A Narrative Review on Functionalized Nanoparticles for the Treatment and Early Detection of Hepatocellular Carcinoma
Abstract
1. Introduction
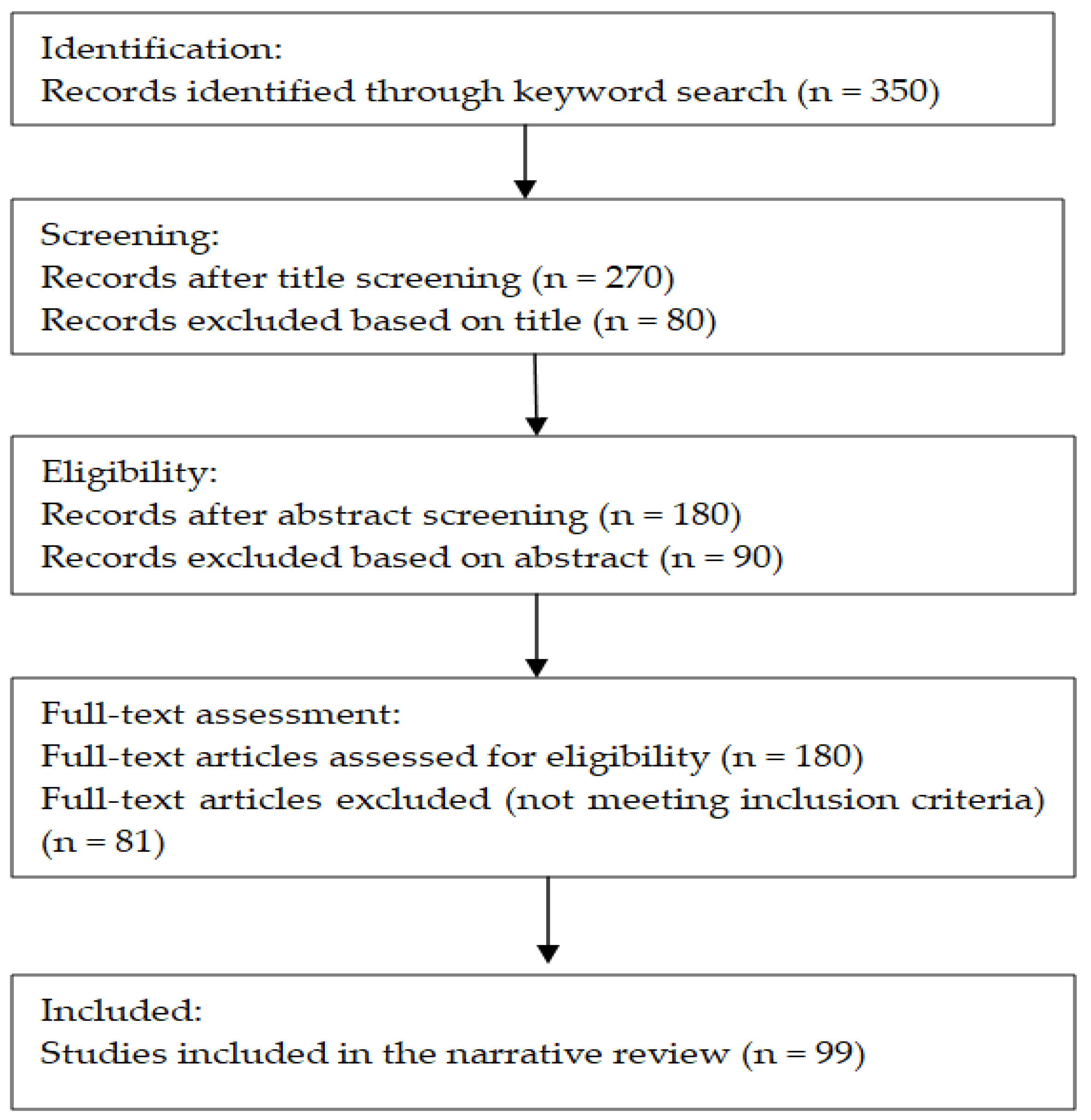
2. Materials and Methods
2.1. Aim of the Study
2.2. Search Strategy
2.3. Inclusion Criteria
- Investigated the use of biofunctionalized nanoparticles in the targeted treatment of HCC;
- Addressed nanoparticle-based strategies involving either active targeting mechanisms (e.g., ligand–receptor interactions) or passive targeting (e.g., the Enhanced Permeability and Retention [EPR] effect);
- Explored gene therapy or immunotherapy approaches facilitated by nanotechnology platforms;
- Examined innovative or emerging therapeutic modalities such as photothermal therapy (PTT) or photodynamic therapy (PDT);
- Focused on the early detection of HCC using nanoparticle-based or nanotechnology-enhanced diagnostic techniques;
- Were published recently (preferably within the last 5–7 years), to ensure the inclusion of the most up-to-date advancements in the field.
2.4. Exclusion Criteria
- Studies primarily focused on cancers other than hepatocellular carcinoma;
- Articles that lacked a clear relevance to nanotechnology or nanoparticle-mediated therapeutic or diagnostic approaches;
- Experimental studies that were outdated or had been superseded by more recent and comprehensive findings;
- Review articles that did not offer novel perspectives or substantially overlapped with previously published content;
- Publications in languages other than English.
3. Results
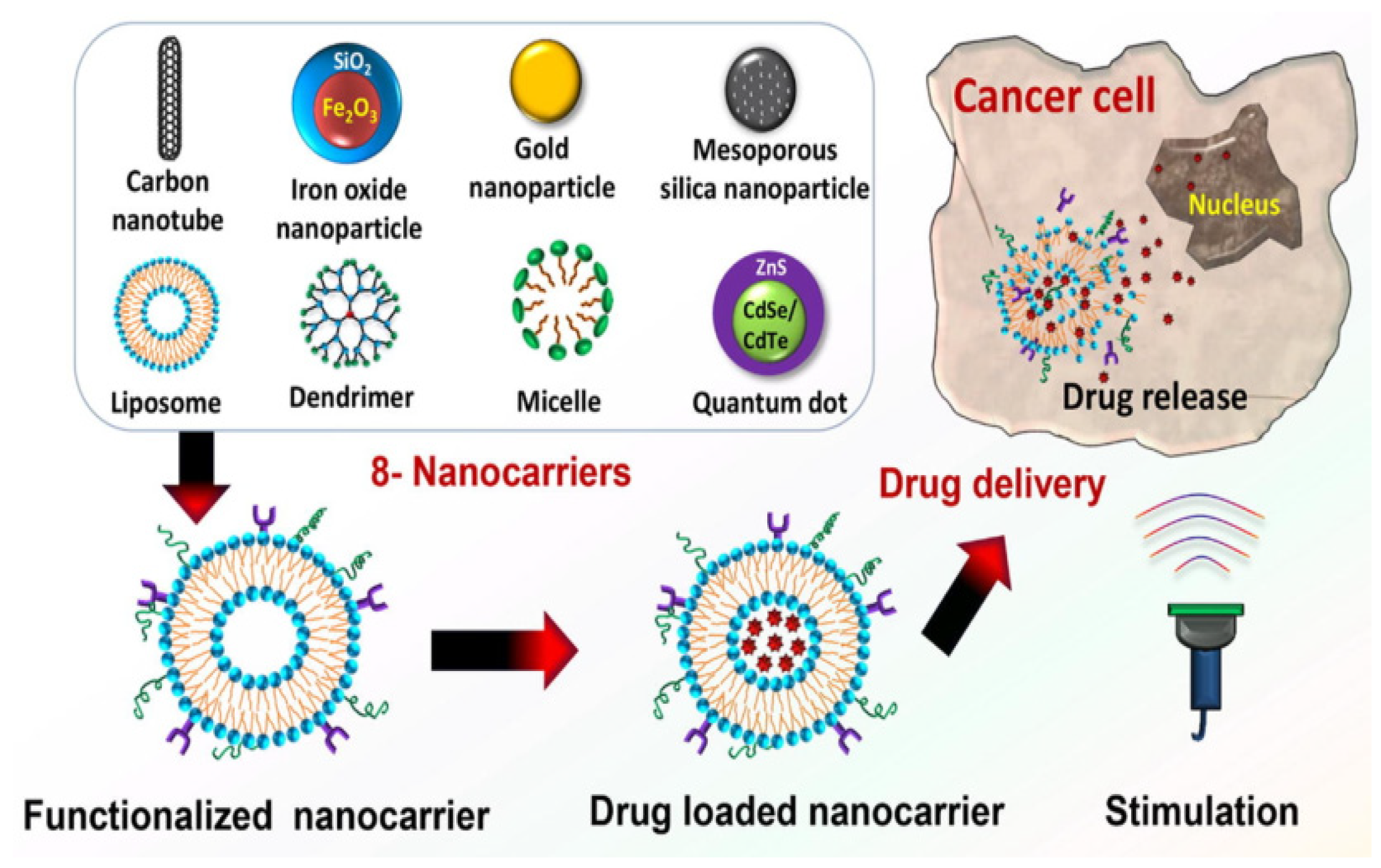
3.1. Multifunctional Nanoparticles for Therapy and Imaging
3.1.1. Nanoparticle Drug Carriers
- Solid Lipid Nanocarriers
- Dendrimers
- Liposomes
- Chitosan
- Micelles
- Carbon Nanotubes
- Gold Nanoparticles
3.1.2. Engineered Nanoparticles for Enhanced Diagnostic Imaging
- Magnetic Nanoparticles
- Quantum Dots
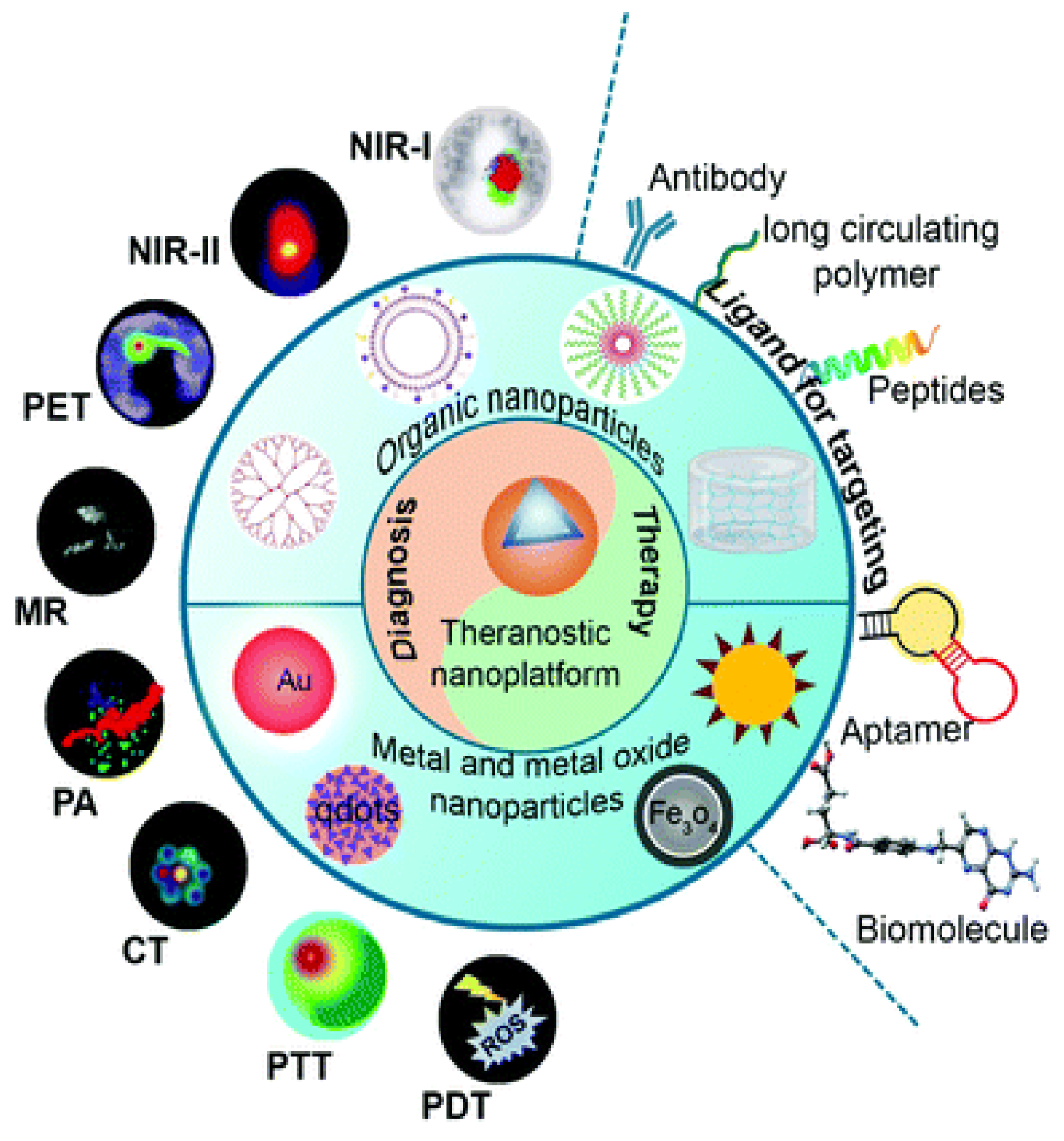
3.1.3. Theranostic Nanoparticles
3.2. Targeted Drug Delivery
3.2.1. Active Targeting
3.2.2. Passive Targeting: The Enhanced Permeability and Retention Effect
3.3. Gene Therapy and RNA Interference (RNAi)
3.3.1. siRNA-Loaded Nanoparticles
3.3.2. CRISPR-Cas9
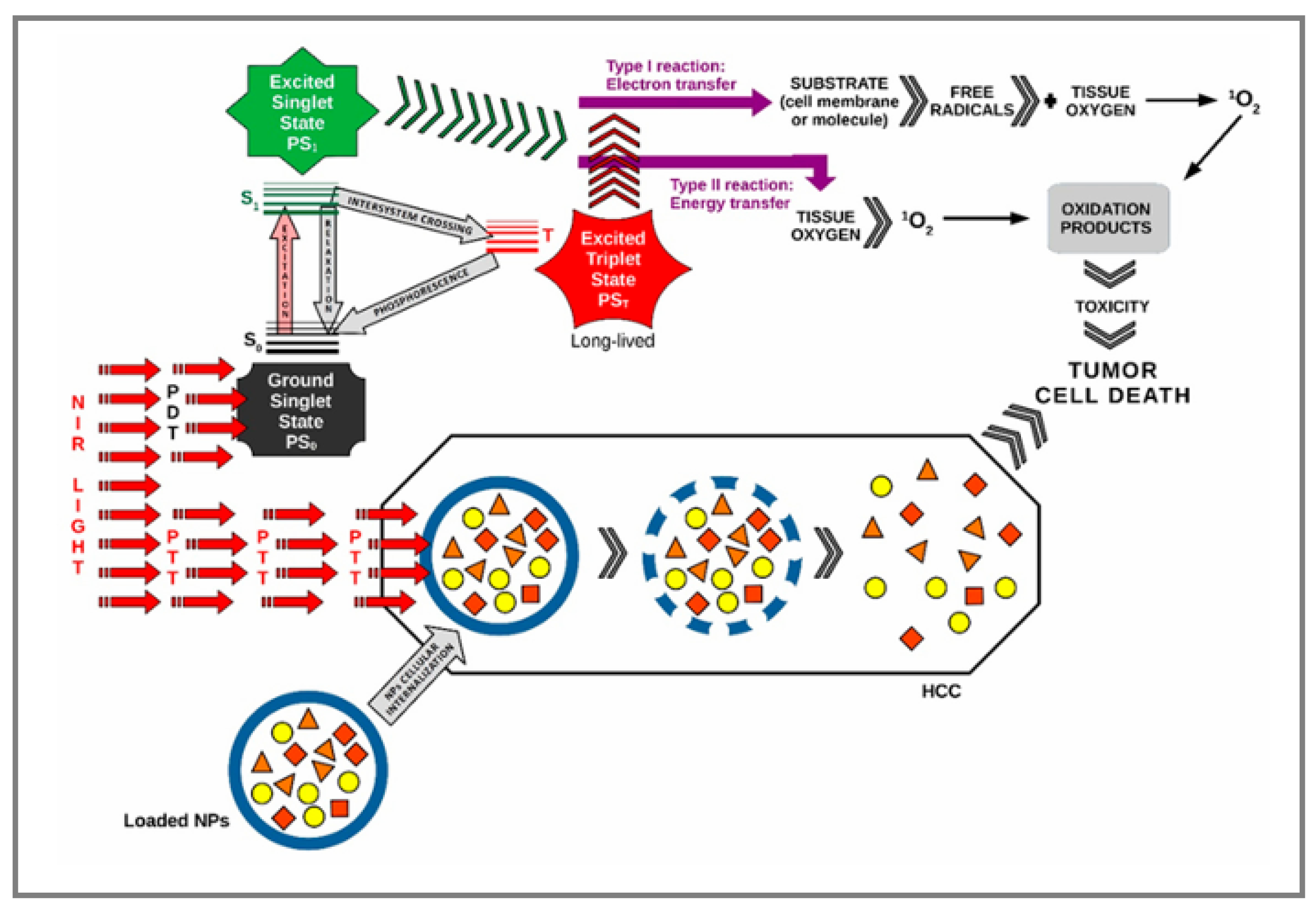
3.4. Photothermal and Photodynamic Therapy
3.4.1. Photothermal Therapy
3.4.2. Photodynamic Therapy
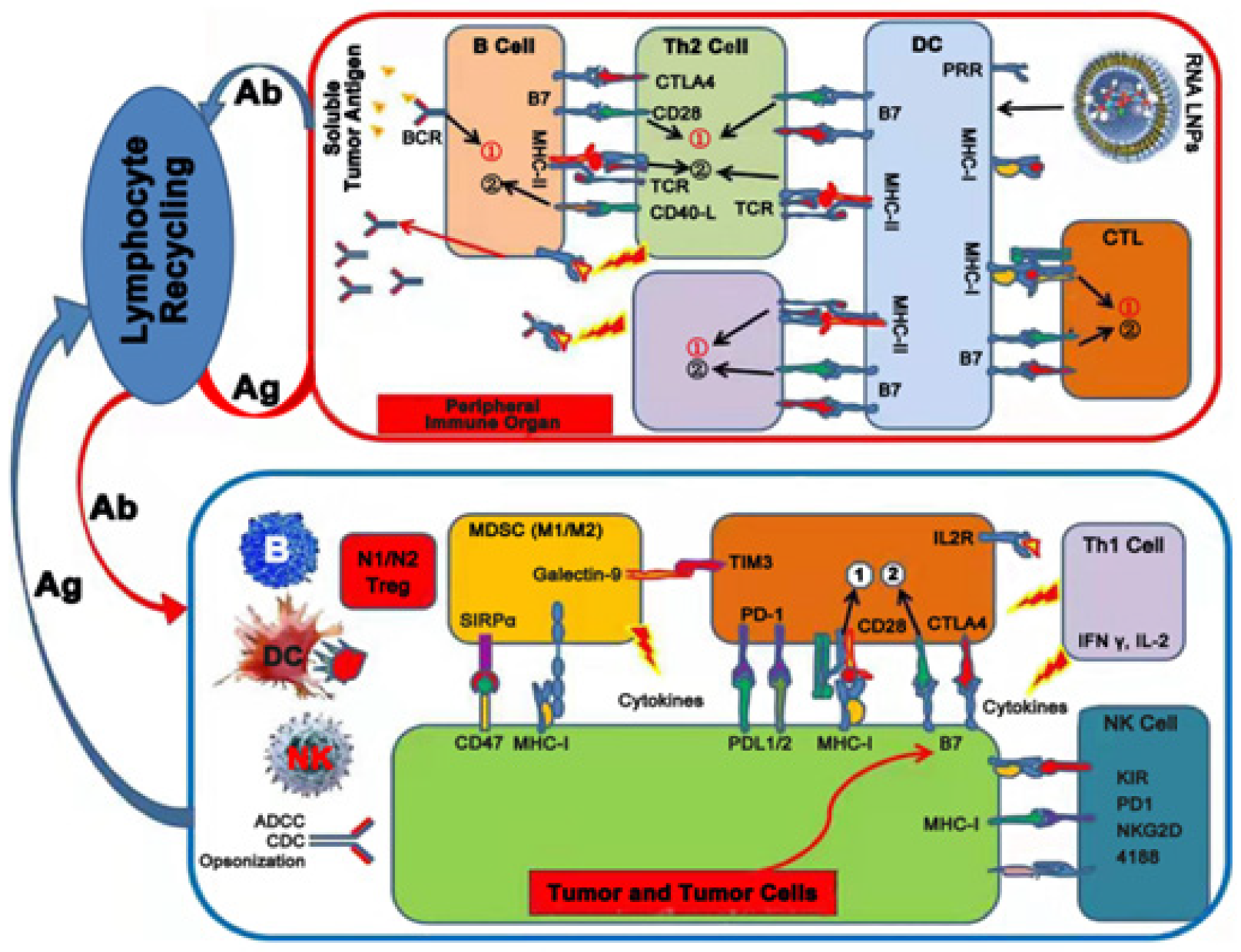
3.5. Immunotherapy
3.5.1. Nanoparticle-Based Vaccines
3.5.2. Nanoparticles Carrying Checkpoint Inhibitors
3.6. Challenges and Future Directions
3.6.1. Toxicity, Biocompatibility, and Immune Clearance
3.6.2. Cost and Scalability
3.6.3. Regulatory Approval
4. Discussion
5. Future Research Trends
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khemlina, G.; Ikeda, S.; Kurzrock, R. The biology of Hepatocellular carcinoma: Implications for genomic and immune therapies. Mol. Cancer 2017, 16, 149. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Global Cancer Observatory: Cancer Today. World Health Organization. Available online: https://gco.iarc.fr/today (accessed on 20 May 2025).
- Bloom, M.; Podder, S.; Dang, H.; Lin, D. Advances in Immunotherapy in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2025, 26, 1936. [Google Scholar] [CrossRef]
- Sequeira, L.M.; Ozturk, N.B.; Sierra, L.; Gurakar, M.; Toruner, M.D.; Zheng, M.; Simsek, C.; Gurakar, A.; Kim, A.K. Hepatocellular Carcinoma and the Role of Liver Transplantation: An Update and Review. J. Clin. Transl. Hepatol. 2025, 13, 327–338. [Google Scholar] [CrossRef]
- Yuan, Y.; Sun, W.; Xie, J.; Zhang, Z.; Luo, J.; Han, X.; Xiong, Y.; Yang, Y.; Zhang, Y. RNA nanotherapeutics for hepatocellular carcinoma treatment. Theranostics 2025, 15, 965–992. [Google Scholar] [CrossRef]
- Chen, Z.; Xie, H.; Hu, M.; Huang, T.; Hu, Y.; Sang, N.; Zhao, Y. Recent progress in treatment of hepatocellular carcinoma. Am. J. Cancer Res. 2020, 10, 2993–3036. [Google Scholar]
- Ruman, U.; Fakurazi, S.; Masarudin, M.J.; Hussein, M.Z. Nanocarrier-Based Therapeutics and Theranostics Drug Delivery Systems for Next Generation of Liver Cancer Nanodrug Modalities. Int. J. Nanomed. 2020, 15, 1437–1456. [Google Scholar] [CrossRef]
- Dadwal, A.; Baldi, A.; Kumar Narang, R. Nanoparticles as carriers for drug delivery in cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, 295–305. [Google Scholar] [CrossRef]
- Yu, X.; Trase, I.; Ren, M.; Duval, K.; Guo, X.; Chen, Z. Design of Nanoparticle-Based Carriers for Targeted Drug Delivery. J. Nanomater. 2016, 2016, 1087250. [Google Scholar] [CrossRef]
- Mudshinge, S.R.; Deore, A.B.; Patil, S.; Bhalgat, C.M. Nanoparticles: Emerging carriers for drug delivery. Saudi Pharm. J. 2011, 19, 129–141. [Google Scholar] [CrossRef]
- Hossen, S.; Hossain, M.K.; Basher, M.K.; Mia, M.N.H.; Rahman, M.T.; Uddin, M.J. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: A review. J. Adv. Res. 2019, 15, 1–18. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, L.; Gu, W.; Gao, Y.; Lin, L.; Zhang, Z.; Xi, Y.; Li, Y. The performance of docetaxel-loaded solid lipid nanoparticles targeted to hepatocellular carcinoma. Biomaterials 2009, 30, 226–232. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, Q.; Li, Y.; Tang, H.; Liu, W.; Yang, X. Doxorubicin and curcumin co-delivery by lipid nanoparticles for enhanced treatment of diethylnitrosamine-induced hepatocellular carcinoma in mice. Eur. J. Pharm. Biopharm. 2015, 93, 27–36. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, M.; Wu, J.; Zou, M.; Wu, D.; Gong, J.; Wang, P.; Yan, H.; Xia, X. Targeted treatment of hepatocellular carcinoma with aptamer-guided solid lipid nanoparticles loaded with norcantharidin. Drug Deliv. 2025, 32, 2519470. [Google Scholar] [CrossRef]
- Fu, F.; Wu, Y.; Zhu, J.; Wen, S.; Shen, M.; Shi, X. Multifunctional Lactobionic Acid-Modified Dendrimers for Targeted Drug Delivery to Liver Cancer Cells: Investigating the Role Played by PEG Spacer. ACS Appl. Mater. Interfaces 2014, 6, 16416–16425. [Google Scholar] [CrossRef]
- Iacobazzi, R.M.; Porcelli, L.; Lopedota, A.A.; Laquintana, V.; Lopalco, A.; Cutrignelli, A.; Altamura, E.; Di Fonte, R.; Azzariti, A.; Franco, M.; et al. Targeting human liver cancer cells with lactobionic acid-G(4)-PAMAM-FITC sorafenib loaded dendrimers. Int. J. Pharm. 2017, 528, 485–497. [Google Scholar] [CrossRef]
- Jędrzak, A.; Grześkowiak, B.F.; Coy, E.; Wojnarowicz, J.; Szutkowski, K.; Jurga, S.; Jesionowski, T.; Mrówczyński, R. Dendrimer based theranostic nanostructures for combined chemo- and photothermal therapy of liver cancer cells in vitro. Colloids Surf. B Biointerfaces 2019, 173, 698–708. [Google Scholar] [CrossRef]
- Zhong, Z.; Liu, Z.; Zhang, X.; Huang, J.; Yu, X.; Li, J.; Xiong, D.; Sun, X.; Luo, Y. Effect of a controlled-release drug delivery system made of oleanolic acid formulated into multivesicular liposomes on hepatocellular carcinoma in vitro and in vivo. Int. J. Nanomed. 2016, 11, 3111–3129. [Google Scholar] [CrossRef]
- Qi, L.; Xu, Z.; Chen, M. In vitro and in vivo suppression of hepatocellular carcinoma growth by chitosan nanoparticles. Eur. J. Cancer 2007, 43, 184–193. [Google Scholar] [CrossRef]
- Bonferoni, M.C.; Gavini, E.; Rassu, G.; Maestri, M.; Giunchedi, P. Chitosan Nanoparticles for Therapy and Theranostics of Hepatocellular Carcinoma (HCC) and Liver-Targeting. Nanomaterials 2020, 10, 870. [Google Scholar] [CrossRef]
- Huang, W.; Wang, W.; Wang, P.; Tian, Q.; Zhang, C.; Wang, C.; Yuan, Z.; Liu, M.; Wan, H.; Tang, H. Glycyrrhetinic acid-modified poly(ethylene glycol)–b-poly(γ-benzyl l-glutamate) micelles for liver targeting therapy. Acta Biomater. 2010, 6, 3927–3935. [Google Scholar] [CrossRef]
- Yang, T.; Lan, Y.; Cao, M.; Ma, X.; Cao, A.; Sun, Y.; Yang, J.; Li, L.; Liu, Y. Glycyrrhetinic acid-conjugated polymeric prodrug micelles co-delivered with doxorubicin as combination therapy treatment for liver cancer. Colloids Surf. B Biointerfaces 2019, 175, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, V.; Yadav, P.; Bhattacharya, S.S.; Mishra, A.K.; Verma, N.; Verma, A.; Pandit, J.K. Carbon Nanotubes: An Emerging Drug Carrier for Targeting Cancer Cells. J. Drug Deliv. 2014, 2014, 670815. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.G.P.; Baburao, C.; Pispati, V.; Pathipati, H.; Muthy, N.; Prassana, S.R.V.; Rathode, B.G. Carbon nanotubes. A novel drug delivery system. Int. J. Res. Pharm. Chem. 2012, 2, 523–532. [Google Scholar]
- Salem, D.S.; Sliem, M.A.; El-Sesy, M.; Shouman, S.A.; Badr, Y. Improved chemo-photothermal therapy of hepatocellular carcinoma using chitosan-coated gold nanoparticles. J. Photochem. Photobiol. B Biol. 2018, 182, 92–99. [Google Scholar] [CrossRef]
- Siddique, S.; Chow, J.C.L. Gold Nanoparticles for Drug Delivery and Cancer Therapy. Appl. Sci. 2020, 10, 3824. [Google Scholar] [CrossRef]
- Ji, M.; Qiu, X.; Hou, L.; Huang, S.; Li, Y.; Liu, Y.; Duan, S.; Hu, Y. Construction and application of a liver cancer-targeting drug delivery system based on core–shell gold nanocages. Int. J. Nanomed. 2018, 13, 1773–1789. [Google Scholar] [CrossRef]
- Grześkowiak, B.F.; Maziukiewicz, D.; Kozłowska, A.; Kertmen, A.; Coy, E.; Mrówczyński, R. Polyamidoamine Dendrimers Decorated Multifunctional Polydopamine Nanoparticles for Targeted Chemo- and Photothermal Therapy of Liver Cancer Model. Int. J. Mol. Sci. 2021, 22, 738. [Google Scholar] [CrossRef]
- Shen, Z.; Li, B.; Liu, Y.; Zheng, G.; Guo, Y.; Zhao, R.; Jiang, K.; Fan, L.; Shao, J. A self-assembly nanodrug delivery system based on amphiphilic low generations of PAMAM dendrimers-ursolic acid conjugate modified by lactobionic acid for HCC targeting therapy. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 227–236. [Google Scholar] [CrossRef]
- Xu, Y.; Guo, X.; Tu, L.; Zou, Q.; Li, Q.; Tang, C.; Chen, B.; Wu, C.; Wei, M. Lactoferrin-modified PEGylated liposomes loaded with doxorubicin for targeting delivery to hepatocellular carcinoma. Int. J. Nanomed. 2015, 5123. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhao, P.; Wu, S.; Yang, T.; Chen, Y.; Zhang, X.; He, C.; Zheng, C.; Li, K.; Ma, X.; et al. Cisplatin and curcumin co-loaded nano-liposomes for the treatment of hepatocellular carcinoma. Int. J. Pharm. 2018, 545, 261–273. [Google Scholar] [CrossRef]
- Hefnawy, A.; Khalil, I.H.; Arafa, K.; Emara, M.; El-Sherbiny, I.M. Dual-Ligand Functionalized Core-Shell Chitosan-Based Nanocarrier for Hepatocellular Carcinoma-Targeted Drug Delivery. Int. J. Nanomed. 2020, 15, 821–837. [Google Scholar] [CrossRef]
- Li, W.; Tan, G.; Zhang, H.; Wang, Z.; Jin, Y. Folate chitosan conjugated doxorubicin and pyropheophorbide acid nanoparticles (FCDP–NPs) for enhance photodynamic therapy. RSC Adv. 2017, 7, 44426–44437. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Zhang, C.; Sun, Q.; Yi, W.; Wang, X.; Cheng, D.; Chen, S.; Liang, B.; Shuai, X. Regulated pH-Responsive Polymeric Micelles for Doxorubicin Delivery to the Nucleus of Liver Cancer Cells. J. Biomed. Nanotechnol. 2016, 12, 1258–1269. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Vinothini, K.; Dou, F.; Jing, Y.; Chuturgoon, A.A.; Arumugam, T.; Rajan, M. Hyper-branched multifunctional carbon nanotubes carrier for targeted liver cancer therapy. Arab. J. Chem. 2022, 15, 103649. [Google Scholar] [CrossRef]
- Meng, L.; Zhang, X.; Lu, Q.; Fei, Z.; Dyson, P.J. Single walled carbon nanotubes as drug delivery vehicles: Targeting doxorubicin to tumors. Biomaterials 2012, 33, 1689–1698. [Google Scholar] [CrossRef] [PubMed]
- Tomuleasa, C.; Soritau, O.; Orza, A.; Dudea, M.; Petrushev, B.; Mosteanu, O.; Susman, S.; Florea, A.; Pall, E.; Aldea, M.; et al. Gold nanoparticles conjugated with cisplatin/doxorubicin/capecitabine lower the chemoresistance of hepatocellular carcinoma-derived cancer cells. J. Gastrointestin Liver Dis. 2012, 21, 187–196. [Google Scholar] [PubMed]
- Wang, J.; Zhang, Y.; Liu, L.; Cui, Z.; Liu, X.; Wang, L.; Li, Y.; Li, Q. Combined chemo/photothermal therapy based on mesoporous silica-Au core-shell nanoparticles for hepatocellular carcinoma treatment. Drug Dev. Ind. Pharm. 2019, 45, 1487–1495. [Google Scholar] [CrossRef]
- Farinha, P.; Coelho, J.M.P.; Reis, C.P.; Gaspar, M.M. A Comprehensive Updated Review on Magnetic Nanoparticles in Diagnostics. Nanomaterials 2021, 11, 3432. [Google Scholar] [CrossRef]
- Ungureanu, B.S.; Teodorescu, C.-M.; Săftoiu, A. Magnetic Nanoparticles for Hepatocellular Carcinoma Diagnosis and Therapy. J. Gastrointest. Liver Dis. 2016, 25, 375–383. [Google Scholar] [CrossRef]
- Guo, W.; Song, X.; Liu, J.; Liu, W.; Chu, X.; Lei, Z. Quantum Dots as a Potential Multifunctional Material for the Enhancement of Clinical Diagnosis Strategies and Cancer Treatments. Nanomaterials 2024, 14, 1088. [Google Scholar] [CrossRef]
- Yu, X.; Chen, L.; Li, K.; Li, Y.; Xiao, S.; Luo, X.; Liu, J.; Zhou, L.; Deng, Y.; Pang, D.; et al. Immunofluorescence detection with quantum dot bioconjugates for hepatoma in vivo. J. Biomed. Opt. 2007, 12, 014008. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.-B.; Rong, Y.; Fang, M.; Yuan, J.-P.; Peng, C.-W.; Liu, S.-P.; Li, Y. Recognition and capture of metastatic hepatocellular carcinoma cells using aptamer-conjugated quantum dots and magnetic particles. Biomaterials 2013, 34, 3816–3827. [Google Scholar] [CrossRef]
- Sharmiladevi, P.; Girigoswami, K.; Haribabu, V.; Girigoswami, A. Nano-enabled theranostics for cancer. Mater. Adv. 2021, 2, 2876–2891. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, X.; Miao, Y.; Li, J.; Gan, Y. Lipid-coated iron oxide nanoparticles for dual-modal imaging of hepatocellular carcinoma. Int. J. Nanomed. 2017, 12, 2033–2044. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Liu, S.; He, J.; Pan, C.C.; Li, H.; Zhou, Z.Y.; Ding, Y.; Huo, D.; Hu, Y. Synthesis and application of strawberry-like Fe3O4-Au nanoparticles as CT-MR dual-modality contrast agents in accurate detection of the progressive liver disease. Biomaterials 2015, 51, 194–207. [Google Scholar] [CrossRef]
- Karahaliloglu, Z.; Kilicay, E.; Hazer, B. PLinaS-g-PEG coated magnetic nanoparticles as a contrast agent for hepatocellular carcinoma diagnosis. J. Biomater. Sci. Polym. Ed. 2020, 31, 1580–1603. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, H.J.; Zamboni, C.G.; Hassan, L.F.; Radant, N.P.; Jacob, D.; Mease, R.C.; Minn, I.; Tzeng, S.Y.; Gabrielson, K.L.; Bhardwaj, P.; et al. Polymeric nanoparticles for dual-targeted theranostic gene delivery to hepatocellular carcinoma. Sci. Adv. 2022, 8, eabo6406. [Google Scholar] [CrossRef]
- Kozenkova, E.; Levada, K.; Efremova, M.V.; Omelyanchik, A.; Nalench, Y.A.; Garanina, A.S.; Pshenichnikov, S.; Zhukov, D.G.; Lunov, O.; Lunova, M.; et al. Multifunctional Fe3O4-Au Nanoparticles for the MRI Diagnosis and Potential Treatment of Liver Cancer. Nanomaterials 2020, 10, 1646. [Google Scholar] [CrossRef]
- Chen, X.; Song, L.; Li, X.; Zhang, L.; Li, L.; Zhang, X.; Wang, C. Co-delivery of hydrophilic/hydrophobic drugs by multifunctional yolk-shell nanoparticles for hepatocellular carcinoma theranostics. Chem. Eng. J. 2020, 389, 124416. [Google Scholar] [CrossRef]
- Yoo, J.; Park, C.; Yi, G.; Lee, D.; Koo, H. Active Targeting Strategies Using Biological Ligands for Nanoparticle Drug Delivery Systems. Cancers 2019, 11, 640. [Google Scholar] [CrossRef]
- Cannito, S.; Bincoletto, V.; Turato, C.; Pontisso, P.; Scupoli, M.T.; Ailuno, G.; Andreana, I.; Stella, B.; Arpicco, S.; Bocca, C. Hyaluronated and PEGylated Liposomes as a Potential Drug-Delivery Strategy to Specifically Target Liver Cancer and Inflammatory Cells. Molecules 2022, 27, 1062. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Hu, L.; Huang, S.; Xu, W.; Wan, J.; Wang, D.; Zheng, G.; Xia, Z. Curcumin-loaded galactosylated BSA nanoparticles as targeted drug delivery carriers inhibit hepatocellular carcinoma cell proliferation and migration. Int. J. Nanomed. 2018, 13, 8309–8323. [Google Scholar] [CrossRef]
- Sun, R.; Fang, L.; Lv, X.; Fang, J.; Wang, Y.; Chen, D.; Wang, L.; Chen, J.; Qi, Y.; Tang, Z.; et al. In vitro and in vivo evaluation of self-assembled chitosan nanoparticles selectively overcoming hepatocellular carcinoma via asialoglycoprotein receptor. Drug Deliv. 2021, 28, 2071–2084. [Google Scholar] [CrossRef] [PubMed]
- Takke, A.; Shende, P. Nanotherapeutic silibinin: An insight of phytomedicine in healthcare reformation. Nanomed. Nanotechnol. Biol. Med. 2019, 21, 102057. [Google Scholar] [CrossRef]
- Ghalehkhondabi, V.; Soleymani, M.; Fazlali, A. Folate-targeted nanomicelles containing silibinin as an active drug delivery system for liver cancer therapy. J. Drug Deliv. Sci. Technol. 2021, 61, 102157. [Google Scholar] [CrossRef]
- Wu, J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Li, Y.; Luo, J.; Lee, J.S.; Xiao, W.; Gonik, A.M.; Agarwal, R.G.; Lam, K.S. The effect of surface charge on in vivo biodistribution of PEG-oligocholic acid based micellar nanoparticles. Biomaterials 2011, 32, 3435–3446. [Google Scholar] [CrossRef]
- Onzi, G.; Guterres, S.S.; Pohlmann, A.R.; Frank, L.A. Passive Targeting and the Enhanced Permeability and Retention (EPR) Effect. In The ADME Encyclopedia; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–13. [Google Scholar] [CrossRef]
- Ferreira, D.D.S.; Lopes, S.C.D.A.; Franco, M.S.; Oliveira, M.C. pH-sensitive Liposomes for Drug Delivery in Cancer Treatment. Ther. Deliv. 2013, 4, 1099–1123. [Google Scholar] [CrossRef]
- Partikel, K.; Korte, R.; Stein, N.C.; Mulac, D.; Herrmann, F.C.; Humpf, H.-U.; Langer, K. Effect of nanoparticle size and PEGylation on the protein corona of PLGA nanoparticles. Eur. J. Pharm. Biopharm. 2019, 141, 70–80. [Google Scholar] [CrossRef]
- Jain, R.K. Transport of molecules in the tumor interstitium: A review. Cancer Res. 1987, 47, 3039–3051. [Google Scholar]
- Subhan, M.A.; Parveen, F.; Filipczak, N.; Yalamarty, S.S.K.; Torchilin, V.P. Approaches to Improve EPR-Based Drug Delivery for Cancer Therapy and Diagnosis. J. Pers. Med. 2023, 13, 389. [Google Scholar] [CrossRef]
- Kong, F.-H.; Ye, Q.-F.; Miao, X.-Y.; Liu, X.; Huang, S.-Q.; Xiong, L.; Wen, Y.; Zhang, Z.-J. Current status of sorafenib nanoparticle delivery systems in the treatment of hepatocellular carcinoma. Theranostics 2021, 11, 5464–5490. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Miao, Y.; Chen, M.; Chen, X.; Li, F.; Zhang, X.; Gan, Y. Stepwise targeting and responsive lipid-coated nanoparticles for enhanced tumor cell sensitivity and hepatocellular carcinoma therapy. Theranostics 2020, 10, 3722–3736. [Google Scholar] [CrossRef] [PubMed]
- Gavrilov, K.; Saltzman, W.M. Therapeutic siRNA: Principles, challenges, and strategies. Yale J. Biol. Med. 2012, 85, 187–200. [Google Scholar]
- Hanahan, D.; Folkman, J. Patterns and Emerging Mechanisms of the Angiogenic Switch during Tumorigenesis. Cell 1996, 86, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.-W.; Lai, Y.-T.; Chern, G.-J.; Huang, S.-F.; Tsai, C.-L.; Sung, Y.-C.; Chiang, C.-C.; Hwang, P.-B.; Ho, T.-L.; Huang, R.-L.; et al. Galactose Derivative-Modified Nanoparticles for Efficient siRNA Delivery to Hepatocellular Carcinoma. Biomacromolecules 2018, 19, 2330–2339. [Google Scholar] [CrossRef]
- Rodponthukwaji, K.; Pingrajai, P.; Jantana, S.; Taya, S.; Duangchan, K.; Nguyen, K.T.; Srisawat, C.; Punnakitikashem, P. Epigallocatechin Gallate Potentiates the Anticancer Effect of AFP-siRNA-Loaded Polymeric Nanoparticles on Hepatocellular Carcinoma Cells. Nanomaterials 2023, 14, 47. [Google Scholar] [CrossRef]
- Xia, Y.; Guo, M.; Xu, T.; Li, Y.; Wang, C.; Lin, Z.; Zhao, M.; Zhu, B. siRNA-loaded selenium nanoparticle modified with hyaluronic acid for enhanced hepatocellular carcinoma therapy. Int. J. Nanomed. 2018, 13, 1539–1552. [Google Scholar] [CrossRef]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A Programmable Dual-RNA–Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Seeger, C.; Sohn, J.A. Targeting Hepatitis B Virus With CRISPR/Cas9. Mol. Ther. Nucleic Acids 2014, 3, e216. [Google Scholar] [CrossRef]
- Song, J.; Zhang, X.; Ge, Q.; Yuan, C.; Chu, L.; Liang, H.; Liao, Z.; Liu, Q.; Zhang, Z.; Zhang, B. CRISPR/Cas9-mediated knockout of HBsAg inhibits proliferation and tumorigenicity of HBV-positive hepatocellular carcinoma cells. J. Cell. Biochem. 2018, 119, 8419–8431. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, F.; Chen, Q.; Wan, C.; Xiong, J.; Xu, J. CRISPR/Cas9-mediated knockout of NSD1 suppresses the hepatocellular carcinoma development via the NSD1/H3/Wnt10b signaling pathway. J. Exp. Clin. Cancer Res. 2019, 38, 467. [Google Scholar] [CrossRef] [PubMed]
- Goh, Z.Y.; Ren, E.C.; Ko, H.L. Intracellular interferon signalling pathways as potential regulators of covalently closed circular DNA in the treatment of chronic hepatitis B. World J. Gastroenterol. 2021, 27, 1369–1391. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Zhuang, C.; Wang, S.; Zhang, Y. Photodynamic and Photothermal Therapy of Hepatocellular Carcinoma. Front. Oncol. 2021, 11, 787780. [Google Scholar] [CrossRef]
- Li, B.; Niu, X.; Xie, M.; Luo, F.; Huang, X.; You, Z. Tumor-Targeting Multifunctional Nanoprobe for Enhanced Photothermal/Photodynamic Therapy of Liver Cancer. Langmuir 2021, 37, 8064–8072. [Google Scholar] [CrossRef]
- Ailioaie, L.M.; Ailioaie, C.; Litscher, G. Synergistic Nanomedicine: Photodynamic, Photothermal and Photoimmune Therapy in Hepatocellular Carcinoma: Fulfilling the Myth of Prometheus? Int. J. Mol. Sci. 2023, 24, 8308. [Google Scholar] [CrossRef]
- Iancu, C.; Mocan, T.; Mocan, L.; Matea, C.; Tabaran, F.; Mosteanu, O.; Pop, T. Photothermal treatment of liver cancer with albumin-conjugated gold nanoparticles initiates Golgi Apparatus–ER dysfunction and caspase-3 apoptotic pathway activation by selective targeting of Gp60 receptor. Int. J. Nanomed. 2015, 10, 5435–5445. [Google Scholar] [CrossRef]
- Huang, A.-T.; Du, J.; Liu, Z.-Y.; Zhang, G.-C.; Abuduwaili, W.; Yan, J.-Y.; Sun, J.-L.; Xu, R.-C.; Liu, T.-T.; Shen, X.-Z.; et al. Sorafenib-Loaded Cu2−xSe Nanoparticles Boost Photothermal–Synergistic Targeted Therapy against Hepatocellular Carcinoma. Nanomaterials 2022, 12, 3191. [Google Scholar] [CrossRef]
- Kumar, A.; Moralès, O.; Mordon, S.; Delhem, N.; Boleslawski, E. Could Photodynamic Therapy Be a Promising Therapeutic Modality in Hepatocellular Carcinoma Patients? A Critical Review of Experimental and Clinical Studies. Cancers 2021, 13, 5176. [Google Scholar] [CrossRef]
- Ogbodu, R.O.; Nitzsche, B.; Ma, A.; Atilla, D.; Gürek, A.G.; Höpfner, M. Photodynamic therapy of hepatocellular carcinoma using tetra-triethyleneoxysulfonyl zinc phthalocyanine as photosensitizer. J. Photochem. Photobiol. B Biol. 2020, 208, 111915. [Google Scholar] [CrossRef]
- Buonaguro, L.; Mauriello, A.; Cavalluzzo, B.; Petrizzo, A.; Tagliamonte, M. Immunotherapy in hepatocellular carcinoma. Ann. Hepatol. 2019, 18, 291–297. [Google Scholar] [CrossRef]
- Chung, S.; Lee, C.M.; Zhang, M. Advances in nanoparticle-based mRNA delivery for liver cancer and liver-associated infectious diseases. Nanoscale Horiz. 2023, 8, 10–28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xie, F.; Yin, Y.; Zhang, Q.; Jin, H.; Wu, Y.; Pang, L.; Li, J.; Gao, J. Immunotherapy of Tumor RNA-Loaded Lipid Nanoparticles Against Hepatocellular Carcinoma. Int. J. Nanomed. 2021, 16, 1553–1564. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Jiang, C.; Wang, P.; Chen, Q.; Wang, B.; Fu, X.; Liang, Y.; Zhang, D.; Zeng, Y.; Liu, X. Caveolin-mediated cytosolic delivery of spike nanoparticle enhances antitumor immunity of neoantigen vaccine for hepatocellular carcinoma. Theranostics 2023, 13, 4166–4181. [Google Scholar] [CrossRef] [PubMed]
- Da, X.; Cao, B.; Mo, J.; Xiang, Y.; Hu, H.; Qiu, C.; Zhang, C.; Lv, B.; Zhang, H.; He, C.; et al. Inhibition of growth of hepatocellular carcinoma by co-delivery of anti-PD-1 antibody and sorafenib using biomimetic nano-platelets. BMC Cancer 2024, 24, 273. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, S.; Zhou, Q.; Zhou, J.; Li, J.; Ma, Y.; Hu, B.; Liu, C.; Zhao, Y. Nanobubble-based anti-hepatocellular carcinoma therapy combining immune check inhibitors and sonodynamic therapy. Nanoscale Adv. 2022, 4, 4847–4862. [Google Scholar] [CrossRef]
- Fiorito, S.; Serafino, A.; Andreola, F.; Togna, A.; Togna, G. Toxicity and Biocompatibility of Carbon Nanoparticles. J. Nanosci. Nanotechnol. 2006, 6, 591–599. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Fan, Y.; Feng, Q.; Cui, F. Biocompatibility and Toxicity of Nanoparticles and Nanotubes. J. Nanomater. 2012, 2012, 548389. [Google Scholar] [CrossRef]
- Liu, X.; Bai, Y.; Zhou, B.; Yao, W.; Song, S.; Liu, J.; Zheng, C. Recent advances in hepatocellular carcinoma-targeted nanoparticles. Biomed. Mater. 2024, 19, 042004. [Google Scholar] [CrossRef]
- Baig, B.; Halim, S.A.; Farrukh, A.; Greish, Y.; Amin, A. Current status of nanomaterial-based treatment for hepatocellular carcinoma. Biomed. Pharmacother. 2019, 116, 108852. [Google Scholar] [CrossRef]
- Li, J.; Chen, C.; Xia, T. Understanding Nanomaterial–Liver Interactions to Facilitate the Development of Safer Nanoapplications. Adv. Mater. 2022, 34, 2106456. [Google Scholar] [CrossRef] [PubMed]
- Tsoi, K.M.; MacParland, S.A.; Ma, X.-Z.; Spetzler, V.N.; Echeverri, J.; Ouyang, B.; Fadel, S.M.; Sykes, E.A.; Goldaracena, N.; Kaths, J.M.; et al. Mechanism of hard-nanomaterial clearance by the liver. Nature Mater. 2016, 15, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, R.; Babu, R.J.; Palakurthi, S. Nanomedicine Scale-up Technologies: Feasibilities and Challenges. Aaps Pharmscitech 2014, 15, 1527–1534. [Google Scholar] [CrossRef]
- Mülhopt, S.; Diabaté, S.; Dilger, M.; Adelhelm, C.; Anderlohr, C.; Bergfeldt, T.; Gómez De La Torre, J.; Jiang, Y.; Valsami-Jones, E.; Langevin, D.; et al. Characterization of Nanoparticle Batch-To-Batch Variability. Nanomaterials 2018, 8, 311. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.M.; Warheit, D.B.; Ng, S.P.; Comfort, K.K.; Grabinski, C.M.; Braydich-Stolle, L.K. At the Crossroads of Nanotoxicology in vitro: Past Achievements and Current Challenges. Toxicol. Sci. 2015, 147, 5–16. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, Q.; Wang, L.; Zhang, Y.; Zhu, L. Engineered nanoparticles for imaging and targeted drug delivery in hepatocellular carcinoma. Exp. Hematol. Oncol. 2025, 14, 62. [Google Scholar] [CrossRef]
- Malik, R.; Patil, S. Nanotechnology: Regulatory Outlook on Nanomaterials and Nanomedicines in United States, Europe and India. Appl. Clin. Res. Clin. Trials Regul. Aff. 2020, 7, 225–236. [Google Scholar] [CrossRef]
| Type | Payload | Targeting Ligand | Target Model | Efficacy Outcomes | Toxicity Outcomes and Limitations | Reference |
|---|---|---|---|---|---|---|
| SLNs | Docetaxel | Galactose | BEL7402 cells; tumor-bearing nude mice (Preclinical study) | Reduced systemic toxicity vs. free docetaxel | No liver damage; long-term toxicity and immunogenicity not evaluated; limited pharmacokinetics (PK) profile. | [12] |
| SLNs | Doxorubicin (DOX) + Curcumin | - | BEL-7402, BEL-7402/5-FU cells; DEN-induced HCC in mice (Preclinical study) | High drug loading; sustained release; enhanced efficacy in drug-resistant cells | Reduced liver toxicity; no long-term toxicity/accumulation data | [13] |
| Poly- dopamine core NPs decorated with PAMAM | Doxorubicin | Folic Acid | HepG2 cells (Preclinical study) | High drug loading; synergistic chemo-photothermal effect | Low toxicity to normal liver cells; PAMAM increases cytotoxicity at high concentrations | [28] |
| Dendrimer based self-assem- bling nanodrug | Ursolic acid (UA) | Lactobionic acid | ASGPR-overexpressing SMMC7721 cells; H22 tumor-bearing mice (Preclinical study) | Enhanced cytotoxicity to SMMC7721 cells; significant tumor suppression in vivo | Reduced cytotoxicity in ASGPR-negative HeLa cells; better safety profile than free UA; limited data on long-term toxicity | [29] |
| MVLs | Oleanolic Acid (OA) | HepG2 cells (in vitro); H22 murine hepatoma (in vivo) (Preclinical study) | Enhanced cellular uptake; apoptosis induction; high tumor inhibition at high dose; prolonged survival | Mild hepatic and pulmonary toxicity at high dose; no renal or hematologic effects; not suitable for IV use due to poor solubility. | [18] | |
| PEGylated liposome (PLS) | Doxorubicin | Lactoferrin (Lf) | ASGPR-positive HCC cells (HepG2, BEL7402, SMMC7721); BALB/c nude mice with HepG2 xenografts (Preclinical study) | Improved therapeutic effect vs. non-targeted formulations | No significant body weight loss; low systemic toxicity; no PK or biodistribution data | [30] |
| Liposomes | Cisplatin (CDDP) + Curcumin (CUR) | - | HepG2 xenografts in BALB/c nude mice; H22 tumors in Kunming mice (Preclinical study) | Enhanced tumor inhibition; synergistic effect; reduced tumor volume; prolonged survival | Reduced nephrotoxicity and hepatotoxicity vs. free CDDP; maintained body weight; normal liver/kidney markers | [31] |
| Core–shell chitosan- based NPs | Doxorubicin | Lactobionic acid, Glycyrrhetinic acid | HepG2 cells; Wistar rats with induced HCC (Preclinical study) | Enhanced cellular uptake; apoptosis induction; and tissue regeneration observed | Reduced nephrotoxicity and cardiotoxicity vs. free DOX; less liver/kidney damage histologically | [32] |
| Chitosan NPs | Doxorubicin | Folic Acid | HepG2 cells (Preclinical study) | Enhanced anti-tumor effect | No major toxic effects observed in vitro | [33] |
| Polymeric micelles | Doxorubicin | Glycyrrhetinic Acid (also therapeutic) | HepG2 cells; HepG2 xenograft in nude mice (Preclinical study) | Synergistic tumor growth inhibition; enhanced apoptosis; improved cytotoxicity prolonged survival | Reduced cardiac accumulation of DOX; no significant body weight loss | [22] |
| Polymeric micelles (PEG-PGA(DIP)) | Doxorubicin | - | Bel-7402 cell line (Preclinical study) | Increased delivery of DOX into the nucleus;improved therapeutic perfor- mance vs. controls | Reduced systemic toxicity due to controlled release in acidic environment; possible instability in bloodstream | [34] |
| MWCNT- based nanocarrier | Doxorubicin | Folic Acid | HepG2 cells; HEK293 cells (Preclinical study) | High DOX loading; pH-sensitive release; high cytotoxicity toward HepG2 cells | Minimal toxicity in HEK293 cells; good biocompatibility; long-term toxicity not assessed | [35] |
| SWCNT- based nanocarrier | Doxorubicin | Folic acid | SMMC-7721 cells; SMMC-7721 xenograft in BALB/c nude mice (Preclinical study) | Superior in vitro cytotoxicity vs. free DOX; enhanced tumor suppression in vivo | No significant weight loss; lower AST/PLT elevation vs. free DOX; minimal renal/liver histological damage | [36] |
| AuNPs | Cisplatin, Doxorubicin, Capecitabine | - | HepG2 cells; CSC (chemotherapy-resistant HCC cells); LIV (normal liver cells) (Preclinical study) | Reduced viability in CSC and HepG2 cells vs. free drugs; enhanced apoptosis in CSC | Slightly increased toxicity in LIV cells vs. free drugs; possible false-positives from sample handling | [37] |
| Gold-coated mesoporous silica nanoparticles (Au-MSNs) | Sorafenib (SO) | - | Huh-7, SMMC-7721, HepG2; L-02 as control (Preclinical study) | Reduced viability in HCC cells, enhanced under NIR; improved uptake and cytotoxicity vs. SO-MSNs or Au-MSNs alone | Moderate toxicity without NIR; increased toxicity with NIR due to hyperthermia | [38] |
| Nanoparticle Classification | Application Type | Diagnostic Agent | Model | Technique | Efficacy Outcomes | Reference |
|---|---|---|---|---|---|---|
| Lipid-coated iron oxide nanoparticles (GPC@IR783-Fe3O4) | Diagnostic | IR-783 (NIR fluorescent dye) + Fe3O4 (superpara- magnetic) | In vitro (Huh-7 cells); In vivo (Huh-7 tumor-bearing nude mice) (Preclinical study) | NIR fluorescence imaging; T2-weighted MRI | Higher tumor uptake; improved MRI contrast; enhanced tumor signal | [45] |
| Hybrid magnetic-metallic nanoparticle (Fe3O4-Au) | Diagnostic | Fe3O4 (T2 MRI contrast) + Au clusters (CT enhancement) | In vitro: HepG2 cells; In vivo: rats (normal, fatty liver, cirrhosis, HCC) (Preclinical study) | 3T MRI; 64-slice CT | Strong T2 and CT contrast; Clear lesion visualization across liver disease stages; good biocompatibility; low cytotoxicity | [46] |
| Poly(linoleic acid)-grafted PEG-coated SPIONs | Diagnostic | Fe3O4 (T2 MRI contrast) | In vitro: HepG2 and L929 fibroblasts; MRI phantom model (Preclinical study) | T2-MRI, TEM, DLS, cytotoxicity assay, confocal microscopy | High T2 relaxivity; selective uptake by cancer cells; minimal toxicity in normal cells | [47] |
| Core–shell quantum dots (CdSe/ZnS) | Diagnostic | Fluorescent anti-AFP immunoprobe | In vitro: HCCLM6 cells; In vivo: nude mice with subcutaneous hepatoma (Preclinical study) | Fluorescence imaging (confocal and spectral) | High specificity for hepatoma cells; strong tumor-site fluorescence; active targeting; demonstrated biocompatibility | [42] |
| Quantum dots + magnetic particles | Detection and capture of metastatic HCC cells | Aptamer LY-1 | HCCLM9 and MHCC97-L cells; mouse xenograft with lung metastasis; (Preclinical study) clinical HCC tissues; spiked human blood (Clinical study) | Cell-SELEX, flow cytometry, fluorescence microscopy, magnetic capture | High specificity and affinity for HCCLM9 cells; detected metastatic cells in tissues; enabled magnetic capture | [43] |
| Poly(beta-amino-ester) | Theranostic | 18F-FHBG (PET radiotracer) | Orthotopic xenograft in NU/J mice (Hep3b HCC cells) (Preclinical study) | PET, IR fluorescence imaging, MRI | PET signal specific to AFP+ HCC; MRI confirmed targeting and size reduction; no liver toxicity; selective in vitro cancer cell killing | [48] |
| Fe3O4-Au hybrid nanoparticles | Theranostic | Fe3O4 (magnetite) | Huh7, PLC/PRF/5- Alexander, HepG2 (Preclinical study) | MRI relaxometry, magnetic hyperthermia, cytotoxicity assays | Effective MRI contrast; potential for heat-based therapy; reduced toxicity in liver cells | [49] |
| Upconversion based on yolk-shell nanoparticles | Theranostic | Yb3+-doped upconversion core | In vitro (HepG2); In vivo (HCC-bearing mice) (Preclinical study) | CT, MRI, UCL imaging, PTT, confocal microscopy | Strong CT/MRI contrast (Yb3+); high UCL signal; pH/NIR-triggered dual drug release (DOX, HCPT); tumor localization; high photothermal efficiency; significant tumor inhibition; minimal systemic toxicity | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cosma, M.; Mocan, T.; Sabau, L.I.; Pop, T.; Mosteanu, O.; Mocan, L. A Narrative Review on Functionalized Nanoparticles for the Treatment and Early Detection of Hepatocellular Carcinoma. Appl. Sci. 2025, 15, 7649. https://doi.org/10.3390/app15147649
Cosma M, Mocan T, Sabau LI, Pop T, Mosteanu O, Mocan L. A Narrative Review on Functionalized Nanoparticles for the Treatment and Early Detection of Hepatocellular Carcinoma. Applied Sciences. 2025; 15(14):7649. https://doi.org/10.3390/app15147649
Chicago/Turabian StyleCosma, Meda, Teodora Mocan, Lavinia Ioana Sabau, Teodora Pop, Ofelia Mosteanu, and Lucian Mocan. 2025. "A Narrative Review on Functionalized Nanoparticles for the Treatment and Early Detection of Hepatocellular Carcinoma" Applied Sciences 15, no. 14: 7649. https://doi.org/10.3390/app15147649
APA StyleCosma, M., Mocan, T., Sabau, L. I., Pop, T., Mosteanu, O., & Mocan, L. (2025). A Narrative Review on Functionalized Nanoparticles for the Treatment and Early Detection of Hepatocellular Carcinoma. Applied Sciences, 15(14), 7649. https://doi.org/10.3390/app15147649






