Systematic Review and Meta-Analysis of Melatonin Quantification in Wine
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion/Exclusion Criteria and Data Extraction
2.3. Statistical Analyses
3. Results and Discussion
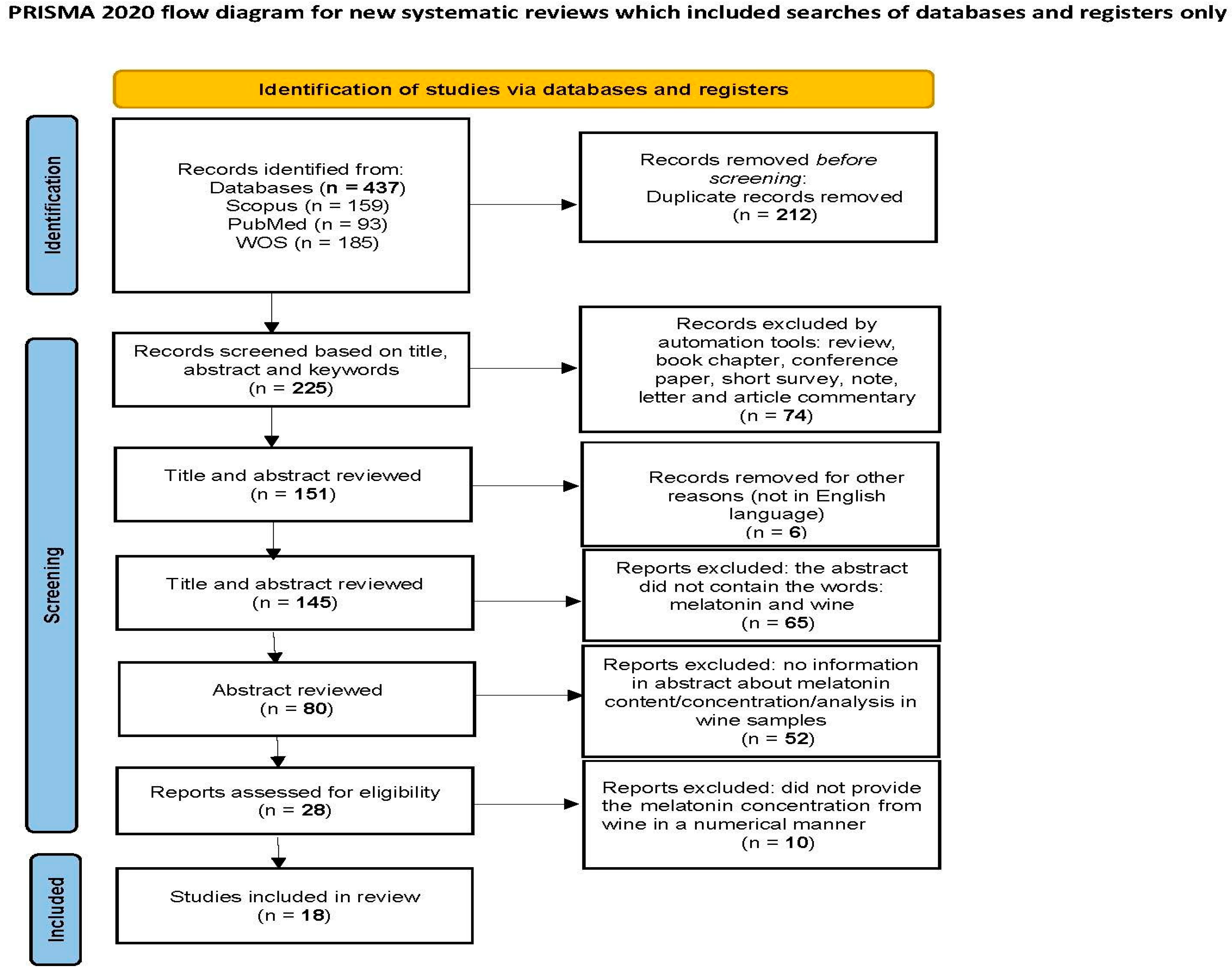
3.1. Selection of Studies
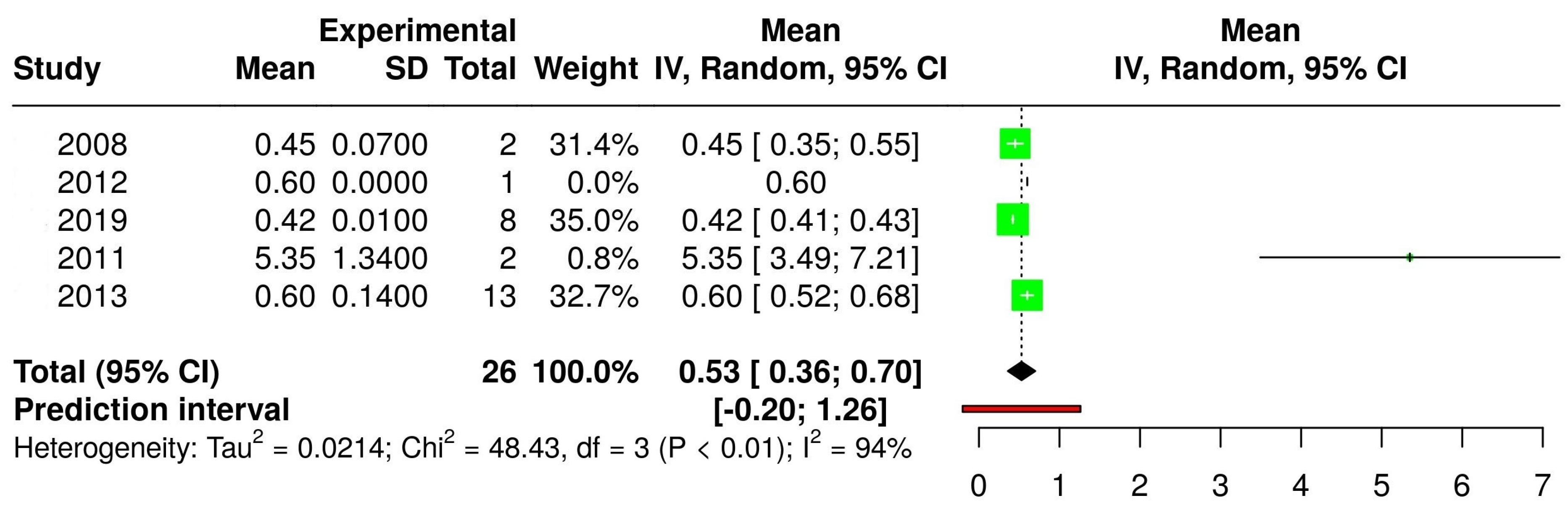
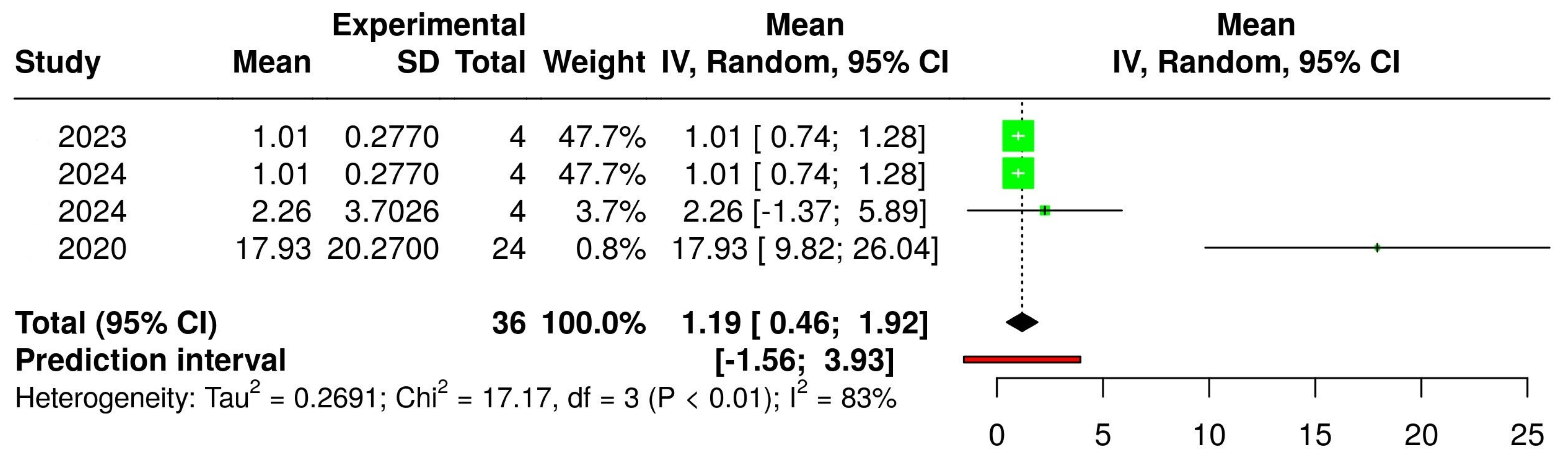
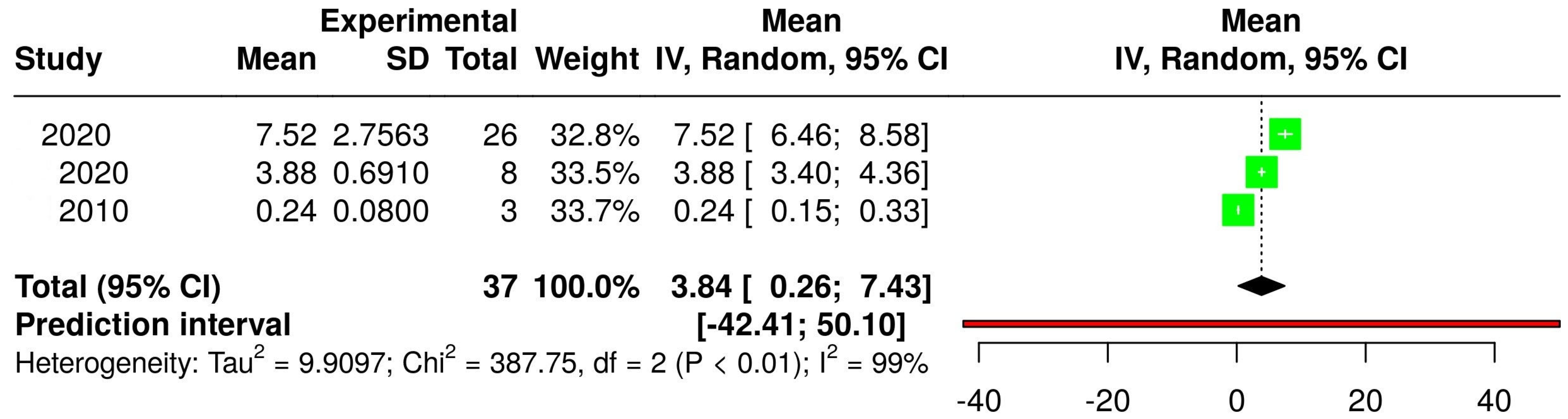
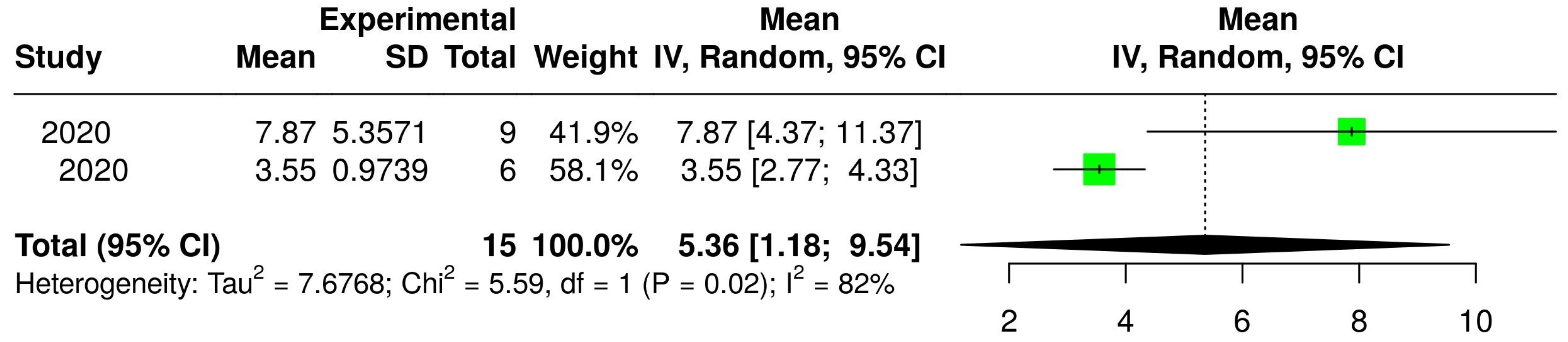
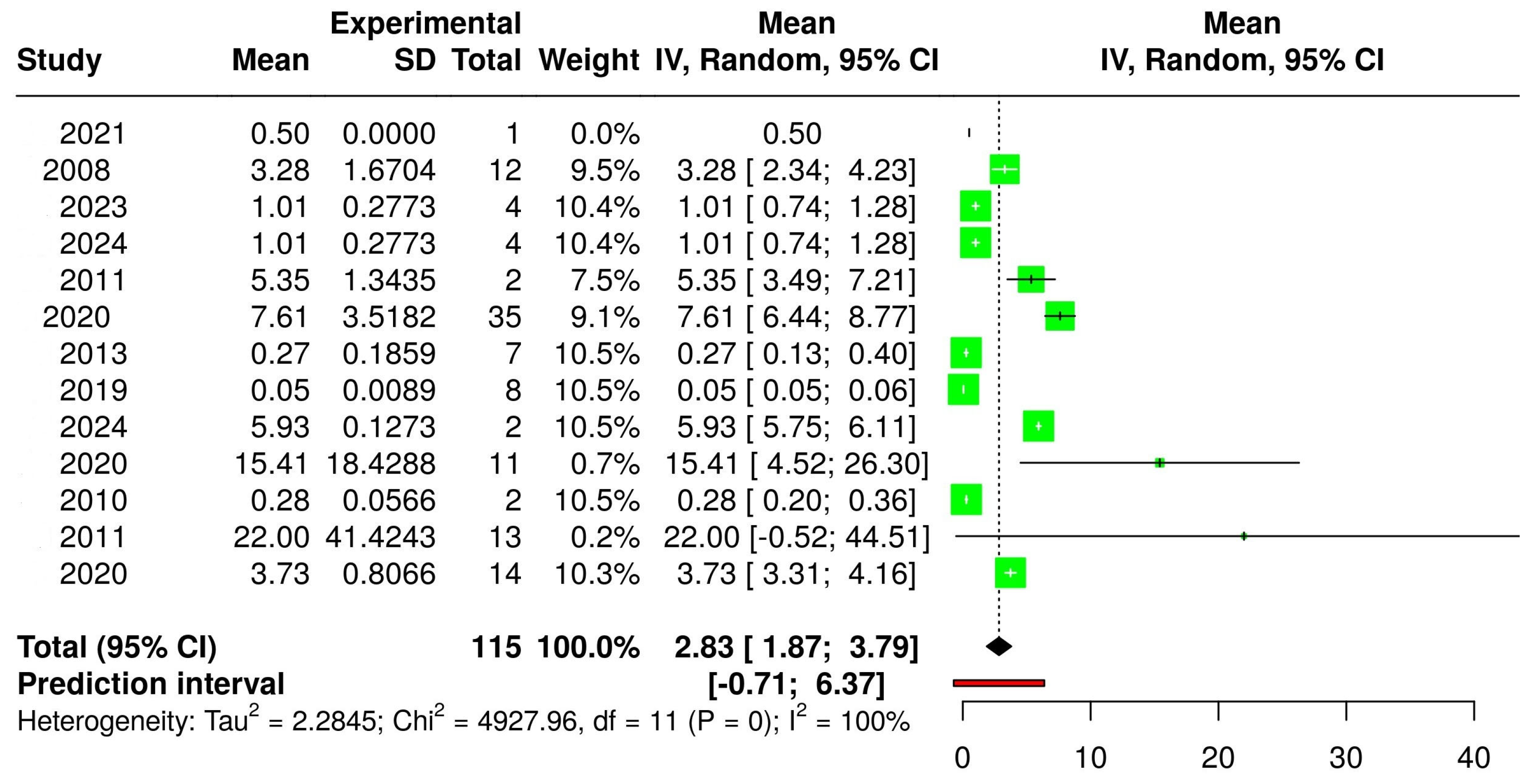
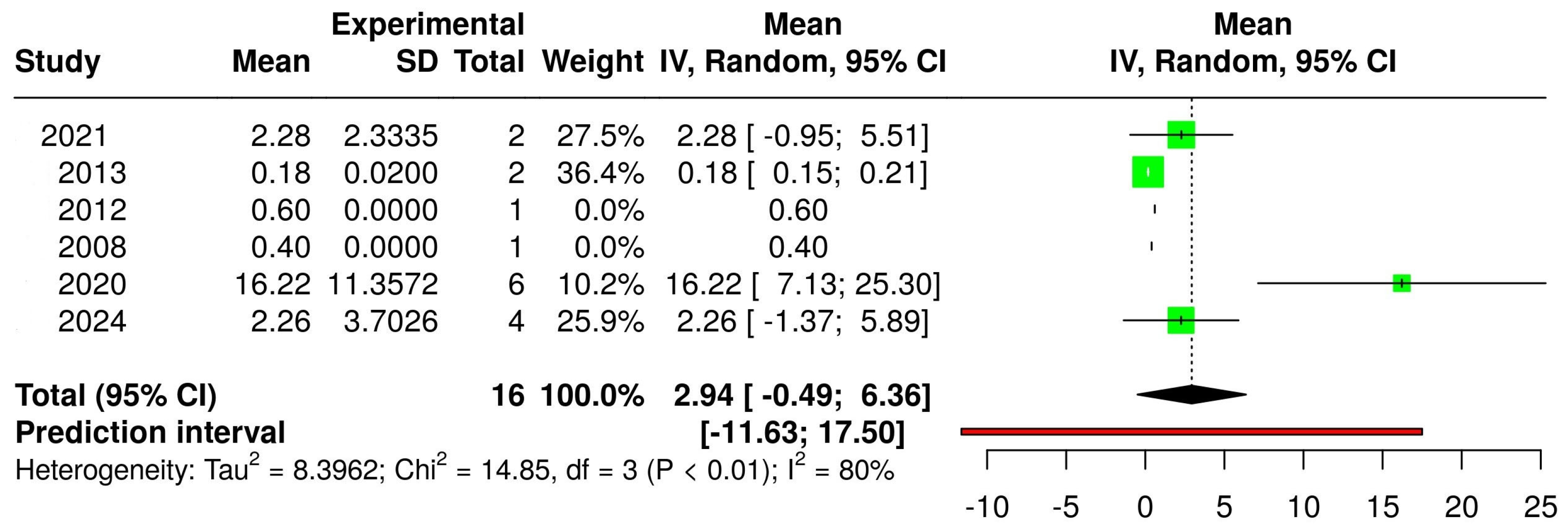
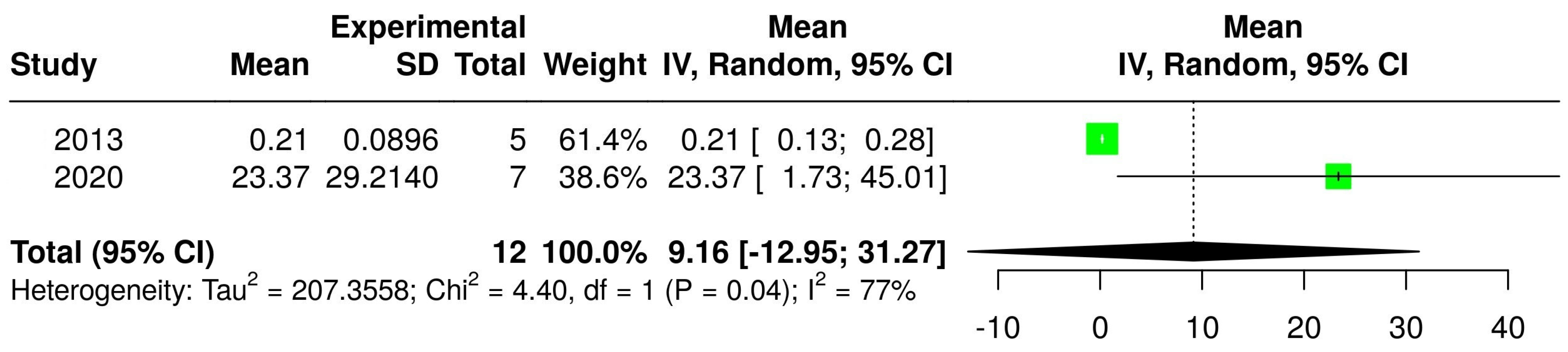
3.2. The Concentration of Melatonin in Wines from Different Countries
3.3. The Methodology of Melatonin Quantification in Wines
3.4. The Concentration of Melatonin According to the Type of Wine
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CEC | Capillary electrochromatography method |
| ELISA | Enzyme-linked immunosorbent assay method |
| HPLC | High performance liquid chromatography |
| DAD | Diode array detection |
| FL | Fluorescence |
| MS | Mass spectrometry |
| SPE | Solid phase extraction |
| DLLME | Dispersive liquid–liquid micro-extraction |
| MEPS | Microextraction by packed sorbent |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| FN | Feteasca Neagra |
| CS | Cabernet Sauvignon |
| MRAW | Summarized raw means |
References
- Cipolla-Neto, J.; Gaspar do Amaral, F. Melatonin as a Hormone: New Physiological and Clinical Insights. Endocr. Rev. 2018, 39, 990–1028. [Google Scholar] [CrossRef] [PubMed]
- Hacışevki, A.; Baba, B. An Overview of Melatonin as an Antioxidant Molecule: A Biochemical Approach, Melatonin—Molecular Biology. In Clinical and Pharmaceutical Approaches, 1st ed.; Dragoi, C.M., Nicolae, A.C., Eds.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Galano, A.; Tan, D.X.; Reiter, R.J. Melatonin as a natural ally against oxidative stress: A physicochemical examination. J. Pineal Res. 2011, 51, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Pieri, C.; Marra, M.; Moroni, F.; Recchioni, R.; Marcheselli, F. Melatonin: A peroxyl radical scavenger more effective than vitamin E. Life Sci. 1994, 55, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Falcón, J.; Besseau, L.; Fuentès, M.; Sauzet, S.; Magnanou, E.; Boeuf, G. Structural and functional evolution of the pineal melatonin system in vertebrates. Ann. N. Y. Acad. Sci. 2009, 1163, 101–111. [Google Scholar] [CrossRef]
- Salehi, B.; Sharopov, F.; Fokou, P.V.T.; Kobylinska, A.; Jonge, L.d.; Tadio, K.; Sharifi-Rad, J.; Posmyk, M.M.; Martorell, M.; Martins, N.; et al. Melatonin in Medicinal and Food Plants: Occurrence, Bioavailability, and Health Potential for Humans. Cells 2019, 8, 681. [Google Scholar] [CrossRef]
- Marhuenda, J.; Villaño, D.; Arcusa, R.; Zafrilla, P. Melatonin in wine and beer: Beneficial effects. Molecules 2021, 26, 343. [Google Scholar] [CrossRef]
- Viegas, O.; Esteves, C.; Rocha, J.; Melo, A.; Ferreira, I.M.P.L.V.O. Simultaneous determination of melatonin and trans-resveratrol in wine by dispersive liquid–liquid microextraction followed by HPLC-FLD. Food Chem. 2021, 339, 128091. [Google Scholar] [CrossRef]
- Carneiro, C.N.; Gomez, F.J.V.; Spisso, A.; Silva, M.F.; Azcarate, S.M.; Dias, F.D.S. Geographical characterization of South America wines based on their phenolic and melatonin composition: An exploratory analysis. Microchem. J. 2020, 158, 105240. [Google Scholar] [CrossRef]
- Meng, X.; Li, Y.; Li, S.; Zhou, Y.; Gan, R.-Y.; Xu, D.-P.; Li, H.-B. Dietary Sources and Bioactivities of Melatonin. Nutrients 2017, 9, 367. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Gitto, E.; Sainz, R.M.; Mayo, J.C.; Leon, J.; Manchester, L.C.; Vijayalaxmi; Kilic, E.; Kilic, U. Pharmacological utility of melatonin in reducing oxidative cellular and molecular damage. Pol. J. Pharmacol. 2004, 56, 159–170. [Google Scholar]
- Garrido, M.; Paredes, S.D.; Cubero, J.; Lozano, M.; Toribio-Delgado, A.F.; Muñoz, J.L.; Reiter, R.J.; Barriga, C.; Rodríguez, A.B. Jerte Valley cherry-enriched diets improve nocturnal rest and increase 6-sulfatoxymelatonin and total antioxidant capacity in the urine of middle-aged and elderly humans. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Iriti, M.; Rossoni, M.; Faoro, F. Melatonin content in grape: Myth or panacea? J. Sci. Food Agri. 2006, 86, 1432–1438. [Google Scholar] [CrossRef]
- Mercolini, L.; Addolorata Saracino, M.; Bugamelli, F.; Ferranti, A.; Malaguti, M.; Hrelia, S.; Raggi, M.A. HPLC-Fl analysis of melatonin and resveratrol isomers in wine using an SPE procedure. J. Separ. Sci. 2008, 31, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Cruz, E.M.A.; Álvarez-Fernández, E.; Valero, A.; Troncoso, M.; García-Parrilla, M.C. Melatonin and derived L-tryptophan metabolites produced during alcoholic fermentation by different wine yeast strains. Food Chem. 2017, 217, 431–437. [Google Scholar] [CrossRef]
- James, A.; Yao, T.; Ke, H.; Wang, Y. Microbiota for production of wine with enhanced functional components. Food Sci. Hum. Wellness 2023, 12, 1481–1492. [Google Scholar] [CrossRef]
- Xiang, G.; Jia, R.; Wang, F.; Wang, S.; Li, Y.; Yao, Y. Exogenous L-tryptophan treatment increases the concentrations of melatonin and aroma compounds in grape berries and wine. Food Qual. Saf. 2024, 8, fyad042. [Google Scholar] [CrossRef]
- Rodriguez-Naranjo, M.I.; Torija, M.J.; Mas, A.; Cantos-Villar, E.; Garcia-Parrilla, C. Production of melatonin by Saccharomyces strains under growth and fermentation conditions. J. Pineal Res. 2012, 53, 219–224. [Google Scholar] [CrossRef]
- Rodriguez-Naranjo, M.I.; Ordóñez, J.L.; Callejón, R.M.; Cantos-Villar, E.; Garcia-Parrilla, M.C. Melatonin is formed during winemaking at safe levels of biogenic amines. Food Chem. Toxicol. 2013, 57, 140–146. [Google Scholar] [CrossRef]
- Fernandez-Mar, M.I.; Mateos, R.; García-Parrilla, M.C.; Puertas, B.; Cantos-Villar, E. Bioactive compounds in wine: Resveratrol, hydroxytyrosol and melatonin: A review. Food Chem. 2012, 130, 797–813. [Google Scholar] [CrossRef]
- Lee, S.; Jin, J.X.; Taweechaipaisankul, A.; Kim, G.A.; Lee, B.C. Synergistic effects of resveratrol and melatonin on in vitro maturation of porcine oocytes and subsequent embryo development. Theriogenology 2018, 114, 191–198. [Google Scholar] [CrossRef]
- Xu, L.; Yue, Q.; Bian, F.; Zhai, H.; Yao, Y. Melatonin Treatment Enhances the Polyphenol Content and Antioxidant Capacity of Red Wine. Hortic. Plant J. 2018, 4, 44–50. [Google Scholar] [CrossRef]
- Eremia, S.A.V.; Albu, C.; Radu, G.-L.; Alecu, A.; Brinduse, E. The Influence of Melatonin Treatment in the Vinification of Feteasca Neagra and Cabernet Sauvignon Wines on the Profile of Polyphenolic Compounds and Antioxidant Activity. Antioxidants 2023, 12, 1214. [Google Scholar] [CrossRef] [PubMed]
- Muñiz-Calvo, S.; Guillamón, J.M.; Domínguez, I.; Doménech-Carbó, A. Detecting and Monitoring the Production of Melatonin and Other Related Indole Compounds in Different Saccharomyces Strains by Solid-State Electrochemical Techniques. Food Anal. Methods 2017, 10, 1408–1418. [Google Scholar] [CrossRef]
- Stage, P.W.; Sombra, L.L.; Messina, G.; Martinez, L.D.; Silya, M.F. Determination of melatonin in wine and plant extracts by capillary electrochromatography with immobilized carboxylic multi-walled carbon nanotubes as stationary phase. Electrophoresis 2010, 31, 2242–2248. [Google Scholar] [CrossRef]
- Rodriguez-Naranjo, M.I.; Gil-Izquierdo, A.; Troncoso, A.M.; Cantos, E.; Garcia-Parrilla, M.C. Melatonin: A new bioactive compound in wine. J. Food Compos. Anal. 2011, 24, 603–608. [Google Scholar] [CrossRef]
- Carneiro, A.F.; Carneiro, C.N.; Gomez, F.J.V.; Spisso, A.; Silva, M.F.; Minho, L.A.C.; dos Santos, W.N.L.; Dias, F.D. Doehlert matrix for the optimization of ultrasound dispersive liquid–liquid microextraction of melatonin in Argentine and Brazilian wine samples. Microchem. J. 2020, 159, 105313. [Google Scholar] [CrossRef]
- Mercolini, L.; Mandrioli, R.; Raggi, M.A. Content of melatonin and other antioxidants in grape-related foodstuffs: Measurement using a MEPS-HPLC-FL method. J. Pineal Res. 2012, 53, 21–28. [Google Scholar] [CrossRef]
- Fracassetti, D.; Vigentini, I.; Lo Faro, A.F.F.; De Nisi, P.; Foschino, R.; Tirelli, A.; Orioli, M.; Iriti, M. Assessment of Tryptophan, Tryptophan Ethylester, and Melatonin Derivatives in Red Wine by SPE-HPLC-FL and SPE-HPLC-MS Methods. Foods 2019, 8, 99. [Google Scholar] [CrossRef]
- Eremia, S.A.V.; Albu, C.; Radu, G.L.; Ion, M. Different Extraction Approaches for the Analysis of Melatonin from Cabernet Sauvignon and Feteasca Neagra Wines Using a Validated HPLC-FL Method. Molecules 2023, 28, 2768. [Google Scholar] [CrossRef]
- Tudela, R.; Ribas-Agustí, A.; Buxaderas, S.; Riu-Aumatell, M.; Castellari, M.; López-Tamames, E. Ultrahigh-Performance Liquid Chromatography (UHPLC)–Tandem Mass Spectrometry (MS/MS) Quantification of Nine Target Indoles in Sparkling Wines. J. Agric. Food Chem. 2016, 64, 4772–4776. [Google Scholar] [CrossRef]
- Albu, C.; Radu, L.E.; Radu, G.L. Assessment of Melatonin and Its Precursors Content by a HPLC-MS/MS Method from Different Romanian Wines. ACS Omega 2020, 5, 27254–27260. [Google Scholar] [CrossRef] [PubMed]
- Gardana, C.; Iriti, M.; Stuknytė, M.; De Noni, I.; Simonetti, P. ‘Melatonin isomer’ in wine is not an isomer of the melatonin but tryptophan-ethylester. J. Pineal Res. 2014, 57, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Grao-Cruces, E.; Calvo, J.R.; Maldonado-Aibar, M.D.; Millan-Linares, M.d.C.; Montserrat-de la Paz, S. Mediterranean Diet and Melatonin: A Systematic Review. Antioxidants 2023, 12, 264. [Google Scholar] [CrossRef]
- Meng, J.-F.; Shi, T.-C.; Song, S.; Zhang, Z.-W.; Fang, Y.-L. Melatonin in grapes and grape-related foodstuffs: A review. Food Chem. 2017, 231, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Kocadağlı, T.; Yılmaz, C.; Gökmen, V. Determination of melatonin and its isomer in foods by liquid chromatography tandem mass spectrometry. Food Chem. 2014, 153, 151–156. [Google Scholar] [CrossRef]
- Gomez, F.J.; Hernández, I.G.; Martinez, L.D.; Silva, M.F.; Cerutti, S. Analytical tools for elucidating the biological role of melatonin in plants by LC-MS/MS. Electrophoresis 2013, 34, 1749–1756. [Google Scholar] [CrossRef]
- Rodriguez-Naranjo, M.I.; Gil-Izquierdo, A.; Troncoso, A.M.; Cantos-Villar, E.; Garcia-Parrilla, M.C. Melatonin is synthesised by yeast during alcoholic fermentation in wines. Food Chem. 2011, 126, 1608–1613. [Google Scholar] [CrossRef]
- Scutarașu, E.C.; Niță, R.G.; Vlase, L.; Zamfir, C.I.; Cioroiu, B.I.; Colibaba, L.C.; Muntean, D.; Luchian, C.E.; Vlase, A.M.; Cotea, V. Maximizing Wine Antioxidants: Yeast’s Contribution to Melatonin Formation. Antioxidants 2024, 13, 916. [Google Scholar] [CrossRef]
- Eremia, S.A.V.; Albu, C.; Radu, G.-L.; Alecu, A.; Stoica, A.G.; Brinduse, E. Winemaking Technologies for the Production of Cabernet Sauvignon and Feteasca Neagra Wines Enriched with Antioxidant Active Principles Due to the Addition of Melatonin. Foods 2024, 13, 884. [Google Scholar] [CrossRef]
- Vitalini, S.; Gardana, C.; Zanzotto, A.; Fico, G.; Faoro, F.; Simonetti, P.; Iriti, M. From vineyard to glass: Agrochemicals enhance the melatonin and total polyphenol contents and antiradical activity of red wines. J. Pineal Res. 2011, 51, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Vitalini, S.; Gardana, C.; Simonetti, P.; Fico, G.; Iriti, M. Melatonin, melatonin isomers and stilbenes in Italian traditional grape products and their antiradical capacity. J. Pineal Res. 2013, 54, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Fekete, J.T.; Gyorffy, B. MetaAnalysisOnline.com: An Online Tool for the Rapid Meta-Analysis of Clinical and Epidemiological Studies. J. Med. Internet Res. 2025, 27, e64016. [Google Scholar] [CrossRef]
- Iriti, M.; Varoni, E.M. The good health of Bacchus: Melatonin in grapes, the unveiled myth. LWT 2016, 65, 758–776. [Google Scholar] [CrossRef]
- Bordet, F.; Roullier-Gall, C.; Ballester, J.; Vichi, S.; Quintanilla-Casas, B.; Gougeon, R.D.; Julien-Ortiz, A.; Kopplin, P.S.; Alexandre, H. Different Wines from Different Yeasts? “Saccharomyces cerevisiae Intraspecies Differentiation by Metabolomic Signature and Sensory Patterns in Wine”. Microorganisms 2021, 9, 2327. [Google Scholar] [CrossRef]
- Monga, G.; Yerram, S.; Koppula, S.; Sindhu, R.K.; Kumar, S. A Brief Assessment of the Bioanalytical Methods by using LC-MS/MS for the Quantitation of Melatonin: A Potential Biomarker for Sleep-Related Disorders. Braz. J. Anal. Chem. 2024, 11, 12–32. [Google Scholar] [CrossRef]
- Spadoni, G.; Diamantini, G.; Bedini, A.; Tarzia, G.; Vacondio, F.; Silva, C.; Rivara, M.; Mor, M.; Plazzi, P.V.; Zusso, M.; et al. Synthesis, antioxidant activity and structure-activity relationships for a new series of 2-(-acylaminoethyl)indoles with melatonin-like cytoprotective activity. J. Pineal Res. 2006, 40, 259–269. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Zhang, Y.; Zhou, Y.; Liu, Z.-F.; Wei, B.-B.; Feng, X.-S. Melatonin in different food samples: Recent update on distribution, bioactivities, pretreatment and analysis techniques. Food Res. Int. 2023, 163, 112272. [Google Scholar] [CrossRef]
- Gomez, F.J.V.; Monasterio, R.P.; Vargas, V.C.S.; Silva, M.F. Analytical Characterization of Wine and Its Precursors by Capillary Electrophoresis. Electrophoresis 2012, 33, 2240–2252. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Nunes, A.R.; Alves, G.; Silva, L.R. Serotonin and Melatonin: Plant Sources, Analytical Methods, and Human Health Benefits. Rev. Bras. Farm. 2021, 31, 162–175. [Google Scholar] [CrossRef]
- Rajotia, M.S.; Sharma, A.; Bhatt, A.; Mangal, V.; Sood, S.; Thakur, A.K.; Kashyap, S.; Verma, L.K. Melatonin Detection and Quantification Techniques. In Melatonin in Plants: A Regulator for Plant Growth and Development, 1st ed.; Kumar, R., Altaf, M.A., Lal, M.K., Tiwari, R.K., Eds.; Springer: Singapore, 2023; pp. 19–38. [Google Scholar] [CrossRef]
- Mehrzadi, S.; Pourhanifeh, M.H.; Mirzaei, A.; Moradian, F.; Hosseinzadeh, A. An updated review of mechanistic potentials of melatonin against cancer: Pivotal roles in angiogenesis, apoptosis, autophagy, endoplasmic reticulum stress and oxidative stress. Cancer Cell Int. 2021, 21, 188. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.-X.; Hardeland, R. Targeting Host Defense System and Rescuing Compromised Mitochondria to Increase Tolerance against Pathogens by Melatonin May Impact Outcome of Deadly Virus Infection Pertinent to COVID-19. Molecules 2020, 25, 4410. [Google Scholar] [CrossRef] [PubMed]
- Borisenkov, M.F.; Popov, S.V.; Smirnov, V.V.; Martinson, E.A.; Solovieva, S.V.; Danilova, L.A.; Gubin, D.G. The Association between Melatonin-Containing Foods Consumption and Students’ Sleep–Wake Rhythm, Psychoemotional, and Anthropometric Characteristics: A Semi-Quantitative Analysis and Hypothetical Application. Nutrients 2023, 15, 3302. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Jurado, A.; Escribano, B.M. Presence of melatonin in foods of daily consumption: The benefit of this hormone for health. Food Chem. 2024, 458, 140172. [Google Scholar] [CrossRef]
| Method | Sampling Method | Country | Red Wine | Conc. ng mL−1 | White Wine | Conc. ng mL−1 | Rose/Dessert Wine | Conc. ng mL−1 | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| HPLC-FL | SPE | Italy | Sangiovese | 0.5 | Trebbiano | 0.4 | - | [14] | |
| HPLC-FL | MEPS | Italy | - | Albana | 0.6 | - | [28] | ||
| HPLC-FL | DLLME | Portugal | Syrah | 4.29 | Multivarietal | 0.63 | - | [8] | |
| Alicante Bouschet | 1.92 | Moscatel Graúdo | 3.93 | ||||||
| Touriga Nacional | 2.71 | - | |||||||
| Castelão | 4.48 | ||||||||
| CS | 3.06 | ||||||||
| Touriga Franca | 1.05 | ||||||||
| Syrah | 2.61 | ||||||||
| CS | 2.85 | ||||||||
| Touriga Nacional | 2.82 | ||||||||
| Castelão | 7.44 | ||||||||
| Aragonez | 4.27 | ||||||||
| Trincadeira | 1.90 | ||||||||
| HPLC-FL | DLLME | Romania | FN | 0.74 | - | - | [30,41] | ||
| CS | 1.09 | ||||||||
| FN | 0.84 | ||||||||
| CS | 1.36 | ||||||||
| HPLC-MS/MS | only filtered | Italy | Groppello | 6.3 | - | - | [42] | ||
| Merlot | 4.4 | ||||||||
| HPLC-MS/MS | only filtered | Argentine | Malbec | 7.47 | - | - | [9] | ||
| 5.88 | |||||||||
| 5.80 | |||||||||
| 6.15 | |||||||||
| 4.67 | |||||||||
| 4.15 | |||||||||
| 4.81 | |||||||||
| 6.56 | |||||||||
| 6.11 | |||||||||
| 9.67 | |||||||||
| 9.46 | |||||||||
| 9.68 | |||||||||
| 7.81 | |||||||||
| 7.33 | |||||||||
| 10.49 | |||||||||
| 8.34 | |||||||||
| 12.53 | |||||||||
| 12.51 | |||||||||
| 4.55 | |||||||||
| 7.80 | |||||||||
| 12.39 | |||||||||
| 10.89 | |||||||||
| 7.62 | |||||||||
| 5.93 | |||||||||
| 3.30 | |||||||||
| 3.56 | |||||||||
| Brazil | Tempranillo, Tannat, Alicante, CS, Egiodola Syrah, Petit Syrah | 12.08 | - | - | |||||
| 4.97 | |||||||||
| 19.89 | |||||||||
| 7.40 | |||||||||
| 4.15 | |||||||||
| 8.06 | |||||||||
| 8.06 | |||||||||
| 3.51 | |||||||||
| 2.74 | |||||||||
| UPLC-TSQ Access Max-MS | only filtered | Romania | - | Aligoté and Fetească albă | 0.22 | - | [40] | ||
| 0.65 | |||||||||
| Sauvignon Blanc | 7.81 | ||||||||
| 0.37 | |||||||||
| HPLC-MS/MS | concentration | Spain | CS | 14.2 | - | [26] | |||
| Petit Verdot | 5.1 | ||||||||
| Prieto Picudo | 49 | ||||||||
| Syrah | 86.5 | ||||||||
| Tempranillo | 129.5 | ||||||||
| HPLC-MS/MS | SPE | Italy | Groppello | 0.35 | Chaudelune | 0.18 | Recioto di Soave | 0.14 | [43] |
| Melag | 0.62 | ||||||||
| Nebbiolo | 0.14 | Santelmo | 0.18 | ||||||
| Terre di Rubinoro | 0.17 | Passito di Pantelleria | 0.31 | ||||||
| Syrah | 0.23 | Marsala | 0.11 | ||||||
| Placido Rizzotto | 0.05 | Moscato di Pantelleria | 0.29 | ||||||
| La Segreta | 0.31 | ||||||||
| UHPLC/ESI-QTRAP | SPE | Italy | Nebbiolo | 0.06 | - | [29] | |||
| 0.06 | |||||||||
| 0.06 | |||||||||
| 0.04 | |||||||||
| 0.05 | |||||||||
| 0.05 | |||||||||
| 0.06 | |||||||||
| 0.04 | |||||||||
| HPLC-QTOF-MS | SPE | China | Marselan | 5.84 | - | [17] | |||
| 6.02 | |||||||||
| HPLC-MS/MS | SPE | Romania | CS | 17.7 | Sparkling | 19.6 | Lidia | 30.8 | [32] |
| Merlot and Pinot Noir | 23.0 | Riesling | 11.8 | CS | 8.4 | ||||
| Merlot | 18.0 | ||||||||
| CS and Merlot | 11.6 | Riesling | 17.0 | Lidia | 82.6 | ||||
| Merlot | 2.0 | Lidia | 32.6 | ||||||
| Babeasca neagra | 3.8 | Noah | 35.4 | Riesling and Chasselas | 2.0 | ||||
| Seibel 1 | 10 | ||||||||
| Isabelle and Babeasca neagra | 11.6 | Riesling and Feteasca | 1.0 | CS | 5.8 | ||||
| Seibel 1 | 1 | ||||||||
| Othello | 66.6 | CS | 12.5 | CS | 1.4 | ||||
| CS | 4.2 | ||||||||
| CEC | LE | Argentine | Malbec | 0.24 | Chardonnay | 0.16 | - | [25] | |
| CS | 0.32 | ||||||||
| ELISA | SPE | Spain | CS | 0.23 | [26] | ||||
| Jaen Tinto | 0.16 | ||||||||
| Merlot | 0.21 | ||||||||
| Palomino Negro | 0.28 | ||||||||
| Petit Verdot | 0.22 | ||||||||
| Prieto Picudo | 0.19 | ||||||||
| Syrah | 0.22 | ||||||||
| Tempranillo | 0.14 | ||||||||
| HPLC-DAD | US-DLLME | Argentine | Malbec | 3.02 | - | [27] | |||
| Malbec | 3.70 | ||||||||
| Malbec | 4.95 | ||||||||
| Malbec | 3.54 | ||||||||
| Brazil | CS | 2.23 | |||||||
| Tannat | 3.54 | ||||||||
| Alicante | 4.56 | ||||||||
| HPLC-MS/MS | US | Argentine | Malbec | 3.3 | |||||
| Malbec | 4.1 | ||||||||
| Malbec | 4.8 | ||||||||
| Malbec | 3.6 | ||||||||
| Brazil | CS | 2.74 | |||||||
| Tannat | 3.51 | ||||||||
| Alicante | 4.7 | ||||||||
| HPLC-MS/MS | concentration | Spain | CS | 74.13 * | Palomino Fino | 390.82 * | - | [39] | |
| Merlot | 241.22 * | ||||||||
| Tempranillo | 77.72 * | ||||||||
| Tintilla de Rota | 322.68 * | ||||||||
| Merlot | 245.46 * | ||||||||
| Syrah | 423.01 * | ||||||||
| Tempranillo | 306.86 * | ||||||||
| HPLC-MS/MS | - | Turkey | - | 170.7 * | - | - | [37] | ||
| HPLC-MS/MS | SPE | Argentine | Tannat | 151.74 * | - | - | [38] | ||
| Merlot | 211.28 * | ||||||||
| Malbec | 145.26 * | ||||||||
| CS | 185.09 * | ||||||||
| Malbec | 60.16 * | ||||||||
| Sample Pretreatment | Cartridge/Sorbent | Elution/Extraction Solvent | Analytical Method | LoD and LoQ ng mL−1 | References |
|---|---|---|---|---|---|
| SPE | C8 cartridges | methanol | HPLC-MS/MS | 0.12 and 0.42 | [38] |
| SPE | Bond Elut C18 | methanol | HPLC-MS/MS | 0.6 | [32] |
| SPE | Strata X-Polymeric Reversed Phase | methanol | HPLC-MS/MS | 2.3 and 18 | [29] |
| SPE | ProElut C18 | methanol | HPLC-MS/MS | - | [17] |
| SPE | HLB Oasys | methanol | HPLC-MS/MS | - | [43] |
| SPE | C18 | methanol | ELISA | - | [26] |
| SPE | BondElut C18 | methanol | HPLC-FL | 0.03 and 0.01 | [14] |
| MEPS | C8 | methanol | HPLC-FL | 0.05 and 0.02 | [28] |
| DLLME | - | chloroform | HPLC-FL | 0.01 and 0.05 | [41] |
| DLLME | chloroform | HPLC-FL | 0.01 and 0.05 | [30] | |
| DLLME | - | chloroform | HPLC-FL | 0.07 and 0.24 | [8] |
| US-DLLME | - | dichloromethane | HPLC-DAD | 230 and 700 | [27] |
| only filtered | - | - | HPLC-MS/MS | 0.059 and 0.12 | [40] |
| only filtered | - | - | HPLC-MS/MS | - | [9] |
| only filtered | - | - | HPLC-MS/MS | - | [42] |
| LE | - | methanol | CEC | 0.01 and 0.03 | [25] |
| concentration | 3:1 (v:v) | methanol | HPLC-MS/MS | - | [39] |
| - | - | - | HPLC-MS/MS | 0.033 and 0.112 | [37] |
| HPLC-MS | 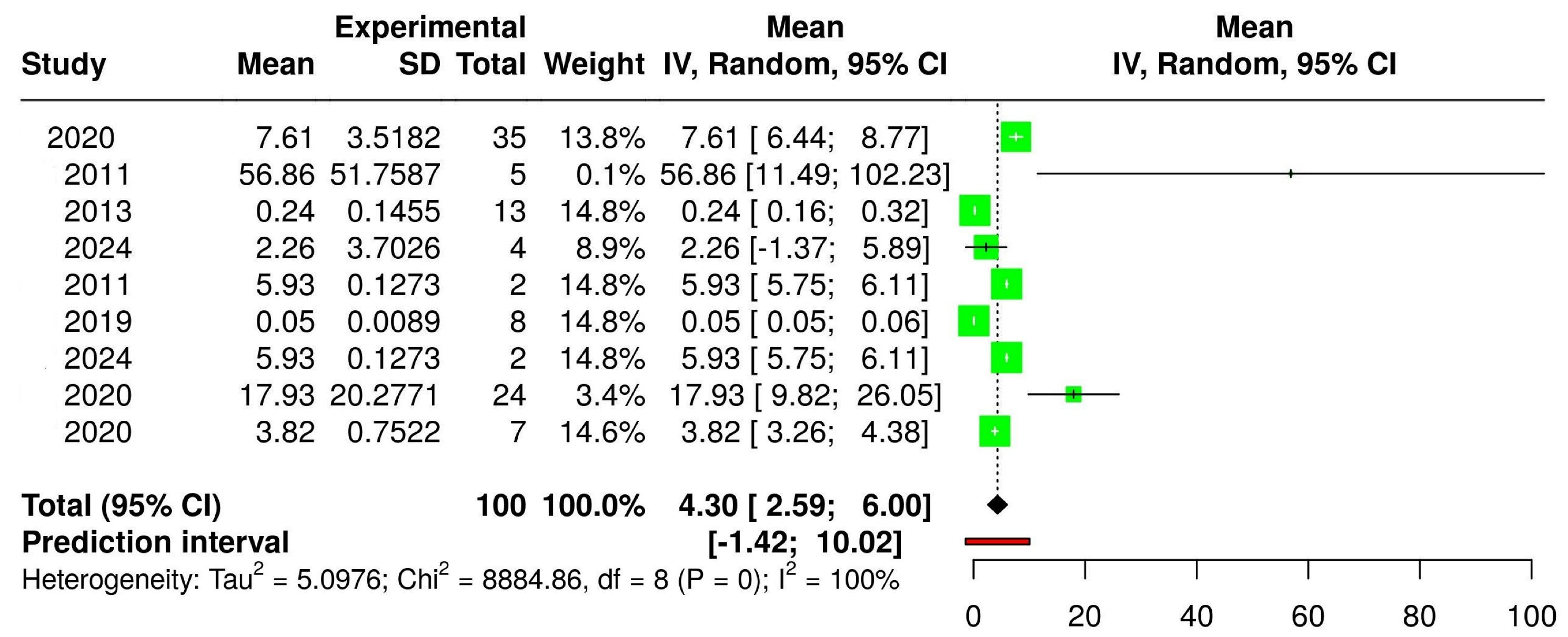 |
| HPLC-FL |  |
| Malbec | 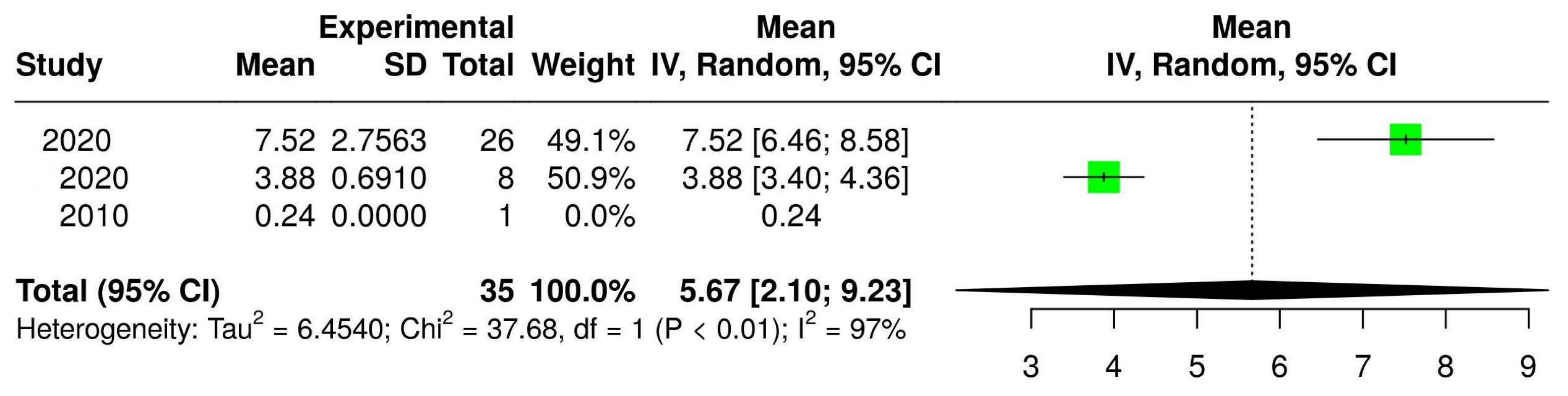 |
| Cabernet Sauvignon | 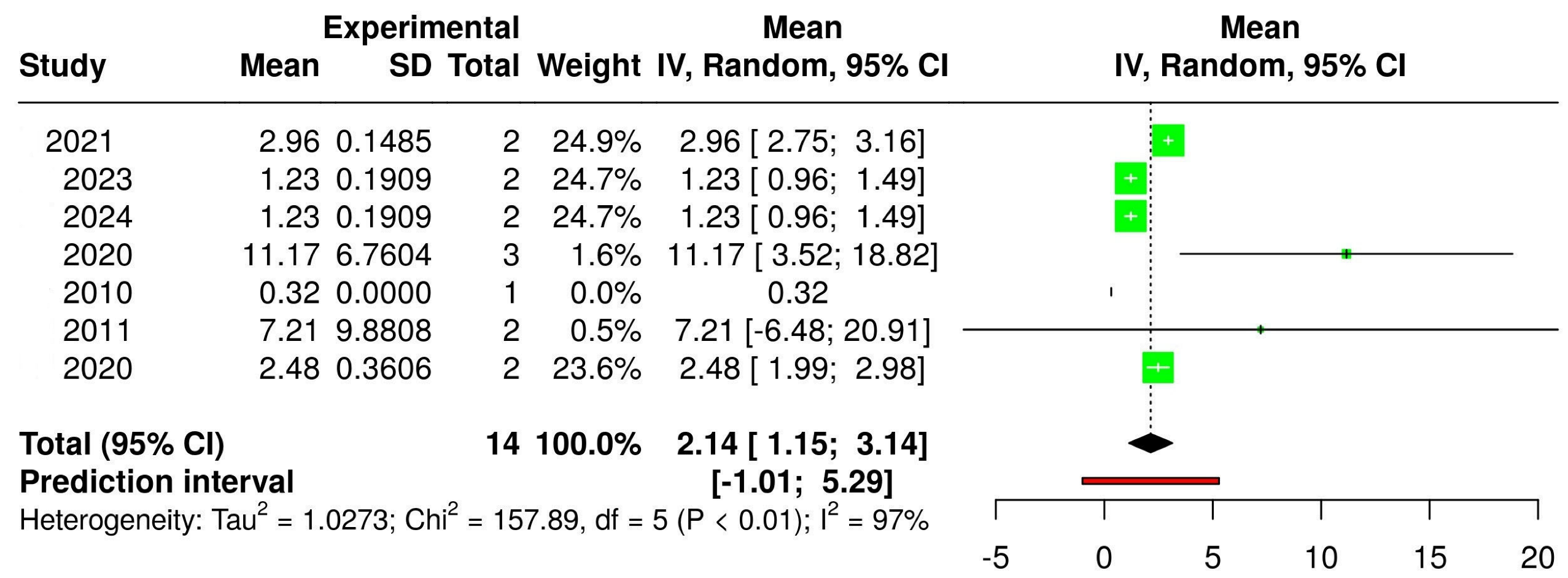 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eremia, S.A.V.; Radu, G.L.; Albu, C. Systematic Review and Meta-Analysis of Melatonin Quantification in Wine. Appl. Sci. 2025, 15, 7755. https://doi.org/10.3390/app15147755
Eremia SAV, Radu GL, Albu C. Systematic Review and Meta-Analysis of Melatonin Quantification in Wine. Applied Sciences. 2025; 15(14):7755. https://doi.org/10.3390/app15147755
Chicago/Turabian StyleEremia, Sandra A. V., Gabriel Lucian Radu, and Camelia Albu. 2025. "Systematic Review and Meta-Analysis of Melatonin Quantification in Wine" Applied Sciences 15, no. 14: 7755. https://doi.org/10.3390/app15147755
APA StyleEremia, S. A. V., Radu, G. L., & Albu, C. (2025). Systematic Review and Meta-Analysis of Melatonin Quantification in Wine. Applied Sciences, 15(14), 7755. https://doi.org/10.3390/app15147755





