Is Ozonation Treatment Efficient to Provide Safe Reclaimed Water? Assessing the Effects of Synthetic Wastewater Effluents in Human Cell Models
Abstract
1. Introduction
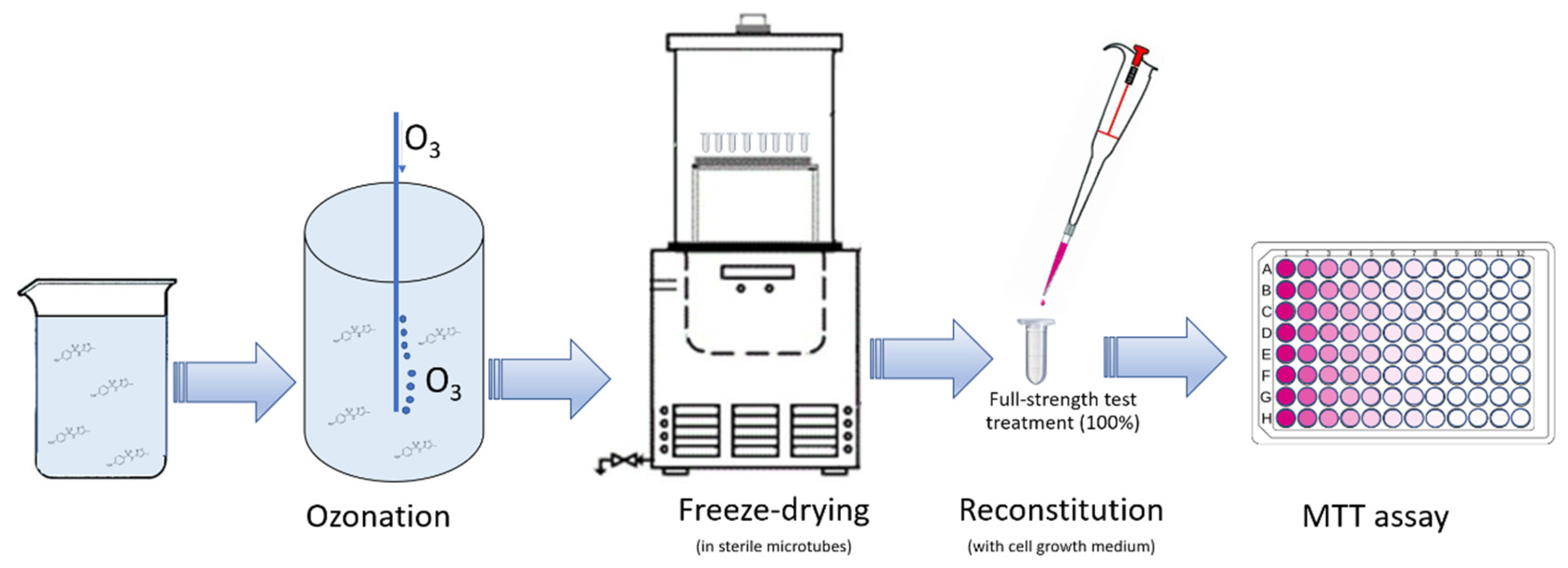
2. Materials and Methods
2.1. Samples Preparation
2.2. Cell Culture and Testing
2.3. Data Analysis
3. Results and Discussion
3.1. Ozonation Treatment Efficacy
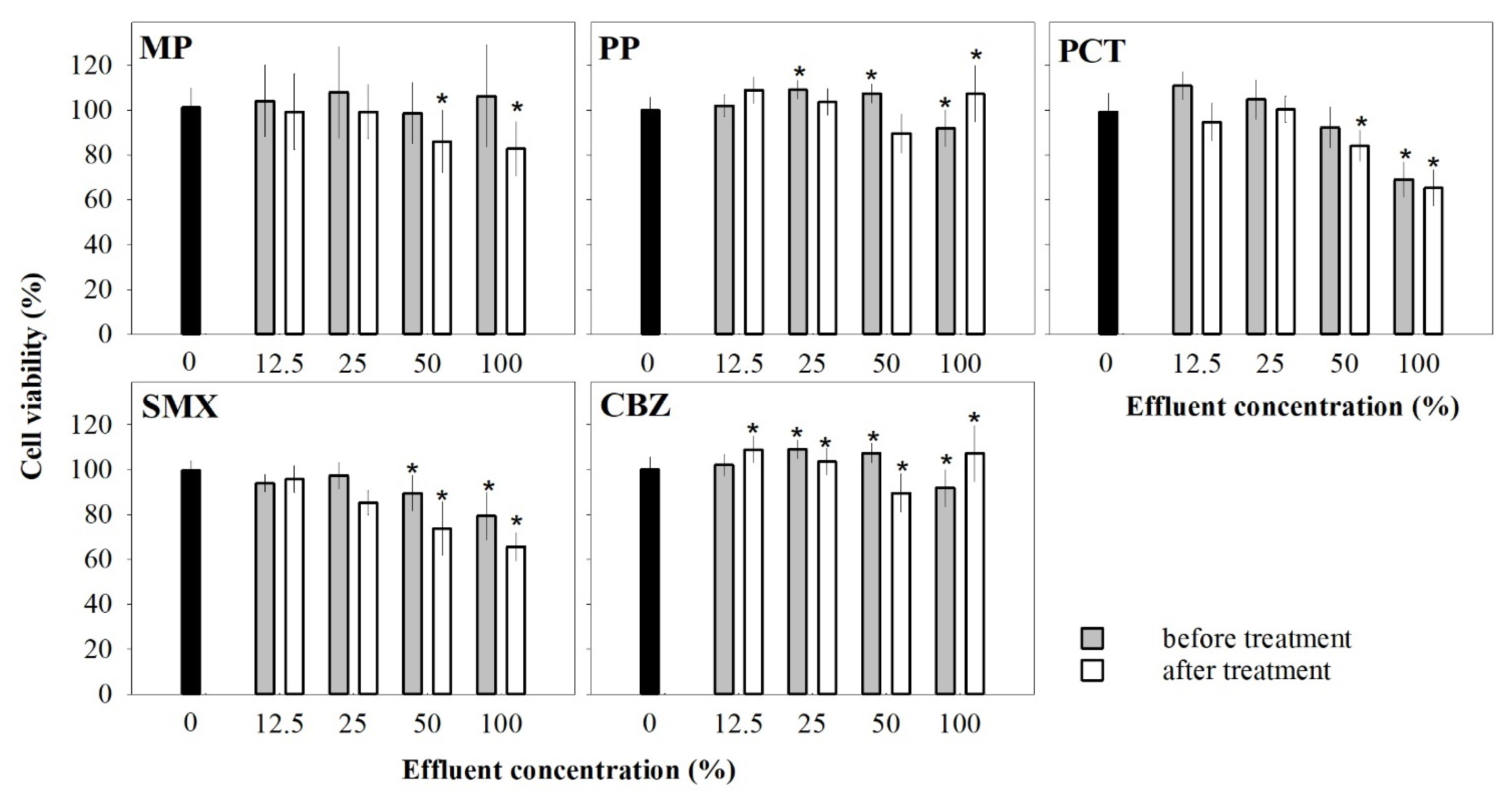
3.2. Potential Cytotoxicity Associated with the Use of Ozonation to Degrade PPCPs
3.2.1. Effects of Single-Chemical Effluents on Cell Viability
3.2.2. Effects of Effluents with Mixed PPCPs on Cell Viability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Santos, A.F.; Alvarenga, P.; Gando-Ferreira, L.M.; Quina, M.J. Urban Wastewater as a Source of Reclaimed Water for Irrigation: Barriers and Future Possibilities. Environments 2023, 10, 17. [Google Scholar] [CrossRef]
- Maryam, B.; Büyükgüngör, H. Wastewater Reclamation and Reuse Trends in Turkey: Opportunities and Challenges. J. Water Process Eng. 2019, 30, 100501. [Google Scholar] [CrossRef]
- Garrido-Cardenas, J.A.; Esteban-García, B.; Agüera, A.; Sánchez-Pérez, J.A.; Manzano-Agugliaro, F. Wastewater Treatment by Advanced Oxidation Process and Their Worldwide Research Trends. Int. J. Environ. Res. Public Health 2020, 17, 170. [Google Scholar] [CrossRef] [PubMed]
- Shahriar, A.; Tan, J.; Sharma, P.; Hanigan, D.; Verburg, P.; Pagilla, K.; Yang, Y. Modeling the Fate and Human Health Impacts of Pharmaceuticals and Personal Care Products in Reclaimed Wastewater Irrigation for Agriculture. Environ. Pollut. 2021, 276, 116532. [Google Scholar] [CrossRef]
- Kumar, S.; Talan, A.; Boyle, K.; Ormeci, B.; Drogui, P.; Tyagi, R.D. Water Recycling: Economic and Environmental Benefits. In Biomass, Biofuels, Biochemicals. Circular Bioeconomy—Current Developments and Future Outlook; Elsevier: Amsterdam, The Netherlands, 2021; pp. 91–120. [Google Scholar] [CrossRef]
- Deng, S.; Yan, X.; Zhu, Q.; Liao, C. The Utilization of Reclaimed Water: Possible Risks Arising from Waterborne Contaminants. Environ. Pollut. 2019, 254, 113020. [Google Scholar] [CrossRef]
- Niemi, L.; Taggart, M.; Boyd, K.; Zhang, Z.; Gaffney, P.P.J.; Pfleger, S.; Gibb, S. Assessing Hospital Impact on Pharmaceutical Levels in a Rural ‘Source-to-Sink’ Water System. Sci. Total Environ. 2020, 737, 139618. [Google Scholar] [CrossRef]
- Silva, C.; Cachada, A.; Gonçalves, F.J.M.; Nannou, C.; Lambropoulou, D.; Patinha, C.; Abrantes, N.; Pereira, J.L. Chemical Characterization of Riverine Sediments Affected by Wastewater Treatment Plant Effluent Discharge. Sci. Total Environ. 2022, 839, 156305. [Google Scholar] [CrossRef]
- Wu, Y.; Song, S.; Chen, X.; Shi, Y.; Cui, H.; Liu, Y.; Yang, S. Source-Specific Ecological Risks and Critical Source Identification of PPCPs in Surface Water: Comparing Urban and Rural Areas. Sci. Total Environ. 2023, 854, 158792. [Google Scholar] [CrossRef]
- Ebele, A.J.; Abou-Elwafa Abdallah, M.; Harrad, S. Pharmaceuticals and Personal Care Products (PPCPs) in the Freshwater Aquatic Environment. Emerg. Contam. 2017, 3, 1–16. [Google Scholar] [CrossRef]
- Kujawska, A.; Kiełkowska, U.; Atisha, A.; Yanful, E.; Kujawski, W. Comparative Analysis of Separation Methods Used for the Elimination of Pharmaceuticals and Personal Care Products (PPCPs) from Water—A Critical Review. Sep. Purif. Technol. 2022, 290, 120797. [Google Scholar] [CrossRef]
- Narayanan, M.; El-sheekh, M.; Ma, Y.; Pugazhendhi, A.; Natarajan, D.; Kandasamy, G.; Raja, R.; Saravana Kumar, R.M.; Kumarasamy, S.; Sathiyan, G.; et al. Current Status of Microbes Involved in the Degradation of Pharmaceutical and Personal Care Products (PPCPs) Pollutants in the Aquatic Ecosystem. Environ. Pollut. 2022, 300, 118922. [Google Scholar] [CrossRef] [PubMed]
- Mellah, A.; Fernandes, S.P.S.; Rodríguez, R.; Otero, J.; Paz, J.; Cruces, J.; Medina, D.D.; Djamila, H.; Espiña, B.; Salonen, L.M. Adsorption of Pharmaceutical Pollutants from Water Using Covalent Organic Frameworks. Chem. A Eur. J. 2018, 24, 10601–10605. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, H. Catalytic Ozonation for Water and Wastewater Treatment: Recent Advances and Perspective. Sci. Total Environ. 2020, 704, 135249. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.; Costa, R.; Quinta-Ferreira, R.M.; Martins, R.C. Application of Ozonation for Pharmaceuticals and Personal Care Products Removal from Water. Sci. Total Environ. 2017, 586, 265–283. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Advanced Oxidation Process-Mediated Removal of Pharmaceuticals from Water: A Review. J. Environ. Manag. 2018, 219, 189–207. [Google Scholar] [CrossRef]
- Potivejkul, S.; Pimdee, P.; Phimolsathien, T. Perceptions on Ozonation Water Treatment Use: An Alternative Idea for Asean Water Resource Sustainability. J. Sustain. Sci. Manag. 2017, 12, 49–59. [Google Scholar]
- Jesus, F.; Bernardo, C.; Martins, R.C.; Gomes, J.; Pereira, J.L. Ecotoxicological Consequences of the Abatement of Contaminants of Emerging Concern by Ozonation—Does Mixture Complexity Matter? Water 2022, 14, 1801. [Google Scholar] [CrossRef]
- Gomes, J.; Frasson, D.; Pereira, J.L.; Gonçalves, F.J.M.; Castro, L.M.; Quinta-Ferreira, R.M.; Martins, R.C. Ecotoxicity Variation through Parabens Degradation by Single and Catalytic Ozonation Using Volcanic Rock. Chem. Eng. J. 2019, 360, 30–37. [Google Scholar] [CrossRef]
- Gomes, J.; Jesus, F.; Domingues, E.; Gonçalves, F.; Pereira, J.L.; Martins, R.C. Photocatalytic Oxidation of Pharmaceutical Contaminants of Emerging Concern Using Sunlight and Visible Radiation: Mechanism and Ecotoxicological Evaluation. J. Water Process Eng. 2021, 43, 102204. [Google Scholar] [CrossRef]
- Pereira, A.; Silva, L.; Lino, C.; Meisel, L.; Pena, A. Assessing Environmental Risk of Pharmaceuticals in Portugal: An Approach for the Selection of the Portuguese Monitoring Stations in Line with Directive 2013/39/EU. Chemosphere 2016, 144, 2507–2515. [Google Scholar] [CrossRef]
- Gomes, J.; Bernardo, C.; Jesus, F.; Pereira, J.L.; Martins, R.C. Ozone Kinetic Studies Assessment for the PPCPs Abatement: Mixtures Relevance. Chem. Eng. 2022, 6, 20. [Google Scholar] [CrossRef]
- Khasawneh, O.F.S.; Palaniandy, P. Occurrence and removal of pharmaceuticals in wastewater treatment plants. Process Saf. Environ. Prot. 2021, 150, 532–556. [Google Scholar] [CrossRef]
- Saeid, S.; Kråkström, M.; Tolvanen, P.; Kumar, N.; Eränen, K.; Mikkola, J.-P.; Kronberg, L.; Eklund, P.; Peurla, M.; Aho, A.; et al. Advanced Oxidation Process for Degradation of Carbamazepine from Aqueous Solution: Influence of Metal Modified Microporous, Mesoporous Catalysts on the Ozonation Process. Catalysts 2020, 10, 90. [Google Scholar] [CrossRef]
- Durgo, K.; Oreščanin, V.; Horvat, T.; Oreščanin, V.; Mikelić, L.; Čolić, J.F.; Lulić, S. Cytotoxicity and Mutagenicity Study of Waste and Purified Water Samples from Electroplating Industries Prepared by Use of Ferrous Sulfate and Wood Fly Ash. J. Environ. Sci. Health Part A 2005, 40, 949–957. [Google Scholar] [CrossRef]
- Orescanin, V.; Kopjar, N.; Durgo, K.; Elez, L.; Gustek, S.F.; Colic, J.F. Citotoxicity Status of Electroplating Wastewater Prior/after Neutralization/Purification with Alkaline Solid Residue of Electric Arc Furnace Dust. J. Environ. Sci. Health Part. A 2009, 44, 273–278. [Google Scholar] [CrossRef]
- Durgo, K.; Oreščanin, V.; Lulić, S.; Kopjar, N.; elježić, D.Ž.; Čolić, J.F. The Assessment of Genotoxic Effects of Wastewater from a Fertilizer Factory. J. Appl. Toxicol. 2009, 29, 42–51. [Google Scholar] [CrossRef]
- Kizhedath, A.; Wilkinson, S.; Glassey, J. Assessment of Hepatotoxicity and Dermal Toxicity of Butyl Paraben and Methyl Paraben Using HepG2 and HDFn In Vitro Models. Toxicol. Vitr. 2019, 55, 108–115. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Moldéus, P. Mechanism of P-Hydroxybenzoate Ester-Induced Mitochondrial Dysfunction and Cytotoxicity in Isolated Rat Hepatocytes. Biochem. Pharmacol. 1998, 55, 1907–1914. [Google Scholar] [CrossRef]
- Yin, M.-C.; Lin, C.-C.; Wu, H.-C.; Tsao, S.-M.; Hsu, C.-K. Apoptotic Effects of Protocatechuic Acid in Human Breast, Lung, Liver, Cervix, and Prostate Cancer Cells: Potential Mechanisms of Action. J. Agric. Food Chem. 2009, 57, 6468–6473. [Google Scholar] [CrossRef]
- Yip, E.C.H.; Chan, A.S.L.; Pang, H.; Tam, Y.K.; Wong, Y.H. Protocatechuic Acid Induces Cell Death in HepG2 Hepatocellular Carcinoma Cells through a C-Jun N-Terminal Kinase-Dependent Mechanism. Cell Biol. Toxicol. 2006, 22, 293–302. [Google Scholar] [CrossRef]
- Kalinowska, M.; Gołębiewska, E.; Świderski, G.; Męczyńska-Wielgosz, S.; Lewandowska, H.; Pietryczuk, A.; Cudowski, A.; Astel, A.; Świsłocka, R.; Samsonowicz, M.; et al. Plant-derived and dietary hydroxybenzoic acids—A comprehensive study of structural, anti-/pro-oxidant, lipophilic, antimicrobial, and cytotoxic activity in mda-mb-231 and mcf-7 cell lines. Nutrients 2021, 13, 3107. [Google Scholar] [CrossRef] [PubMed]
- Wróbel, A.; Gregoraszczuk, E.L. Effects of Single and Repeated In Vitro Exposure of Three Forms of Parabens, Methyl-, Butyl- and Propylparabens on the Proliferation and Estradiol Secretion in MCF-7 and MCF-10A Cells. Pharmacol. Rep. 2013, 65, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Klopčič, I.; Kolšek, K.; Dolenc, M.S. Glucocorticoid-like Activity of Propylparaben, Butylparaben, Diethylhexyl Phthalate and Tetramethrin Mixtures Studied in the MDA-Kb2 Cell Line. Toxicol. Lett. 2015, 232, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Szelag, S.; Zabłocka, A.; Trzeciak, K.; Drozd, A.; Baranowska-Bosiacka, I.; Kolasa, A.; Goschorska, M.; Chlubek, D.; Gutowska, I. Propylparaben-Induced Disruption of Energy Metabolism in Human HepG2 Cell Line Leads to Increased Synthesis of Superoxide Anions and Apoptosis. Toxicol. Vitr. 2016, 31, 30–34. [Google Scholar] [CrossRef]
- Fouyet, S.; Olivier, E.; Leproux, P.; Dutot, M.; Rat, P. Pregnant Women and Endocrine Disruptors: Role of P2X7 Receptor and Mitochondrial Alterations in Placental Cell Disorders. Cells 2022, 11, 495. [Google Scholar] [CrossRef]
- Ottaviano, E.; Baron, G.; Fumagalli, L.; Leite, J.; Colombo, E.A.; Artasensi, A.; Aldini, G.; Borghi, E. Candida Albicans Biofilm Inhibition by Two Vaccinium Macrocarpon (Cranberry) Urinary Metabolites: 5-(3′,4′-DihydroxyPhenyl)-γ-Valerolactone and 4-Hydroxybenzoic Acid. Microorganisms 2021, 9, 1492. [Google Scholar] [CrossRef]
- Mittraphab, Y.; Amen, Y.; Nagata, M.; Matsumoto, M.; Wang, D.; Shimizu, K. Anti-Phototoxicity Effect of Phenolic Compounds from Acetone Extract of Entada Phaseoloides Leaves via Activation of COX-2 and INOS in Human Epidermal Keratinocytes. Molecules 2022, 27, 440. [Google Scholar] [CrossRef]
- Shin, S.W.; Jung, E.; Kim, S.; Lee, K.-E.; Youm, J.-K.; Park, D. Antagonist Effects of Veratric Acid against UVB-Induced Cell Damages. Molecules 2013, 18, 5405–5419. [Google Scholar] [CrossRef]
- Behrends, V.; Giskeødegård, G.F.; Bravo-Santano, N.; Letek, M.; Keun, H.C. Acetaminophen Cytotoxicity in HepG2 Cells Is Associated with a Decoupling of Glycolysis from the TCA Cycle, Loss of NADPH Production, and Suppression of Anabolism. Arch. Toxicol. 2019, 93, 341–353. [Google Scholar] [CrossRef]
- Guo, C.; McMartin, K.E. The Cytotoxicity of Oxalate, Metabolite of Ethylene Glycol, Is Due to Calcium Oxalate Monohydrate Formation. Toxicology 2005, 208, 347–355. [Google Scholar] [CrossRef]
- Schepers, M.S.J.; Van Ballegooijen, E.S.; Bangma, C.H.; Verkoelen, C.F. Oxalate Is Toxic to Renal Tubular Cells Only at Supraphysiologic Concentrations. Kidney Int. 2005, 68, 1660–1669. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.; Enguita, F.J.; Ferreira, J.; Leitão, A.L. DNA Damage Induced by Hydroquinone Can Be Prevented by Fungal Detoxification. Toxicol. Rep. 2014, 1, 1096–1105. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A. Preparação e Caracterização de Nanofibras Magnéticas Para Liberação Controlada de Fármacos e Hipertemia. Ph.D Thesis, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil, 2018. [Google Scholar]
- Al-Musawi, M.; Durham, J.; Whitworth, J.M.; Stone, S.J.; Nixdorf, D.R.; Valentine, R.A. Effect of Topical Neuromodulatory Medications on Oral and Skin Keratinocytes. J. Oral. Pathol. Med. 2017, 46, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Ma, M.; Li, N.; Hou, R.; Huang, C.; Oda, Y.; Wang, Z. Chlorination, Chloramination and Ozonation of Carbamazepine Enhance Cytotoxicity and Genotoxicity: Multi-Endpoint Evaluation and Identification of Its Genotoxic Transformation Products. J. Hazard. Mater. 2018, 342, 679–688. [Google Scholar] [CrossRef]
- Gomes, J.F.; Lopes, A.; Gmurek, M.; Quinta-Ferreira, R.M.; Martins, R.C. Study of the influence of the matrix characteristics over the photocatalytic ozonation of parabens using Ag-TiO2. Sci. Total Environ. 2019, 646, 1468–1477. [Google Scholar] [CrossRef]

| ID | Solution Composition | Reaction Time (min) | TOD (mg O3/L) |
|---|---|---|---|
| MP | MP | 10 | 7.56 |
| PP | PP | 8 | 10.85 |
| PCT | PCT | 20 | 16.70 |
| SMX | SMX | 6 | 6.53 |
| CBZ | CBZ | 1.5 | 2.10 |
| Mix 2 | MP + PP | 12 | 12.39 |
| Mix 3 | MP + PP + PCT | 30 | 14.17 |
| Mix 4 | MP + PP + PCT + SMX | 40 | 20.85 |
| Mix 5 | MP + PP + PCT + SMX + CBZ | 60 | 25.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocha, A.T.; Jesus, F.; Oliveira, H.; Gomes, J.; Pereira, J.L. Is Ozonation Treatment Efficient to Provide Safe Reclaimed Water? Assessing the Effects of Synthetic Wastewater Effluents in Human Cell Models. Appl. Sci. 2025, 15, 7784. https://doi.org/10.3390/app15147784
Rocha AT, Jesus F, Oliveira H, Gomes J, Pereira JL. Is Ozonation Treatment Efficient to Provide Safe Reclaimed Water? Assessing the Effects of Synthetic Wastewater Effluents in Human Cell Models. Applied Sciences. 2025; 15(14):7784. https://doi.org/10.3390/app15147784
Chicago/Turabian StyleRocha, Ana Teresa, Fátima Jesus, Helena Oliveira, João Gomes, and Joana Luísa Pereira. 2025. "Is Ozonation Treatment Efficient to Provide Safe Reclaimed Water? Assessing the Effects of Synthetic Wastewater Effluents in Human Cell Models" Applied Sciences 15, no. 14: 7784. https://doi.org/10.3390/app15147784
APA StyleRocha, A. T., Jesus, F., Oliveira, H., Gomes, J., & Pereira, J. L. (2025). Is Ozonation Treatment Efficient to Provide Safe Reclaimed Water? Assessing the Effects of Synthetic Wastewater Effluents in Human Cell Models. Applied Sciences, 15(14), 7784. https://doi.org/10.3390/app15147784









