Featured Application
The right approach and use of Japanese knotweed resources, a plant considered invasive and harmful to the natural environment in many regions of the world, as an additional source of health-promoting substances is an opportunity for sustainable development and ecological and economic benefits, which are so desirable today.
Abstract
Due to its health-promoting properties, resveratrol is one of the most desirable compounds in many industries. Hence, this work focused on finding the conditions of its extraction from Japanese knotweed which could be used on an industrial scale to obtain extracts with the best antioxidant properties. The contribution of polyphenolics to the activity of the obtained isolates was also assessed in this study. Ultrasound-assisted solvent extraction was used to prepare extracts in various solvents under conditions differing in extraction time, temperature, and ultrasound frequency. The extracts were tested for their ability to neutralize radicals and reduce metal ions. It was shown that although the best extractant was the same water–alcohol mixture, the optimal conditions for the extraction of resveratrol and polyphenols were different: 10 min, 50 °C and 80 kHz for resveratrol (for which the highest contents of resveratrol equals 0.91 mg/g was obtained) and 20 min, 25 °C and 37 kHz for polyphenolics (for which the total phenolic content equals 31.28 mg of gallic acid/g was determined) Under the latter conditions, one of the best antioxidant activities was also obtained. The results confirm that Japanese knotweed, despite its bad reputation in Europe as a very invasive species, can be used as a source of sought-after resveratrol and polyphenols.
1. Introduction
In times of increased exposure of the body to highly reactive forms of oxygen and nitrogen, the innate defense mechanisms are not sufficient to neutralize them effectively. This leads to their excessive accumulation in the body and the formation of so-called oxidative stress, which promotes damage to cellular DNA, protein molecules, fatty acids, and cell membranes. As a result, cell and tissue dysfunction occur, and many diseases develop, including cancer [1]. One of the relatively simple ways to combat oxidative stress is to ensure a balanced diet [2]. Nature provides many substances with antioxidant properties [3]. These include products rich in polyunsaturated fatty acids, fiber, and polyphenols [4]. Research conducted especially on the latter has shown that they protect cellular components from oxidative damage and help prevent degenerative diseases [5].
Taking the above into account, research is being conducted worldwide on effective methods of isolating polyphenolic compounds from natural sources. The importance of this topic is enhanced by the fact that for many of them, chemical synthesis is an unprofitable undertaking, among others, due to the diversity and complexity of their structure. It is also important that many polyphenols are unstable compounds, and during extraction from plant material, they can be transformed/degraded into other substances, often with unknown biological effects on the human body. As a result, the efficiency of isolation of a given group of compounds is the result of two parallel and opposing processes, i.e., the release of compounds from the plant matrix and their degradation. Both depend on the extraction conditions used.
Currently, various approaches are used to isolate compounds from plants. These include simple techniques useful on a laboratory scale for assessing the actual content of antioxidants in plant material, as well as more advanced ones, consistent with the principles of green chemistry, which can be used in the industrial isolation of natural compounds. The latter, commonly referred to as “assisted extraction techniques”, are usually more convenient to use, more economical, more environmentally friendly, and faster. One such technique is ultrasound-assisted solvent extraction (UASE), in which the effects related to the cavitation phenomenon increase the extraction efficiency per unit of time [6].
This work is part of the trend of research on natural polyphenolic compounds. Its main goal is to find the most effective conditions of resveratrol extraction from Japanese knotweed (Polygonum cuspidatum), ensuring high antioxidant activity of the extract, thus extending the data discussed in [7]. Japanese knotweed is considered in many countries, especially in Europe, to be not only an invasive species, but also expansive and therefore undesirable in the natural environment [8]. It is recommended to remove it before the flowering period and then mechanically destroy it because it grows back easily. Nevertheless, despite its bad reputation, it is a valuable source of polyphenolic compounds and especially resveratrol [7]. This compound has many health-promoting properties, including preventing the development of serious diseases [9,10]. For this reason, it is highly desirable in many industries. On an industrial scale, it is typically obtained from dried red grape cuticles. Thus, finding effective conditions for isolating resveratrol from knotweed will allow the use of this undesirable weed as an alternative source of this valuable compound for the production of supplements and food additives, contributing to balanced development and increasing the ecological and economic benefits of many economies. In this study, UASE was used to prepare extracts, a technique that can be easily scaled up to industrial needs while respecting pro-ecological principles. As extraction solvents, in place of expensive ethanol, its substitute, i.e., methanol, water, and a mixture of equal volumes of these two components, was used. The ability to neutralize reactive forms, adopted as the main criterion for assessing the quality of extracts, was determined using methods using colored radicals: 2,2′-diphenylpicrylhydrazyl (DPPH) in the so-called DPPH method and 2′-azinobis(3-ethylbenzenethiazoline-6-sulfonate) (ABTS) in the so-called ABTS method. The ability to reduce metal ions was estimated using the ferric reducing antioxidant parameter method (shortly the FRAP method) and the cupric reducing antioxidant capacity (shortly the CUPRAC method).
2. Materials and Methods
2.1. Chemicals and Materials
2,2′-Azinobis(3-ethylbenzothiazoline-6-sulfonic acid diammonium salt (ABTS), 2,2′-diphenyl-1-picrylhydrazyl (DPPH), 2,4,6-tri(2-pyridyl)-s-triazine (TPTZ), 2,9-dimethyl-1,10-phenanthroline, (neocupreine, Nc), 3,4′,5-trihydroxy-trans-stilbene (resveratrol), Folin–Ciocalteu phenol reagent, and potassium persulfate (disodipotassium di-persulfate) were purchased from Sigma Aldrich (Poznań, Poland). Acetic acid, acetonitrile (Super Gradient), copper(II) chloride, iron(III) chloride hexahydrate, hydrochloric acid, ammonium acetate, ethanol, ethyl acetate, methanol, sodium acetate, and sodium carbonate were supplied by Avantor Performance Materials Poland (Gliwice, Poland). Water was purified using the Milli-Q system from Millipore (Millipore, Bedford, MA, USA). Ground knotweed rhizomes (Polygonum cuspidatum) were purchased from a local store (Lublin, Poland). According to the manufacturer’s declaration on the packaging, it contained only dried and ground knotweed rhizomes. Accurately weighed portions of the material were used for extraction.
2.2. Preparation of Extracts
To prepare solutions for determining resveratrol content and testing antioxidant properties, the extraction of plant material was performed. For this purpose, ultrasound-assisted solvent extraction (UASE) and different extraction conditions were used. In each case, 0.4 g of herb and 50 mL of a given solvent (water, methanol, or methanol/water 50%/50% v/v) were used. The proportions given include the amounts suggested by the manufacturer for preparing the infusion. The UASE process was performed using a thermostatically controlled ultrasonic bath Elmasonic P (Elma, Singen, Germany) under the following controlled conditions:
- temperature: 25 °C or 50 °C
- ultrasonic frequency: 37 kHz or 80 kHz
- generator power: 100% of maximum power (720 W)
- extraction time: 10 min or 20 min
- ratio of plant material to volume of extraction mixture: 0.4 g/50 mL.
The resulting extracts were filtered through Whatman No. 4 paper, made up to a known volume, and then diluted with an appropriate solvent prior to HPLC and antioxidant measurements. Each extract was prepared in the same way.
2.3. Chromatographic Analysis
Chromatographic measurements were performed using a Dionex DX600 liquid chromatograph (Dionex Corp., Sunnyvale, CA, USA), which contained the following elements: an automatic injector equipped with a 25 µL dosing loop, a gradient pump (GP50), a UV-VIS detector (AD25) and a DAD detector (PDA100) and a thermostated Prodigy ODS-2 column (5 µm, 250 × 4.6 mm ID, Phenomenex, Torrance, CA, USA). All chromatographic separations were performed using the following measurement conditions:
- -
- temperature: 25 °C
- -
- mobile phase composition: a mixture of acetonitrile (solvent B) and deionized Milli-Q water with acetic acid (5% v/v, solvent A). Each separation was performed first using isocratic elution for 15 min (85% A and 15% B), after which the measurement was carried out in gradient conditions with linear increase (increase of B = 2.5%/min, t = 10 min, final content of component B = 40%)
- -
- mobile phase flow: F = 1 mL/min
- -
- detection at a wavelength of 330 nm.
The qualitative analysis of resveratrol was performed based on a comparison of the retention times and absorption spectrum of the trans-resveratrol standard with the substances analyzed in the extracts tested. The quantitative analysis was performed based on the calibration curve for resveratrol standard solutions (concentration range 0.001–0.01 mg/mL). The characteristics of the obtained curve were as follows:
- -
- equation of the calibration curve: y = 240.80x − 0.1374 (where: y—area under the peak [mAU*min], x—concentration [mg/mL]
- -
- limit of quantification: LOQ = 0.001 mg/mL
- -
- coefficient of quantification: R2 = 0.9995. The LOQ was assumed to be 10 × Sy/slope.
The measurement for each extract was repeated three times [7].
2.4. Determination of Antioxidant Properties
The antioxidant activity tests were performed using selected and most commonly used colorimetric methods. Extracts were used for measurements, which were appropriately diluted so that the final extract concentration was 0.8 mg/mL. A UV Probe-2550 spectrophotometer (Shimadzu, Kyoto, Japan) was used to measure antioxidant properties.
2.4.1. Free Radical Neutralization Capacity Assessed Using ABTS and DPPH Methods
The ability of the tested extracts to neutralize radicals was determined using methods using colored radicals: 2,2′-diphenylpicrylhydrazyl (DPPH) and 2′-azinobis(3-ethylbenzenethiazoline-6-sulfonate (ABTS). The latter was prepared during a 12-h oxidation reaction of ABTS with persulfate [11]. Before the measurement, the starting solutions of radicals/cation radicals were diluted with methanol to an initial absorbance of about 0.7 AU. The color change of the measuring solutions was determined at a wavelength of 516 nm (in the case of the DPPH method) [12] and 744 nm (in the case of the ABTS method). For this purpose, 2900 μL of the radical or cation radical solution and 100 μL of extract (prepared in methanol, water, or water–methanol (50/50, v/v)) were used each time. The percentage inhibition (%I) was calculated after 60 min of reaction from the equation:
where A60 and A0 are the absorbance values of ABTS+● or DPPH● at times 0 and 60, respectively.
(%) = (1 − A60/A0) × 100%
2.4.2. Ability to Reduce Metal Ions, Assessed Using FRAP and CUPRAC Methods
The ability to reduce iron III ions was tested using the method developed by Benzie and Strain [13]. The following substances were used to prepare the FRAP solution: FeCl3·6H2O solution (final Fe(III) concentration was 20 mM), TPTZ in 40 mM HCl (final TPTZ concentration was 10 mM), and acetate buffer at pH = 3.6 (0.3 M). The above-mentioned solutions were mixed in a 1:1:10 ratio. 2900 µL of the FRAP solution and 100 µL of the extract were used to assess antioxidant properties. In the case of the CUPRAC method [14], the ability to reduce copper ions was measured in a system containing: 740 µL of CuCl2 (final concentration of Cu(II) in the solution is 10 mM), 740 µL of neocuproine in ethanol with a final concentration of 7.5 mM), 740 µL of 1.0 M CH3COOH/CH3COONH4 buffer solution at pH = 7.0, 680 µL of water and 100 µL of the extract samples tested. The absorbance of the formed complexes was measured at a wavelength of 593 nm (for FRAP) and 450 nm (for CUPRAC). In each of the methods tested, a mixture containing all the reagents and 100 µL of extraction solvent was used to zero the spectrophotometer. Antioxidant activity was expressed in mg of Trolox per 1 g of sample.
2.5. Determination of Polyphenolic Compounds
The content of polyphenolic compounds in the tested samples was determined using the Folin–Ciocalteu method with some modifications [15]. For this purpose, 1580 µL of water and 100 µL of Folin–Ciocalteu reagent were mixed with 100 µL of the tested extract. After waiting 5 min, 300 µL of 20% w/v aqueous sodium carbonate solution was added to the mixture. Each sample was incubated for 2 h, and after this time, the absorbance of the resulting colored complex (λ = 765 nm) was measured. The results are expressed as mg of gallic acid (GAE) per gram of dry plant material. For this purpose, a calibration curve was prepared for gallic acid in the concentration range of 0.005–1.0 mg/mL. The presented result is the average value of three independent measurements.
3. Results and Discussion
3.1. Determination of Resveratrol Content
In order to isolate resveratrol from the Polygonum cuspidatum herb, the UASE technique was applied, which is quite popular among currently used assisted extraction techniques, especially in the food and pharmaceutical industries [16]. The extraction is facilitated by the phenomenon of cavitation. It occurs during the propagation of ultrasound, i.e., a series of compression and dilution waves, in liquid systems. As a result, cavitation bubbles are induced in the liquid [17]. Within a few hundred microseconds, the bubbles grow in a pulsating manner to about 150 μm, and after reaching a critical point, they collapse and release a large amount of energy. This causes the generation of extreme temperatures (5000 °C) and pressures (2000 atm) and mechanical waves propagating at a speed of 150 m/s [18]. During extraction, these extreme conditions (high temperature and pressure) are responsible for the destruction of cell walls, which facilitates the release of bioactive components and increases mass transport per unit of time [19]. It should be added that in this technique, the extraction efficiency depends on the frequency of ultrasound, the intensity of cavitation bubbles, the power of the ultrasound generator, as well as the temperature and time of exposure of the extracted material to ultrasound, and the type of solvent used for extraction. With this in mind, this study investigated the effect of changing these experimental parameters on the extraction efficiency of resveratrol from Polygonum cuspidatum. The results of this series of studies are presented in Figure 1.
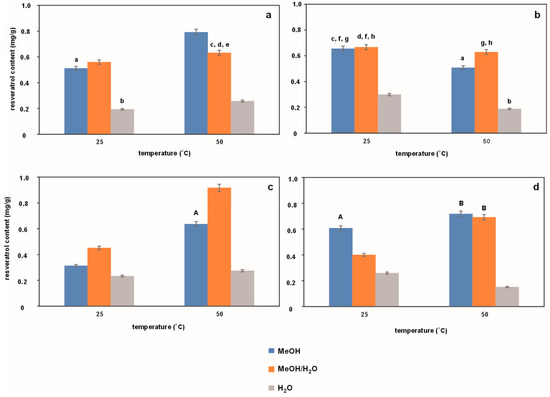
Figure 1.
Effect of temperature on the resveratrol content in extracts from Japanese knotweed (Polygonum cuspidatum) obtained by the UASE technique during 10 and 20 min at a frequency of 37 kHz (a,b), respectively, and during 10 and 20 min at a frequency of 80 kHz (c,d), respectively. The same letters indicate data for which the results are not statistically significant.
Figure 1 compares the content of resveratrol in extracts of Japanese knotweed obtained under different conditions of the UASE process. Three extractants were used for extraction: methanol (MeOH), methanol/water (MeOH/H2O), and water (H2O). Figure 1a collects data for extracts obtained at temperatures of 25 °C and 50 °C during 10 min of extraction carried out at a frequency of 37 kHz. In turn, Figure 1b presents the results for extracts obtained under conditions analogous to the previous ones, with the difference that the extraction time was extended to 20 min. Figure 1c,d shows the resveratrol content in extracts obtained similarly as before at temperatures of 25 °C and 50 °C during 10 and 20 min of extraction, but at a frequency of 80 kHz. For the purposes of statistical analysis, Figure 1a was compared with Figure 1b and Figure 1c with Figure 1d. Lowercase letters (a–h) and uppercase letters (A–B) in Figure 1a,b and Figure 1c,d, respectively, indicate a lack of statistical significance.
As can be seen, the extraction temperature affects the efficiency of resveratrol isolation. The data in Figure 1a show that the resveratrol content in the extracts tested is higher at higher temperatures. This effect is visible in the case of each extraction solvent used. These data are confirmed by the literature. The use of UASE for the isolation of resveratrol from Polygonum cuspidatum is examined in [20]. The authors of the cited paper obtained extracts with a relatively high content of resveratrol when the extraction was carried out at a higher temperature (45 °C) and at an ultrasound frequency of 37 kHz.
The situation changes when the extraction time is extended to 20 min. The data in Figure 1b indicate that extending the extraction time causes an increase in the amount of resveratrol at 25 °C (see data for 25 °C in Figure 1a,b) and a decrease in its content at 50 °C with time, clearly visible for the methanol and water extract (see data for 50 °C in Figure 1a,b, blue and gray bars). Thus, it seems that extending the extraction time favors the reduction of resveratrol amounts at higher extraction temperatures, which may be related to the transformation and/or degradation of this compound during extraction. At the higher frequency of 80 kHz (Figure 1c,d), lower resveratrol contents are observed than at a lower frequency of 37 kHz (Figure 1a,b), which is visible both at shorter and longer extraction times for the temperature of 25 °C.
As is known from the literature [21], the frequency has a great influence on the cavitation effect. In general, a lower frequency results in a larger bubble radius and stronger mechanical waves and a pronounced cavitation effect, which should result in a more efficient extraction. In our case, this general trend is also visible. An exception is the extract obtained using a methanol/water mixture at 50 °C and 10 min extraction time at 80 kHz, for which the extraction efficiency of resveratrol is the highest. However, similar cases can be found in the literature (an increase in efficiency with the increase of frequency). Liao et al. [22] observed that when the ultrasound frequency increased from 18 to 54 kHz, the extraction efficiency of anthocyanins also increased significantly. Yet, it is difficult to clearly explain what caused the highest content of resveratrol in the extract obtained under the above-mentioned conditions because it is a result of many experimental variables: frequency, temperature, time, and the extraction solvent used. One should not forget about the opposite parallel process of possible degradation of the analyte released from the plant matrix.
3.2. Ability to Neutralize Free Radicals (ABTS and DPPH Methods)
Antioxidant activity can be studied using various analytical techniques, referring, on the one hand, to different properties of the tested compounds and, on the other hand, to different stages of the oxidation process and a different understanding of the role of antioxidants (different types of antioxidant activity) [23]. Particularly popular are studies using stable free radicals ABTS and DPPH, which can be easily determined spectrophotometrically, and the antioxidant properties of the substance can be established based on the color change. Whenever possible, it is advisable to use both of the above methods to determine the antiradical activity of the tested samples because the radicals used in them are not chemically identical. In the literature [24], one can find data on antioxidant properties determined using DPPH and ABTS for extracts obtained in various ways from Polygonum cuspidatum. Hsu et al. [24] studied the antioxidant properties of extracts from knotweed, which were obtained by maceration with 50% ethanol. The results obtained by them showed that the IC50 values of the Polygonum cuspidatum extract were about 110 µg/mL, while the IC50 values of (+)-catechin and ascorbic acid were about 35 µg/mL and about 50 µg/mL, respectively. This indicates that the tested extract has a lower ability to neutralize the DPPH radical than these standard compounds. The antioxidant properties of ethanolic extracts of Polygonum cuspidatum were also confirmed in experiments performed by Choi et al. [25]. As in the previous studies, the ability to neutralize the ABTS and DPPH color radicals of the extracts they tested was, however, much lower than the standard antioxidants they used (such as BHA and vitamin C).
Figure 2 and Figure 3 show the antioxidant activity expressed as % inhibition of the ABTS cation radical (see Figure 2) and the DPPH radical (see Figure 3) for Japanese knotweed extracts obtained under UASE conditions with different ultrasound time and frequency (10 or 20 min and 37 or 80 kHz, respectively) using methanol, water and a mixture of these two liquids as an extraction medium at temperatures of 25 °C and 50 °C. As before, for statistical analysis, Figure 2a (Figure 3a) was compared with Figure 2b (Figure 3b), and Figure 2c (Figure 3c) with Figure 2d (Figure 3d). Lowercase letters a–g (a–h) and uppercase letters A–F (A–J) in the figures indicate a lack of statistical significance. Comparing the data in both figures, it is clear at first glance that the tested extracts exhibit different antioxidant activity. Higher % inhibition values are observed in the ABTS method than in the DPPH method. It should be noted here that this is most likely related to the availability of antioxidants in the sample to the sterically protected DPPH radical [26] and, consequently, poorer neutralization of this radical during the measurement (60 min). Moreover, in almost every case studied, the greatest antioxidant properties are shown by water–methanol extracts and the weakest by water extracts. However, there is no clear trend regarding the influence of individual variables on the antioxidant properties of extracts. An exception in this respect is the effect of temperature in the DPPH method. In this case, we can say that the higher the temperature, the better the antioxidant properties. Undoubtedly, this part of the research should be summed up as follows: the greatest antioxidant properties are not observed in the case of the extract characterized by the highest resveratrol content (i.e., methanol–water extract, extraction temperature of 50 °C, time of 10 min, and frequency of 80 kHz), so not only resveratrol is responsible for the antioxidant properties.
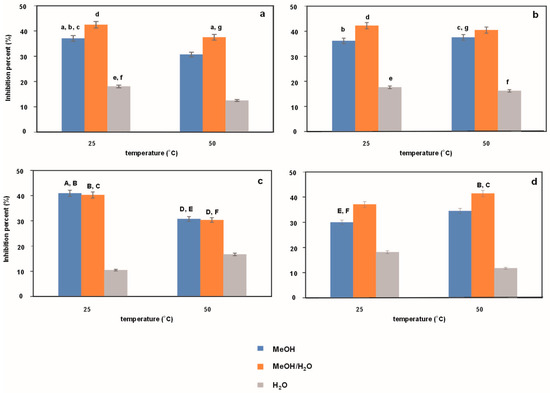
Figure 2.
Antioxidant activity determined using ABTS method for extracts obtained from Japanese knotweed at 25 and 50 °C using UASE under the following extraction conditions: (a) extraction time of 10 min and a frequency of 37 kHz; (b) extraction time of 20 min and a frequency of 37 kHz; (c) extraction time of 10 min and a frequency of 80 kHz; (d) extraction time of 20 min and a frequency of 80 kHz. Using methanol, methanol/water mixture (50/50%, v/v), and water as reaction solvents. The same letters indicate data for which the results are not statistically significant.
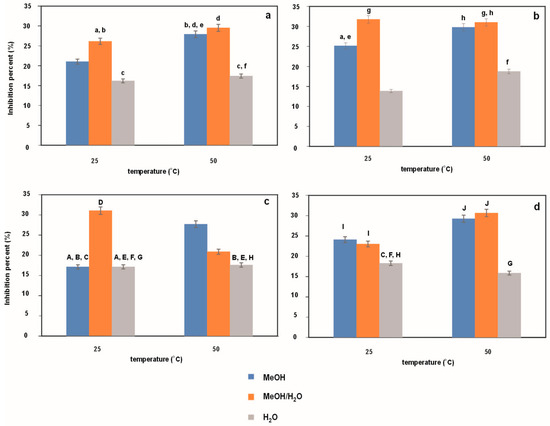
Figure 3.
Antioxidant activity determined using DPPH method for extracts obtained from Japanese knotweed at 25 and 50 °C using UASE under the following extraction conditions: (a) extraction time of 10 min and a frequency of 37 kHz; (b) extraction time of 20 min and a frequency of 37 kHz; (c) extraction time of 10 min and a frequency of 80 kHz; (d) extraction time of 20 min and a frequency of 80 kHz. Using methanol, methanol/water mixture (50/50%, v/v), and water as reaction solvents. The same letters indicate data for which the results are not statistically significant.
3.3. Ability to Reduce Metal Ions (FRAP and CUPRAC Methods)
The FRAP and CUPRAC spectroscopic methods, measuring ferric reduction as antioxidant power and cupric reducing antioxidant capacity, respectively, determine the ability of a given antioxidant to reduce an oxidant. The measure of this ability, as a result of a chemical reaction, is the change in the oxidant’s color. The degree of color change is correlated with the concentration of total antioxidant capacity [27].
As can be seen from the data in Figure 4 and Figure 5, the examined extracts demonstrate the ability to reduce metal ions (both iron and copper). It should be added here that the methods used, although measuring the same type of antioxidant capacity (reduction capacity), are not identical. The measurements are performed under different pH conditions (FRAP—acidic, CUPRAC—alkaline) [27]. What is more, the CUPRAC method is dedicated to both hydrophilic and lipophilic antioxidants, while FRAP is more for hydrophilic ones. Our results are in agreement with the literature [7,25,28,29]. The ability to reduce iron ions using the potassium ferricyanide method for ethanolic extracts of Polygonum cuspidatum was determined by Pan et al. [28]. Based on the performed experiments, the authors found a positive correlation between the reducing power and antioxidant activity of the tested extract, which was comparable to resveratrol and butylated hydroxytoluene (BHT) [28]. Moreover, Lee et al. [29] observed that the extract of Polygonum cuspidatum obtained by the supercritical fluid extraction technique with carbon dioxide also reduced metal ions in a concentration-dependent manner. When the concentration was increased to 250 mg/mL, this reducing ability was remarkable.
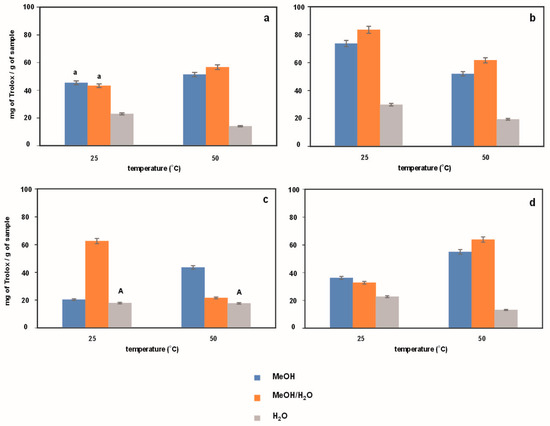
Figure 4.
Antioxidant activity determined using FRAP method for extracts obtained from Japanese knotweed at 25 and 50 °C using UASE under the following extraction conditions: (a) extraction time of 10 min and a frequency of 37 kHz; (b) extraction time of 20 min and a frequency of 37 kHz; (c) extraction time of 10 min and a frequency of 80 kHz; (d) extraction time of 20 min and a frequency of 80 kHz. Using methanol, methanol/water mixture (50/50%, v/v), and water as reaction solvents. The same letters indicate data for which the results are not statistically significant.
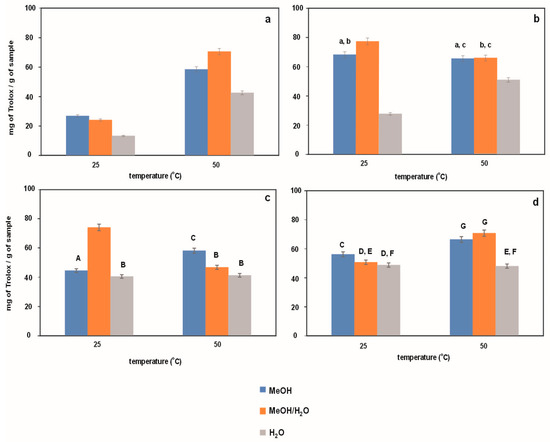
Figure 5.
Antioxidant activity determined using CUPRAC method for extracts obtained from Japanese knotweed at 25 and 50 °C using UASE under the following extraction conditions: (a) extraction time of 10 min and a frequency of 37 kHz; (b) extraction time of 20 min and a frequency of 37 kHz; (c) extraction time of 10 min and a frequency of 80 kHz; (d) extraction time of 20 min and a frequency of 80 kHz. Using methanol, methanol/water mixture (50/50%, v/v), and water as reaction solvents. The same letters indicate data for which the results are not statistically significant.
The results from the data show that, in most cases, methanol–water extracts have the best antioxidant properties, while aqueous extracts have the weakest. Extracts obtained at higher frequencies have lower antioxidant properties than extracts obtained at lower frequencies. This trend is visible to a greater or lesser extent in both methods. In most cases, the best antioxidant properties are shown by methanol–water extracts, while the weakest are in water, as in the previous two methods. It seems that the methanol/water mixture is the best extraction medium for isolating compounds responsible for antioxidant properties, mainly polyphenolic compounds. In order to confirm the validity of this hypothesis, in the next step of the research, it was decided to determine the content of polyphenolic compounds in the examined extracts.
3.4. Determination of Polyphenolic Content
Polyphenolic compounds are a group of health-promoting compounds that are widely represented in plants. As a result, these secondary metabolites, often used in medicines, nutraceuticals, food additives, and fine chemicals, are responsible for the therapeutic effects of plants. Among them are compounds with different properties from the point of view of physicochemistry and biological activity. Hence, the spectrum of their beneficial effects on the human body is very wide. Many of them determine the antioxidant activity of plant extracts.
Figure 6 compares the content of polyphenolic compounds in extracts obtained from Japanese knotweed using methanol, water and a mixture of these two liquids as an extraction medium at temperatures of 25 °C and 50 °C and different extraction times (10 min and 20 min) and at different ultrasound frequencies, i.e., 37 kHz and 80 kHz. Based on the presented results, it can be concluded that the most polyphenolic compounds are contained in methanol–water extracts (orange bars), and the lowest content is characteristic of water extracts (gray bars). This fact is related to the better solubility of this class of compounds in alcohol-water mixtures, which directly translates into the antioxidant properties of the extracts. As for the effect of UASE conditions, the use of a higher ultrasound frequency results in weaker isolation of polyphenolic compounds (compare the heights of the bars for a given extractant and temperature in Figure 6a,b with those in Figure 6c,d). In general, an increase in temperature to 50 °C leads to lower efficiency of polyphenol isolation as a result of their very probable degradation. As is known, during the thermal isolation of natural substances, two competitive processes take place—extraction and degradation/transformation of the released substances into others. The validity of this statement is confirmed by the data in Figure 6d, which shows that at a less effective ultrasound frequency in isolation, an increase in temperature reveals a higher yield of polyphenols. In summary, the highest content of these compounds is observed in extracts obtained using a methanol/water mixture at a temperature of 25 °C, a longer extraction time (20 min), and a lower frequency (37 kHz). It should be noted here that the content of polyphenols obtained by us for the given conditions (31.28 mg/g) is consistent with the literature. Gan et al. [30] determined the content of polyphenolic compounds in various medicinal plants, including knotweed, giving a value of 34.91 mg/g. In the literature, one can also find papers in which the authors obtained higher contents of polyphenolic compounds [24,25,31]. Such differences are the result of the diversity of the plants tested, resulting from, among others, the cultivation conditions or the harvest period.
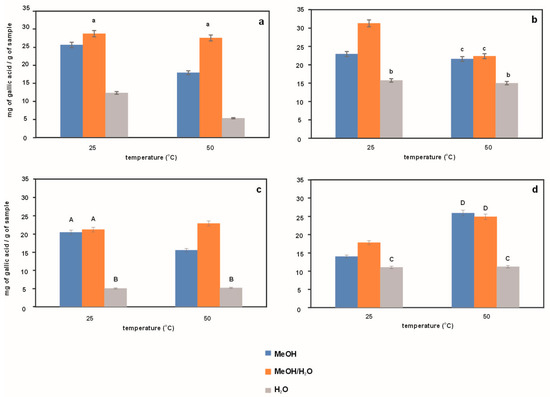
Figure 6.
Effect of temperature on the content of polyphenols in extracts from Japanese knotweed (Polygonum cuspidatum) obtained by the UASE technique during 10 and 20 min at a frequency of 37 kHz (a,b), respectively, and during 10 and 20 min at a frequency of 80 kHz (c,d), respectively. The same letters indicate data for which the results are not statistically significant.
By relating the data from the last figure (Figure 6) to those presented in Figure 2, Figure 3, Figure 4 and Figure 5, it is noted that the antioxidant properties of the extract obtained in this way are very high (or the highest among those compared). Therefore, the antioxidant properties of the extracts are the result of the action of its components, mainly polyphenols, and not only resveratrol itself (the extract that contains the most of it does not show the highest antioxidant properties at all), although undoubtedly resveratrol as one of a polyphenols is an important, characteristic for knotweed, contribution to the properties of the extract.
4. Conclusions
The results discussed in this paper prove that Japanese knotweed is a valuable and alternative source of resveratrol and many other health-promoting substances sought by many industries. Due to the lack of profitability of technological production of resveratrol, the best conditions for its extraction using the UASE technique were indicated, which can be easily scaled up to the needs of industrial isolation of this compound. The highest resveratrol content was determined in methanol/water extracts using the following UASE parameters: extraction time of 10 min, temperature of 50 °C, and frequency of 80 kHz. The mixture of methanol and water was also the best extraction medium for the isolation of the total polyphenolics. Interestingly, however, the optimal conditions for their extraction were different: extraction time of 20 min, temperature of 25 °C, and frequency of 37 kHz. The latter conditions were also conducive to obtaining extracts with the highest antioxidant capacity for neutralizing radicals and reducing metal ions. In the final conclusion, the presented results indicate a valuable way of managing the Japanese knotweed herb, which constitutes an opportunity for sustainable development and the ecological and economic benefits so desired today.
Author Contributions
Conceptualization and methodology, M.O.-T.; writing—original draft preparation, M.O.-T.; writing—review and editing, D.W.; supervision, D.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors would like to thank the Institute of Chemical Sciences of the Maria Curie-Skłodowska University in Lublin for creating the research infrastructure, without which this research would not be possible.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ABTS | 2,2′-Azinobis(3-ethylbenzothiazoline-6-sulfonic acid diammonium salt |
| CUPRAC | Cupric Reducing Antioxidant Capacity |
| DPPH | 2,2′-diphenyl-1-picrylhydrazyl |
| FRAP | Ferric Reducing Antioxidant Parameter |
| GAE | Gallic Acid Equivalent |
| Nc | 2,9-dimethyl-1,10-phenanthroline, (neocupreine) |
| TPTZ | 2,4,6-tri(2-pyridyl)-s-triazine |
| UASE | ultrasound-assisted solvent extraction |
References
- Shadyro, O.; Lisovskaya, A. ROS-induced lipid transformations without oxygen participation. Chem. Phys. Lipids 2019, 221, 176–183. [Google Scholar] [CrossRef]
- Ilari, S.; Proietti, S.; Milani, F.; Vitiello, L.; Muscoli, C.; Russo, P.; Bonassi, S. Dietary Patterns, Oxidative Stress, and Early Inflammation: A Systematic Review and Meta-Analysis Comparing Mediterranean, Vegan, and Vegetarian Diets. Nutrients 2025, 17, 548. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.-J.; Li, H.-B. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrova, K.; Koelman, L.; Rodrigues, C.E. Dietary patterns and biomarkers of oxidative stress and inflammation: A systematic review of observational and intervention studies. Redox Biol. 2021, 42, 101869. [Google Scholar] [CrossRef] [PubMed]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 14, 806470. [Google Scholar] [CrossRef]
- Moreda-Piñeiro, J.; Moreda-Piñeiro, A. Combined assisted extraction techniques as green sample pre-treatments in food analysis. Trends Anal. Chem. 2019, 118, 1–18. [Google Scholar] [CrossRef]
- Olszowy-Tomczyk, M.; Wianowska, D. A Comparative Evaluation of the Antioxidant Ability of Polygonum cuspidatum Extracts with That of Resveratrol Itself. Processes 2025, 13, 9. [Google Scholar] [CrossRef]
- Quinty, V.; Colas, C.; Nasreddine, R.; Nehmé, R.; Piot, C.; Draye, M.; Destandau, E.; Da Silva, D.; Chatel, G. Screening and Evaluation of Dermo-Cosmetic Activities of the Invasive Plant Species Polygonum cuspidatum. Plants 2022, 12, 83. [Google Scholar] [CrossRef]
- Constantinescu, T.; Mihis, A.G. Resveratrol as a privileged molecule with antioxidant activity. Food Chem. Adv. 2023, 3, 100539. [Google Scholar] [CrossRef]
- Tao, W.; Zhang, H.; Jiang, X.; Chen, N. Resveratrol combats chronic diseases through enhancing mitochondrial quality. Sci. Human. Wellness 2024, 13, 597–610. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Garcia, E.J.; Oldoni, T.; de Alencar, S.M.; Reis, A.; Loguercio, A.D.; Grande, R.H.M. Antioxidant activity by DPPH assay of potential solutions to be applied on bleached teeth. Braz. Dent. J. 2012, 23, 22–27. [Google Scholar] [CrossRef]
- Spiegel, M.; Kapusta, K.; Kołodziejczyk, W.; Saloni, J.; Zbikowska, B.; Hill, G.A.; Sroka, Z. Antioxidant activity of selected phenolic acids–Ferric Reducing Antioxidant Power Assay and QSAR analysis of the structural features. Molecules 2020, 25, 3088. [Google Scholar] [CrossRef] [PubMed]
- Özyürek, M.; Güçlü, K.; Tütem, E.; Başkan, K.S.; Erçağ, E.; Çelik, S.E.; Baki, S.; Yildis, L.; Karaman, S.; Apak, R. A comprehensive review of CUPRAC methodology. Anal. Methods 2011, 3, 2439–2453. [Google Scholar] [CrossRef]
- Olszowy-Tomczyk, M.; Wianowska, D. Comparison of the Antioxidant Properties of Extracts Obtained from Walnut Husks as well as the Influence of Juglone on Their Evaluation. Appl. Sci. 2024, 14, 2972. [Google Scholar] [CrossRef]
- Esclapez, M.D.; Garcia-Perez, J.V.; Mulet, A.; Carcel, J.A. Ultrasound-assisted extraction of natural products. Food Eng. Rev. 2011, 3, 108–120. [Google Scholar] [CrossRef]
- Soria, A.C.; Villamiel, M. Effect of ultrasound on the technological properties and bioactivity of food: A review. Trends Food Sci. Technol. 2010, 21, 323–331. [Google Scholar] [CrossRef]
- Chemat, F.; Zille-Huma; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem 2011, 18, 813–835. [Google Scholar] [CrossRef]
- Talmaciu, A.I.; Volf, I.; Popa, V.I. A comparative analysis of the “green” techniques applied for polyphenols extraction from bioresources. Chem. Biodivers. 2015, 12, 1635–1651. [Google Scholar] [CrossRef]
- Fletes-Vargas, G.; Rodríguez-Rodríguez, R.; Pacheco, N.; Pérez-Larios, A.; Espinosa-Andrews, H. Evaluation of the Biological Properties of an Optimized Extract of Polygonum cuspidatum Using Ultrasonic-Assisted Extraction. Molecules 2023, 28, 4079. [Google Scholar] [CrossRef]
- Shen, L.; Pang, S.; Zhong, M.; Sun, Y.; Qayum, A.; Liu, X.; Rashid, A.; Xu, B.; Liang, Q.; Ma, H.; et al. A comprehensive review of ultrasonic assisted extraction (UAE) for bioactive components: Principles, advantages, equipment, and combined technologies. Ultrason. Sonochem 2023, 101, 106646. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Xu, H.; Li, J.; Peng, L. Effects of ultrasound frequency and process variables of modified ultrasound-assisted extraction on the extraction of anthocyanin from strawberry fruit. Food Sci. Technol. Campinas 2022, 42, 1–8. [Google Scholar] [CrossRef]
- Wołosiak, R.; Druzyńska, B.; Derewiaka, D.; Piecyk, M.; Majewska, E.; Ciecierska, M.; Worobiej, E.; Pakosz, P. Verification of the Conditions for Determination of Antioxidant Activity by ABTS and DPPH Assays—A Practical Approach. Molecules 2022, 27, 50. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Chan, Y.P.; Chang, J. Antioxidant activity of extract from Polygonum cuspidatum. Biol. Res. 2007, 40, 13–21. [Google Scholar] [CrossRef]
- Choi, D.H.; Han, J.H.; Yu, K.H.; Hong, M.; Lee, S.Y.; Park, K.H.; Lee, S.L.; Kwon, T.H. Antioxidant and Anti-Obesity Activities of Polygonum cuspidatum Extract through Alleviation of Lipid Accumulation on 3T3-L1 Adipocytes. J. Microbiol. Biotechnol. 2020, 30, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Olszowy-Tomczyk, M.; Wianowska, D. Antioxidant Properties of Selected Flavonoids in Binary Mixtures—Considerations on Myricetin, Kaempferol and Quercetin. Int. J. Mol. Sci. 2023, 24, 10070. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, X.; Wang, H.; Liang, Y.; Zhu, J.; Li, H.; Zhang, Z.; Wu, Q. Antioxidant potential of ethanolic extract of Polygonum cuspidatum and application in peanut oil. Food Chem. 2007, 105, 1518–1524. [Google Scholar] [CrossRef]
- Lee, C.C.; Chen, Y.T.; Chiu, C.C.; Liao, W.T.; Liu, Y.C.; David Wang, H.M. Polygonum cuspidatum extracts as bioactive antioxidaion, anti-tyrosinase, immune stimulation and anticancer agents. J. Biosci. Bioeng. 2015, 119, 464–469. [Google Scholar] [CrossRef]
- Gan, R.Y.; Xu, X.R.; Song, F.L.; Kuang, L.; Li, H.B. Antioxidant activity and total phenolic content of medicinal plants associated with prevention and treatment of cardiovascular and cerebrovascular diseases. J. Med. Plants Res. 2010, 4, 2438–2444. [Google Scholar]
- Ardelean, F.; Moacă, E.A.; Păcurariu, C.; Antal, D.S.; Dehelean, C.; Toma, C.C.; Drăgan, S. Invasive Polygonum cuspidatum: Physico-chemical analysis of a plant extract with pharmaceutical potential. Stud. Univ. “Vasile Goldiş” Ser. Ştiinţele Vieţii 2016, 26, 415–421. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).