Molecular Response of Bacteria Exposed to Wastewater-Borne Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Al2O3NPs and Bulk Al2O3 Suspensions
2.2. Lab-Scale Wastewater Treatment Plant (WWTP)
2.3. Growth Inhibition and Toxicity Assessment in Pseudomonas putida
2.4. Gene Expression Exposures
2.5. RNA Extraction, Reverse Transcription, and Real-Time Quantitative PCR
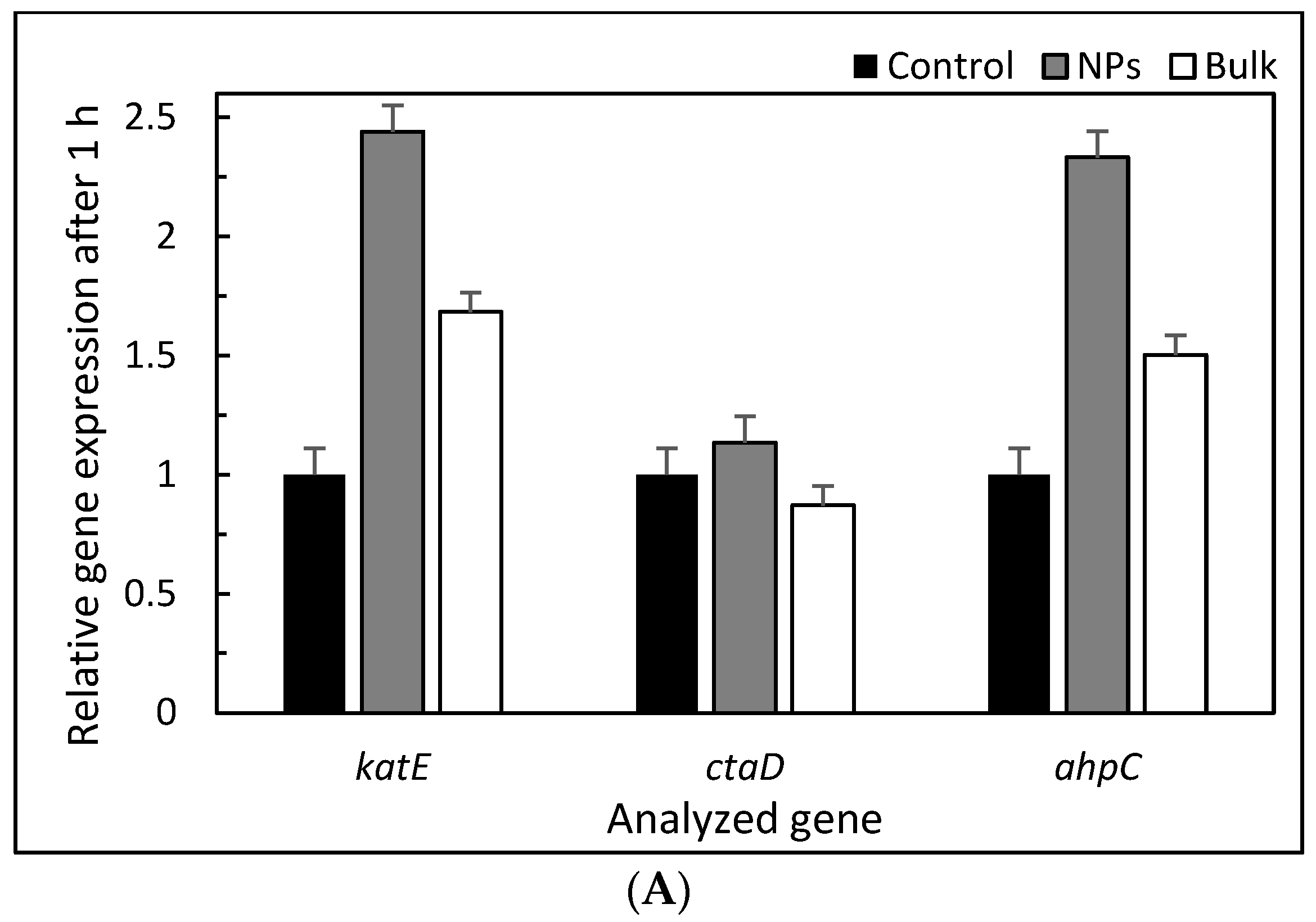
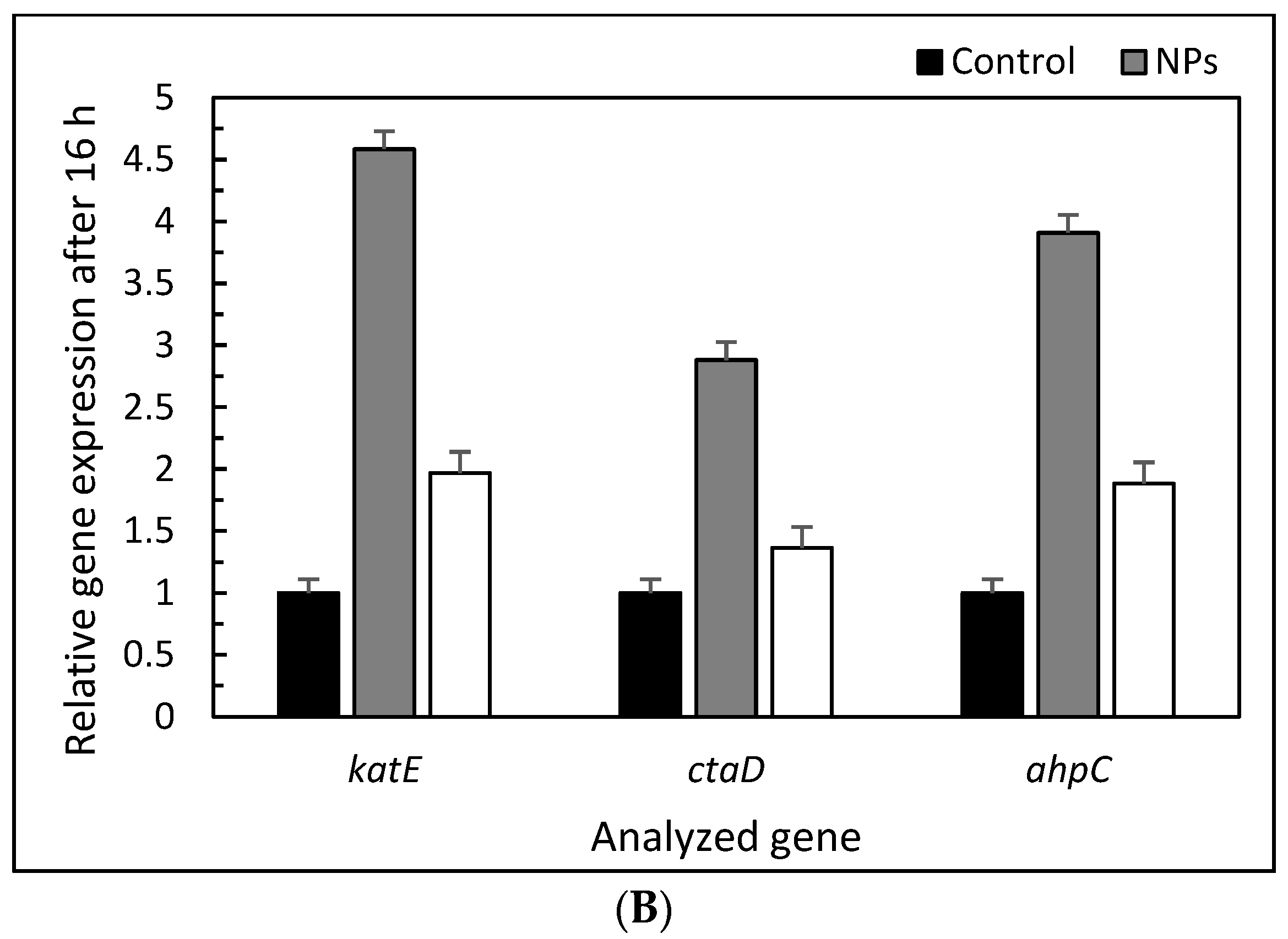
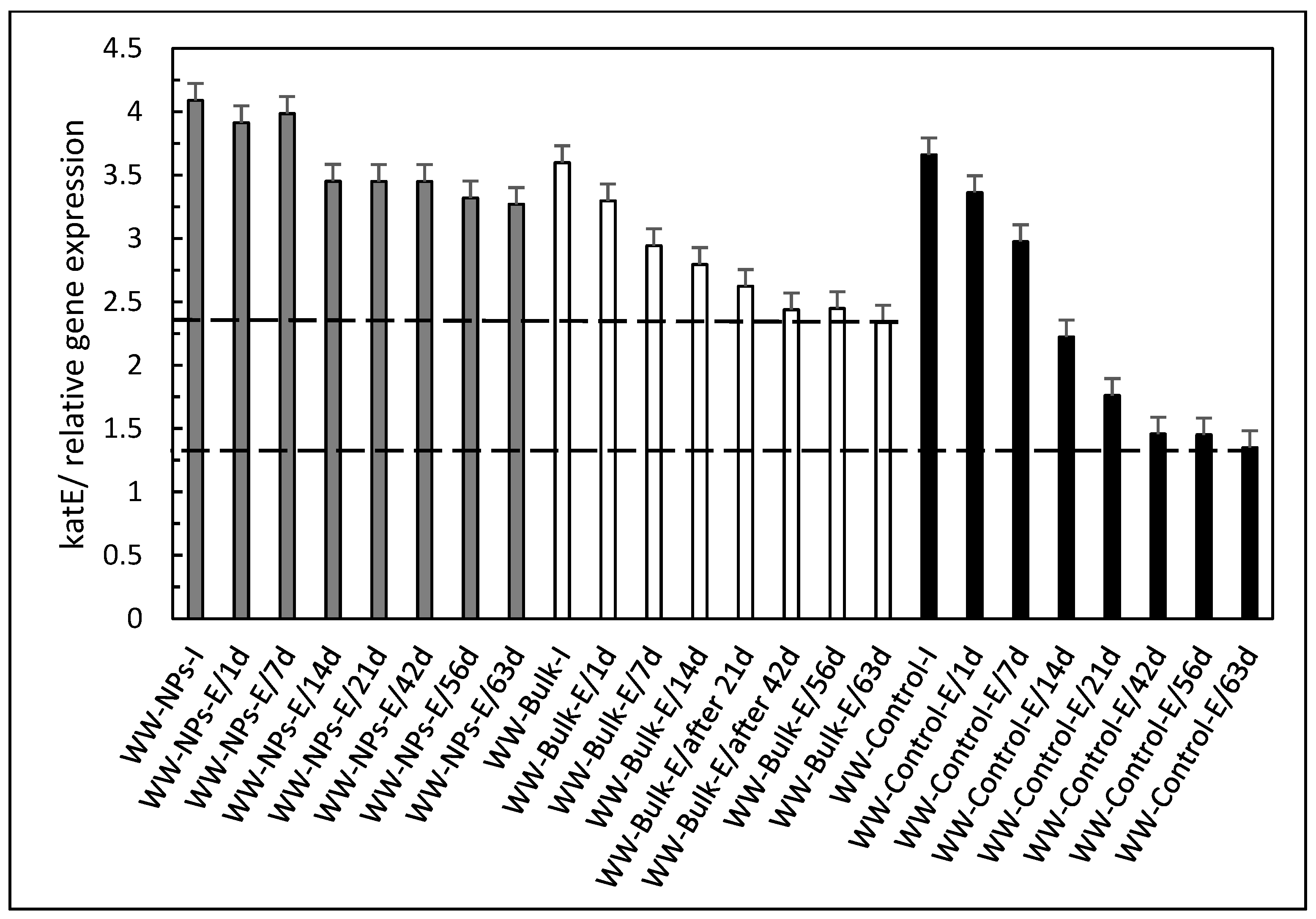
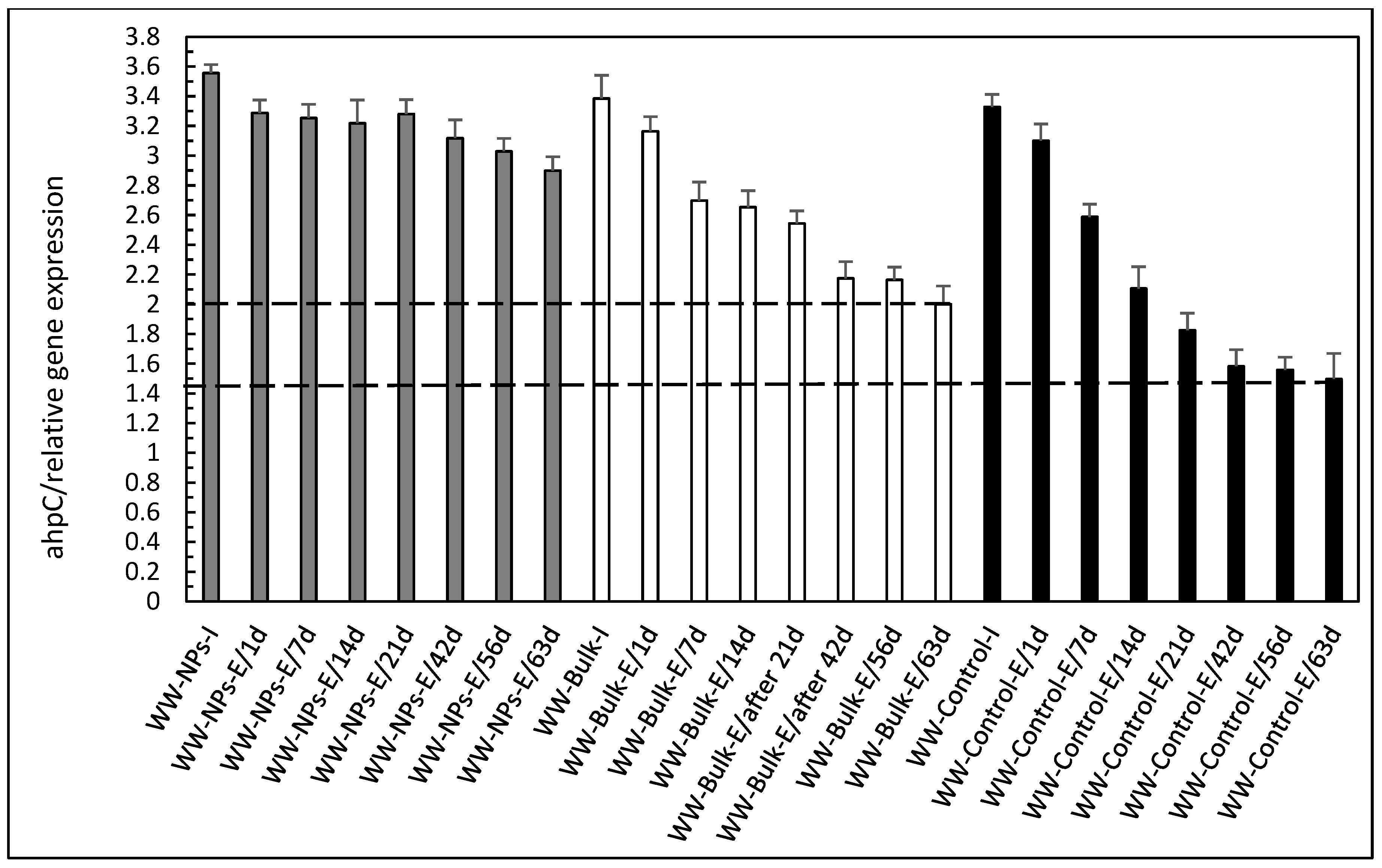
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Al2O3NPs | Aluminum oxide nanoparticles |
| TUa | Acute Toxicity Unit |
| EC50 | Effective Concentration for 50% of the population |
| qPCR | Quantitative Polymerase Chain Reaction |
| RT-qPCR | Quantitative Reverse Transcription Polymerase Chain Reaction |
| ROS | Reactive Oxygen Species |
| WWTP | Wastewater Treatment Plant |
| SBR | Sequencing Batch Reactor |
| Ct | Cycle threshold (in qPCR) |
| RNA | Ribonucleic Acid |
| DNA | Deoxyribonucleic Acid |
| rRNA | Ribosomal RNA |
| ISO | International Organization for Standardization |
| NP | Nanoparticle |
| CAS | Chemical Abstracts Service (Registry Number) |
| PCR | Polymerase Chain Reaction |
| OD | Optical Density |
| DSMZ | Deutsche Sammlung von Mikroorganismen und Zellkulturen |
References
- Rodríguez, J.A.; Fernández-García, M. Synthesis, Properties, and Applications of Oxide Nanomaterials; Wiley: Hoboken, NJ, USA, 2006. [Google Scholar] [CrossRef]
- Raczyński, P.; Górny, K.; Bełdowski, P.; Marciniak, B.; Pöschel, T.; Dendzik, Z. Influence of silicon nanocone on cell membrane self-sealing capabilities for targeted drug delivery—Computer simulation study. Arch. Biochem. Biophys. 2023, 749, 109802. [Google Scholar] [CrossRef] [PubMed]
- Lammers, T.; Hennink, W.E.; Storm, G. Tumour-targeted nanomedicines: Principles and practice. Br. J. Cancer 2008, 99, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Mohapatra, S.; Mishra, H.; Farooq, U.; Kumar, K.; Ansari, M.J.; Aldawsari, M.F.; Alalaiwe, A.S.; Mirza, M.A.; Iqbal, Z. Nanotechnology in cosmetics and cosmeceuticals—A review of latest advancements. Gels 2022, 8, 173. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, E.K.; Golemanov, K.; Dufils, P.-E.; Wilson, J.; Ahuja, R.; Heux, L.; Berret, J.-F. Nanorods of natural polysaccharides: Formation mechanisms and rheology. ACS Appl. Polym. Mater. 2021, 3, 3009–3018. [Google Scholar] [CrossRef]
- Zajkowska-Pietrzak, W.; Turczyński, J.; Kurowska, B.; Teisseyre, H.; Fronc, K.; Dąbrowski, J.; Kret, S. ZnO nanowires grown on Al2O3–ZnAl2O4 nanostructure using solid–vapor mechanism. Arch. Metall. Mater. 2023, 68, 1177–1182. [Google Scholar] [CrossRef]
- Som, C.; Wick, P.; Krug, H.; Nowack, B. Environmental and health effects of nanomaterials in nanotextiles and facade coatings. Environ. Int. 2011, 37, 1131–1142. [Google Scholar] [CrossRef]
- Mushtaq, F.; Asani, A.; Hoop, M.; Chen, X.-Z.; Ahmed, D.; Nelson, B.J.; Pané, S. Highly efficient coaxial TiO2–PtPd tubular nanomachines for photocatalytic water purification with multiple locomotion strategies. Adv. Funct. Mater. 2016, 26, 6995. [Google Scholar] [CrossRef]
- Bottero, J.Y.; Rose, J.; Wiesner, M.R. Nanotechnologies: Tools for sustainability in a new wave of water treatment processes. Integr. Environ. Assess. Manag. 2006, 2, 391–395. [Google Scholar] [CrossRef]
- Pak, T.; Luz, L.F.L., Jr.; Tosco, T.; Costa, G.S.R.; Rosa, P.R.R.; Archilha, N.L. Pore-scale investigation of the use of reactive nanoparticles for in situ remediation of contaminated groundwater source. Proc. Natl. Acad. Sci. USA 2020, 117, 13366–13373. [Google Scholar] [CrossRef]
- Kumah, E.A.; Fopa, R.D.; Harati, S.; Boadu, P.; Zohoori, F.V.; Pak, T. Human and environmental impacts of nanoparticles: A scoping review of the current literature. BMC Public Health 2023, 23, 1059. [Google Scholar] [CrossRef]
- Sadiq, I.M.; Chowdhury, B.; Chandrasekaran, N.; Mukherjee, A. Antimicrobial sensitivity of Escherichia coli to alumina nanoparticles. Nanomedicine 2009, 5, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, C.; Saccà, M.L.; Costa, G.; Nande, M.; Martin, M. Impact of Ag and Al2O3 nanoparticles on soil organisms: In vitro and soil experiments. Sci. Total Environ. 2014, 473–474, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Khan, H.M.; Khan, A.A.; Pal, R.; Cameotra, S.S. Antibacterial potential of Al2O3 nanoparticles against multidrug resistance strains of Staphylococcus aureus isolated from skin exudates. J. Nanoparticle Res. 2013, 15, 1970. [Google Scholar] [CrossRef]
- Bakand, S.; Hayes, A. Toxicological considerations, toxicity assessment, and risk management of inhaled nanoparticles. Int. J. Mol. Sci. 2016, 17, 929. [Google Scholar] [CrossRef]
- Chain, J.J.F.; Finlayson, S.; Crease, T.; Cristescu, M. Variation in transcriptional responses to copper exposure across Daphnia pulex lineages. Aquat. Toxicol. 2019, 210, 85–97. [Google Scholar] [CrossRef]
- Qiu, T.A.; Bozich, J.S.; Lohse, S.E.; Vartanian, A.M.; Jacob, L.M.; Meyer, B.M.; Gunsolus, I.L.; Niemuth, N.J.; Murphy, C.J.; Haynes, C.L.; et al. Gene expression as an indicator of the molecular response and toxicity in the bacterium Shewanella oneidensis and the water flea Daphnia magna exposed to functionalized gold nanoparticles. Environ. Sci. Nano 2015, 2, 615–629. [Google Scholar] [CrossRef]
- Poynton, H.C.; Loguinov, A.V.; Varshavsky, J.R.; Chan, S.; Perkins, E.J.; Vulpe, C.D. Gene expression profiling in Daphnia magna Part I: Concentration-dependent profiles provide support for the no observed transcriptional effect level. Environ. Sci. Technol. 2008, 42, 6250–6256. [Google Scholar] [CrossRef]
- Poynton, H.C.; Lazorchak, J.M.; Impellitteri, C.A.; Smith, M.E.; Rogers, K.; Patra, M.; Hammer, K.A.; Allen, H.J.; Vulpe, C.D. Differential gene expression in Daphnia magna suggests distinct modes of action and bioavailability for ZnO nanoparticles and Zn ions. Environ. Sci. Technol. 2011, 45, 762–768. [Google Scholar] [CrossRef]
- Doskocz, N.; Affek, K.; Matczuk, M.; Załęska-Radziwiłł, M. Effect of Al2O3NPs on gene expression in Daphnia magna: Implications for environmental risk assessment. Desalin. Water Treat. 2025, 321, 101000. [Google Scholar] [CrossRef]
- Yamini, V.; Shanmugam, V.; Rameshpathy, M.; Venkatraman, G.; Ramanathan, G.; Al Garalleh, H.; Hashmi, A.; Brindhadevi, K.; Devi Rajeswari, V. Environmental effects and interaction of nanoparticles on beneficial soil and aquatic microorganisms. Environ. Res. 2023, 236, 116776. [Google Scholar] [CrossRef]
- Das, P.; Xenopoulos, M.A.; Williams, C.J.; Hoque, M.E.; Metcalfe, C.D. Effects of silver nanoparticles on bacterial activity in natural waters. Environ. Toxicol. Chem. 2012, 31, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Ameen, F.; Alsamhary, K.; Alabdullatif, J.A.; AlNadhari, S. A review on metal-based nanoparticles and their toxicity to beneficial soil bacteria and fungi. Ecotoxicol. Environ. Saf. 2021, 213, 112027. [Google Scholar] [CrossRef] [PubMed]
- Bharti, S.; Mukherji, S.; Mukherji, S. Enhanced antibacterial activity of decahedral silver nanoparticles. J. Nanopart. Res. 2021, 23, 36. [Google Scholar] [CrossRef]
- Venkatasubbu, G.D.; Baskar, R.; Anusuya, T.; Seshan, C.A.; Chelliah, R. Toxicity mechanism of titanium dioxide and zinc oxide nanoparticles against food pathogens. Colloids Surf. B Biointerfaces 2016, 148, 600–606. [Google Scholar] [CrossRef]
- González, G.; Herrera, G.; García, M.T.; Peña, M. Biodegradation of phenolic industrial wastewater in a fluidized bed bioreactor with immobilized cells of Pseudomonas putida. Bioresour. Technol. 2001, 80, 137–142. [Google Scholar] [CrossRef]
- Weimer, A.; Kohlstedt, M.; Volke, D.C.; Nikel, P.I.; Wittmann, C. Industrial biotechnology of Pseudomonas putida: Advances and prospects. Appl. Microbiol. Biotechnol. 2020, 104, 7745–7766. [Google Scholar] [CrossRef]
- Peng, J.; Miao, L.; Chen, X.; Liu, P. Comparative transcriptome analysis of Pseudomonas putida KT2440 revealed its response mechanisms to elevated levels of zinc stress. Front. Microbiol. 2018, 9, 1669. [Google Scholar] [CrossRef]
- Ouyang, K.; Mortimer, M.; Holden, P.A.; Cai, P.; Wu, Y.; Gao, C.; Huang, Q. Towards a better understanding of Pseudomonas putida biofilm formation in the presence of ZnO nanoparticles (NPs): Role of NP concentration. Environ. Int. 2020, 137, 105485. [Google Scholar] [CrossRef]
- Li, Y.; Park, J.S.; Deng, J.H.; Bai, Y. Cytochrome c oxidase subunit IV is essential for assembly and respiratory function of the enzyme complex. J. Bioenerg. Biomembr. 2006, 38, 283–291. [Google Scholar] [CrossRef]
- Doskocz, N.; Affek, K.; Matczuk, M.; Drozd, M.; Załęska-Radziwiłł, M. Nanoparticles in wastewater: A comprehensive approach to understanding their ecotoxicity and genotoxicity. Desalin. Water Treat. 2025, 321, 100988. [Google Scholar] [CrossRef]
- ISO 107122-1994; Water quality–Pseudomonas putida growth inhibition test (Pseudomonas cell multiplication test). ISO: Geneva, Switzerland, 1994.
- Weber, E. Grundriss der Biologischen Statistik für Naturwissenschaftler, Landwirte und Mediziner; VEB Fischer: Jena, Germany, 1972. [Google Scholar]
- Persoone, G.; Marsalek, B.; Blinova, I.; Törökne, A.; Zarina, D.; Manusadzianas, L.; Nałęcz-Jawecki, G.; Tofan, L.; Stepanova, N.; Tothova, L.; et al. A practical and user-friendly toxicity classification system with microbiotests for natural waters and wastewaters. Environ. Toxicol. 2003, 18, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Abdal Dayem, A.; Hossain, M.K.; Lee, S.B.; Kim, K.; Saha, S.K.; Yang, G.-M.; Choi, H.Y.; Cho, S.-G. The Role of Reactive Oxygen Species (ROS) in the Biological Activities of Metallic Nanoparticles. Int. J. Mol. Sci. 2017, 18, 120. [Google Scholar] [CrossRef]
- Zielińska, A.; Costa, B.; Ferreira, M.V.; Miguéis, D.; Louros, J.M.S.; Durazzo, A.; Lucarini, M.; Eder, P.; Chaud, M.V.; Morsink, M.; et al. Nanotoxicology and nanosafety: Safety-by-design and testing at a glance. Int. J. Environ. Res. Public Health 2020, 17, 4657. [Google Scholar] [CrossRef]
- Joudeh, N.; Linke, D. Nanoparticle classification, physicochemical properties, characterization, and applications: A comprehensive review for biologists. J. Nanobiotechnol. 2022, 20, 262. [Google Scholar] [CrossRef]
- Xie, Y.; He, Y.; Irwin, P.L.; Jin, T.; Shi, X. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl. Environ. Microbiol. 2011, 77, 2325–2331. [Google Scholar] [CrossRef]
- Metryka, O.; Wasilkowski, D.; Mrozik, A. Evaluation of the Effects of Ag, Cu, ZnO and TiO2 Nanoparticles on the Expression Level of Oxidative Stress-Related Genes and the Activity of Antioxidant Enzymes in Escherichia coli, Bacillus cereus and Staphylococcus epidermidis. Int. J. Mol. Sci. 2022, 23, 4966. [Google Scholar] [CrossRef]
- Cadenas, S. Mitochondrial uncoupling, ROS generation and cardioprotection. Biochim. Biophys. Acta–Bioenerg. 2018, 1859, 940–950. [Google Scholar] [CrossRef]
- Ouyang, K.; Walker, S.L.; Yu, X.; Gao, C.; Huang, Q.; Cai, P. Metabolism, Survival, and Gene Expression of Pseudomonas putida to Hematite Nanoparticles Mediated by Surface-Bound Humic Acid. Environ. Sci. Nano 2018, 5, 682–695. [Google Scholar] [CrossRef]
- Morales, G.; Ugidos, A.; Rojo, F. Inactivation of the Pseudomonas putida Cytochrome o Ubiquinol Oxidase Leads to a Significant Change in the Transcriptome and to Increased Expression of the CIO and cbb3-1 Terminal Oxidases. Environ. Microbiol. 2006, 8, 1764–1774. [Google Scholar] [CrossRef] [PubMed]
- Levard, C.; Hotze, E.M.; Lowry, G.V.; Brown, G.E., Jr. Environmental Transformations of Silver Nanoparticles: Impact on Stability and Toxicity. Environ. Sci. Technol. 2012, 46, 6900–6914. [Google Scholar] [CrossRef] [PubMed]
- Adeleye, A.S.; Conway, J.R.; Garner, K.; Huang, Y.; Su, Y.; Keller, A.A. Engineered Nanomaterials for Water Treatment and Remediation: Costs, Benefits, and Applicability. Chem. Eng. J. 2016, 286, 640–662. [Google Scholar] [CrossRef]
- Abbas, Q.; Yousaf, B.; Ullah, H.; Ali, M.U.; Ok, Y.S.; Rinklebe, J. Environmental Transformation and Nano-Toxicity of Engineered Nanoparticles (ENPs) in Aquatic and Terrestrial Organisms. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2523–2581. [Google Scholar] [CrossRef]
- Borisov, V.B.; Siletsky, S.A.; Paiardini, A.; Hoogewijs, D.; Forte, E.; Giuffrè, A.; Poole, R.K. Bacterial oxidases of the cytochrome bd family: Redox enzymes of unique structure, function, and utility as drug targets. Antioxid. Redox Signal. 2021, 34, 1280–1318. [Google Scholar] [CrossRef]
- Dinamarca, M.A.; Ruiz-Manzano, A.; Rojo, F. Inactivation of cytochrome o ubiquinol oxidase relieves catabolic repression of the Pseudomonas putida GPo1 alkane degradation pathway. J. Bacteriol. 2002, 184, 3785–3793. [Google Scholar] [CrossRef]
- Joseph, T.M.; Al-Hazmi, H.E.; Śniatała, B.; Esmaeili, A.; Habibzadeh, S. Nanoparticles and Nanofiltration for Wastewater Treatment: From Polluted to Fresh Water. Environ. Res. 2023, 238, 117114. [Google Scholar] [CrossRef]
- Akdemir, T. Trace Element Concentrations in Effluent of Municipal Wastewater Treatment Plants Along the Turkish Coasts and Assessment of Human Health Risk. Front. Mar. Sci. 2024, 11, 1521449. [Google Scholar] [CrossRef]
- Solanki, S.; Sinha, S.; Tyagi, S.; Singh, R. Nanomaterials for Efficient Removal of Heavy Metals. In Advances in Chemical Pollution, Environmental Management and Protection; Kumar, A., Bilal, M., Ferreira, L.F.R., Eds.; Elsevier BV: Amsterdam, Netherlands, 2024; Volume 10, pp. 117–136. [Google Scholar] [CrossRef]
- Richardson, D.J. Structural and Functional Flexibility of Bacterial Respiromes. In Bacterial Physiology: A Molecular Approach; El-Sharoud, W., Ed.; Springer: Heidelberg, Berlin, 2008; pp. 97–128. [Google Scholar] [CrossRef]
- Molina-Henares, M.A.; Ramos-González, M.I.; Rinaldo, S.; Espinosa-Urgel, M. Gene Expression Reprogramming of Pseudomonas alloputida in Response to Arginine through the Transcriptional Regulator ArgR. Microbiology 2024, 170, 001449. [Google Scholar] [CrossRef]
- Planchon, M.; Léger, T.; Spalla, O.; Huber, G.; Ferrari, R. Metabolomic and Proteomic Investigations of Impacts of Titanium Dioxide Nanoparticles on Escherichia coli. PLoS ONE 2017, 12, e0178437. [Google Scholar] [CrossRef]
- Rihacek, M.; Kosaristanova, L.; Fialova, T.; Rypar, T.; Sterbova, D.S.; Adam, V.; Zurek, L.; Cihalova, K. Metabolic Adaptations of Escherichia coli to Extended Zinc Exposure: Insights into Tricarboxylic Acid Cycle and Trehalose Synthesis. BMC Microbiol. 2024, 24, 384. [Google Scholar] [CrossRef] [PubMed]
- Meli, K.; Kamika, I.; Keshri, J.; Momba, M.N.B. The Impact of Zinc Oxide Nanoparticles on the Bacterial Microbiome of Activated Sludge Systems. Sci. Rep. 2016, 6, 39176. [Google Scholar] [CrossRef]
- Phan, D.; Pasha, A.B.M.T.; Carwile, N.; Kapoor, V. Effect of Zinc Oxide Nanoparticles on Physiological Activities and Gene Expression of Wastewater Nitrifying Bacteria. Environ. Eng. Sci. 2020, 37, 328–336. [Google Scholar] [CrossRef]
- Luche, S.; Steunou, A.L.; Mounier, S.; Chaspoul, F.; Walcarius, A.; Rols, J.L. ZnO Nanoparticles Induce Stringent Response and Metabolic Pathway Alterations in Bacillus subtilis. Nanotoxicology 2018, 12, 392–408. [Google Scholar] [CrossRef]
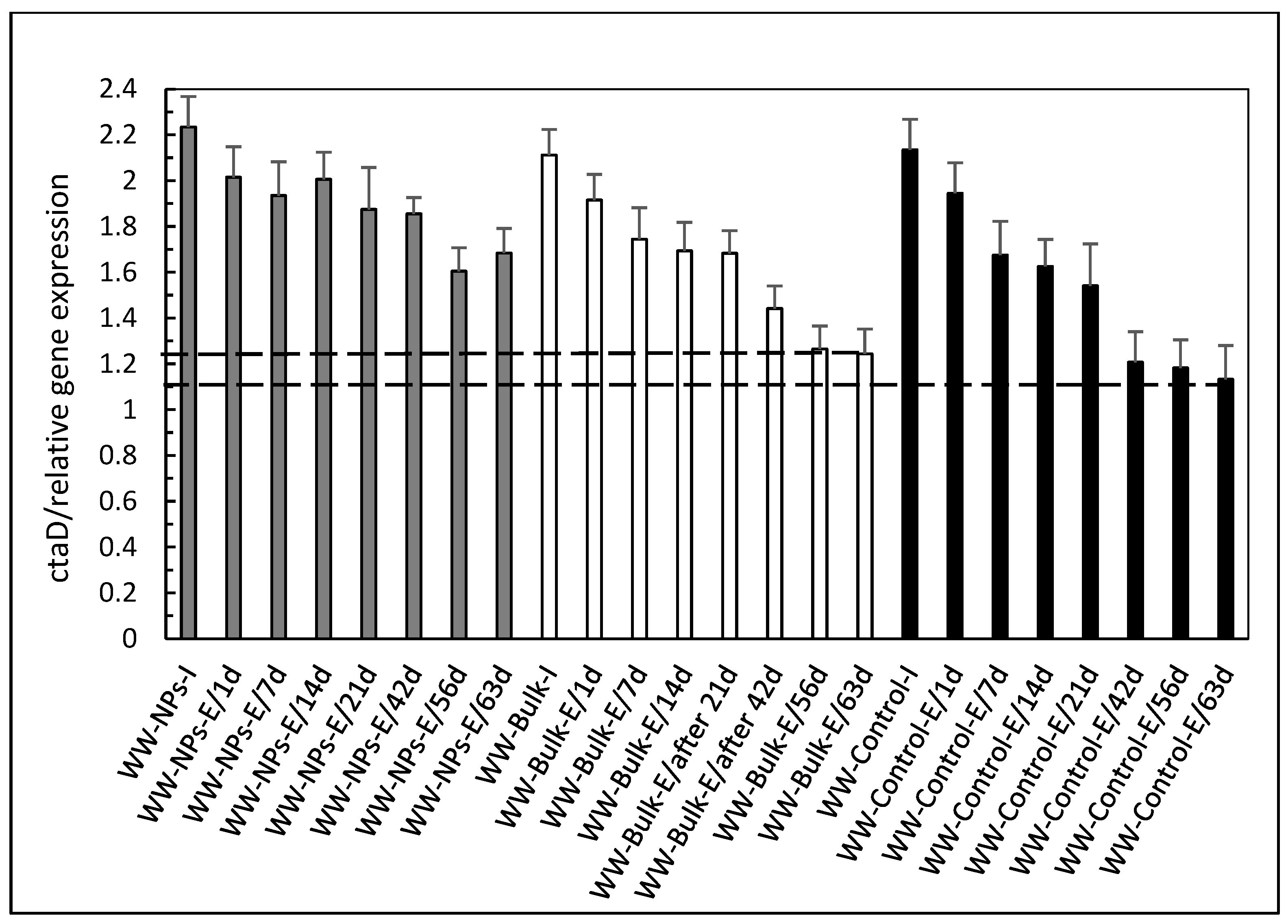
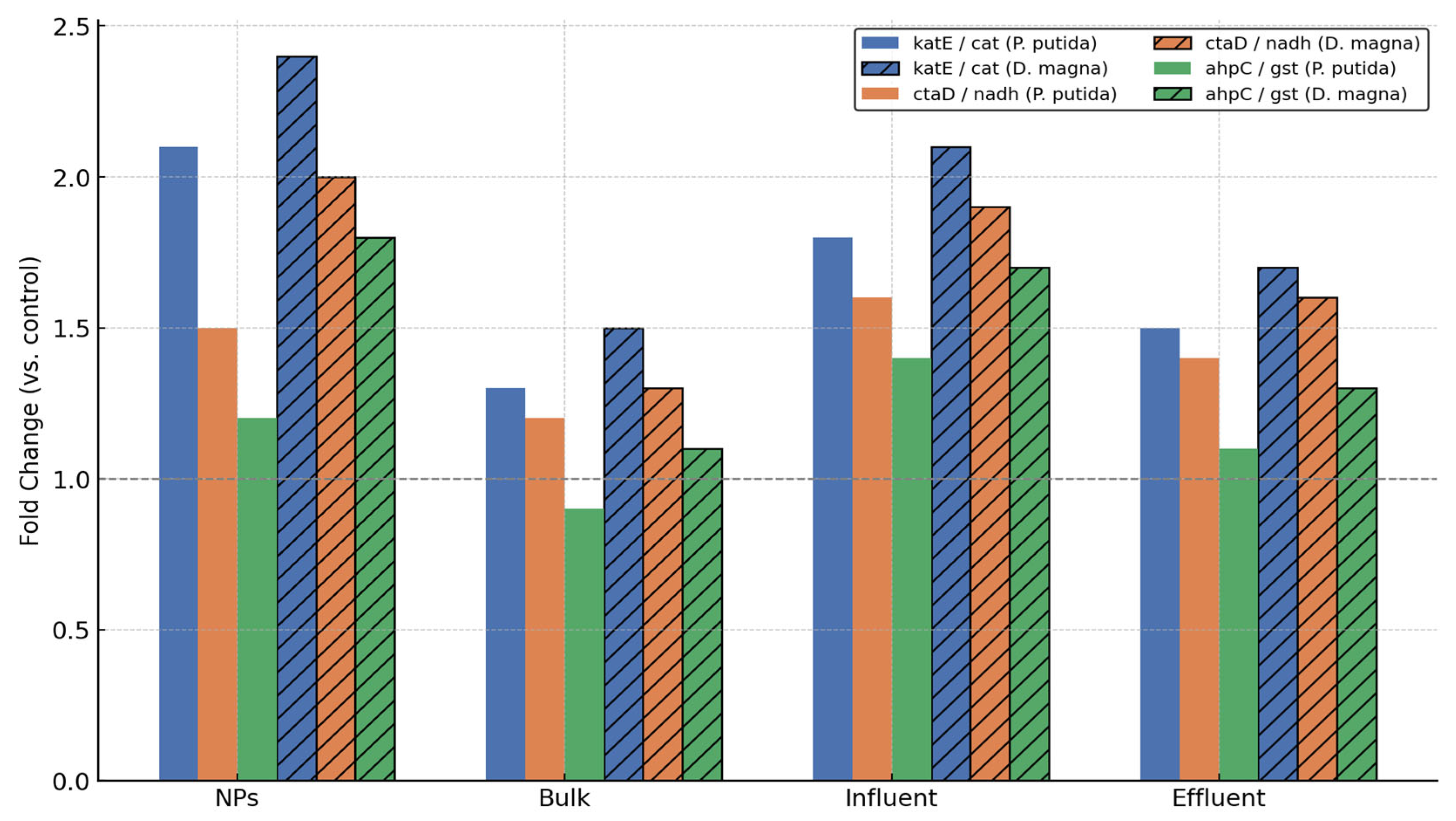
| No. | Samples Collected for Analysis | Sample Name |
|---|---|---|
| 1 | Al2O3NPs (10 mg/L) | NPs |
| 2 | Bulk Al2O3 (10 mg/L) | Bulk |
| 3 | Control (water) | Control |
| 4 | Synthetic domestic wastewater-borne 10 mg/L Al2O3NPs, fed into the SBR reactor (Influent) | WW-NPs-I |
| 5 | Synthetic domestic wastewater-borne bulk Al2O3 (10mg/L), fed into the SBR reactor (Influent) | WW-Bulk-I |
| 6 | Synthetic domestic wastewater (control), fed into the SBR reactor (Influent) | WW-Control-I |
| 7 | Synthetic domestic wastewater-borne 10 mg/L Al2O3NPs, treated by activated sludge method in SBR reactor (Effluent) | WW-NPs-E |
| 8 | Synthetic domestic wastewater-borne bulk Al2O3 (10mg/L), treated by activated sludge method in SBR reactor (Effluent) | WW-Bulk-E |
| 9 | Synthetic domestic wastewater (control), treated by activated sludge method in SBR reactor (Effluent) | WW-Control-E |
| Genes | Reverse Starter (5′–3′) | Forward Starter (5′–3′) | Function |
|---|---|---|---|
| catE | CTT GAT ACC CAC CGA ACC TG | CTC GCC AAC ATC GAC CTG AAG | Xenobiotic detoxification |
| ctaD | GCA GGT TGA GGA TGG TGG C | CCA GCC AGC GTC ACC TTC T | Electron transport and energy production |
| ahpC | GGC AGC CTT GAC CTT ACG C | ATC AAG ATT GTC GAG CTG AAC G | Oxidative stress |
| 16S ribosomal RNA (16S) | GAA ATT CCA CCA CCC TCT ACC | TAC CTT GCT GTT TTG ACG TTA CC | Component of prokaryotic ribosomes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doskocz, N.; Affek, K.; Załęska-Radziwiłł, M. Molecular Response of Bacteria Exposed to Wastewater-Borne Nanoparticles. Appl. Sci. 2025, 15, 7746. https://doi.org/10.3390/app15147746
Doskocz N, Affek K, Załęska-Radziwiłł M. Molecular Response of Bacteria Exposed to Wastewater-Borne Nanoparticles. Applied Sciences. 2025; 15(14):7746. https://doi.org/10.3390/app15147746
Chicago/Turabian StyleDoskocz, Nina, Katarzyna Affek, and Monika Załęska-Radziwiłł. 2025. "Molecular Response of Bacteria Exposed to Wastewater-Borne Nanoparticles" Applied Sciences 15, no. 14: 7746. https://doi.org/10.3390/app15147746
APA StyleDoskocz, N., Affek, K., & Załęska-Radziwiłł, M. (2025). Molecular Response of Bacteria Exposed to Wastewater-Borne Nanoparticles. Applied Sciences, 15(14), 7746. https://doi.org/10.3390/app15147746





