Diagnostic Accuracy and Agreement Between AI and Clinicians in Orthodontic 3D Model Analysis
Abstract
1. Introduction
2. Materials and Method
2.1. Study Design
2.2. Inclusion Criteria
- Presence of fully erupted permanent teeth from central incisors to first molars
- High-quality intraoral scans showing properly captured teeth and gingiva
2.3. Exclusion Criteria
- Presence of primary teeth or extensively decayed teeth
- Crowns, bridges, or restorative dental work affecting tooth anatomy
- Models with faulty occlusion or incomplete scans
- Scans containing major defects or large gaps
2.4. Measurements
- Bolton analysis (overall and anterior)
- Overjet and overbite measurements
- Space analysis
- Angle classification (right and left)
2.5. Ethical Approval
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
- AI-based Titan Dental Design showed almost perfect agreement with orthodontists in molar classification, indicating strong reliability for simple categorical assessments.
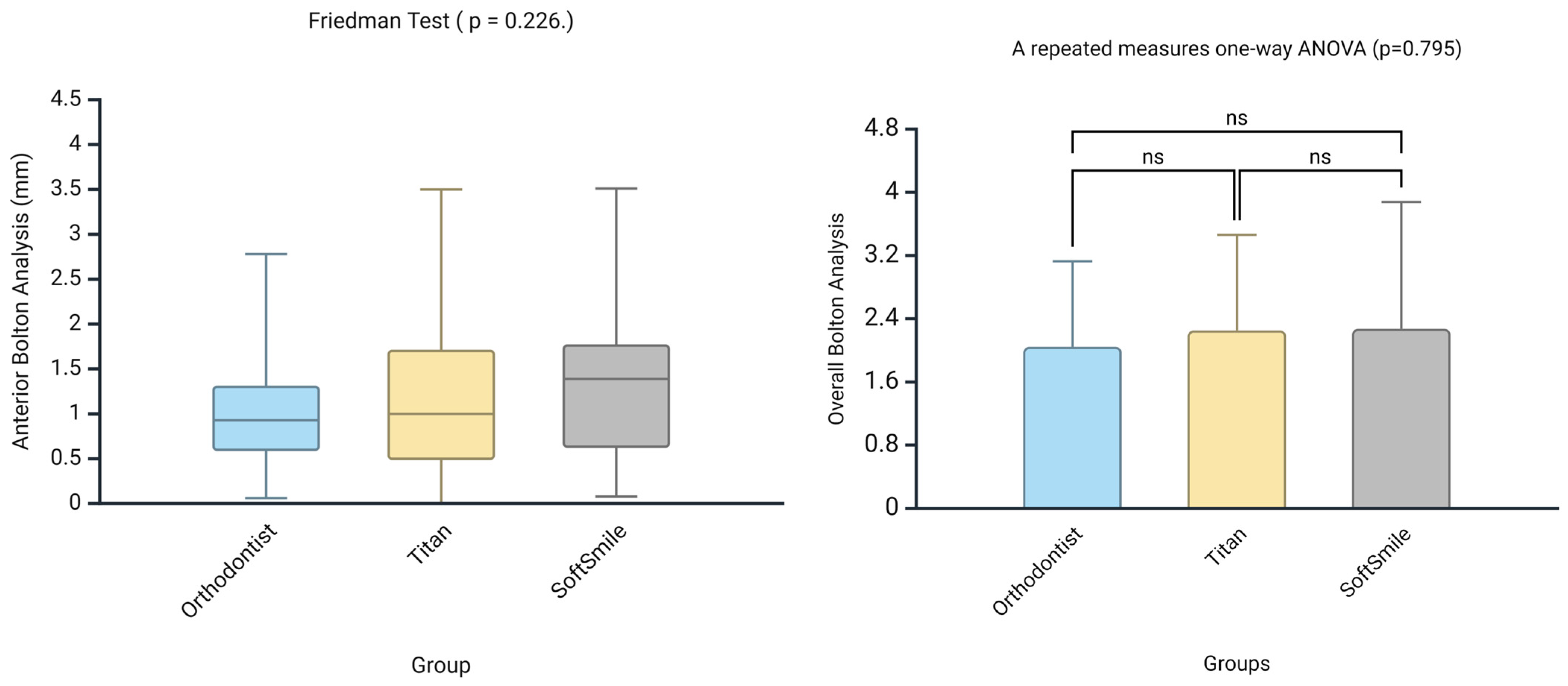
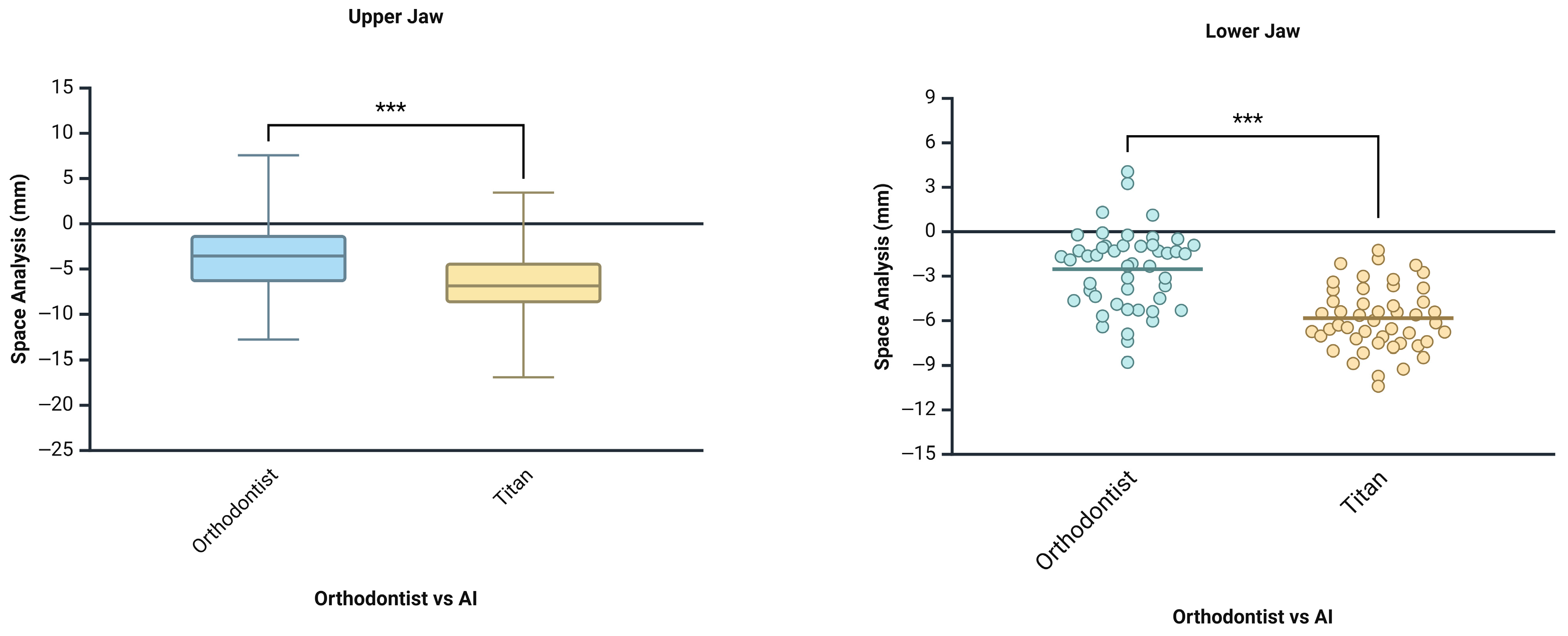
- Although both AI systems showed limited agreement with the orthodontist in identifying the location of Bolton discrepancies, when the location of tooth size excess (maxillary or mandibular) was consistent across all groups, the numerical measurements were comparable, with no statistically significant differences observed.
- AI platforms may have limitations in accurately detecting key measurement points, such as mesiodistal tooth widths, which can affect the precision of tooth size discrepancy analyses.
- Until further improvements are made, such AI-based analyses should be interpreted with caution and should not replace clinical judgment in orthodontic decision-making.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaushik, K.; Bhatt, S.; Gupta, R.Y.; Patadiya, H.H.; Luthra, A.; Rao, K.A. Intraoral Scanner in Dentistry: A Comprehensive Review. J. Adv. Med. Dent. Sci. Res. 2025, 13, 57–61. [Google Scholar]
- Rheude, B.; Sadowsky, P.L.; Ferriera, A.; Jacobson, A. An evaluation of the use of digital study models in orthodontic diagnosis and treatment planning. Angle Orthod. 2005, 75, 300–304. [Google Scholar]
- Sehrawat, S.; Kumar, A.; Grover, S.; Dogra, N.; Nindra, J.; Rathee, S.; Dahiya, M.; Kumar, A. Study of 3D scanning technologies and scanners in orthodontics. Mater. Today Proc. 2022, 56, 186–193. [Google Scholar] [CrossRef]
- Fujiyama, K.; Honjo, T.; Suzuki, M.; Matsuoka, S.; Deguchi, T. Analysis of pain level in cases treated with Invisalign aligner: Comparison with fixed edgewise appliance therapy. Prog. Orthod. 2014, 15, 1–7. [Google Scholar] [CrossRef]
- Miller, K.B.; McGorray, S.P.; Womack, R.; Quintero, J.C.; Perelmuter, M.; Gibson, J.; Dolan, T.A.; Wheeler, T.T. A comparison of treatment impacts between Invisalign aligner and fixed appliance therapy during the first week of treatment. Am. J. Orthod. Dentofac. Orthop. 2007, 131, 302.e1–302.e9. [Google Scholar] [CrossRef]
- Ruiz, D.C.; Mureșanu, S.; Du, X.; Elgarba, B.M.; Fontenele, R.C.; Jacobs, R. Unveiling the role of artificial intelligence applied to clear aligner therapy: A scoping review. J. Dent. 2025, 154, 105564. [Google Scholar] [CrossRef]
- Anwar, S.M.; Majid, M.; Qayyum, A.; Awais, M.; Alnowami, M.; Khan, M.K. Medical image analysis using convolutional neural networks: A review. J. Med. Syst. 2018, 42, 1–13. [Google Scholar] [CrossRef]
- Yamashita, R.; Nishio, M.; Do, R.K.G.; Togashi, K. Convolutional neural networks: An overview and application in radiology. Insights Imaging 2018, 9, 611–629. [Google Scholar] [CrossRef]
- Park, Y.H.; Kim, S.H.; Choi, Y.Y. Prediction models of early childhood caries based on machine learning algorithms. Int. J. Environ. Res. Public Health 2021, 18, 8613. [Google Scholar] [CrossRef]
- Kim, S.; Lee, Y.H.; Noh, Y.K.; Park, F.C.; Auh, Q.S. Age-group determination of living individuals using first molar images based on artificial intelligence. Sci. Rep. 2021, 11, 1073. [Google Scholar] [CrossRef]
- Tarce, M.; Zhou, Y.; Antonelli, A.; Becker, K. The Application of Artificial Intelligence for Tooth Segmentation in CBCT Images: A Systematic Review. Appl. Sci. 2024, 14, 6298. [Google Scholar] [CrossRef]
- Wang, X.; Alqahtani, K.A.; Van den Bogaert, T.; Shujaat, S.; Jacobs, R.; Shaheen, E. Convolutional Neural Network for Automated Tooth Segmentation on Intraoral Scans. BMC Oral Health 2024, 24, 804. [Google Scholar] [CrossRef]
- Vaughan, M.; Mheissen, S.; Cobourne, M.; Ahmed, F. Diagnostic accuracy of artificial intelligence for dental and occlusal parameters using standardized clinical photographs. Am. J. Orthod. Dentofac. Orthop. 2025, 167, 733–740. [Google Scholar] [CrossRef]
- Gracea, R.S.; Winderickx, N.; Vanheers, M.; Hendrickx, J.; Preda, F.; Shujaat, S.; de Llano-Perula, M.C.; Jacobs, R. Artificial intelligence for orthodontic diagnosis and treatment planning: A scoping review. J. Dent. 2024, 152, 105442. [Google Scholar] [CrossRef]
- Retrouvey, J.M. The role of AI and machine learning in contemporary orthodontics. APOS Trends Orthod. 2021, 11, 74–80. [Google Scholar] [CrossRef]
- Leifert, M.F.; Leifert, M.M.; Efstratiadis, S.S.; Cangialosi, T.J. Comparison of Space Analysis Evaluations with Digital Models and Plaster Dental Casts. Am. J. Orthod. Dentofac. Orthop. 2009, 136, 16.e1–16.e4. [Google Scholar] [CrossRef]
- Park, S.H.; Byun, S.H.; Oh, S.H.; Lee, H.L.; Kim, J.W.; Yang, B.E.; Park, I.Y. Evaluation of the reliability, reproducibility and validity of digital orthodontic measurements based on various digital models among young patients. J. Clin. Med. 2020, 9, 2728. [Google Scholar] [CrossRef]
- Nance, H. The Limitation of Orthodontic Treatment. Am. J. Orthod. 1947, 33, 253–301. [Google Scholar] [CrossRef]
- Bolton, W.A. The Clinical Application of a Tooth-Size Analysis. Am. J. Orthod. 1962, 48, 504–529. [Google Scholar] [CrossRef]
- Ülgen, M. Ortodonti: Anomaliler, Sefalometri, Etioloji, Büyüme ve Gelişim, Tanı; Yeditepe Üniversitesi: İstanbul, Türkiye, 2000. [Google Scholar]
- Nalcaci, R.; Topcuoglu, T.; Ozturk, F. Comparison of Bolton Analysis and Tooth Size Measurements Obtained Using Conventional and Three-Dimensional Orthodontic Models. Eur. J. Dent. 2013, 7 (Suppl. 1), S066–S070. [Google Scholar] [CrossRef]
- Akyalcin, S.; Dyer, D.J.; English, J.D.; Sar, C. Comparison of 3-Dimensional Dental Models from Different Sources: Diagnostic Accuracy and Surface Registration Analysis. Am. J. Orthod. Dentofac. Orthop. 2013, 144, 831–837. [Google Scholar] [CrossRef]
- Angle, E.H. Classification of Malocclusion. Dent. Cosm. 1899, 41, 350–357. [Google Scholar]
- Dewey, M. Classification of Malocclusion. Int. J. Orthod. 1915, 1, 133–147. [Google Scholar] [CrossRef]
- Quimby, M.L.; Vig, K.W.; Rashid, R.G.; Firestone, A.R. The Accuracy and Reliability of Measurements Made on Computer-Based Digital Models. Angle Orthod. 2004, 74, 298–303. [Google Scholar]
- Mok, C.W.; Zhou, L.; Mcgrath, C.; Hägg, U.; Bendeus, M. Digital Images as an Alternative to Orthodontic Casts in Assessing Malocclusion and Orthodontic Treatment Need. Acta Odontol. Scand. 2007, 65, 362–368. [Google Scholar] [CrossRef]
- Lang, F.A.; Lang, N.A.; Vorloeper, J.; Niederau, C.; Craveiro, R.B.; Knaup, I.; Wolf, M. Validation of a digital, partly automated three-dimensional cast analysis for evaluation of orthodontic treatment assessment. Head Face Med. 2025, 21, 1–14. [Google Scholar] [CrossRef]
- Faber, J.; Faber, C.; Faber, P. Artificial Intelligence in Orthodontics. APOS Trends Orthod. 2019, 9, 201–205. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, Y.; Li, S.; Zhao, Z.; Wu, Z. Machine Learning in Orthodontics: Challenges and Perspectives. Adv. Clin. Exp. Med. 2021, 30, 1065–1074. [Google Scholar] [CrossRef]
- Yu, J.H.; Kim, J.H.; Liu, J.; Mangal, U.; Ahn, H.K.; Cha, J.Y. Reliability and time-based efficiency of artificial intelligence-based automatic digital model analysis system. Eur. J. Orthod. 2023, 45, 712–721. [Google Scholar] [CrossRef]
- Bossuyt, P.M.; Reitsma, J.B.; Bruns, D.E.; Gatsonis, C.A.; Glasziou, P.P.; Irwig, L.; Lijmer, J.G.; Moher, D.; Rennie, D.; de Vet, H.C.; et al. STARD 2015: An updated list of essential items for reporting diagnostic accuracy studies. Radiology 2015, 277, 826–832. [Google Scholar] [CrossRef]
- Camardella, L.T.; Ongkosuwito, E.M.; Penning, E.W.; Kuijpers-Jagtman, A.M.; Vilella, O.V.; Breuning, K.H. Accuracy and reliability of measurements performed using two different software programs on digital models generated using laser and computed tomography plaster model scanners. Korean J. Orthod. 2020, 50, 13. [Google Scholar] [CrossRef]
- Camardella, L.; Breuning, H.; Vasconcellos Vilella, O. Accuracy and reproducibility of measurements on plaster models and digital models created using an intraoral scanner. J. Orofac. Orthop. 2017, 78, 211–220. [Google Scholar] [CrossRef]
- Cohen, J. A coefficient of agreement for nominal scales. Educ. Psychol. Meas. 1960, 20, 37–46. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Bootvong, K.; Liu, Z.; McGrath, C.; Hägg, U.; Wong, R.W.; Bendeus, M.; Yeung, S. Virtual Model Analysis as an Alternative Approach to Plaster Model Analysis: Reliability and Validity. Eur. J. Orthod. 2010, 32, 589–595. [Google Scholar] [CrossRef]
- Rossini, G.; Parrini, S.; Castroflorio, T.; Deregibus, A.; Debernardi, C.L. Diagnostic Accuracy and Measurement Sensitivity of Digital Models for Orthodontic Purposes: A Systematic Review. Am. J. Orthod. Dentofac. Orthop. 2016, 149, 161–170. [Google Scholar] [CrossRef]
- Fleming, P.; Marinho, V.; Johal, A. Orthodontic Measurements on Digital Study Models Compared with Plaster Models: A Systematic Review. Orthod. Craniofac. Res. 2011, 14, 1–16. [Google Scholar] [CrossRef]
- Hirogaki, Y.; Sohmura, T.; Satoh, H.; Takahashi, J.; Takada, K. Complete 3-D Reconstruction of Dental Cast Shape Using Perceptual Grouping. IEEE Trans. Med. Imaging 2001, 20, 1093–1101. [Google Scholar] [CrossRef]
- Bor, S.; Ciğerim, S.Ç.; Kotan, S. Comparison of AI-Assisted Cephalometric Analysis and Orthodontist-Performed Digital Tracing Analysis. Prog. Orthod. 2024, 25, 41. [Google Scholar] [CrossRef]
- Chandrashekar, G.; AlQarni, S.; Bumann, E.E.; Lee, Y. Collaborative Deep Learning Model for Tooth Segmentation and Identification Using Panoramic Radiographs. Comput. Biol. Med. 2022, 148, 105829. [Google Scholar] [CrossRef]
- Wang, L.; Gao, Y.; Shi, F.; Li, G.; Chen, K.C.; Tang, Z.; Xia, J.J.; Shen, D. Automated Segmentation of Dental CBCT Image with Prior-Guided Sequential Random Forests. Med. Phys. 2016, 43, 336–346. [Google Scholar] [CrossRef]
- Miranda, F.; Barone, S.; Gillot, M.; Baquero, B.; Anchling, L.; Hutin, N.; Gurgel, M.; Al Turkestani, N.; Huang, Y.; Massaro, C.; et al. Artificial Intelligence Applications in Orthodontics. J. Calif. Dent. Assoc. 2023, 51, 2195585. [Google Scholar] [CrossRef]
- Oğuz, F.; Bor, S.; Khanmohammadi, A.; Kıranşal, M. Evaluation of Condylar and Airway Volume in Skeletal Class I Patients with Different Vertical Growth Patterns. Appl. Sci. 2025, 15, 2794. [Google Scholar] [CrossRef]
- Im, J.; Kim, J.Y.; Yu, H.S.; Lee, K.J.; Choi, S.H.; Kim, J.H.; Ahn, H.K.; Cha, J.Y. Accuracy and Efficiency of Automatic Tooth Segmentation in Digital Dental Models Using Deep Learning. Sci. Rep. 2022, 12, 9429. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.; Galkin, S.; Teredesai, M.; Nicolay, O.F.; Cangialosi, T.J. Comparison of Measurements Made on Digital and Plaster Models. Am. J. Orthod. Dentofac. Orthop. 2003, 124, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.R.; Flores-Mir, C.; Nebbe, B.; Raboud, D.W.; Heo, G.; Major, P.W. Validity, Reliability, and Reproducibility of Plaster vs Digital Study Models: Comparison of Peer Assessment Rating and Bolton Analysis and Their Constituent Measurements. Am. J. Orthod. Dentofac. Orthop. 2006, 129, 794–803. [Google Scholar] [CrossRef]
- Casko, J.S.; Vaden, J.L.; Kokich, V.G.; Damone, J.; James, R.D.; Cangialosi, T.J.; Riolo, M.L.; Owens, S.E.; Bills, E.D. Objective Grading System for Dental Casts and Panoramic Radiographs. Am. J. Orthod. Dentofac. Orthop. 1998, 114, 589–599. [Google Scholar] [CrossRef]
- Ryu, J.; Kim, Y.H.; Kim, T.W.; Jung, S.K. Evaluation of artificial intelligence model for crowding categorization and extraction diagnosis using intraoral photographs. Sci. Rep. 2023, 13, 5177. [Google Scholar] [CrossRef]
- Wu, T.-H.; Lian, C.; Lee, S.; Pastewait, M.; Piers, C.; Liu, J.; Wang, F.; Chiu, C.Y.; Wang, W.; Jackson, C.; et al. Two-Stage Mesh Deep Learning for Automated Tooth Segmentation and Landmark Localization on 3D Intraoral Scans. IEEE Trans. Med. Imaging 2022, 41, 3158–3166. [Google Scholar] [CrossRef]

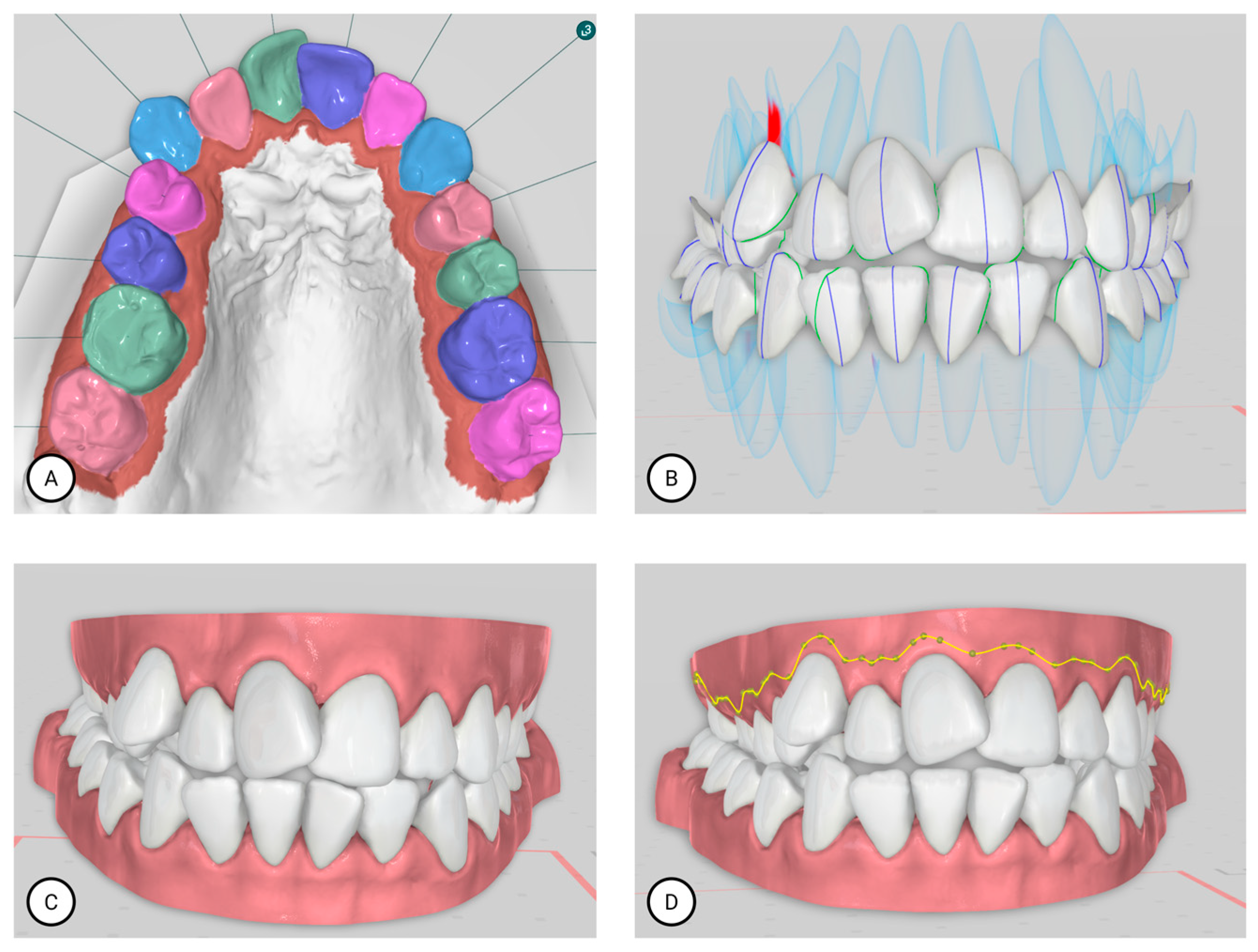
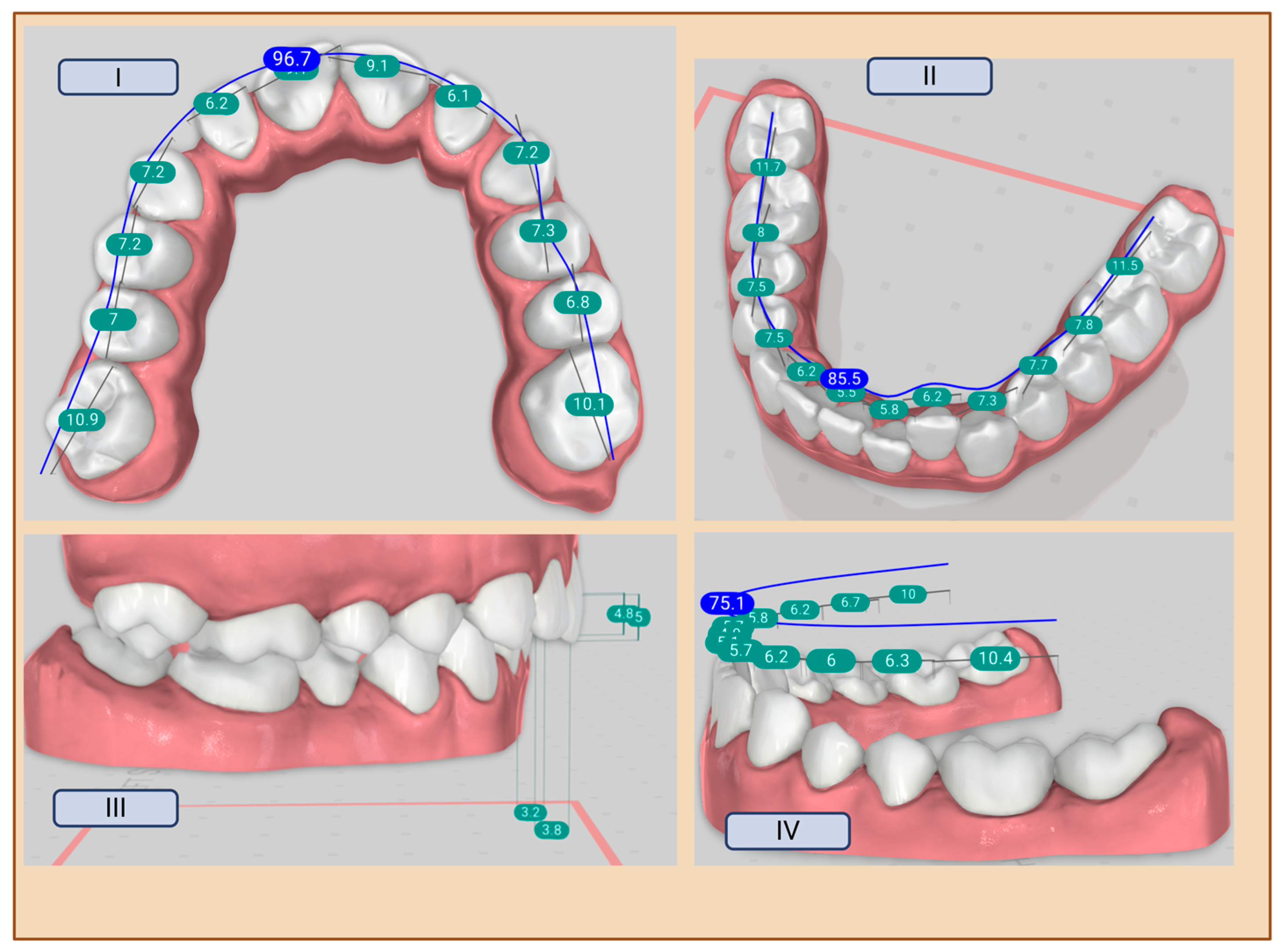
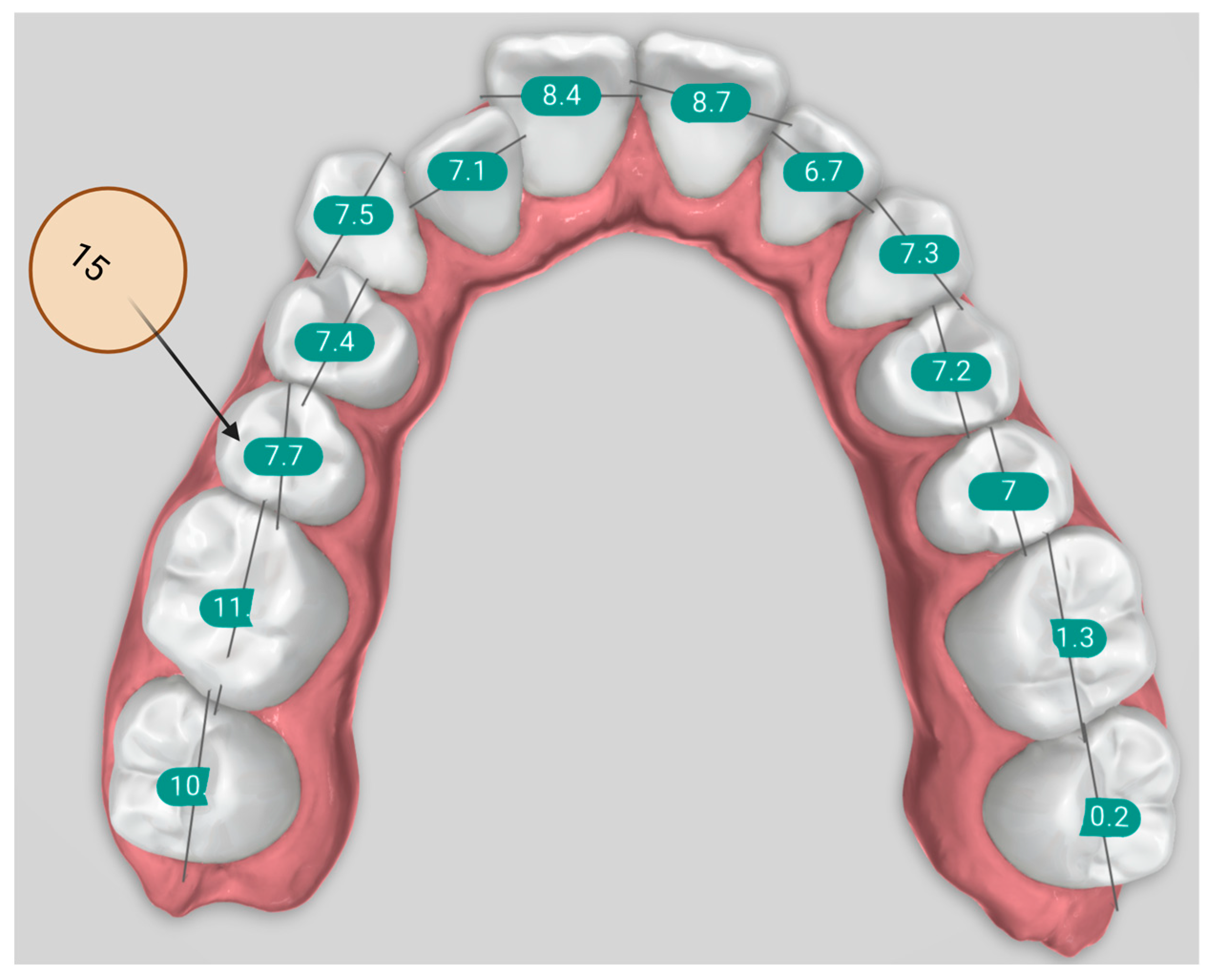
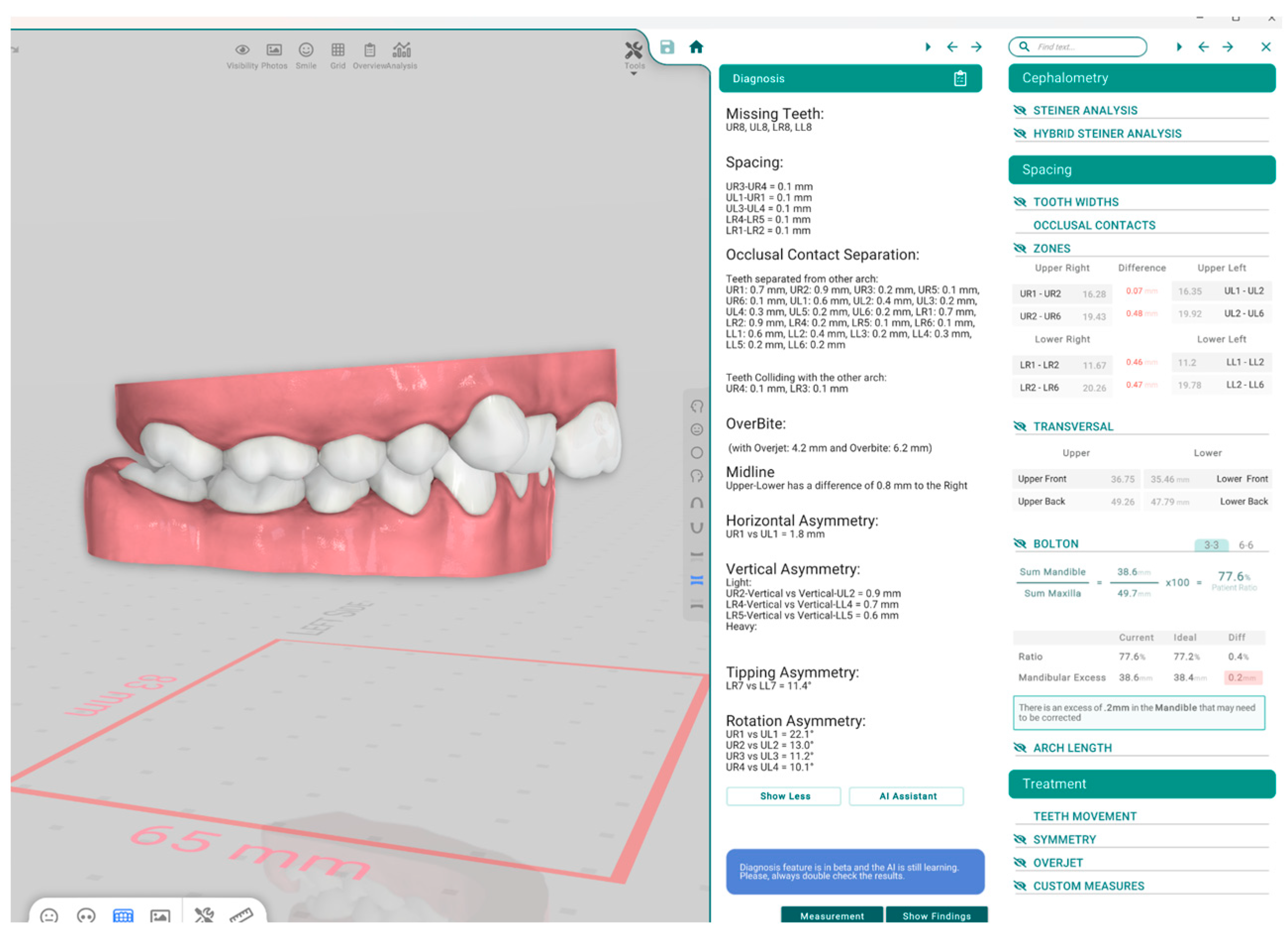
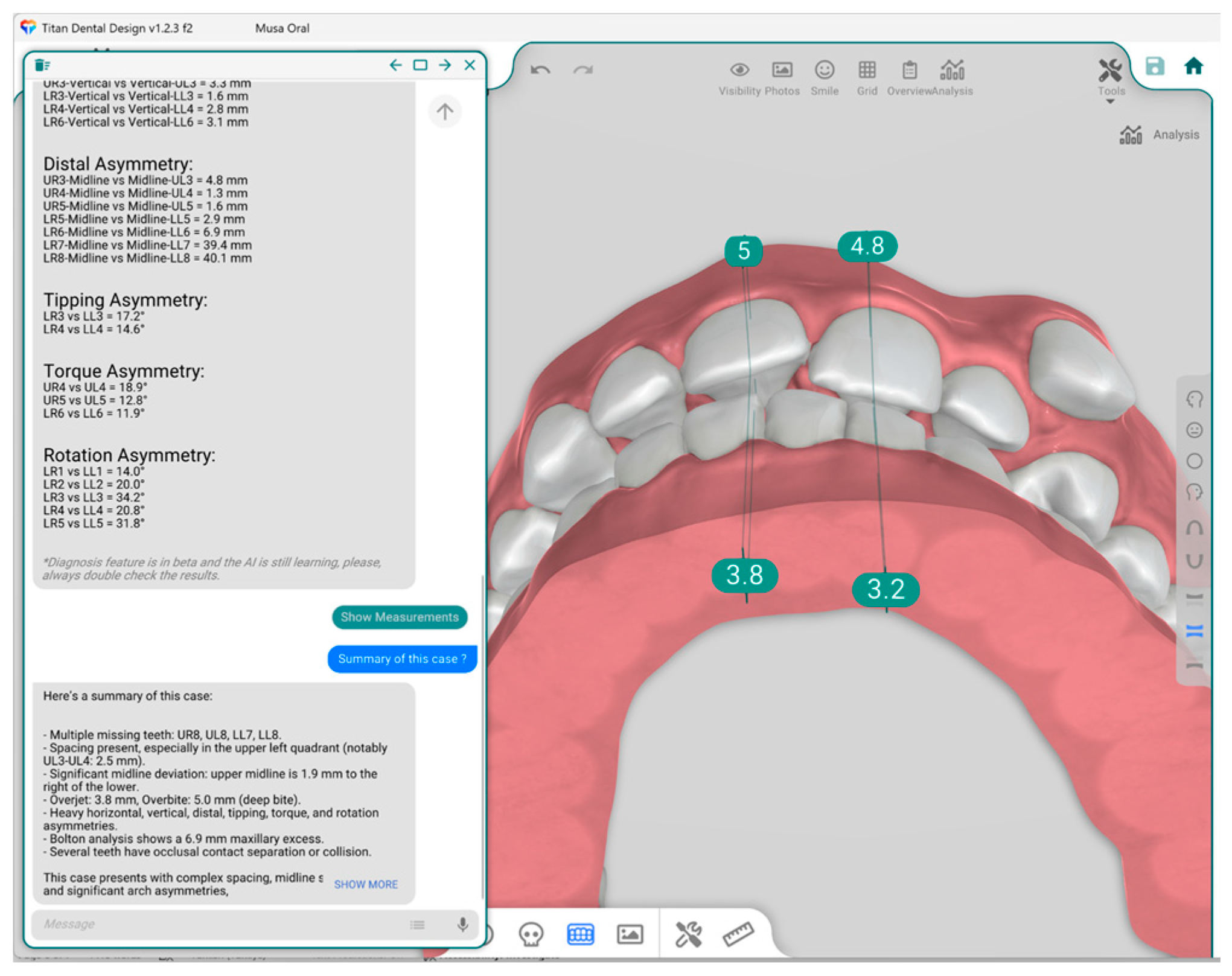
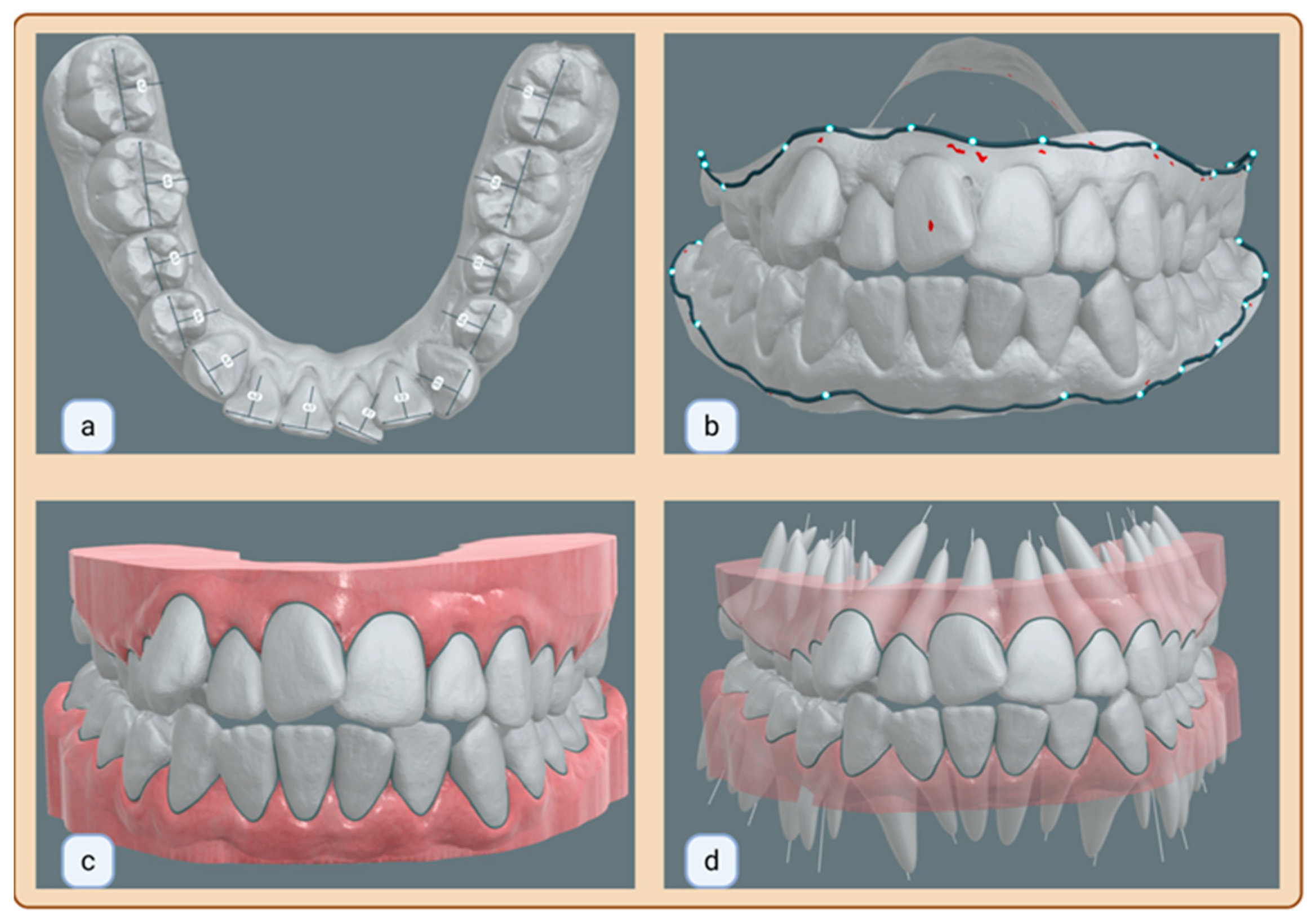

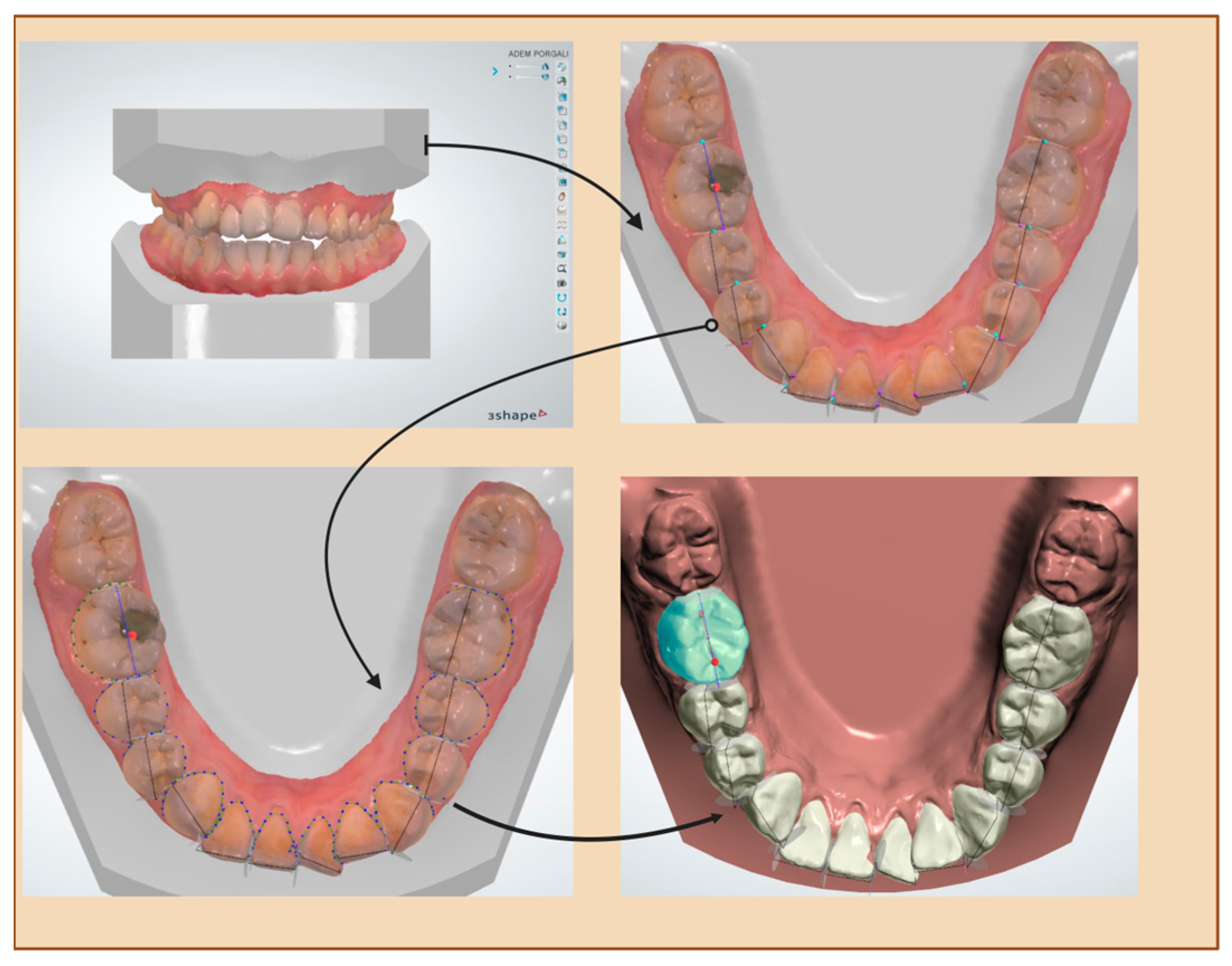
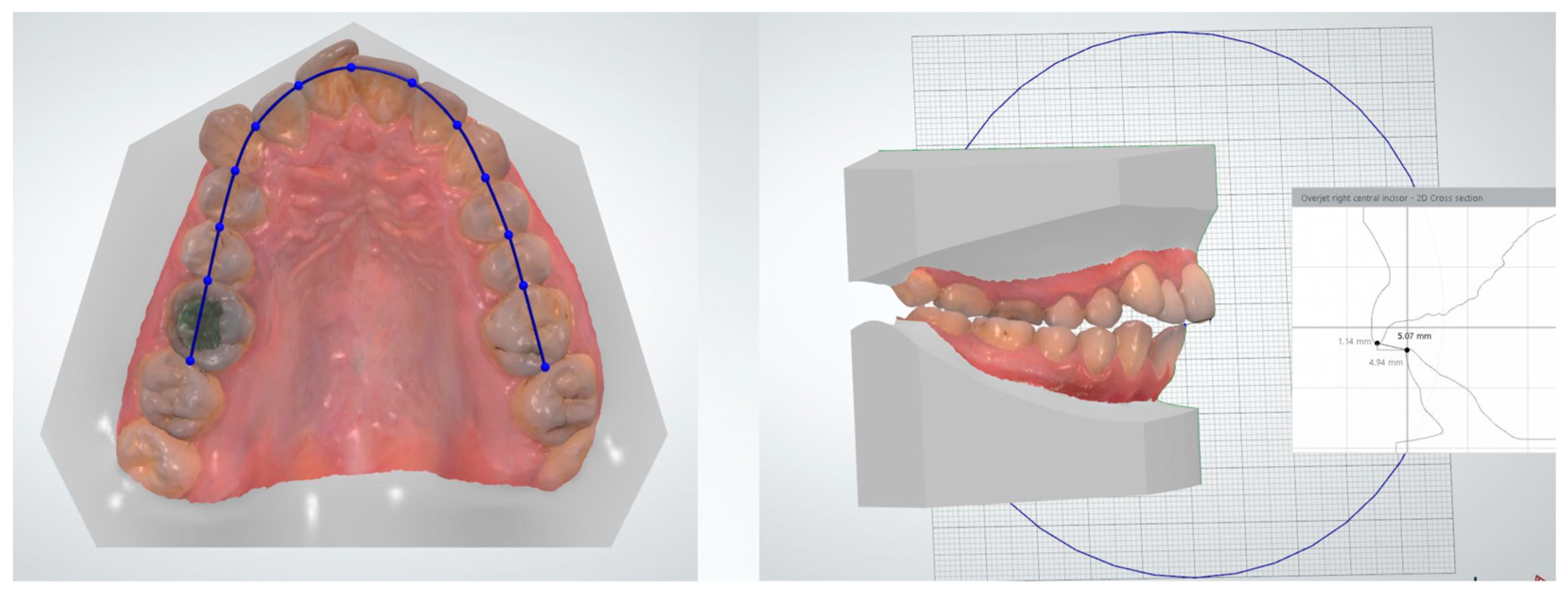

| Term | Description | Objective | Groups |
|---|---|---|---|
| Bolton Analysis | Compares the mesiodistal widths of maxillary and mandibular teeth to determine the tooth size discrepancy between arches. It consists of two key components: Overall Bolton Ratio and Anterior Bolton Ratio. | Helps identify size imbalances between upper and lower teeth, which can affect occlusion and treatment planning. | Orthodontist, Titan Dental Design, SoftSmile |
| Overall Bolton Analysis Compares the total mesiodistal width of all 12 mandibular teeth (first molar to first molar) to that of the corresponding 12 maxillary teeth. If the actual mandibular width exceeds the ideal value based on the maxillary width, a mandibular excess is present; conversely, if the mandibular width is less, a maxillary excess is indicated. | Ensures overall compatibility of tooth size between upper and lower arches, aiding in proper occlusion and treatment. | Orthodontist, Titan Dental Design, SoftSmile | |
| Anterior Bolton Analysis Compares the mesiodistal widths of the six anterior mandibular teeth (canine to canine) to the six anterior maxillary teeth. As with the overall ratio, discrepancies can be expressed in millimeters as either mandibular or maxillary excess, depending on which arch has the surplus tooth material. | Identifies size imbalances in the anterior teeth, which can affect anterior occlusion and aesthetics. | Orthodontist, Titan Dental Design, SoftSmile | |
| Space Analysis | Measures the amount of space available in the dental arch and compares it to the space required for proper alignment. | Assists in determining if there is crowding or spacing in the dental arch. | Orthodontist, Titan Dental Design |
| Overbite | Refers to the vertical overlap of the upper front teeth over the lower front teeth. | Used to assess the degree of vertical discrepancy between upper and lower teeth. | Orthodontist, Titan Dental Design |
| Overjet | Refers to the horizontal distance between the upper and lower front teeth. | Helps in assessing the forward positioning of the upper front teeth relative to the lower front teeth. | Orthodontist, Titan Dental Design |
| Angle Classification (Molar Relationship) | Angle Classification (Molar Relationship) is a system used to classify malocclusion based on the relationship between the upper and lower first molars. The upper first molar’s position is fixed, and classification is based on its relation to the lower molars. | Used for diagnosing the type of malocclusion according to the positioning of molars in the dental arches. | Orthodontist, Titan Dental Design |
| Comparison | Cohen’s Kappa | Level of Agreement | p-Value |
|---|---|---|---|
| Location 1 (Anterior Bolton Analysis)—Ortho vs. Titan | −0.051 | None | 0.716 |
| Location 1 (Anterior Bolton Analysis)—Ortho vs. SoftSmile | 0.113 | Minimal | 0.43 |
| Location 1 (Anterior Bolton Analysis)—Titan vs. SoftSmile | 0.496 | Moderate | <0.001 |
| Location 2 (Overall Bolton Analysis)—Ortho vs. Titan | −0.077 | None | 0.489 |
| Location 2 (Overall Bolton Analysis)—Ortho vs. SoftSmile | −0.124 | None | 0.382 |
| Location 2 (Overall Bolton Analysis)—Titan vs. SoftSmile | 0.406 | Moderate | 0.001 |
| Molar Classification—Right (Ortho vs. Titan) | 0.955 | Almost perfect | <0.001 |
| Molar Classification—Left (Ortho vs. Titan) | 0.9 | Almost perfect | <0.001 |
| Location 1 (Anterior Bolton Analysis)—Ortho1 vs. Ortho2 | 0.71 | Substantial | <0.001 |
| Location 2 (Overall Bolton Analysis)—Ortho1 vs. Ortho2 | 0.467 | Moderate | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bor, S.; Oğuz, F.; Khanmohammadi, A. Diagnostic Accuracy and Agreement Between AI and Clinicians in Orthodontic 3D Model Analysis. Appl. Sci. 2025, 15, 7786. https://doi.org/10.3390/app15147786
Bor S, Oğuz F, Khanmohammadi A. Diagnostic Accuracy and Agreement Between AI and Clinicians in Orthodontic 3D Model Analysis. Applied Sciences. 2025; 15(14):7786. https://doi.org/10.3390/app15147786
Chicago/Turabian StyleBor, Sabahattin, Fırat Oğuz, and Ayla Khanmohammadi. 2025. "Diagnostic Accuracy and Agreement Between AI and Clinicians in Orthodontic 3D Model Analysis" Applied Sciences 15, no. 14: 7786. https://doi.org/10.3390/app15147786
APA StyleBor, S., Oğuz, F., & Khanmohammadi, A. (2025). Diagnostic Accuracy and Agreement Between AI and Clinicians in Orthodontic 3D Model Analysis. Applied Sciences, 15(14), 7786. https://doi.org/10.3390/app15147786







