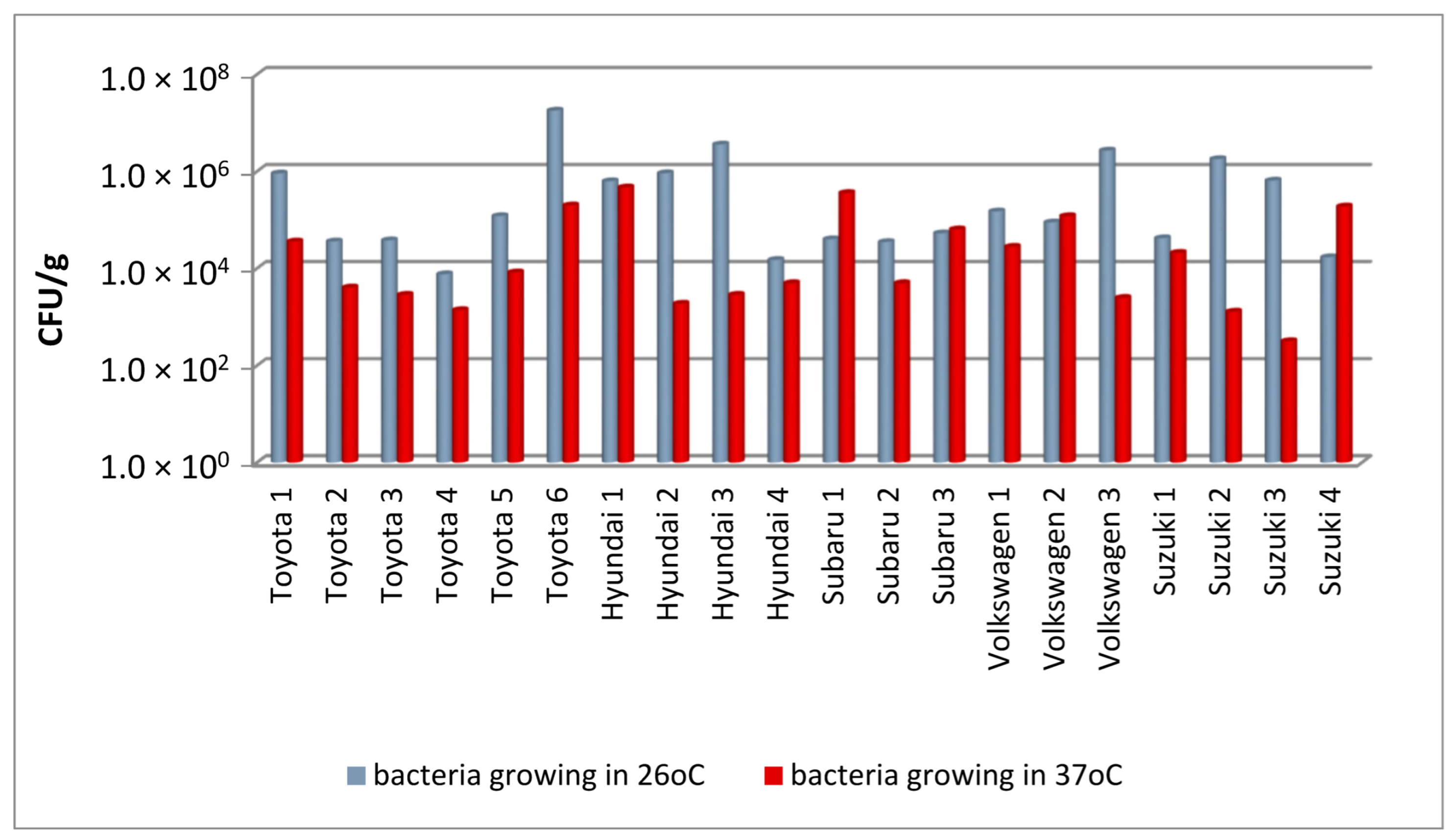
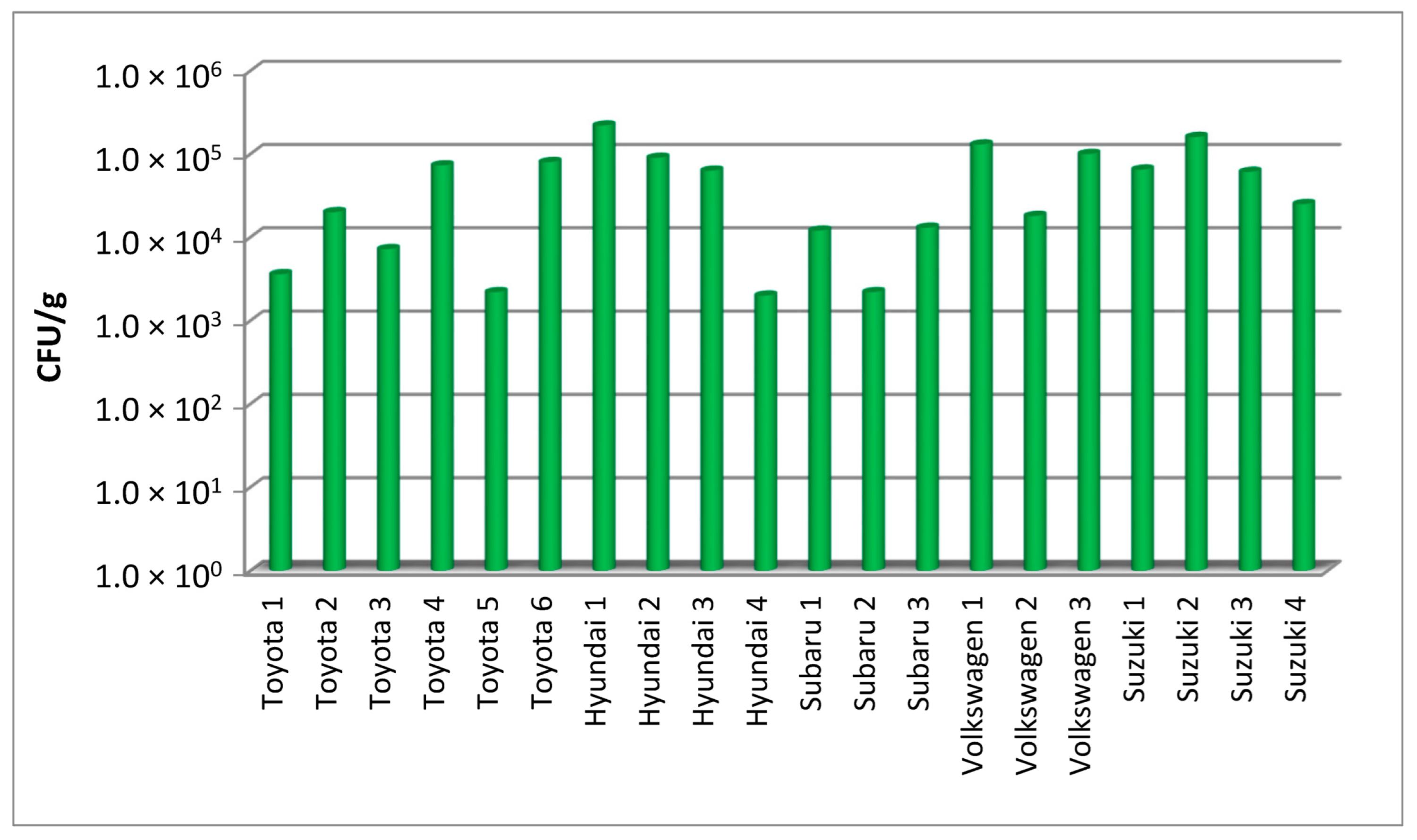
In this study, filters from cars of five different brands, obtained during periodic replacement of filters, were examined. The results of the quantitative assessment of the number of bacteria and fungi in the filters from each vehicle are shown in
Figure 1 and
Figure 2, while their summary for each car brand is shown in
Table 1.
A qualitative analysis showed that the number of bacteria growing at 26 °C varied greatly, even in the case of filters from vehicles of the same brand (
Figure 1). For example, in Toyota cars, it ranged from 7.6 × 10
3 to 1.8 × 10
7 CFU/g of filter material (these were both the highest and lowest values of this parameter in this assessment). The bacterial concentrations were significantly higher than the bacterial numbers detected in cabin filters by Viegas et al. [
32], which did not exceed 4.7 × 10
4 CFU/m
2 of the filter. Similar discrepancies in terms of the level of microbial contamination were recorded for bacteria capable of growing at human body temperature, the content of which can provide indirect information on the presence of potentially pathogenic microorganisms. The abundance of bacteria growing at 37 °C was between 3.2 × 10
2 and 4.7 × 10
5 CFU/g. The large discrepancies in the results obtained for individual samples are reflected by the high standard deviation values for all tested car brands (
Table 1). The quantitative analysis also covered the content of molds in the filters, showing the contamination of the filters with these microorganisms at a level of 10
3–10
5 CFU/g, which is slightly lower than for the bacteria cultured at the same temperature (
Figure 2).
Existing literature data indicate that the dust collected from filters from air conditioning units may serve as test material providing important information on the nature and quantity of airborne pollutants [
37]. It was shown that for automotive ventilation and air-conditioning systems, the culturable microbial counts in the dust were 4 × 10
6 for bacteria and 5 × 10
5 CFU/g for fungi [
35]. The analogous values obtained by Li et al. [
8] were 2.6 × 10
4 CFU/g and 1.3 × 10
4 CFU/g, respectively. The results obtained in the present study show that the analysis of fragments of filter material as a whole makes it possible to detect even more microorganisms than when analyzing dust from air-conditioning systems alone. A similar microflora extraction technique was used successfully in a study on cabin filters carried out by Viegas et al. [
32].
In Poland, it is recommended to replace cabin filters after a mileage of 10–15 km or once a year [
12]. The service life of the filters used in this study corresponds to these guidelines, as they were obtained during periodic (annual) vehicle inspections at authorized service centers for each brand. This makes it possible to estimate the comparatively highest level of filter contamination, at the end of their life, and possibly assess the resulting risks.
The main objective of the present study was to assess the presence of bacteria showing resistance to several selected antibiotics in the tested cabin filters. The study used the culture method, which, allowing to determine the abundance of culturable bacteria, is effectively used in quantitative studies of airborne microorganisms in the indoor environment, including the vehicles cabins [
6,
9,
19,
21,
29]. Standard spread plate method was used by Zaman et al. [
35], among others, to study the microflora present in dust from automotive cabin filters. They observed that bacteria isolated from filter dust from air conditioning system of cars operated in an urban area in Lahore, Pakistan, showed resistance to ampicillin and cefoxitin, while they were sensitive to ciprofloxacin [
35].
Admittedly, a culture-based method, unlike molecular techniques, does not allow the assessment of the potential of spreading drug resistance due to gene transfer from dead bacterial cells [
1]. It may also cause some problems with distinguishing between the transient and resident populations of antibiotic-resistant microorganisms [
38]. On the other hand, there is evidence that the presence of a specific resistance determinant gene does not always result in a specific phenotypic effect. For example, it was found that a strain of
Pseudomonas fluorescens bacteria carrying the
vanB gene did not phenotypically show resistance to vancomycin, while
P. fluorescens and
P. aeruginosa equipped with the
qepA gene nevertheless did not show phenotypic resistance to ciprofloxacin [
6]. Obtaining antibiotic-resistant bacterial isolates in culture allows for their further testing to determine the resistance profile against selected antibiotics, which was also one of the areas of the present study.
However, the optimal way seems to be a combination of the two approaches, which is confirmed by studies on not only cabin filters, but also filters from air conditioning systems in various types of objects. Viegas et al. [
32] used a culture-based method combined with molecular analysis of fungal isolates in a study of automotive cabin filters (from 28 private cars and 19 cabs) used in urban areas in Portugal (three cities, including Lisbon). They revealed that slightly higher levels of bacterial contamination were found in cabs (up to 4.7 × 10
4 CFU/m
2) than in private cars (up to1.7 × 10
4 CFU/m
2), with bacteria detected in more than 60% of taxis and more than 25% of private cars. Interestingly, no Gram-negative bacteria were detected in the filters tested. However, studying the fungal microfloraallowed the authors to isolate seven azole-resistant fungal strains belonging to the genera
Penicillium,
Cladosporium, and
Aspergillus.
The combination of culture-based methods and molecular analysis of antibiotic resistance genes was also applied by Li et al. [
34] during the study of dust samples from air conditioning systems in hospitals. They revealed the contamination of the samples with antibiotic resistance genes. In parallel, they obtained some isolates of resistant bacteria on media with antibiotics such as ampicillin, chloromycetin, meropenem, kanamycin, tetracycline or erythromycin. The study confirmed the presence of both resistant bacteria and antibiotic resistance genes in the samples and the possibility of their accumulation in hospital air conditioning systems.
Molecular analysis in the form of high-throughput qPCR with illumina sequencing was used by Li et al. [
33] in a study including 12 samples of dust collected from air conditioning filters from different environments: hospitals, urban and rural residences, and farms. The techniques they used allowed them to determine the antibiotic resistance profile in the samples, including the frequency of resistance genes in each type of facility. A total of 177 different antibiotic resistance determinant genes were detected, mostly in samples from farms and rural buildings. Molecular biodiversity studies revealed a predominance of Proteobacteria in dust from tested air conditioning systems. Bacteria belonging to Proteobacteria, Actinobacteria, and Chloroflexi were identified as the most common hosts of antibiotic-resistant genes, with Proteobacteria and Actinobacteria being the most frequently involved in multi-resistance.
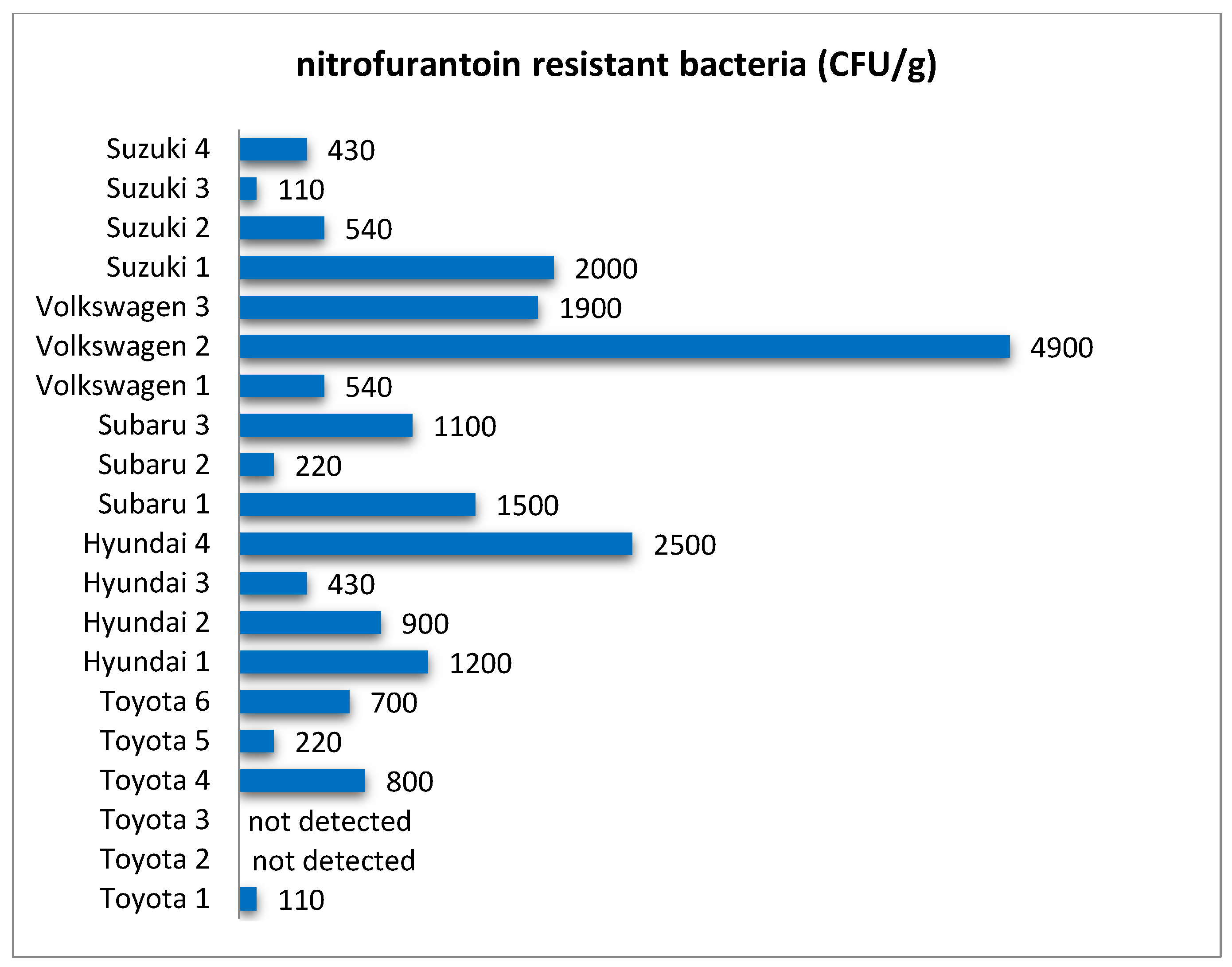
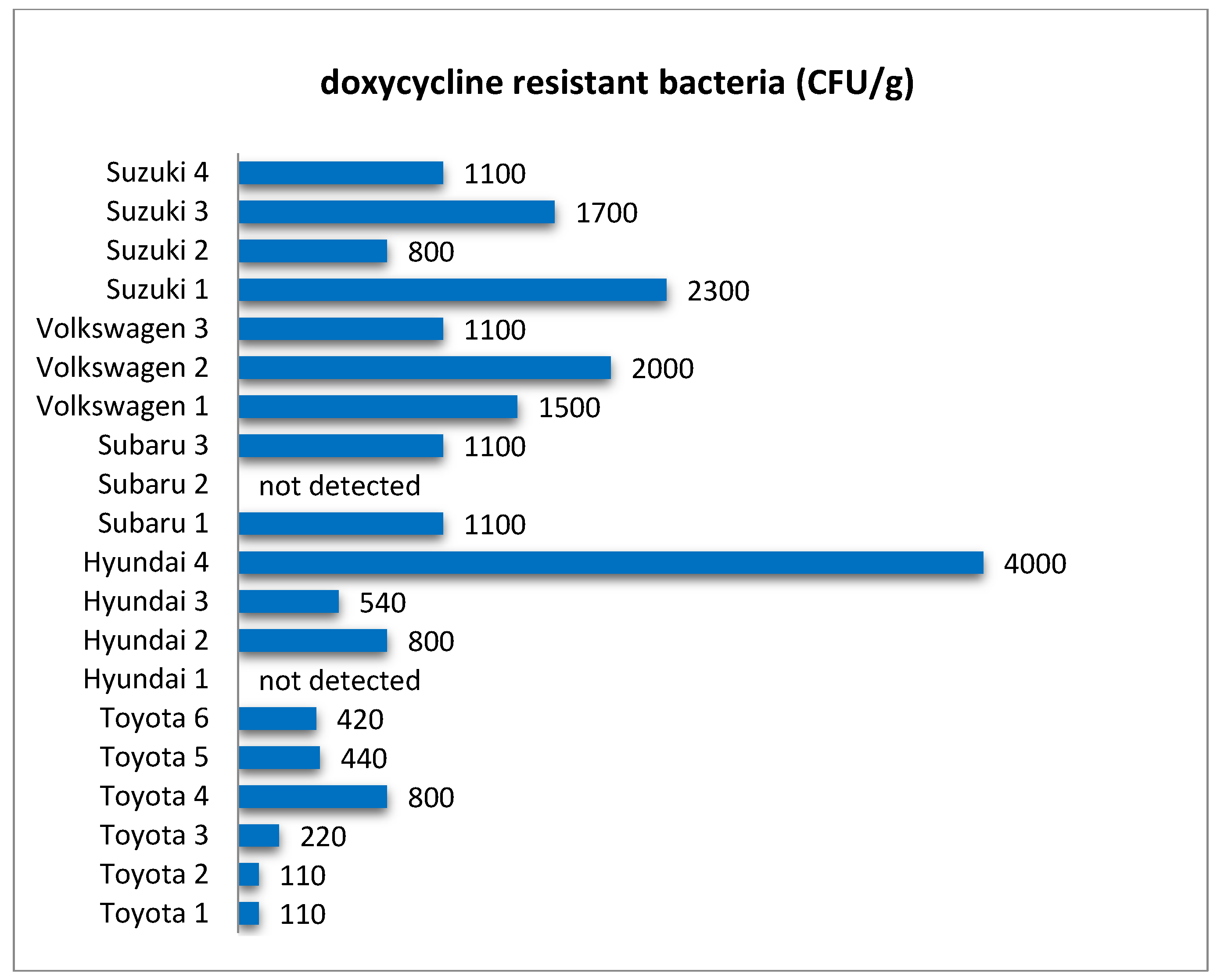
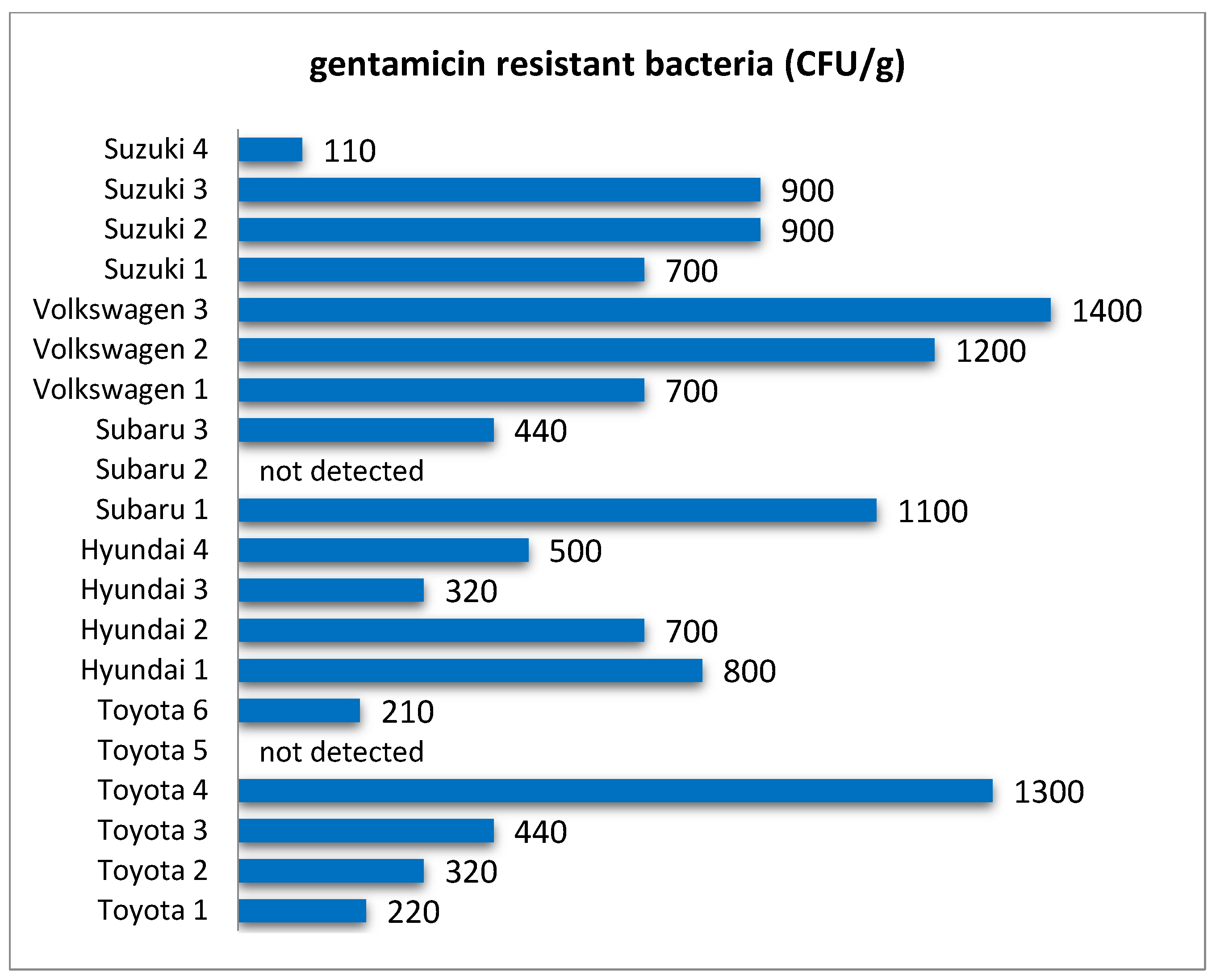
In this research, quantitative analysis of bacteria resistant to selected antibiotics showed that these microorganisms were common in filters from all analyzed car brands (
Figure 3,
Figure 4,
Figure 5,
Figure 6 and
Figure 7) and their abundance was at the level of 10
2–10
3 CFU/g. One of the reasons for this can be the relatively high level of standardization in the production of cabin filters and the fact that a given manufacturer offers filters with similar properties but dedicated to different brands of vehicles. This makes the possible level of contamination a matter of how the vehicle is used rather than the brand. As in the case of the total number of bacteria and fungi, there were quite large differences in the concentration of antibiotic-resistant bacteria in filters from individual vehicles (
Table 2). Both the total level of microbial contamination of filter materials and the content of antibiotic-resistant bacteria turned out to be a very individual matter, and in fact, each vehicle could be considered as a separate case. It is likely that much here is due to the varying conditions and locations of each vehicle’s operation. In particular, it is difficult to explain the incidentally occurring exceptionally high numbers of bacteria resistant to the antibiotics tested in individual vehicles, such as for nitrofurantoin in Volkswagen 2, rifampicin in Volkswagen 1, doxycycline in Hyundai 4, or penicillin in Subaru 3.
In some cases, these bacteria were not detected, which may have been due to the range of detection resulting from the culture technique. Such results were marked in the figures as “not detected”, while also including the situation when the abundance of bacteria tested was below 100 CFU/g.
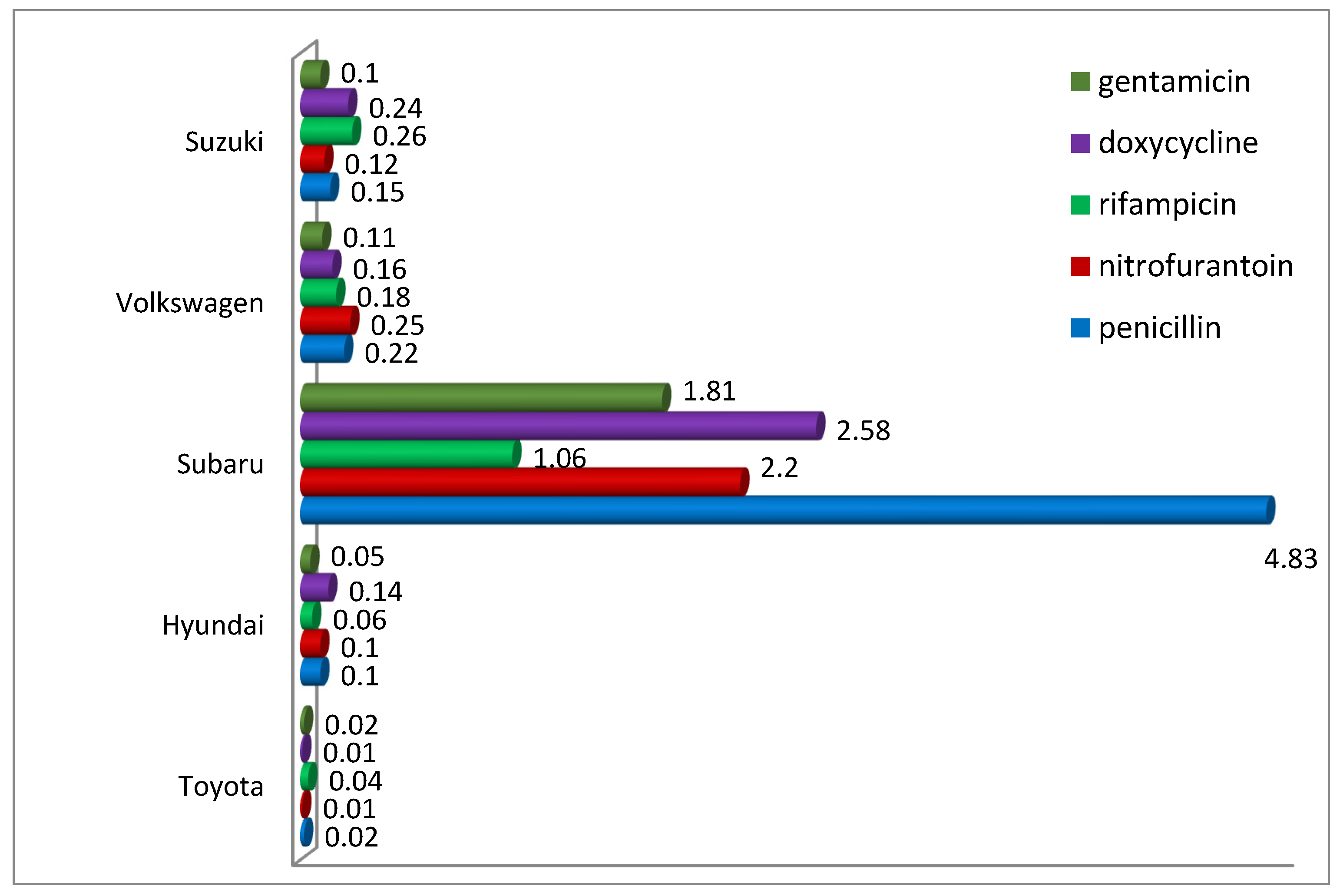
Analysis of the percentage of antibiotic-resistant bacteria in the total number of bacteria provided interesting results (
Figure 8). It showed significant differences between filters from tested car brands. For example, penicillin-resistant bacteria accounted for 0.02% in filters from Toyota cars to as much as 4.83% in filters from Subaru, while it should be noted that the observed differences were mainly due to the difference in the total number of bacteria and not to a higher number of resistant microorganisms. It is also confirmed by comparing the filters from Subaru with those from Hyundai and Volkswagen, where the abundance of penicillin-resistant bacteria was similar, while their percentage differed even several times. A similar relationship was also observed for bacteria resistant to other antibiotics. This may suggest that in some situations, despite the limited abundance of bacteria in the filter material as a result of the technical solutions used, we may still have to deal with relatively abundant bacteria showing antibiotic-resistant characteristics.
As confirmed by numerous literature data, filter materials used in air conditioning systems can harbor a diverse bacterial microflora including representatives of Proteobacteria, Firmicutes, Bacteroidetes, Actinobacteria, and Cyanobacteria [
31]. Bacterial taxa that are indicated to be associated with air conditioning systems include the genera
Sphingomonas,
Burkholderia,
Bacillus,
Alcanivorax,
Stenotrophomonas [
26],
Pseudomonas [
39], and
Bacillus [
31]. The latter, due to their relatively large size, can be effectively retained and accumulated in the filter material [
29].
Studies of the indoor air of vehicles confirm the presence of a diverse microflora, with bacteria from the genera
Micrococcus,
Kocuria,
Staphylococcus, and
Bacillus appearing as constant bioaerosol components [
30], as well as Gram-negative bacteria from the genera
Comamonas,
Pseudomonas, and
Serratia [
29]. Li et al. [
8] found that bacteria of the genus
Pseudomonas were the dominant microflora in dust from the air conditioning system. This is consistent with the results obtained in the present study, where identification using biochemical tests and molecular analysis confirmed that the isolated antibiotic-resistant bacteria belonged to the genera
Pseudomonas and
Brevundimonas.
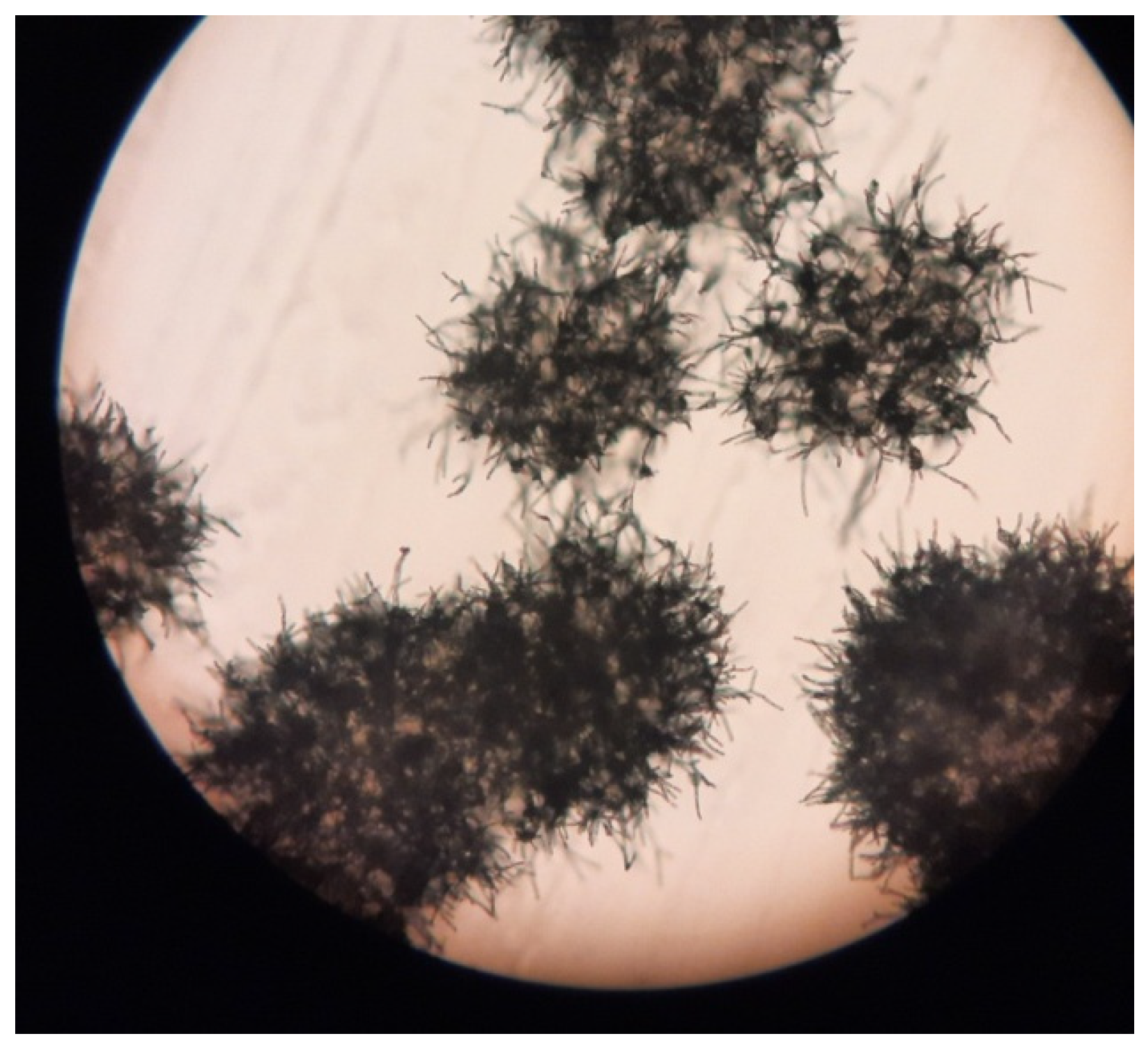
In addition, antibiotic-resistant actinomycetes were also found in the filter material (
Figure 9); however, it was not possible to obtain pure strains, so further studies focused on isolates of
Pseudomonas sp.
It should be pointed out that the environment of a cabin filter is very specific, due to highly variable operating conditions, sometimes a significant degree of air pollution and air flow—both fresh and recycled. This creates very specific conditions for the growth of microorganisms, particularly limiting their viability, especially in the case of pathogenic microorganisms, which usually live in more stable conditions. Therefore, over the course of the study, we were able to isolate and purify only a few strains, as most of them were passable with very low efficiency. Furthermore, only a few of them were able to be maintained in good enough condition to obtain meaningful results from biochemical tests and to keep pure isolates as a source of DNA for sequencing.
In our research, we mainly focused on the environmental context and the possibility of the presence of antibiotic-resistant bacteria in car cabin filters. However, it should be noted that in our research, the bacteria of the genus
Pseudomonas appeared to be dominant and most resistant not only to antibiotics but also to environmental conditions. This is of particular importance from the point of view of health risks, since it is among the representatives of this genus that there are numerous microorganisms which are opportunistic pathogens of humans, animals, and plants. Moreover, they are also characterized by high genetic potential and adaptability to different environments, and, importantly from the point of view of their role in the spread of antibiotic resistance—they present great ease when it comes to horizontal gene transfer [
40].
Selected isolates of antibiotic-resistant bacteria belonging to the genus
Pseudomonas were used to estimate the values of concentrations of tested antibiotics that cause inhibition of the growth of these microorganisms. Minimal inhibition concentrations were determined, and the results are presented in
Table 3. The results clearly demonstrate that the strains capable of colonizing filter materials were able to grow in the presence of antibiotic concentrations even higher than those used for their isolation in the culture tests (100 mg/L), particularly with respect to resistance to penicillin and nitrofurantoin. A strain obtained from a Subaru-derived filter proved to be more resistant, especially towards rifampicin. The obtained values were also much higher than the minimum inhibitory concentrations of antibiotics for
Pseudomonas spp. strains according to EUCAST, which are in the range of 2–8 mg/L for penicillins, 0.5–2 mg/L for fluoroquinolones, 2–16 for aminoglycosides.
The use of filters to treat the air inside the air conditioning system is currently one of the simplest solutions when it comes to ensuring adequate air quality in the internal environment of the car cabin [
36]. Cabin filters should be characterized by high efficiency in eliminating contaminants present in the air, low pressure drop, low sensitivity to changes in temperature and humidity [
41]. A factor that increases the filtration surface and the ability to remove very fine particles is the use of a layer containing activated carbon in the filter material [
22,
41]. Additionally, limiting the growth of microorganisms can be achieved by coating the filter surface with specially adapted nanocomponents containing, among other things, metal oxides such as CuO. The latter solution was proposed by Perelshtein et al. [
42], proving the effectiveness of such a prepared filter against
Escherichia coli and
Staphylococcus aureus bacteria as well as some viruses, including H1N1 influenza virus and SARS-CoV-2.
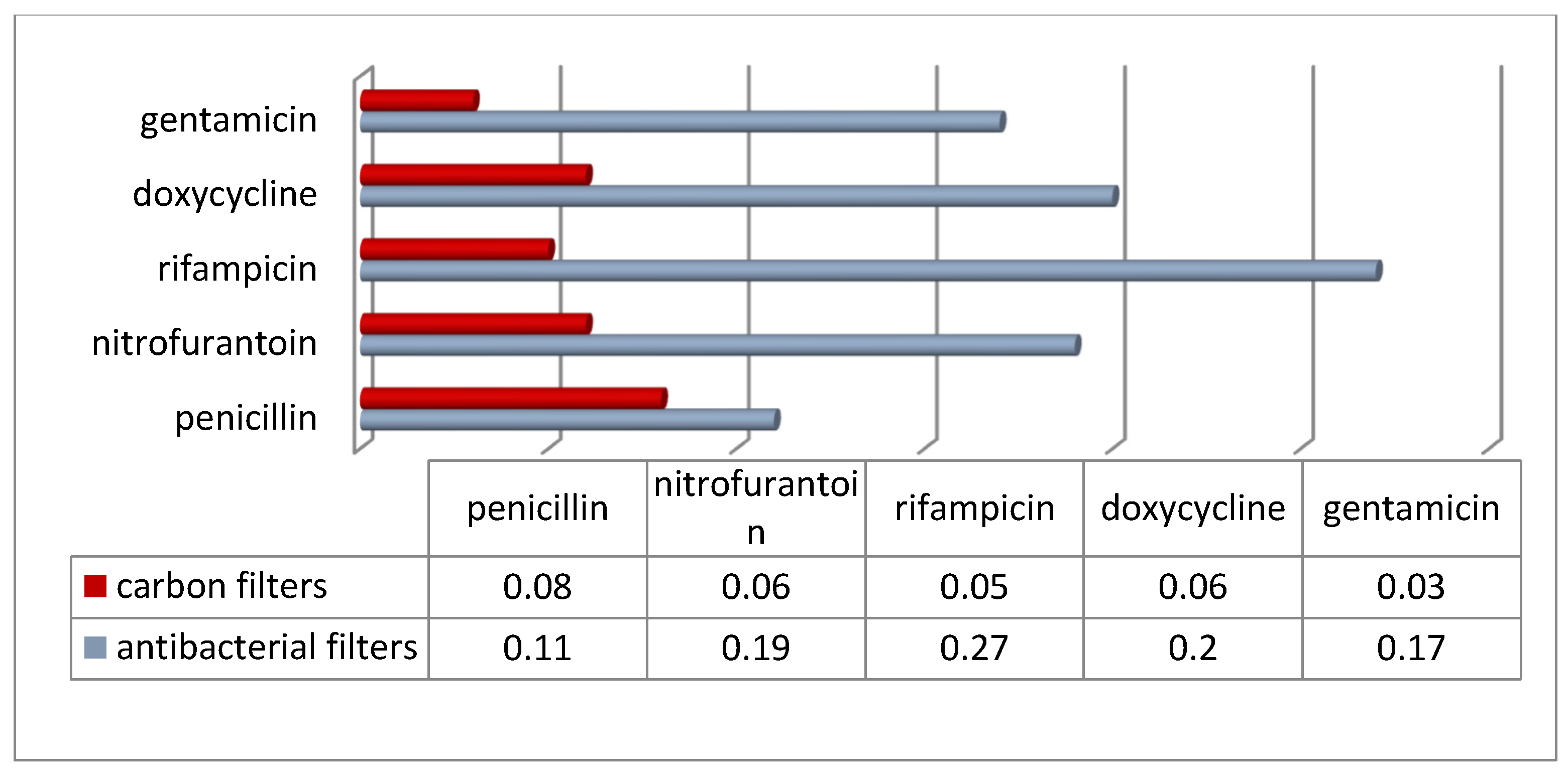
The structure and composition of filter materials is usually the subject of a patent claim [
43], which makes it difficult to interpret the experimental data obtained in the context of conditioning the performance of commercially available filters. The present study attempted to compare the abundance of antibiotic-resistant bacteria depending on the type of cabin filter. Two groups of filters were analyzed: those with activated carbon (referred to as “carbon” in the following description) and those equipped (according to the manufacturer’s declaration) with antibacterial and anti-allergic properties (referred to as “antibacterial”). The results obtained are shown in
Figure 10 and
Figure 11. The abundance of antibiotic-resistant bacteria in filters with antibacterial properties was lower compared to filters equipped only with a layer of activated carbon. On the other hand, interesting results were obtained when trying to compare the percentage of bacteria resistant to particular antibiotics in relation to the total number of bacteria. Namely, it was observed that in view of the significantly lower total bacterial count in antibacterial filters (4.4 × 10
5 CFU/g compared to 2.2 × 10
6 CFU/g in carbon filters), the percentage of antibiotic-resistant bacteria in antibacterial filters turned out to be even several times higher. This may suggest that techniques to reduce the number of microorganisms in cabin filters may not be as effective at eliminating drug-resistant microorganisms.