Image Quality Assessment of Augmented Reality Glasses as Medical Display Devices (HoloLens 2)
Abstract
Featured Application
Abstract
1. Introduction
1.1. Use and Evaluation of Medical Display Devices
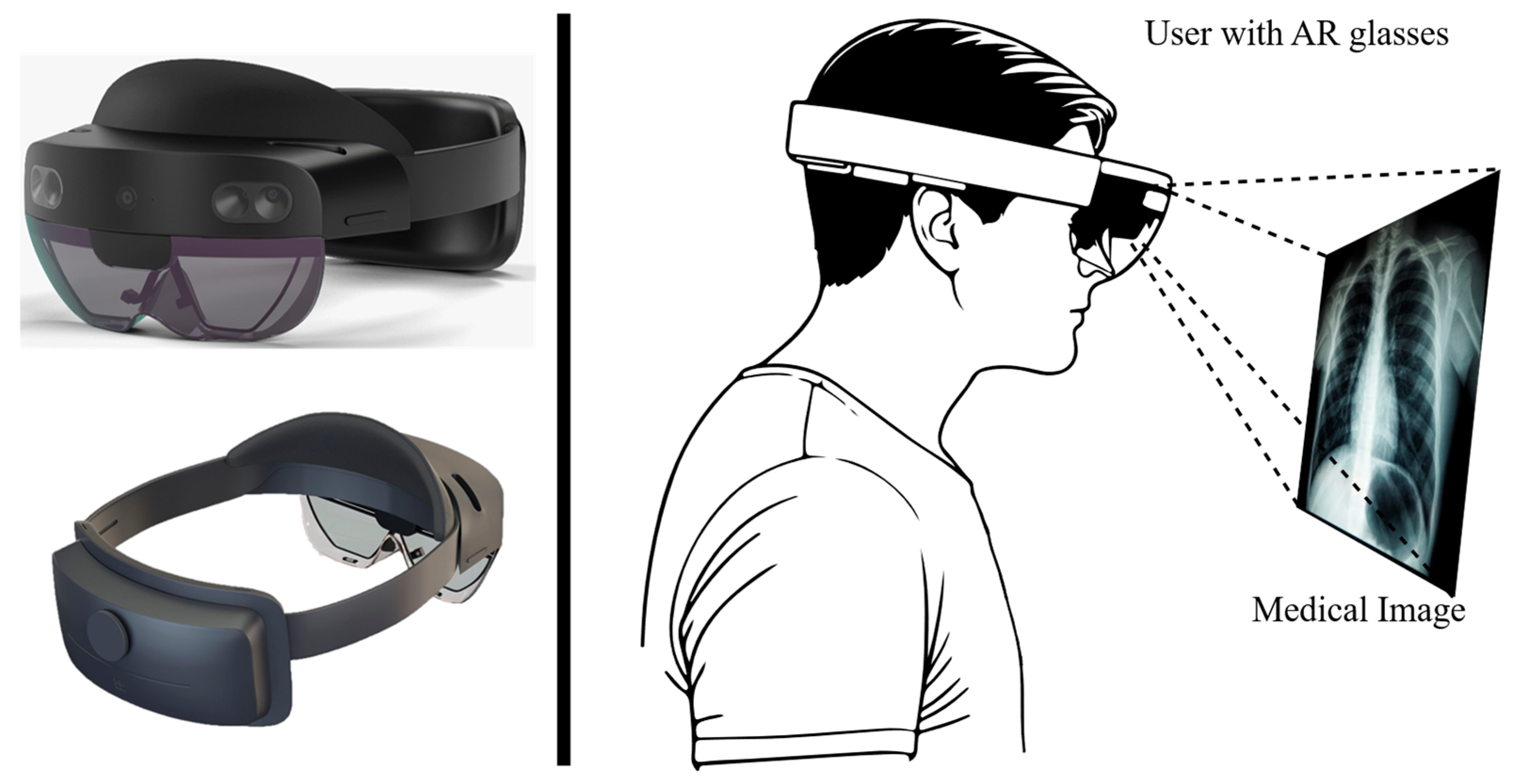
1.2. Use of Augmented Reality Glasses as Medical Display Device
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Evaluation of Overall Image Quality
2.2.2. Evaluation of Minimal and Maximal Luminance
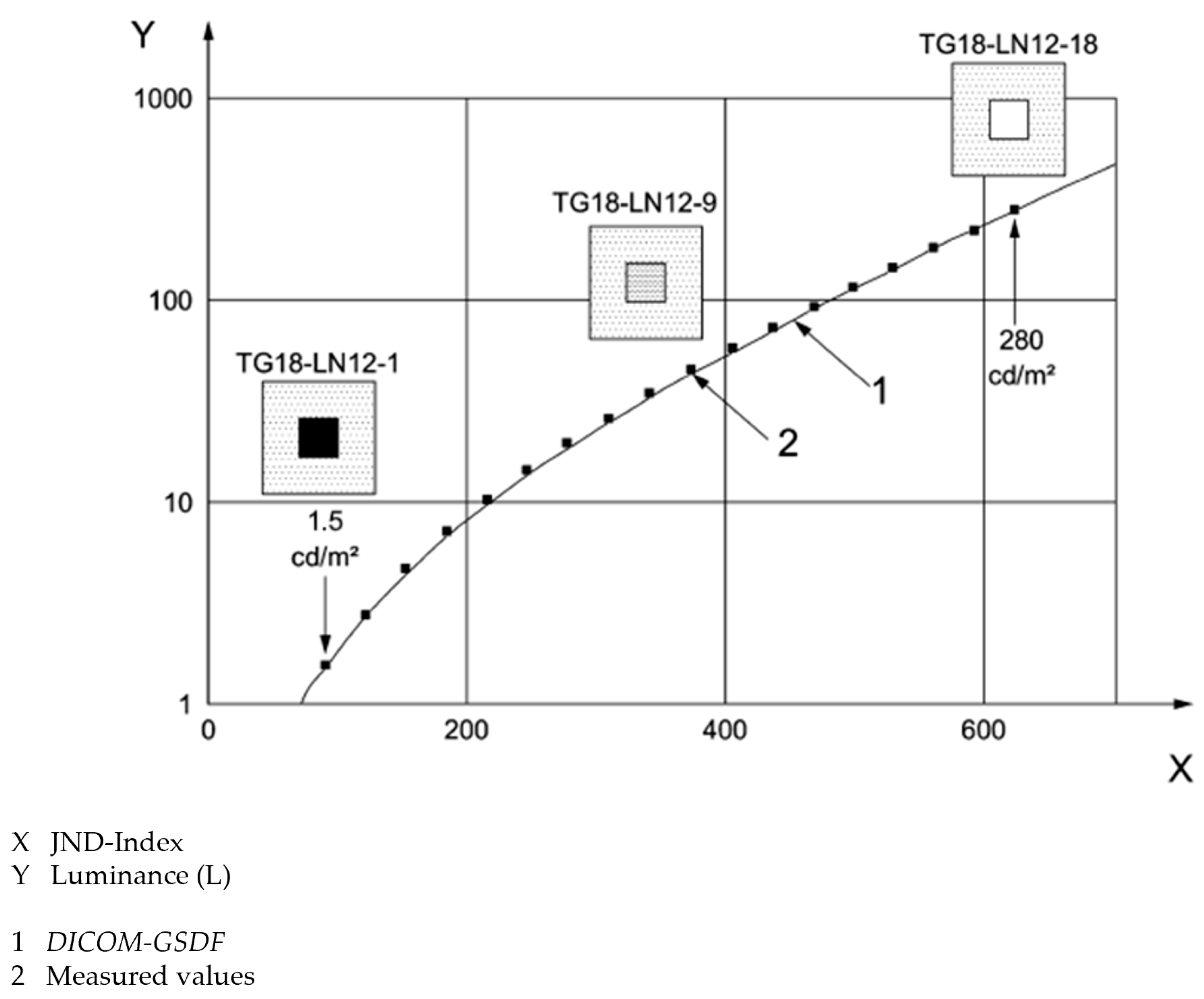
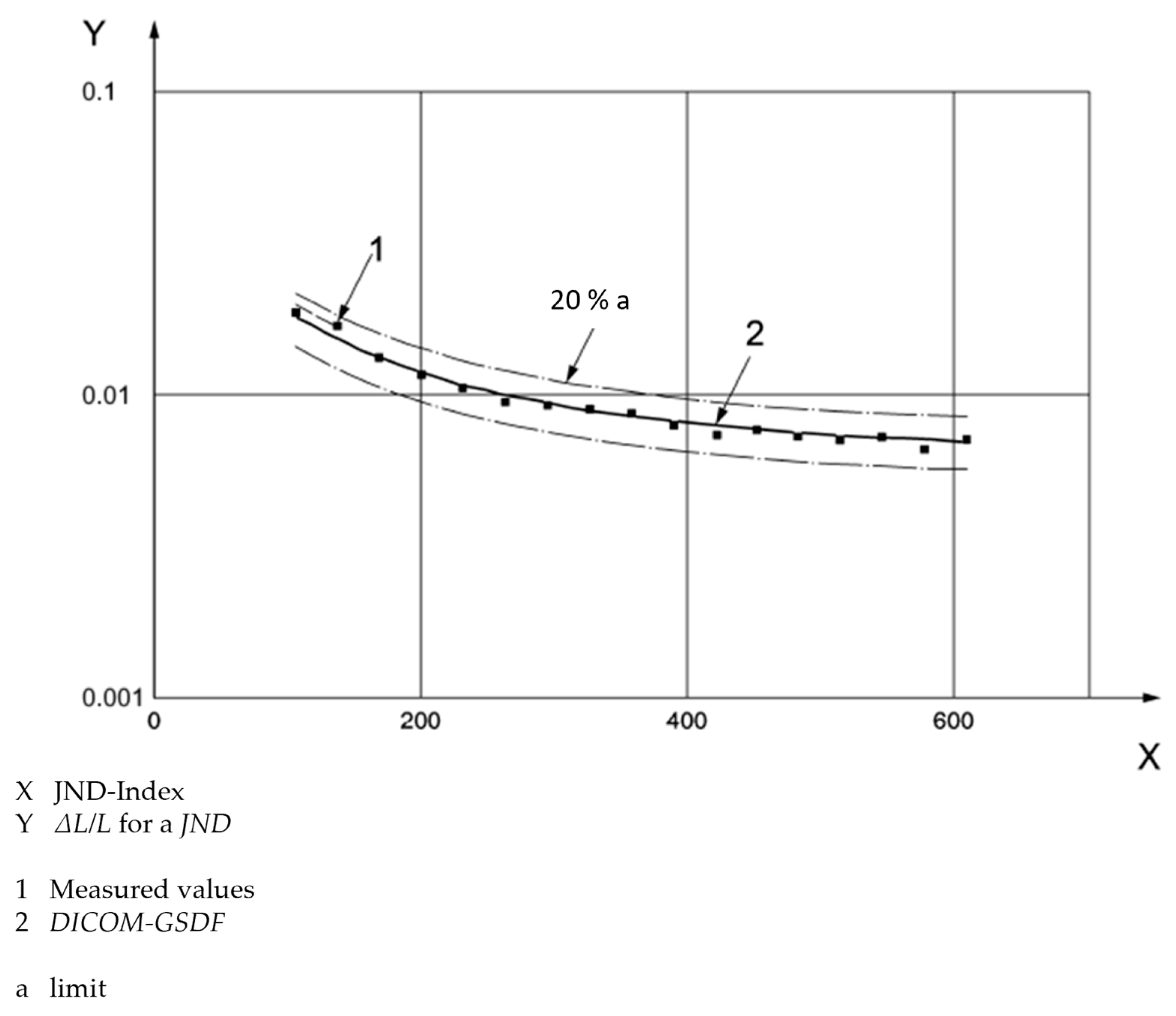
2.2.3. Evaluation of Luminance-to-Grayscale Conformance
2.2.4. Evaluation of Color Uniformity
3. Results
3.1. Evaluation of the Overall Image Quality
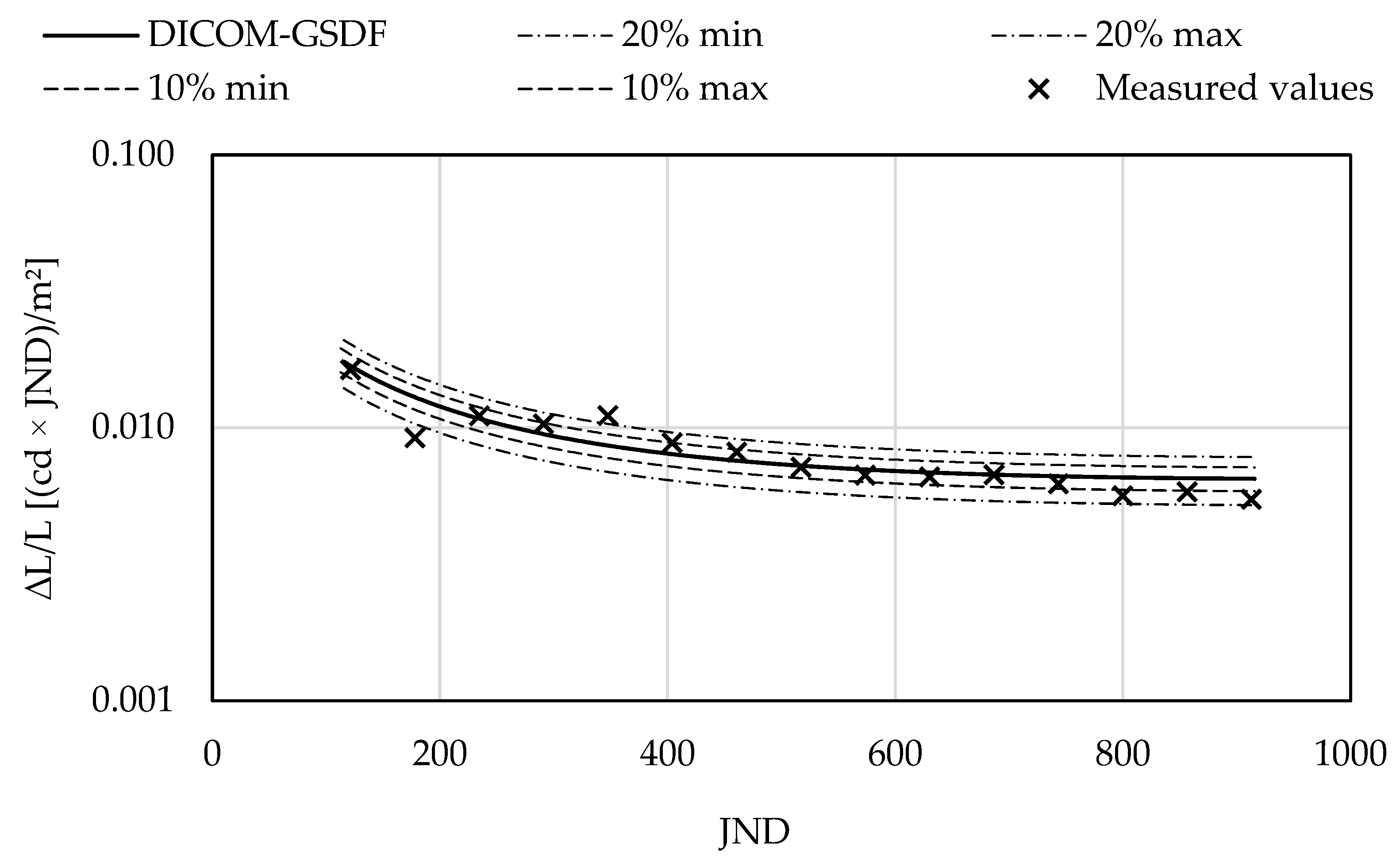
3.2. Evaluation of Minimal and Maximal Lumnance
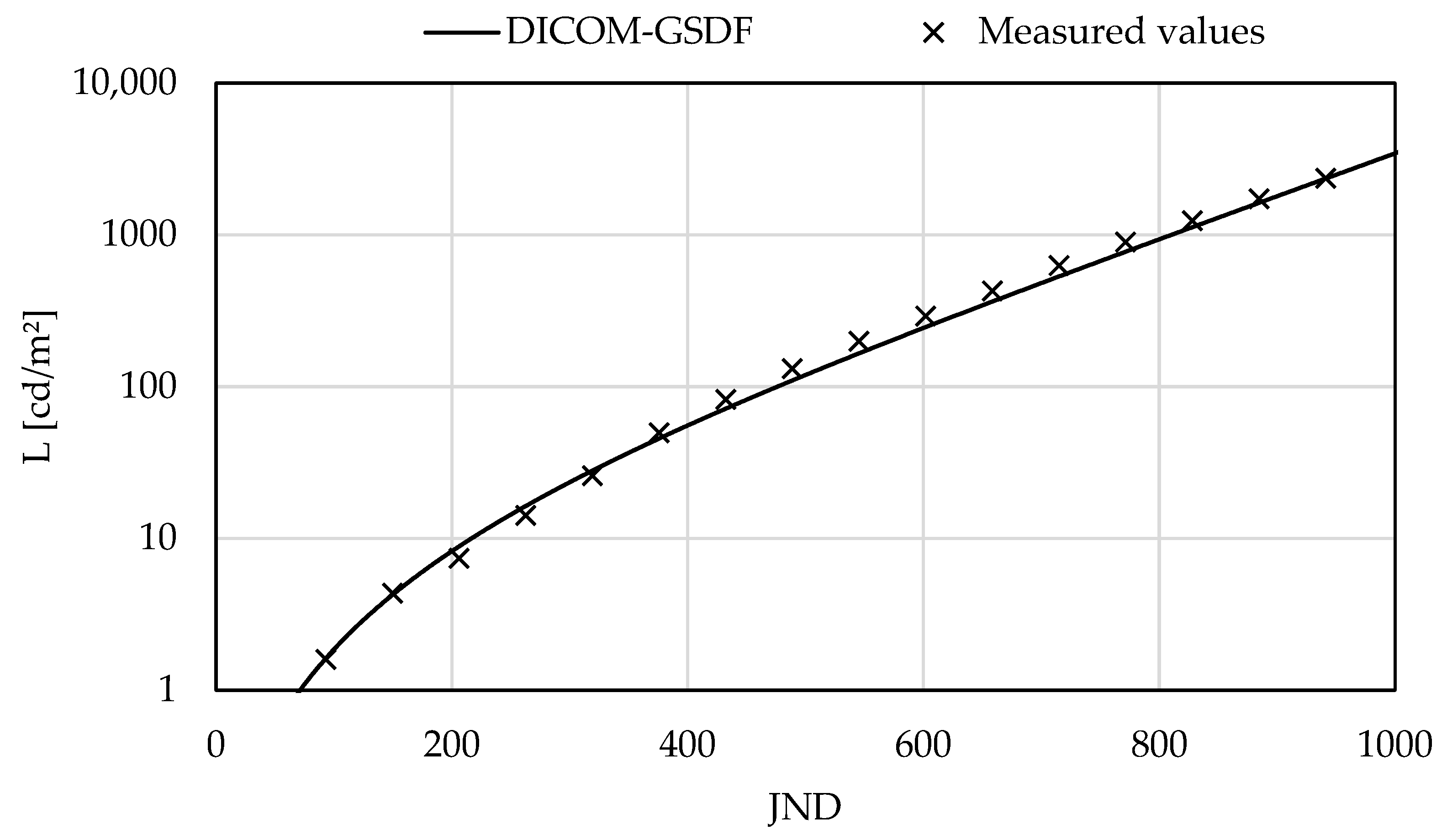
3.3. Evaluation of Luminance-to-Grayscale Conformance
3.4. Evaluation of the Color Uniformity
4. Discussion
4.1. Evaluation of the Overall Image Quality
4.2. Evaluation of Luminance and Luminance-to-Grayscale Conformance
4.3. Evaluation of Color Uniformity
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ackerman, S.J.; Gitlin, J.N.; Gayler, R.W.; Flagle, C.D.; Bryan, R.N. Receiver operating characteristic analysis of fracture and pneumonia detection: Comparison of laser-digitalized workstations and conventional analog radiographs. Radiology 1993, 186, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Scott, W.W.; Bluemke, D.A.; Mysko, W.K.; Weller, G.E.R.; Kelen, G.D.; Reichle, R.L.; Weller, J.C.; Gitlin, J.N. Interpretation of emergency department radiographs by radiologists and emergency medicine physicians: Teleradiology workstation versus radiograph readings. Radiology 1995, 195, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.; Mahoney, M.; Savage, N.W. The impact of computer display performance on the quality of digital radiographs: A review. Aust. Dent. J. 2012, 57, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Samei, E.; Badano, A.; Chakraborty, D.; Compton, K.; Cornelius, C.; Corrigan, K.; Flynn, M.J.; Hemminger, B.; Hangiandreou, N.; Johnson, J.; et al. Assessment of display performance for medical imaging systems: Executive summary of AAPM TG18 Report. Med. Phys. 2005, 32, 1205–1225. [Google Scholar] [CrossRef] [PubMed]
- IEC 62563–1:2009+A1:2016+A2:2021 CSV; Medical Electrical Equipment—Medical Image Display Systems—Part 1: Evaluation Methods. VDE VERLAG GmbH: Berlin, Germany, 2021.
- DIN 6868-157:2022; Image Quality Assurance in Diagnostic Xray Departments—Part 157. X-ray Ordinance Acceptance and Constancy Test Image Display Systems in Their Environment. Beuth Verlag GmbH: Berlin, Germany, 2022.
- NEMA PS 3.14-2006; Digital Image Communications in Medicine (DICOM)—Part 14: Grayscale Standard Display Function. National Electrical Manufactures Association (NEMA): Rosslyn, VA, USA, 2006.
- US Food and Drug Administration (FDA). Display Devices for Diagnostic Radiology—Guidance for Industry and Food and Drug Administration Staff; FDA: Silver Spring, MD, USA, 2022. Available online: https://www.fda.gov/media/95527/download (accessed on 2 June 2025).
- ISO/CIE 11664-5:2016; Colorimetry—Part 5: CIE 1976 L*u*v* Colour Space and u′, v′ Uniform Chromaticity Scale Diagram. International Organization for Standardization (ISO): Genf, Switzerland, 2016.
- European Commission. Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on medical devices, amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and repealing Council Directives 90/385/EEC and 93/42/EEC. Off. J. Eur. Union. 2017, 117, 1–175. [Google Scholar]
- Palumbo, A. Microsoft HoloLens 2 in Medical and Healthcare Context: State of the Art and Future Prospects. Sensors 2022, 22, 7709. [Google Scholar] [CrossRef] [PubMed]
- Southworth, M.K.; Silva, J.N.A.; Blume, W.M.; Van Hare, G.F.; Dalal, A.S.; Silva, J.R. Performance evaluation of mixed reality display for guidance during transcatheter cardiac mapping and ablation. IEEE J. Transl. Eng. Health Med. 2020, 8, 1900810. [Google Scholar] [CrossRef] [PubMed]
- Balci, D.; Kirimker, E.O.; Raptis, D.A.; Gao, Y.; Kow, A.W.C. Uses of a dedicated 3D reconstruction software with augmented and mixed reality in planning and performing advanced liver surgery and living donor liver transplantation (with videos). Hepatobiliary Pancreat. Dis. Int. 2022, 21, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Andersen, D.; Popescu, V.; Cabrera, M.E.; Shanghavi, A.; Mullis, B.; Marley, S.; Gomez, G.; Wachs, J.P. An Augmented Reality-Based Approach for Surgical Telementoring in Austere Environments. Mil. Med. 2017, 182, 310–315. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Andrews, C.M.; Henry, A.B.; Soriano, I.M.; Southworth, M.K.; Silva, J.R. Registration Techniques for Clinical Applications of Three-Dimensional Augmented Reality Devices. IEEE J. Transl. Eng. Health Med. 2021, 9, 4900214. [Google Scholar] [CrossRef] [PubMed]
- Soares, I.; Sousa, R.B.; Petry, M.R.; Moreira, A.P. Accuracy and repeatability tests on HoloLens 2 and HTC vive. Multimodal Technol. Interact. 2021, 5, 47. [Google Scholar] [CrossRef]
- Beams, R.; Brown, E.; Cheng, W.C.; Joyner, J.S.; Kim, A.S.; Kontson, K.; Amiras, D.; Baeuerle, T.; Greenleaf, W.; Grossmann, R.J.; et al. Evaluation Challenges for the Application of Extended Reality Devices in Medicine. J. Digit. Imaging 2022, 35, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, P.; Guo, H.J. HoloLens 2 Technical Evaluation as Mixed Reality Guide. In Proceedings of the 16th International Conference on Virtual, Augmented and Mixed Reality (VAMR 2024), Held as Part of the 26th HCI International Conference (HCII 2024), Washington, DC, USA, 29 June–4 July 2024. [Google Scholar] [CrossRef]
- Cheng, D.; Wang, Q.; Liu, Y.; Chen, H.; Ni, D.; Wang, X.; Yao, C.; Hou, Q.; Hou, W.; Luo, G.; et al. Design and manufacture of AR head-mounted displays: A review and outlook. Light. Adv. Manuf. 2021, 2, 24. [Google Scholar] [CrossRef]
- Penczek, J.; Boynton, P.A.; Beams, R.; Sriram, R.D. Measurement challenges for medical image display devices. J. Digit. Imaging 2021, 34, 458–472. [Google Scholar] [CrossRef] [PubMed]
- Mou, X.; Peng, X.; Mou, T. 38-2: Invited Paper: Evaluating optical performance and image quality in augmented reality eyewear: Standardization, challenges, and measurement methods. Symp. Dig. Tech. Pap. 2024, 55, 324–326. [Google Scholar] [CrossRef]
- de Cunsel, S. Evaluation of augmented reality (AR) displays performance based on human visual perception. In Proceedings of the Digital Optics for Immersive Displays II, 1135008, Virtual Event, 30 March 2020; SPIE: Bellingham, WA, USA, 2020; Volume 11350. [Google Scholar] [CrossRef]
- Wang, J.; Mou, X. The choice of the entrance pupil location in optical measurement of eyewear displays: Theoretical and experimental analyses. J. Soc. Inf. Disp. 2022, 30, 547–555. [Google Scholar] [CrossRef]
- Barten, P.G.J. Physical model for Contrast Sensitivity of the human eye. In Proceedings of the Human Vision, Visual Processing, and Digital Display III, San Jose, CA, USA, 27 August 1992; SPIE: Bellingham, WA, USA, 1992; Volume 1666. [Google Scholar] [CrossRef]
- Zhao, C.; Beams, R.; Johnson, M.; Badano, A. 18-2: Assessment of image quality in augmented reality displays using a computational model of target detectability. Symp. Dig. Tech. Pap. 2022, 53, 194–197. [Google Scholar] [CrossRef]
- Erickson, A.; Kim, K.; Bruder, G.; Welch, G.F. Exploring the Limitations of Environment Lighting on Optical See-Through Head-Mounted Displays. In Proceedings of the 2020 ACM Symposium on Spatial User Interaction (SUI ‘20), Virtual Event, 31 October–1 November 2020. [Google Scholar] [CrossRef]
- Laudien, T.; Ernst, J.M.; Schmerwitz, S. Bringing a colored head-down display symbology heads up: Display fidelity review of a low-cost see-through HMD. In Proceedings of the Artificial Intelligence and Machine Learning for Multi-Domain Operations Applications V, 125380S, Orlando, FL, USA, 12 June 2023; SPIE: Bellingham, WA, USA, 2023; Volume 12538. [Google Scholar] [CrossRef]
- Johnson, M.; Zhao, C.; Varshney, A.; Beams, R. Digital Precompensation for Luminance Nonuniformities in Augmented Reality Head Mounted Displays. In Proceedings of the 2022 IEEE International Symposium on Mixed and Augmented Reality Adjunct (ISMAR-Adjunct), Singapore, 17–21 October 2022. [Google Scholar] [CrossRef]
- McKendrick, A.M.; Johnson, C.A. Temporal properties of vision. In Adler’s Physiology of the Eye—Clinical Application, 10th ed.; Kaufman, P.L., Alm, A., Eds.; Mosby: St. Louis, MO, USA, 2003; pp. 511–530. [Google Scholar]
- IEC/TR 62977-2-5:2018; Electronic Display Devices—Part 2–5: Transparent Displays—Measurements of Optical Characteristics. VDE VERLAG GmbH: Berlin, Germany, 2018.
- US Food and Drug Administration (FDA). 510(k) Premarket Notification: K200384—HipXpert System; FDA: Silver Spring, MD, USA, 2021. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf20/K200384.pdf (accessed on 2 June 2025).
- US Food and Drug Administration (FDA). 510(k) Premarket Notification: K213215—VSI HoloMedicine; FDA: Silver Spring, MD, USA, 2022. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf21/K213215.pdf (accessed on 2 June 2025).
- US Food and Drug Administration (FDA). 510(k) Premarket Notification K222510—Blueprint Mixed Reality System; FDA: Silver Spring, MD, USA, 2023. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf22/K222510.pdf (accessed on 2 June 2025).
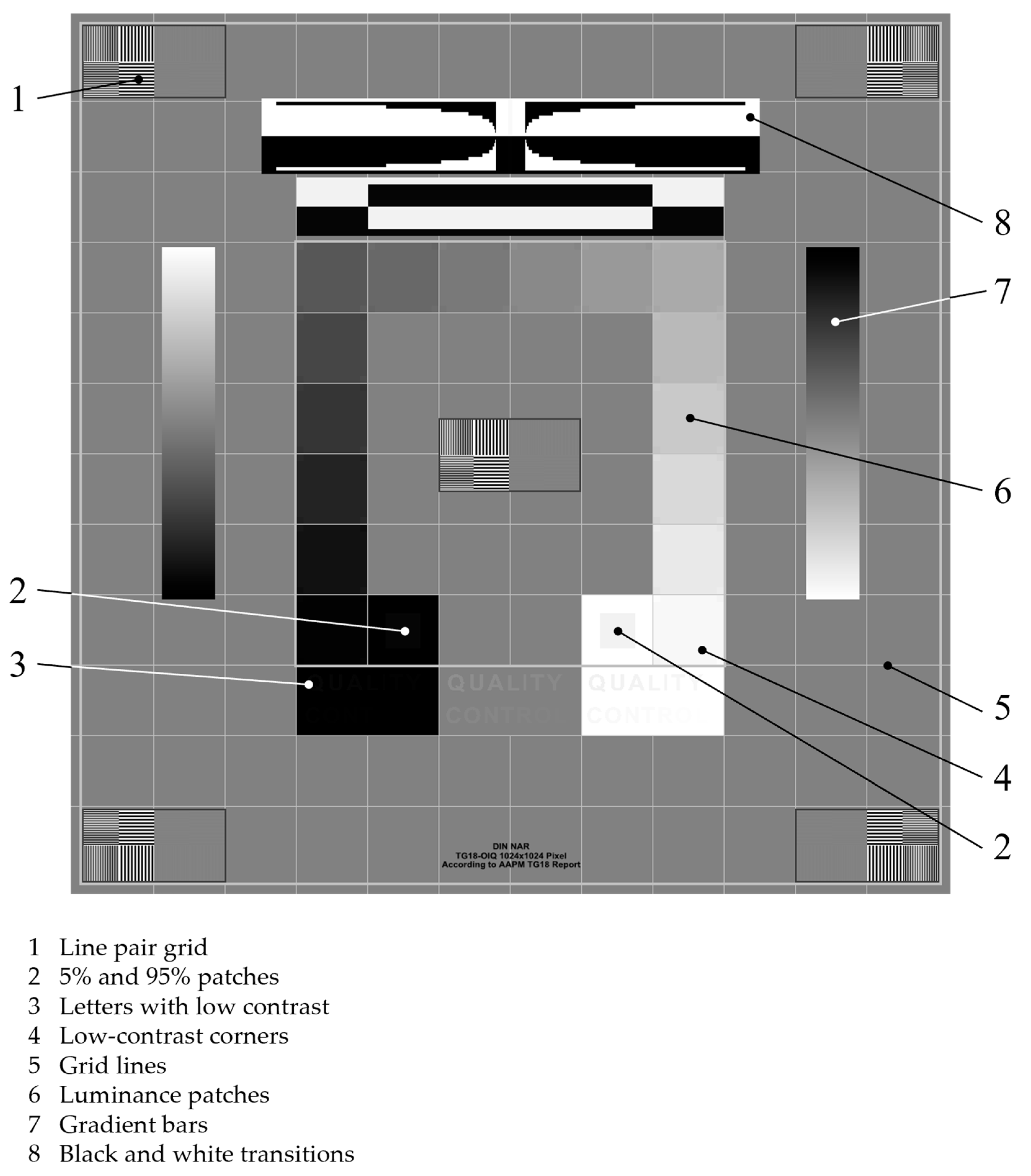
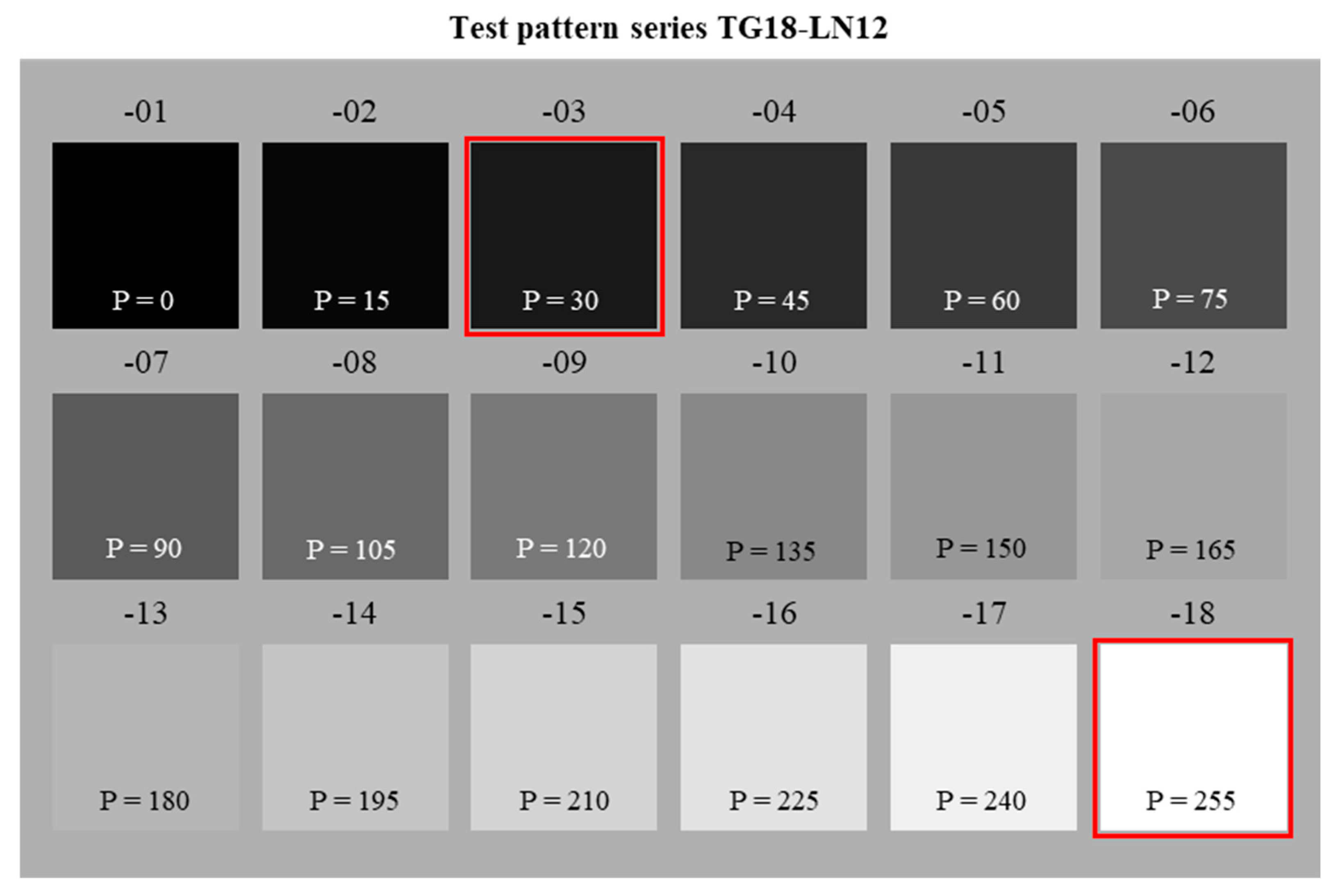
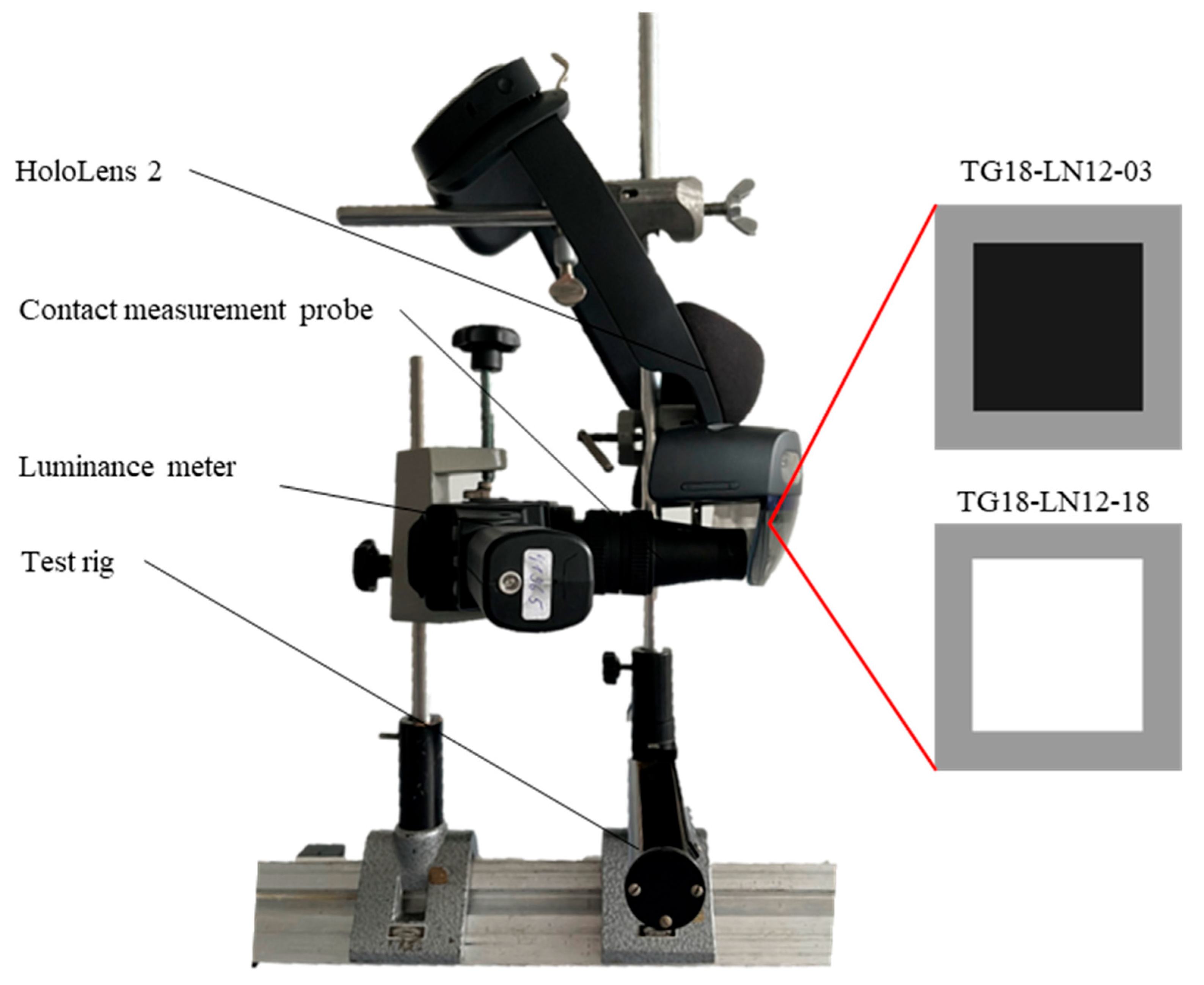
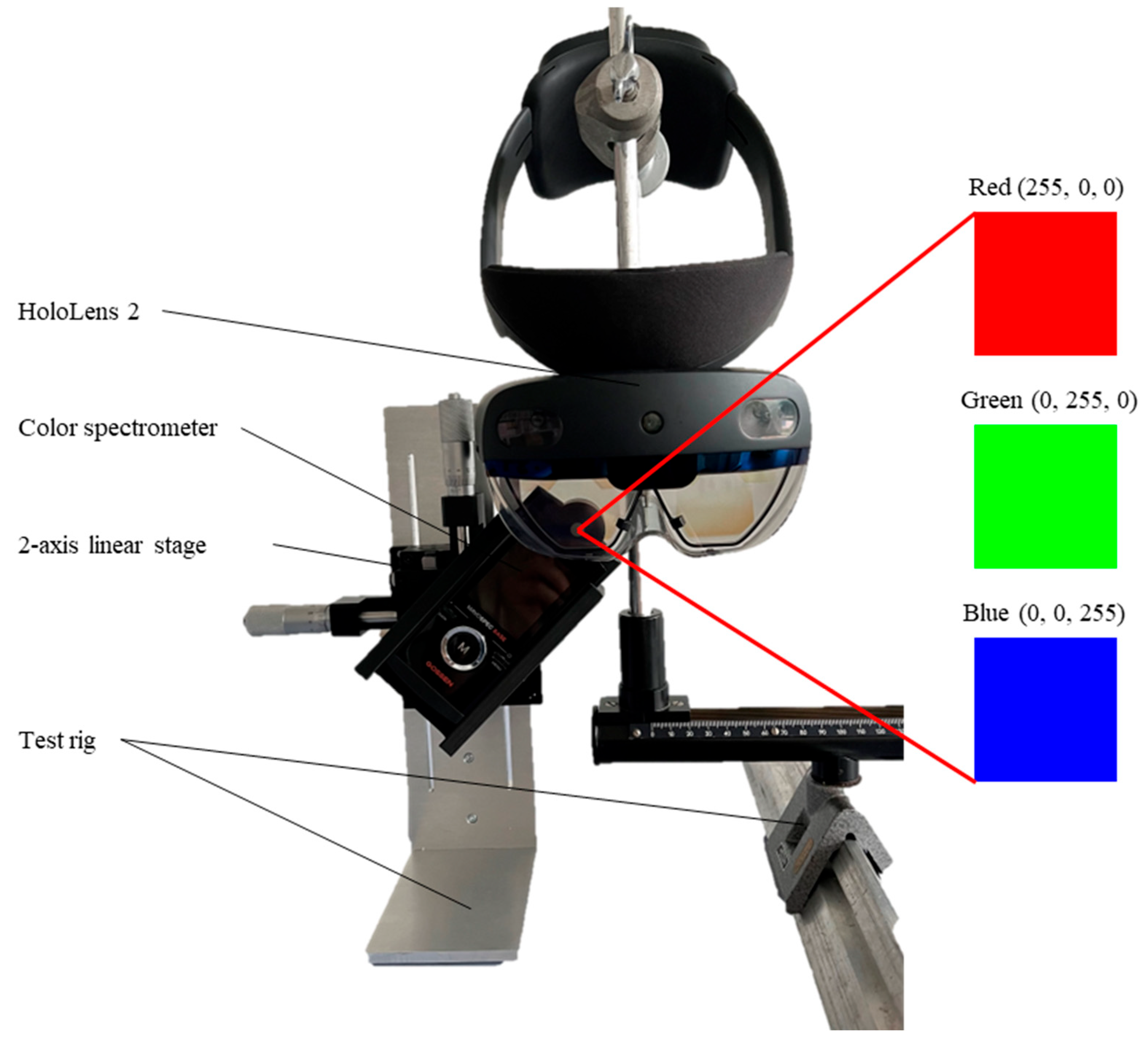
| Category | Material |
|---|---|
| Test device | HoloLens 2 |
| Display software | VSI HoloMedicine (apoQlar GmbH, Hamburg, Germany) |
| Test patterns (AAPM): | TG18-OIQ TG18-LN12-03 to TG18-LN12-18 |
| Other test patterns | Red (RGB: 255, 0, 0) Green (RGB: 0, 255, 0) Blue (RGB: 0, 0, 255) |
| Measuring devices | Luminance: MAVO SPOT 2 with contact measurement probe (Gossen Foto- und Lichtmesstechnik GmbH, Nürnberg, Germany) Illuminance: MAVOLUX (Gossen Foto- und Lichtmesstechnik GmbH, Nürnberg, Germany) Color coordinates: MAVOSPEC BASE (Gossen Foto- und Lichtmesstechnik GmbH, Nürnberg, Germany) |
| Image Element | Control Questions | Primary Display Devices | Secondary Display Devices |
|---|---|---|---|
| 1 |
| Yes | Yes |
| Yes | Yes | |
| 2 |
| Yes | No |
| 3 |
| Yes | Yes |
| Yes | No | |
| 4 |
| Yes | No |
| 5 |
| Yes | Yes |
| 6 |
| 16/16 | 16/16 |
| 7 |
| Yes | Yes |
| 8 |
| Yes | Yes |
| Image Element | Control Question | Diagnostic Room (2.4 lx) | Office (138.7 lx) | Rating | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | P5 | P1 | P2 | P3 | P4 | P5 | Primary | Secondary | ||
| 1 |
| Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | ✓ | ✓ |
| Yes | No | No | Yes | No | Yes | No | No | Yes | No | ✗ | ✗ | |
| 2 |
| No | No | No | No | No | No | No | No | No | No | ✗ | - |
| 3 |
| Yes | Yes | No | Yes | No | Yes | Yes | Yes | No | No | ✓ | ✓ |
| No | No | No | No | No | No | No | No | No | No | ✗ | - | |
| 4 |
| No | No | No | No | No | No | No | No | No | No | ✗ | - |
| 5 |
| Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | ✓ | ✓ |
| 6 |
| 15/16 | 15/16 | 15/16 | 14/16 | 14/16 | 15/16 | 15/16 | 14/16 | 13/16 | 14/16 | ✗ | ✗ |
| 7 |
| No | No | No | No | No | No | No | No | No | No | ✗ | ✗ |
| 8 |
| Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | ✓ | ✓ |
| Color | Position | Color Coordinates | Pairing | Distance | |
|---|---|---|---|---|---|
| u′ | v′ | Δu′v′ | |||
| Red | TL | 0.4434 | 0.5220 | TL/TR | 0.016 |
| TR | 0.4589 | 0.5195 | TL/CE | 0.037 | |
| CE | 0.4802 | 0.5188 | TL/BL | 0.064 | |
| BL | 0.5070 | 0.5184 | TL/BR | 0.033 | |
| BR | 0.4764 | 0.5200 | TR/CE | 0.021 | |
| TR/BL | 0.048 | ||||
| TR/BR | 0.018 | ||||
| CE/BL | 0.027 | ||||
| CE/BR | 0.004 | ||||
| BL/BR | 0.031 | ||||
| Green | TL | 0.1064 | 0.5568 | TL/TR | 0.011 |
| TR | 0.1169 | 0.5556 | TL/CE | 0.031 | |
| CE | 0.1374 | 0.5555 | TL/BL | 0.025 | |
| BL | 0.1312 | 0.5578 | TL/BR | 0.065 | |
| BR | 0.1713 | 0.5545 | TR/CE | 0.021 | |
| TR/BL | 0.014 | ||||
| TR/BR | 0.054 | ||||
| CE/BL | 0.007 | ||||
| CE/BR | 0.034 | ||||
| BL/BR | 0.040 | ||||
| Blue | TL | 0.1789 | 0.1314 | TL/TR | 0.005 |
| TR | 0.1806 | 0.1270 | TL/CE | 0.017 | |
| CE | 0.1749 | 0.1475 | TL/BL | 0.032 | |
| BL | 0.1703 | 0.1625 | TL/BR | 0.022 | |
| BR | 0.1726 | 0.1525 | TR/CE | 0.021 | |
| TR/BL | 0.037 | ||||
| TR/BR | 0.027 | ||||
| CE/BL | 0.016 | ||||
| CE/BR | 0.006 | ||||
| BL/BR | 0.010 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
König, S.; Siebers, S.; Backhaus, C. Image Quality Assessment of Augmented Reality Glasses as Medical Display Devices (HoloLens 2). Appl. Sci. 2025, 15, 7648. https://doi.org/10.3390/app15147648
König S, Siebers S, Backhaus C. Image Quality Assessment of Augmented Reality Glasses as Medical Display Devices (HoloLens 2). Applied Sciences. 2025; 15(14):7648. https://doi.org/10.3390/app15147648
Chicago/Turabian StyleKönig, Simon, Simon Siebers, and Claus Backhaus. 2025. "Image Quality Assessment of Augmented Reality Glasses as Medical Display Devices (HoloLens 2)" Applied Sciences 15, no. 14: 7648. https://doi.org/10.3390/app15147648
APA StyleKönig, S., Siebers, S., & Backhaus, C. (2025). Image Quality Assessment of Augmented Reality Glasses as Medical Display Devices (HoloLens 2). Applied Sciences, 15(14), 7648. https://doi.org/10.3390/app15147648





