A State-of-the-Art Review on the Potential of Waste Cooking Oil as a Sustainable Insulating Liquid for Green Transformers
Abstract
1. Introduction
2. Overview of Plant-Based Oils and Waste Cooking Oils
2.1. Plant-Based Oils
| Property | Natural Ester | Mineral Oil |
|---|---|---|
| Nature | Renewable and biodegradable | Non-renewable and petroleum-derived |
| Emission profile | Acceptable | Unacceptable |
| Cost | High | Prices may increase in the long term |
| Flash point (°C) | 310–343 | 154 |
| Fire point (°C) | 300–365 | 110–185 |
| Viscosity (cSt) at 40 °C | 37.6 | 13 |
| Density (kg/m3) at 20 °C | 0.87–0.62 | 0.83–0.89 |
| Breakdown voltage (kV) | 60 | 45 |
| Dissipation factor (%) 25 °C | 0.08 | 0.02 |
| Dielectric constant 25 °C | 3.3 | 2.4 |
| Pour point (°C) | −22 | −40 |
2.2. Waste Cooking Oils
3. Chemical Properties of Waste Cooking Oils and Purification Process
3.1. Purification of Waste Cooking Oil
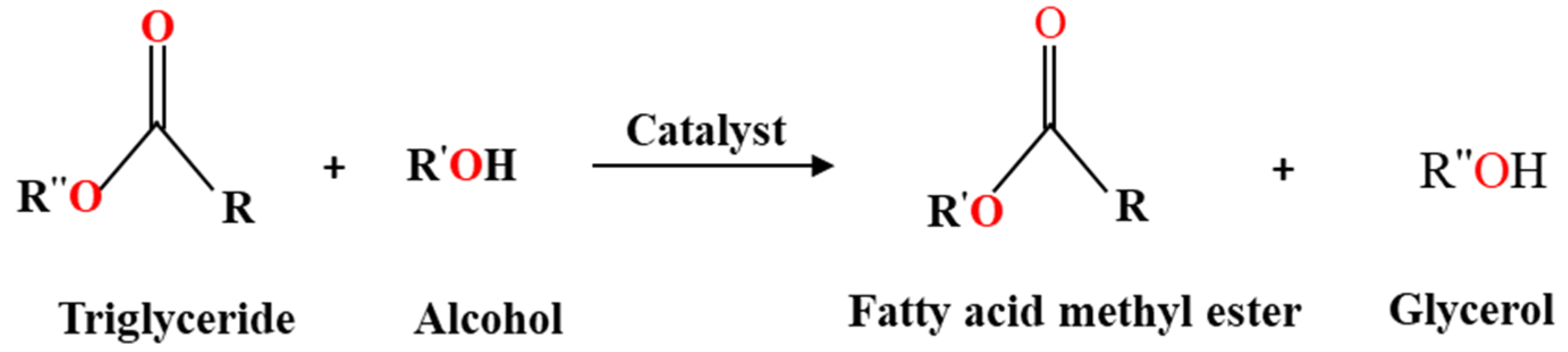
3.2. Transesterification of Waste Cooking Oil
| S/n | Transesterification Process | Reaction Temperature and Time | Percentage Yield (%) | Reference |
|---|---|---|---|---|
| Homogenous catalysis | ||||
| 1. | The reaction was performed using an alkali catalyst (NaOH, NaOCH3, and KOH) and an enzyme (free and immobilized lipase produced from Bacillus subtilus) with methanol | 50 °C/1.5 h for alkaline catalyst and 40 °C/72 h for enzyme | 1% KOH yields 97.01% for alkaline catalyst, while 4% w/w enzyme yielded a maximum of 91.61% | [98] |
| 2. | Potassium hydroxide was used as a catalyst, considering molar ratios of 8:1, 10:1, and 12:1 | 60 °C/1 h at 1000 rpm | The optimal percentage yield of 92.4% was obtained when 0.5 wt.% of KOH and 90 min reaction time were considered | [99] |
| Heterogenous catalysis | ||||
| 3 | 2.5 wt.% catalyst (NaOH/CaO, AlCl3, and NaOH) in methanol (oil-to-methanol ratio 6:1). Excess methanol removed via rotary evaporation | Stirred at 600 rpm for 2 h at 60–65 °C | i. CaO-supported catalyst (from carbide slurry waste): 92.2 ± 0.31 ii. AlCl3: 90.2 ± 0.57 iii. NaOH: 89.7 ± 0.16 | [92] |
| 4 | Transesterification reaction using CaO and considering the molar ratio of 6:1 | Stirred at a speed of 300–400 rpm and 65 °C for 3 h | - | [76] |
| 5 | Transesterification reaction using a heterogeneous catalyst, calcium diglyceroxide, with a methanol to WCO molar ratio of 7.46:1 | 62 °C in the presence of a microwave | Catalyst loading of 1.03% (w/w of WCO) yields biodiesel of 94.86% | [119] |
| 6 | 7% of CaO (w/w) for the transesterification of sunflower oil with a methanol-to-oil ratio of 6:1 | 65 °C and 60 min of reaction time, stirred at 500 rpm | Almost 100% yield was reported | [120] |
| 7 | 10% wt.% of TiO2-MgO with a 50:1 methanol-to-oil ratio | Stirred at 1500 rpm at 160 °C for 6 h | 92.3 | [121] |
| 8 | 6wt.% of SO22−/SnO2/SiO2 | Stirred at 350 rpm at 150 °C for 1 h | 81.4 | [122] |
| Enzymatic catalysis | ||||
| 9 | Transesterification of waste baked duck oil with methanol using Novozym 435 in tert-butyl medium as a catalyst. | 45 °C | 85.4 | [112] |
| 10 | 50 wt.% of Novozym 435 in waste fish oil with an ethanol-to-oil ratio of 35.45:1 | 35 °C for 8 h | 82.91 | [123] |
| 11 | 40 wt.% of Novozym 435® in WCO with a 6:1 methanol-to-oil ratio. | 50 °C for 14 h | 72.0 | [124] |
| 12 | 7.5 wt.% of lipase from porcine pancreas in WCO at a methanol-to-oil ratio of 9:1 | 40 °C for 10 h | 92.33 | [125] |
| Supercritical reaction | ||||
| 13 | Canola oil at a 40:1 ethanol-to-oil ratio | 350 °C for 30 min. and 200 bar | 93.7 | [126] |
| 14 | WCO at 41:1 methanol-to-oil ratio | 287 °C for 30 min | 99.6 | [127] |
| 15 | Rapeseed oil at a 42:1 methanol-to-oil ratio | 350 °C for 15 min and 120 bar | 93.0 | [128] |
| Properties | Homogeneous Base Catalysis (e.g., NaOH, KOH) | Heterogeneous Base Catalysis (e.g., CaO, MgO, SrO, TiO2) | Enzyme Catalysis (e.g., Lipase, Novozym 435) | Supercritical Method (e.g., Supercritical Methanol) |
|---|---|---|---|---|
| Catalyst Solubility | Soluble | Insoluble | Immobilized or soluble (enzyme) | No catalyst required |
| Reaction rate | Very fast | Moderate–fast | Slow–moderate | Very fast (under high temp/pressure) |
| Operating Conditions | Mild temperature (60–70 °C), low pressure | Higher temperature (>80 °C) | Mild temperature (25–45 °C), atmospheric pressure | High temp (200–350 °C), high pressure (100–200 bar) |
| FFA Tolerance | Poor tolerance (<2% FFA) | Poor to moderate (<2% FFA ideal) | Excellent tolerance (high FFA tolerated) | Excellent (not affected by FFA or water) |
| Soap Formation | High if FFA >2% | Possible at high FFA | None | None |
| Product Purity | Affected by soap, impurities, and moisture | Higher purity | Very high purity | Very high purity |
| Catalyst Recovery | Not reusable, requires extensive washing | Easily separable and reusable | Reusable after immobilization | Not applicable |
| Environmental Impact | Generates soap, wastewater | Low (less effluent generation) | Eco-friendly and clean process | Eco-friendly, no catalyst waste |
| Corrosion Risk | High (residual alkali, moisture) | Negligible | None | None |
| Cost Implication | Low catalyst cost, high purification cost | Higher catalyst cost, lower operational cost | High enzyme cost, requires immobilization | High energy and equipment cost |
| Reusability | Not reusable | Reusable for several cycles | Requires immobilization for reusability | Not applicable |
| Separation of Products | Difficult (due to soap, water) | Easy | Very easy (no side products) | Easy due to one-step conversion |
| Industrial Viability | Widely used, but purification is a challenge | Promising, but the temperature requirements are high | Limited by cost, still under development | Technically efficient but expensive |
| Advancements | Limited | Metal-doped heterogeneous catalysts (e.g., TiO2–MgO, SO42−/ZrO2/SiO2) | Immobilized enzymes, membrane reactor, ultrasound/microwave-assisted | Use of ultrasound, reactive distillation, and membrane reactors |
| Possible Variable | Sub-Categories/Conditions | Remarks/Effects | Reference |
|---|---|---|---|
| Temperature | Ambient (20 to 25 °C) | Temperature range affects reaction rate | |
| Moderate (60–100 °C) | [130,131,132] | ||
| High (>100 but <200) | |||
| Pressure | Ambient (101.3 kPa) | Higher pressure may improve reaction yield | [20] |
| High (10 MPa) | |||
| Supercritical (>100 MPa) | |||
| Mixing rate | Low, Medium, High, Static, Ultrasonic | Affects mass transfer and uniformity | [118] |
| Reaction time | Total, Continuous, and Batch | Influences the extent of conversion | [133,134] |
| Molar ratio | Alcohol-to-Glycerol, Alcohol-to-Oil, Alcohol-to-Reagent | Affects the ester yield and cost | [118,135] |
| Catalyst type | Acid, Alkali, and Enzyme | Different catalysts influence conversion | [136] |
4. Properties of Treated Liquids from Waste Cooking Oil as an Alternative Insulating Liquid
4.1. Influence of WCO Treatment on Thermal Properties
4.1.1. Cooling Efficiency
4.1.2. Fire Safety

| References | Flash Point of Waste WCO (°C) | Flash Point After Modification (°C) | Catalyst | Enhancement (%) |
|---|---|---|---|---|
| [82] | 290 | 220 | NaoH | 24.14 |
| [76] | 230 | 140 | CaO | 39.13 |
| [46] | 269 | 184 | KOH | 31.59 |
| [140] | 265 | 194 | CaO | 26.79 |
| [100] | 225 | 210 | Coal ash | 6.67 |
| [141] | 129.6 | 235.7 | - | 81.87 increase |
| [142] | 308.03 | 125 | KOH | 59.42 |
4.1.3. Cold Region Application of WCO-FAME
4.1.4. Acidity of Treated WCO
4.1.5. Breakdown Voltage (BDV) of WCO
4.1.6. Oxidation Stability of WCO
5. Compatibility of WCO with Solid Insulators (Papers)
6. Sustainability and Economic Viability of Waste Cooking Oil Valorization
Challenges Associated with Waste Cooking Oil Valorization and Future Direction
- i.
- Supply chain constraints.
- ii.
- Variability in oil properties.
- iii.
- Cost of Collection, Transportation, and Processing
- iv.
- Carbon Emissions During the Conversion Process
- v.
- Research and Development Needs
- vi.
- Future directions
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Portfolio, E. Transmission reliability and performance: 37.002, transformer life extension. DEC Accessed 2007, 12, 2020. [Google Scholar]
- Setayeshmehr, A.; Fofana, I.; Eichler, C.; Akbari, A.; Borsi, H.; Gockenbach, E. Dielectric spectroscopic measurements on transformer oil-paper insulation under controlled laboratory conditions. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 1100–1111. [Google Scholar] [CrossRef]
- Fofana, I. 50 years in the development of insulating liquids. IEEE Electr. Insul. Mag. 2013, 29, 13–25. [Google Scholar] [CrossRef]
- Lukic, J.; Deville, K.; Lessard, M.; Dreier, L.; Hohlein, I.; Vrsaljko, D.; Peixoto, A.; Melzer, L.; Lewand, L.; Ding, H. Changes of new unused insulating kraft paper properties during drying-Impact on degree of polymerization. Electra 2020, 312, 76–81. [Google Scholar]
- Tang, C.; Chen, R.; Zhang, J.; Peng, X.; Chen, B.; Zhang, L. A review on the research progress and future development of nano-modified cellulose insulation paper. IET Nanodielectr. 2022, 5, 63–84. [Google Scholar] [CrossRef]
- Suhaimi, N.S.; Ishak, M.T.; Rahman, A.R.; Muhammad, F.; Abidin, M.Z.; Khairi, A.K. A review on palm oil-based nanofluids as a future resource for green transformer insulation system. IEEE Access 2022, 10, 103563–103586. [Google Scholar] [CrossRef]
- Hao, J.; Zhang, J.; Ye, W.; Liao, R.; Yang, L. Development of Mixed Insulation Oil as Alternative Liquid Dielectric: A Review. CSEE J. Power Energy Syst. 2024, 10, 1242–1258. [Google Scholar]
- Oparanti, S.O.; Rao, U.M.; Fofana, I. Natural Esters for Green Transformers: Challenges and Keys for Improved Serviceability. Energies 2023, 16, 61. [Google Scholar] [CrossRef]
- Oparanti, S.O.; Fofana, I.; Jafari, R.; Zarrougui, R.; Abdelmalik, A. Canola oil: A renewable and sustainable green dielectric liquid for transformer insulation. Ind. Crops Prod. 2024, 215, 118674. [Google Scholar] [CrossRef]
- Karthik, M.; Narmadhai, N. Transformer insulation-based vegetable seed oil for power system analysis. Biomass Convers. Biorefin. 2024, 14, 21565–21578. [Google Scholar] [CrossRef]
- Oparanti, S.O.; Salaudeen, I.K.; Adekunle, A.A.; Oteikwu, V.E.; Galadima, A.I.; Abdelmalik, A.A. Physicochemical and Dielectric Study on Nigerian Thevetia Peruviana as a Potential Green Alternative Fluid for Transformer Cooling/Insulation. Waste Biomass Valorization 2022, 14, 1693–1703. [Google Scholar] [CrossRef]
- Oparanti, S.O.; Adekunle, A.A.; Oteikwu, V.E.; Galadima, A.I.; Abdelmalik, A.A. An experimental investigation on composite methyl ester as a solution to environmental threat caused by mineral oil in transformer insulation. Biomass Convers. Biorefin. 2022, 14, 12933–12943. [Google Scholar] [CrossRef]
- Pagger, E.P.; Pattanadech, N.; Uhlig, F.; Muhr, M. Biological Insulating Liquids; Springer: Berlin/Heidelberg, Germany, 2023. [Google Scholar]
- Farade, R.A.; Wahab, N.I.A.; Mansour, D.-E.A. The Effect of Nano-Additives in Natural Ester Dielectric Liquids: A Comprehensive Review on Dielectric Properties. IEEE Trans. Dielectr. Electr. Insul. 2023, 30, 1502–1516. [Google Scholar] [CrossRef]
- Masra, S.; Arief, Y.; Sahari, S.; Muhammad, M.; Rigit, A.; Rahman, M. A systematic review on promising development of palm oil and its nanofluid as a biodegradable oil insulation alternative. IEEE Trans. Dielectr. Electr. Insul. 2022, 29, 302–318. [Google Scholar] [CrossRef]
- Das, A.K.; Shill, D.C.; Chatterjee, S. Exploration of electrochemical and thermal attributes to evaluate the performance of novel natural esters. IEEE Trans. Dielectr. Electr. Insul. 2023, 31, 322–329. [Google Scholar] [CrossRef]
- Cilliyuz, Y.; Bicen, Y.; Aras, F.; Aydugan, G. Measurements and performance evaluations of natural ester and mineral oil-immersed identical transformers. Int. J. Electr. Power Energy Syst. 2021, 125, 106517. [Google Scholar] [CrossRef]
- Ye, W.; Hao, J.; Zhang, J.; Li, H.; Zhang, J.; Liao, R. Lightning impulse breakdown behavior of natural ester and modified natural ester under long oil gap: An experiment and first-principles simulation analysis. Ind. Crops Prod. 2024, 210, 118087. [Google Scholar] [CrossRef]
- Abdelmalik, A. Chemically modified palm kernel oil ester: A possible sustainable alternative insulating fluid. Sustain. Mater. Technol. 2014, 1, 42–51. [Google Scholar] [CrossRef]
- Raymon, A. Transesterification Approaches to Natural Esters for Transformer Insulating Fluids–a review. IEEE Trans. Dielectr. Electr. Insul. 2023, 31, 607–614. [Google Scholar] [CrossRef]
- Oparanti, S.O.; Fofana, I.; Zarrougui, R.; Jafari, R.; Yapi, K.M.L. Improving some physicochemical characteristics of environmentally friendly insulating liquids for enhanced sustainability in subpolar transformer applications. Sustain. Mater. Technol. 2024, 41, e00996. [Google Scholar] [CrossRef]
- Yang, T.; Wang, F.; Yao, D.; Li, J.; Zheng, H.; Yao, W.; Lv, Z.; Huang, Z. Low-temperature property improvement on green and low-carbon natural ester insulating oil. IEEE Trans. Dielectr. Electr. Insul. 2022, 29, 1459–1464. [Google Scholar] [CrossRef]
- Oparanti, S.O.; Fofana, I.; Jafari, R.; Zarrougui, R. Optimizing the Impact of Pour Point Depressants on Natural Ester Properties Using Taguchi-Grey Relational Analysis. In Proceedings of the 2024 IEEE Electrical Insulation Conference (EIC), Minneapolis, MN, USA, 2–5 June 2024; pp. 247–250. [Google Scholar]
- Raymon, A.; Pakianathan, P.S.; Rajamani, M.; Karthik, R. Enhancing the critical characteristics of natural esters with antioxidants for power transformer applications. IEEE Trans. Dielectr. Electr. Insul. 2013, 20, 899–912. [Google Scholar] [CrossRef]
- Deepa, S.; Srinivasan, A.; Anusha, M.; Veeramanju, K.; Chaitra, M. Review on the Addition of Antioxidants and Nanoparticles to Natural Ester as an Alternative to Transformer Oil. In Proceedings of the International Conference on Advances in Renewable Energy and Electric Vehicles, Nitte, India, 22–23 December 2022; pp. 217–258. [Google Scholar]
- Karthik, M.; Nuvvula, R.S.; Dhanamjayulu, C.; Khan, B. Appropriate analysis on properties of various compositions on fluids with and without additives for liquid insulation in power system transformer applications. Sci. Rep. 2024, 14, 17814. [Google Scholar] [CrossRef]
- Srinivasa, D.M.; Surendra, U. Investigation of electrical properties of developed indigenous natural ester liquid used as alternate to transformer insulation. Indones. J. Electr. Eng. Comput. Sci. 2023, 29, 609–617. [Google Scholar]
- Wardoyo; Widodo, A.S.; Wijayanti, W.; Wardana, I. The Role of Areca catechu Extract on Decreasing Viscosity of Vegetable Oils. Sci. World J. 2021, 2021, 8827427. [Google Scholar] [CrossRef]
- Idrees, S.A.; Mustafa, L.L.; Saleem, S.S. Improvement viscosity index of lubricating engine oil using low molecular weight compounds. Sci. J. Univ. Zakho 2019, 7, 14–17. [Google Scholar] [CrossRef]
- Oparanti, S.; Abdelmalik, A. Natural Ester Blended with Dielectric Nanoparticles: A Promising Solution to Sustainable Development Threat. In Proceedings of the 2022 IEEE Conference on Electrical Insulation and Dielectric Phenomena (CEIDP), Denver, CO, USA, 30 October 2022–2 November 2022; pp. 277–280. [Google Scholar]
- Oparanti, S.; Abdelmalik, A.; Khaleed, A.; Abifarin, J.; Suleiman, M.; Oteikwu, V. Synthesis and characterization of cooling biodegradable nanofluids from non-edible oil for high voltage application. Mater. Chem. Phys. 2022, 277, 125485. [Google Scholar] [CrossRef]
- Oparanti, S.; Khaleed, A.; Abdelmalik, A. Nanofluid from palm kernel oil for high voltage insulation. Mater. Chem. Phys. 2021, 259, 123961. [Google Scholar] [CrossRef]
- Oparanti, S.; Khaleed, A.; Abdelmalik, A.; Chalashkanov, N. Dielectric characterization of palm kernel oil ester-based insulating nanofluid. In Proceedings of the 2020 IEEE Conference on Electrical Insulation and Dielectric Phenomena (CEIDP), East Rutherford, NJ, USA, 18–30 October 2020; pp. 211–214. [Google Scholar]
- Oparanti, S.; Tambuwal, F.; Khaleed, A.; Abdelmalik, A. DC and AC Breakdown Analysis of Neem Ester/SiO2 Nanofluid for High Voltage Insulation. In Proceedings of the 2021 IEEE Conference on Electrical Insulation and Dielectric Phenomena (CEIDP), Vancouver, BC, Canada, 12–15 December 2021; pp. 383–386. [Google Scholar]
- Oparanti, S.O.; Fofana, I.; Jafari, R.; Zarrougui, R. A state-of-the-art review on green nanofluids for transformer insulation. J. Mol. Liq. 2024, 396, 124023. [Google Scholar] [CrossRef]
- Hussain, M.; Mir, F.A.; Ansari, M. Nanofluid transformer oil for cooling and insulating applications: A brief review. Appl. Surf. Sci. Adv. 2022, 8, 100223. [Google Scholar] [CrossRef]
- Kalakonda, S.P.; Ghassemi, M. Nanodielectric Fluids for Power Transformer Insulation and Cooling: A Review Identifying Challenges and Future Research Needs. IEEE Trans. Dielectr. Electr. Insul. 2024, 32, 349–366. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, D.; Soni, S.; Inda, C.S.; Sharma, S.; Sharma, P.K.; Jhalani, A. A comprehensive review of biodiesel production from waste cooking oil and its use as fuel in compression ignition engines: 3rd generation cleaner feedstock. J. Clean. Prod. 2021, 307, 127299. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, X.; Adamu, A.; Zhu, Z. Economic evaluation and production process simulation of biodiesel production from waste cooking oil. Curr. Res. Green Sustain. Chem. 2021, 4, 100091. [Google Scholar] [CrossRef]
- Fangfang, F.; Alagumalai, A.; Mahian, O. Sustainable biodiesel production from waste cooking oil: ANN modeling and environmental factor assessment. Sustain. Energy Technol. Assess. 2021, 46, 101265. [Google Scholar] [CrossRef]
- Parandi, E.; Safaripour, M.; Abdellattif, M.H.; Saidi, M.; Bozorgian, A.; Nodeh, H.R.; Rezania, S. Biodiesel production from waste cooking oil using a novel biocatalyst of lipase enzyme immobilized magnetic nanocomposite. Fuel 2022, 313, 123057. [Google Scholar] [CrossRef]
- Deraman, M.N.; Bakar, N.A.; Ab Aziz, N.H.; Chairul, I.S.; Ab Ghani, S. The experimental study on the potential of waste cooking oil as a new transformer insulating oil. J. Adv. Res. Fluid Mech. Therm. Sci. 2020, 69, 74–84. [Google Scholar] [CrossRef]
- Chairul, I.S.; Bakar, N.A.; Othman, M.N.; Ab Ghani, S.; Khiar, M.S.A.; Talib, M.A. Potential of used cooking oil as dielectric liquid for oil-immersed power transformers. IEEE Trans. Dielectr. Electr. Insul. 2021, 28, 1400–1407. [Google Scholar] [CrossRef]
- Mehta, D.M.; Kundu, P.; Chowdhury, A.; Lakhiani, V.; Jhala, A. A review on critical evaluation of natural ester vis-a-vis mineral oil insulating liquid for use in transformers: Part 1. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 873–880. [Google Scholar] [CrossRef]
- Raeisian, L.; Niazmand, H.; Ebrahimnia-Bajestan, E.; Werle, P. Feasibility study of waste vegetable oil as an alternative cooling medium in transformers. Appl. Therm. Eng. 2019, 151, 308–317. [Google Scholar] [CrossRef]
- Chairul, I.S.; Bakar, N.A.; Othman, M.N.; Ghani, S.; Deraman, M.N. Development of waste cooking oil methyl ester as potential electrical insulating fluid for power transformer. ARPN J. Eng. Appl. Sci. 2018, 13, 8154–8162. [Google Scholar]
- Junaid, P.M.; Dar, A.H.; Dash, K.K.; Ghosh, T.; Shams, R.; Khan, S.A.; Singh, A.; Pandey, V.K.; Nayik, G.A.; Bhagya Raj, G.V.S. Advances in seed oil extraction using ultrasound assisted technology: A comprehensive review. J. Food Process Eng. 2023, 46, e14192. [Google Scholar] [CrossRef]
- Kong, S.; Keang, T.; Bunthan, M.; Say, M.; Nat, Y.; Tan, C.P.; Tan, R. Hydraulic Cold-Pressed Extraction of Sacha Inchi Seeds: Oil Yield and Its Physicochemical Properties. ChemEngineering 2023, 7, 69. [Google Scholar] [CrossRef]
- Geow, C.H.; Tan, M.C.; Yeap, S.P.; Chin, N.L. A review on extraction techniques and its future applications in industry. Eur. J. Lipid Sci. Technol. 2021, 123, 2000302. [Google Scholar] [CrossRef]
- Thilakarathna, R.; Siow, L.F.; Tang, T.-K.; Chan, E.-S.; Lee, Y.-Y. Physicochemical and antioxidative properties of ultrasound-assisted extraction of mahua (Madhuca longifolia) seed oil in comparison with conventional Soxhlet and mechanical extractions. Ultrason. Sonochem. 2023, 92, 106280. [Google Scholar] [CrossRef]
- Shen, L.; Pang, S.; Zhong, M.; Sun, Y.; Qayum, A.; Liu, Y.; Rashid, A.; Xu, B.; Liang, Q.; Ma, H. A comprehensive review of ultrasonic assisted extraction (UAE) for bioactive components: Principles, advantages, equipment, and combined technologies. Ultrason. Sonochem. 2023, 101, 106646. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, W.; Zhao, S.; Yang, X.; Xu, W.; Guo, M.; Xu, E.; Ding, T.; Ye, X.; Liu, D. Ultrasound-assisted extraction of lipids as food components: Mechanism, solvent, feedstock, quality evaluation and coupled technologies–A review. Trends Food Sci. Technol. 2022, 122, 83–96. [Google Scholar] [CrossRef]
- Girotto, F.; Esposito, M.; Piazza, L. Ultrasound-assisted extraction of oil from hempseed (Cannabis sativa L.): Part 2. J. Sci. Food Agric. 2023, 103, 924–932. [Google Scholar] [CrossRef]
- Esposito, M.; Piazza, L. Ultrasound-assisted extraction of oil from hempseed (Cannabis sativa L.): Part 1. J. Sci. Food Agric. 2022, 102, 732–739. [Google Scholar] [CrossRef]
- Fan, L.; Fan, W.; Mei, Y.; Liu, L.; Li, L.; Wang, Z.; Yang, L. Mechanochemical assisted extraction as a green approach in preparation of bioactive components extraction from natural products-A review. Trends Food Sci. Technol. 2022, 129, 98–110. [Google Scholar] [CrossRef]
- Picot-Allain, C.; Mahomoodally, M.F.; Ak, G.; Zengin, G. Conventional versus green extraction techniques—A comparative perspective. Curr. Opin. Food Sci. 2021, 40, 144–156. [Google Scholar] [CrossRef]
- Buvaneshwaran, M.; Radhakrishnan, M.; Natarajan, V. Influence of ultrasound-assisted extraction techniques on the valorization of agro-based industrial organic waste–A review. J. Food Process Eng. 2023, 46, e14012. [Google Scholar] [CrossRef]
- Peng, X.; Wang, Q.; Kang, S.; Chen, C.; Li, G.; Wang, K.; Liao, R.; Zhao, X. Research progress in modified mineral oil, natural ester and mixed oil in transformers. Electr. Mater. Appl. 2024, 1, e12005. [Google Scholar] [CrossRef]
- Raof, N.A.; Yunus, R.; Rashid, U.; Azis, N.; Yaakub, Z. Effect of molecular structure on oxidative degradation of ester based transformer oil. Tribol. Int. 2019, 140, 105852. [Google Scholar] [CrossRef]
- Shinde, R. Global Footprint of Free Breathing Transformer with Natural Ester. In Proceedings of the 2019 IEEE 20th International Conference on Dielectric Liquids (ICDL), Roma, Italy, 23–27 June 2019; pp. 1–4. [Google Scholar]
- Martin, R.; Athanassatou, H.; Duart, J.C.; Perrier, C.; Sitar, I.; Walker, J.; Claiborne, C.; Boche, T.; Cherry, D.; Darwin, A.; et al. Experiences in Service with New Insulating Liquids; CIGRÉ Technical Brochure No. 436; International Council on Large Electric Systems (CIGRÉ): Paris, France, 2010; pp. 1–95. [Google Scholar]
- Rao, U.M.; Fofana, I.; Sarathi, R. Alternative Liquid Dielectrics for High Voltage Transformer Insulation Systems: Performance Analysis and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Rao, U.M.; Fofana, I.; Jaya, T.; Rodriguez-Celis, E.M.; Jalbert, J.; Picher, P. Alternative dielectric fluids for transformer insulation system: Progress, challenges, and future prospects. IEEE Access 2019, 7, 184552–184571. [Google Scholar] [CrossRef]
- Atanasova-Höhlein, I. IEC 60296 (Ed. 5)–A standard for classification of mineral insulating oil on performance and not on the origin. Transform. Mag. 2021, 8, 86–91. [Google Scholar]
- Jacob, J.; Preetha, P.; Thiruthi Krishnan, S. Review on natural ester and nanofluids as an environmental friendly alternative to transformer mineral oil. IET Nanodielectr. 2019, 3, 33–43. [Google Scholar] [CrossRef]
- Azahar, W.N.A.W.; Bujang, M.; Jaya, R.P.; Hainin, M.R.; Mohamed, A.; Ngad, N.; Jayanti, D.S. The potential of waste cooking oil as bio-asphalt for alternative binder—An overview. J. Teknol. Sci. Eng. 2016, 78, 111–116. [Google Scholar]
- Khedaywi, T.; Melhem, M. Effect of waste vegetable oil on properties of asphalt cement and asphalt concrete mixtures: An overview. Int. J. Pavement Res. Technol. 2024, 17, 280–290. [Google Scholar] [CrossRef]
- Ganesan, K.; Sukalingam, K.; Xu, B. Impact of consumption and cooking manners of vegetable oils on cardiovascular diseases-A critical review. Trends Food Sci. Technol. 2018, 71, 132–154. [Google Scholar] [CrossRef]
- Hosseinzadeh-Bandbafha, H.; Nizami, A.-S.; Kalogirou, S.A.; Gupta, V.K.; Park, Y.-K.; Fallahi, A.; Sulaiman, A.; Ranjbari, M.; Rahnama, H.; Aghbashlo, M.; et al. Environmental life cycle assessment of biodiesel production from waste cooking oil: A systematic review. Renew. Sustain. Energy Rev. 2022, 161, 112411. [Google Scholar] [CrossRef]
- Lombardi, L.; Mendecka, B.; Carnevale, E. Comparative life cycle assessment of alternative strategies for energy recovery from used cooking oil. J. Environ. Manag. 2018, 216, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Khodadadi, M.R.; Malpartida, I.; Tsang, C.-W.; Lin, C.S.K.; Len, C. Recent advances on the catalytic conversion of waste cooking oil. Mol. Catal. 2020, 494, 111128. [Google Scholar] [CrossRef]
- Ortner, M.E.; Müller, W.; Schneider, I.; Bockreis, A. Environmental assessment of three different utilization paths of waste cooking oil from households. Resour. Conserv. Recycl. 2016, 106, 59–67. [Google Scholar] [CrossRef]
- Singh-Ackbarali, D.; Maharaj, R.; Mohamed, N.; Ramjattan-Harry, V. Potential of used frying oil in paving material: Solution to environmental pollution problem. Environ. Sci. Pollut. Res. 2017, 24, 12220–12226. [Google Scholar] [CrossRef] [PubMed]
- Nanda, S.; Rana, R.; Hunter, H.N.; Fang, Z.; Dalai, A.K.; Kozinski, J.A. Hydrothermal catalytic processing of waste cooking oil for hydrogen-rich syngas production. Chem. Eng. Sci. 2019, 195, 935–945. [Google Scholar] [CrossRef]
- Hingu, S.M.; Gogate, P.R.; Rathod, V.K. Synthesis of biodiesel from waste cooking oil using sonochemical reactors. Ultrason. Sonochem. 2010, 17, 827–832. [Google Scholar] [CrossRef]
- Jayaraman, K.; Rajendran, V.; Jayakumar, S.; Attia, M.E. Characterization of Properties of Used Cooking Oil-Derived Biodiesel and Liquid Insulation towards Sustainable and Environmentally Friendly Reclaimed Products. Iran. J. Chem. Chem. Eng. IJCCE Res. Artic. Vol. 2024, 43, 3357–3377. [Google Scholar]
- Mirhashemi, F.S.; Sadrnia, H. NOX emissions of compression ignition engines fueled with various biodiesel blends: A review. J. Energy Inst. 2020, 93, 129–151. [Google Scholar] [CrossRef]
- Chen, H.; Xie, B.; Ma, J.; Chen, Y. NOx emission of biodiesel compared to diesel: Higher or lower? Appl. Therm. Eng. 2018, 137, 584–593. [Google Scholar] [CrossRef]
- Manikandan, G.; Kanna, P.R.; Taler, D.; Sobota, T. Review of waste cooking oil (WCO) as a Feedstock for Biofuel—Indian perspective. Energies 2023, 16, 1739. [Google Scholar] [CrossRef]
- Zhang, H.; Ozturk, U.A.; Wang, Q.; Zhao, Z. Biodiesel produced by waste cooking oil: Review of recycling modes in China, the US and Japan. Renew. Sustain. Energy Rev. 2014, 38, 677–685. [Google Scholar] [CrossRef]
- Goh, B.H.H.; Chong, C.T.; Ge, Y.; Ong, H.C.; Ng, J.-H.; Tian, B.; Ashokkumar, V.; Lim, S.; Seljak, T.; Józsa, V. Progress in utilisation of waste cooking oil for sustainable biodiesel and biojet fuel production. Energy Convers. Manag. 2020, 223, 113296. [Google Scholar] [CrossRef]
- Hemalatha, N.; Kamaraja, A.; Bhuvanesh, A.; Karthik Kumar, K. Exploring the insulating characteristics of transesterified used cooking oil and blends with corn oil infused with natural antioxidants. Biomass Convers. Biorefin. 2023, 15, 3385–3400. [Google Scholar] [CrossRef]
- Mehta, D.M.; Kundu, P.; Chowdhury, A.; Lakhiani, V.; Jhala, A. A review of critical evaluation of natural ester vis-a-vis mineral oil insulating liquid for use in transformers: Part II. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 1705–1712. [Google Scholar] [CrossRef]
- ASTM D1298-12b; Standard Test Method for Density, Relative Density, or API Gravity of Crude Petroleum and Liquid Petroleum Products by Hydrometer Method. ASTM International: West Conshohocken, PA, USA, 2012.
- IEEE Std C57.147-2008; IEEE Guide for Acceptance and Maintenance of Natural Ester Fluids in Transformers. IEEE: New York, NY, USA, 2008.
- ASTM D6871-17; Standard Specification for Natural (Vegetable Oil) Ester Fluids Used in Electrical Apparatus. ASTM International: West Conshohocken, PA, USA, 2017.
- IEC 62770:2013; Fluids for Electrotechnical Applications—Unused Natural Esters for Transformers and Similar Electrical Equipment. International Electrotechnical Commission (IEC): Geneva, Switzerland, 2013.
- ASTM D1816-12; Standard Test Method for Dielectric Breakdown Voltage of Insulating Liquids Using VDE Electrodes. ASTM International: West Conshohocken, PA, USA, 2012.
- Arenas, E.; Villafán-Cáceres, S.M.; Rodríguez-Mejía, Y.; García-Loyola, J.A.; Masera, O.; Sandoval, G. Biodiesel dry purification using unconventional bioadsorbents. Processes 2021, 9, 194. [Google Scholar] [CrossRef]
- Jamaluddin; Yuliet; Musnina, W.O.S.; Yuyun, Y.; Shaldan, S.; Malasugi, S.; Arta, P.A.; Putri, G.N.; Maryani, A.; Haq, M.F.; et al. The Effect of Adsorbents on the Quality of Refined Eel Fish (Anguilla marmorata [Q.] Gaimard) Oil as a Raw Material for Pharmaceutical Preparations. J. Penelit. Pendidik. IPA 2024, 10, 10771–10792. [Google Scholar] [CrossRef]
- Jayasree, T.; Fofana, I.; Celis, E.R.; Picher, P.; Brettschneider, S. Reclamation of Synthetic Ester Dielectric Liquids by Pressure and Gravity Percolation Methods. IEEE Trans. Dielectr. Electr. Insul. 2023, 15, 3385–3400. [Google Scholar] [CrossRef]
- Michael, A.T.; Ajibola, V.; Agbaji, E.; Yusuf, J. Methanolic synthesis of fatty acid methyl esters (FAME) from Waste Materials. Chem. Sci. Int. J 2019, 26, 1–14. [Google Scholar] [CrossRef]
- Thirumarimurugan, M.; Sivakumar, V.; Xavier, A.M.; Prabhakaran, D.; Kannadasan, T. Preparation of biodiesel from sunflower oil by transesterification. Int. J. Biosci. Biochem. Bioinform. 2012, 2, 441–444. [Google Scholar] [CrossRef]
- Cerón Ferrusca, M.; Romero, R.; Martínez, S.L.; Ramírez-Serrano, A.; Natividad, R. Biodiesel production from waste cooking oil: A perspective on catalytic processes. Processes 2023, 11, 1952. [Google Scholar] [CrossRef]
- Verma, P.; Sharma, M.; Dwivedi, G. Impact of alcohol on biodiesel production and properties. Renew. Sustain. Energy Rev. 2016, 56, 319–333. [Google Scholar] [CrossRef]
- Yang, Z.; Chang, G.; Xia, Y.; He, Q.; Zeng, H.; Xing, Y.; Gui, X. Utilization of waste cooking oil for highly efficient recovery of unburned carbon from coal fly ash. J. Clean. Prod. 2021, 282, 124547. [Google Scholar] [CrossRef]
- Susilowati, E.; Hasan, A.; Syarif, A. Free fatty acid reduction in a waste cooking oil as a raw material for biodiesel with activated coal ash adsorbent. In Proceedings of the 2nd Forum in Research, Science, and Technology, Palembang, Indonesia, 30–31 October 2018; p. 012035. [Google Scholar]
- Haq, I.u.; Akram, A.; Nawaz, A.; Abbas, S.Z.; Xu, Y.; Rafatullah, M. Comparative analysis of various waste cooking oils for esterification and transesterification processes to produce biodiesel. Green Chem. Lett. Rev. 2021, 14, 462–473. [Google Scholar] [CrossRef]
- Suherman, S.; Abdullah, I.; Sabri, M.; Silitonga, A.S. Evaluation of physicochemical properties composite biodiesel from waste cooking oil and Schleichera oleosa oil. Energies 2023, 16, 5771. [Google Scholar] [CrossRef]
- Durairaj, K.P.; Jaya Christa, S.T.; Seetharaman, S.; Ananthan, B. Assessing biorefinery-derived used cooking oil methyl esters for compatibility with paper insulations in transformers. Energy Sources Part A Recovery Util. Environ. Eff. 2025, 47, 4997–5026. [Google Scholar] [CrossRef]
- Abdelmalik, A.A. The Feasibility of Using a Vegetable Oil-Based Fluid as Electrical Insulating Oil. Ph.D. Thesis, University of Leicester, Leicester, UK, 2012. [Google Scholar]
- Dijkstra, A.J.; Van Opstal, M. The total degumming process. J. Am. Oil Chem. Soc. 1989, 66, 1002–1009. [Google Scholar] [CrossRef]
- Dijkstra, A.J. Oil refining. In Sunflower; Elsevier: Amsterdam, The Netherlands, 2015; pp. 227–258. [Google Scholar]
- Sathivel, S.; Prinyawiwatkul, W.; Negulescu, I.I.; King, J.M.; Basnayake, B. Effects of purification process on rheological properties of catfish oil. J. Am. Oil Chem. Soc. 2003, 80, 829–832. [Google Scholar] [CrossRef]
- Chairul, I.S.; Ab Ghani, S.; Zainuddin, H.; Rahim, N.H.; Talib, M.A.; Rahman, N.H.N. Exploration of the potential of reclaimed waste cooking oil for oil-immersed power transformers. TELKOMNIKA Telecommun. Comput. Electron. Control 2017, 15, 957–963. [Google Scholar] [CrossRef]
- Oparanti, S.O.; Khaleed, A.A.; Abdelmalik, A.A. AC breakdown analysis of synthesized nanofluids for oil-filled transformer insulation. Int. J. Adv. Manuf. Technol. 2021, 117, 1395–1403. [Google Scholar] [CrossRef]
- Li, Q.; Xu, J.; Du, W.; Li, Y.; Liu, D. Ethanol as the acyl acceptor for biodiesel production. Renew. Sustain. Energy Rev. 2013, 25, 742–748. [Google Scholar] [CrossRef]
- Dwivedi, G.; Sharma, M. Application of Box–Behnken design in optimization of biodiesel yield from Pongamia oil and its stability analysis. Fuel 2015, 145, 256–262. [Google Scholar] [CrossRef]
- Gaur, A.; Mishra, S.; Chowdhury, S.; Baredar, P.; Verma, P. A review on factor affecting biodiesel production from waste cooking oil: An Indian perspective. Mater. Today Proc. 2021, 46, 5594–5600. [Google Scholar] [CrossRef]
- Dwivedi, G.; Sharma, M. Prospects of biodiesel from Pongamia in India. Renew. Sustain. Energy Rev. 2014, 32, 114–122. [Google Scholar] [CrossRef]
- Iso, M.; Chen, B.; Eguchi, M.; Kudo, T.; Shrestha, S. Production of biodiesel fuel from triglycerides and alcohol using immobilized lipase. J. Mol. Catal. B Enzym. 2001, 16, 53–58. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, H.; Zhang, X.; Yan, Y.; Hameed, B. Effect of monohydric alcohols on enzymatic transesterification for biodiesel production. Chem. Eng. J. 2010, 157, 223–229. [Google Scholar] [CrossRef]
- Muppaneni, T.; Reddy, H.K.; Ponnusamy, S.; Patil, P.D.; Sun, Y.; Dailey, P.; Deng, S. Optimization of biodiesel production from palm oil under supercritical ethanol conditions using hexane as co-solvent: A response surface methodology approach. Fuel 2013, 107, 633–640. [Google Scholar] [CrossRef]
- Sitorus, H.B.; Setiabudy, R.; Bismo, S.; Beroual, A. Jatropha curcas methyl ester oil obtaining as vegetable insulating oil. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 2021–2028. [Google Scholar] [CrossRef]
- Nabi, M.N.; Hoque, S.N.; Akhter, M.S. Karanja (Pongamia Pinnata) biodiesel production in Bangladesh, characterization of karanja biodiesel and its effect on diesel emissions. Fuel Process. Technol. 2009, 90, 1080–1086. [Google Scholar] [CrossRef]
- Raj, R.A.; Murugesan, S.; Ramanujam, S.; Stonier, A.A. Empirical model application to analyze reliability and hazards in Pongamia oil using breakdown voltage characteristics. IEEE Trans. Dielectr. Electr. Insul. 2022, 29, 1948–1957. [Google Scholar] [CrossRef]
- Abbah, E.; Nwandikom, G.; Egwuonwu, C.; Nwakuba, N. Effect of reaction temperature on the yield of biodiesel from neem seed oil. Am. J. Energy Sci. 2016, 3, 16–20. [Google Scholar]
- Yin, X.; Ma, H.; You, Q.; Wang, Z.; Chang, J. Comparison of four different enhancing methods for preparing biodiesel through transesterification of sunflower oil. Appl. Energy 2012, 91, 320–325. [Google Scholar] [CrossRef]
- Gupta, A.R.; Rathod, V.K. Calcium diglyceroxide catalyzed biodiesel production from waste cooking oil in the presence of microwave: Optimization and kinetic studies. Renew. Energy 2018, 121, 757–767. [Google Scholar] [CrossRef]
- Calero, J.; Luna, D.; Sancho, E.D.; Luna, C.; Bautista, F.M.; Romero, A.A.; Posadillo, A.; Verdugo, C. Development of a new biodiesel that integrates glycerol, by using CaO as heterogeneous catalyst, in the partial methanolysis of sunflower oil. Fuel 2014, 122, 94–102. [Google Scholar] [CrossRef]
- Wen, Z.; Yu, X.; Tu, S.-T.; Yan, J.; Dahlquist, E. Biodiesel production from waste cooking oil catalyzed by TiO2–MgO mixed oxides. Bioresour. Technol. 2010, 101, 9570–9576. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T. Mixed methanol–ethanol technology to produce greener biodiesel from waste cooking oil: A breakthrough for SO42−/SnO2–SiO2 catalyst. Fuel Process. Technol. 2011, 92, 1639–1645. [Google Scholar] [CrossRef]
- Marín-Suárez, M.; Méndez-Mateos, D.; Guadix, A.; Guadix, E.M. Reuse of immobilized lipases in the transesterification of waste fish oil for the production of biodiesel. Renew. Energy 2019, 140, 1–8. [Google Scholar] [CrossRef]
- Taher, H.; Nashef, E.; Anvar, N.; Al-Zuhair, S. Enzymatic production of biodiesel from waste oil in ionic liquid medium. Biofuels 2019, 10, 463–472. [Google Scholar] [CrossRef]
- Khan, N.; Maseet, M.; Basir, S.F. Synthesis and characterization of biodiesel from waste cooking oil by lipase immobilized on genipin cross-linked chitosan beads: A green approach. Int. J. Green Energy 2020, 17, 84–93. [Google Scholar] [CrossRef]
- Farobie, O.; Sasanami, K.; Matsumura, Y. A novel spiral reactor for biodiesel production in supercritical ethanol. Appl. Energy 2015, 147, 20–29. [Google Scholar] [CrossRef]
- Demirbas, A. Biodiesel from waste cooking oil via base-catalytic and supercritical methanol transesterification. Energy Convers. Manag. 2009, 50, 923–927. [Google Scholar] [CrossRef]
- Kiss, F.E.; Micic, R.D.; Tomić, M.D.; Nikolić-Djorić, E.B.; Simikić, M.Đ. Supercritical transesterification: Impact of different types of alcohol on biodiesel yield and LCA results. J. Supercrit. Fluids 2014, 86, 23–32. [Google Scholar] [CrossRef]
- Raqeeb, M.A.; Bhargavi, R. Biodiesel production from waste cooking oil. J. Chem. Pharm. Res. 2015, 7, 670–681. [Google Scholar]
- Sánchez, M.; Bergamin, F.; Peña, E.; Martínez, M.; Aracil, J. A comparative study of the production of esters from Jatropha oil using different short-chain alcohols: Optimization and characterization. Fuel 2015, 143, 183–188. [Google Scholar] [CrossRef]
- Murugesan, S.; Raj, R.A.; Sarathi, R.; Amizhtan, S. Experimental investigation of electrical and rheological properties of modified Punga oil. IEEE Trans. Dielectr. Electr. Insul. 2023, 30, 1422–1431. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, F.; Wang, C.; Shu, Y.; Ouyang, L.; Wang, Q.; Huang, Z.; Li, J. Optimizing Molecular Structure for Trimethylolpropane Ester-Insulating Oil: Achieving High Fluidity and Stability. IEEE Trans. Dielectr. Electr. Insul. 2023, 30, 1432–1440. [Google Scholar] [CrossRef]
- Borugadda, V.B.; Goud, V.V. Epoxidation of castor oil fatty acid methyl esters (COFAME) as a lubricant base stock using heterogeneous ion-exchange resin (IR-120) as a catalyst. Energy Procedia 2014, 54, 75–84. [Google Scholar] [CrossRef]
- Borugadda, V.B.; Goud, V.V. Thermal, oxidative and low temperature properties of methyl esters prepared from oils of different fatty acids composition: A comparative study. Thermochim. Acta 2014, 577, 33–40. [Google Scholar] [CrossRef]
- Brunschwig, C.; Moussavou, W.; Blin, J. Use of bioethanol for biodiesel production. Prog. Energy Combust. Sci. 2012, 38, 283–301. [Google Scholar] [CrossRef]
- Sanli, H.; Canakci, M. Effects of different alcohol and catalyst usage on biodiesel production from different vegetable oils. Energy Fuels 2008, 22, 2713–2719. [Google Scholar] [CrossRef]
- Foo, W.H.; Koay, S.S.N.; Chia, S.R.; Chia, W.Y.; Tang, D.Y.Y.; Nomanbhay, S.; Chew, K.W. Recent advances in the conversion of waste cooking oil into value-added products: A review. Fuel 2022, 324, 124539. [Google Scholar] [CrossRef]
- Vajjha, R.S.; Das, D.K. A review and analysis on influence of temperature and concentration of nanofluids on thermophysical properties, heat transfer and pumping power. Int. J. Heat Mass Transf. 2012, 55, 4063–4078. [Google Scholar] [CrossRef]
- ASTM D445-18; Standard Test Method for Kinematic Viscosity of Transparent and Opaque Liquids (and Calculation of Dynamic Viscosity). ASTM International: West Conshohocken, PA, USA, 2018.
- Prasad, R.; Sreekanth, C.; Muniraj, R.; Jarin, T. Characterization and aging impact study on antioxidants with CaO catalyzed methyl ester from waste cooking oil as sustainable insulation as biorefinery approach. Biomass Convers. Biorefin. 2024, 15, 10043–10060. [Google Scholar] [CrossRef]
- Mandour, H.A.S.; Khalaf Allah, A.K.; Taha, G.M.; Nasrat, L.; Zahran, H. Transforming Sustainability: Blending Chemically Enhanced Soybean Oil with Used Transformer Oil for Eco-Friendly Recycling. Aswan Univ. J. Environ. Stud. 2023, 4, 471–483. [Google Scholar] [CrossRef]
- Sinha, P.; Madavi, A.S. Study on yield percentage of biodiesel from waste cooking oil using transesterification. Int. J. Appl. Eng. Res. 2021, 16, 154–160. [Google Scholar] [CrossRef]
- Grace, I. Transformer Explosion Kills 14 at Wedding. Daily Times, 1 November 2017. [Google Scholar]
- Khoirudin; Kristiawan, B.; Sukarman; Abdulah, A.; Santoso, B.; Wijayanta, A.T.; Aziz, M. Flash Point Improvement of Mineral Oil Utilizing Nanoparticles to Reduce Fire Risk in Power Transformers: A Review. Fire 2024, 7, 305. [Google Scholar] [CrossRef]
- Rafiq, M.; Shafique, M.; Azam, A.; Ateeq, M.; Khan, I.A.; Hussain, A. Sustainable, renewable and environmental-friendly insulation systems for high voltages applications. Molecules 2020, 25, 3901. [Google Scholar] [CrossRef]
- Ashraful, A.M.; Masjuki, H.H.; Kalam, M.A.; Fattah, I.R.; Imtenan, S.; Shahir, S.; Mobarak, H. Production and comparison of fuel properties, engine performance, and emission characteristics of biodiesel from various non-edible vegetable oils: A review. Energy Convers. Manag. 2014, 80, 202–228. [Google Scholar] [CrossRef]
- Alviso, D.; Saab, E.; Clevenot, P.; Romano, S.D. Flash point, kinematic viscosity and refractive index: Variations and correlations of biodiesel–diesel blends. J. Braz. Soc. Mech. Sci. Eng. 2020, 42, 347. [Google Scholar] [CrossRef]
- ASTM D3487-16e1; Standard Specification for Mineral Insulating Oil Used in Electrical Apparatus. ASTM International: West Conshohocken, PA, USA, 2009.
- ASTM D6871-03; Standard Specification for Natural (Vegetable Oil) Ester Fluids Used in Electrical Apparatus. Electrical Insulating Liquids and Gases-Electrical Protective Equipment. ASTM International: West Conshohocken, PA, USA, 2008.
- Lee, J.-G.; Choi, H.-H. Development of real-scale transformer fire test technology and evaluation of a solid aerosol-based fire suppression system. Fire Saf. J. 2025, 153, 104376. [Google Scholar] [CrossRef]
- Obebe, E.O.; Hadjadj, Y.; Oparanti, S.O.; Fofana, I. Enhancing the Performance of Natural Ester Insulating Liquids in Power Transformers: A Comprehensive Review on Antioxidant Additives for Improved Oxidation Stability. Energies 2025, 18, 1690. [Google Scholar] [CrossRef]
- Moore, S.P.; Wangard, W.; Rapp, K.J.; Woods, D.L.; Del Vecchio, R.M. Cold start of a 240-MVA generator step-up transformer filled with natural ester fluid. IEEE Trans. Power Deliv. 2014, 30, 256–263. [Google Scholar] [CrossRef]
- Su, B.; Wang, L.; Xue, Y.; Yan, J.; Dong, Z.; Lin, H.; Han, S. Effect of pour point depressants combined with dispersants on the cold flow properties of biodiesel-diesel blends. J. Am. Oil Chem. Soc. 2021, 98, 163–172. [Google Scholar] [CrossRef]
- Kuzmin, K.A.; Kosolapova, S.M.; Rudko, V.A. Investigating the mechanism of action of polymer pour point depressants on cold flow properties of biodiesel fuels. Colloids Surf. A Physicochem. Eng. Asp. 2024, 702, 134971. [Google Scholar] [CrossRef]
- Xue, Y.; Zhao, Z.; Xu, G.; Lian, X.; Yang, C.; Zhao, W.; Ma, P.; Lin, H.; Han, S. Effect of poly-alpha-olefin pour point depressant on cold flow properties of waste cooking oil biodiesel blends. Fuel 2016, 184, 110–117. [Google Scholar] [CrossRef]
- Ma, P.; Xue, Y.; Zhao, W.; Lan, G.; Hang, Z.; Liu, F.; Han, S. Study on the performance mechanism of methacrylate pour point depressant in soybean biodiesel blends. RSC Adv. 2015, 5, 90144–90149. [Google Scholar] [CrossRef]
- Xue, Y.; Yang, C.; Xu, G.; Zhao, Z.; Lian, X.; Sheng, H.; Lin, H. The influence of polymethyl acrylate as a pour point depressant for biodiesel. Energy Sources Part A Recovery Util. Environ. Eff. 2017, 39, 17–22. [Google Scholar] [CrossRef]
- Wan, F.; Du, L.; Chen, W.; Wang, P.; Wang, J.; Shi, H. A novel method to directly analyze dissolved acetic acid in transformer oil without extraction using Raman spectroscopy. Energies 2017, 10, 967. [Google Scholar] [CrossRef]
- Zhang, E.; Liu, J.; Zhang, C.; Zheng, P.; Nakanishi, Y.; Wu, T. State-of-art review on chemical indicators for monitoring the aging status of oil-immersed transformer paper insulation. Energies 2023, 16, 1396. [Google Scholar] [CrossRef]
- Kouassi, K.D.; Fofana, I.; Cissé, L.; Hadjadj, Y.; Yapi, K.M.L.; Diby, K.A. Impact of low molecular weight acids on oil impregnated paper insulation degradation. Energies 2018, 11, 1465. [Google Scholar] [CrossRef]
- Subramaniam, S.; Ahmad, H.; Bakar, N.A.; Haris, F.A.; Rahman, R.A. The Investigation Of The Synthesis Cooking Oil As A New Power Transformer Oil. In Proceedings of the 18th International Engineering Research Conference 2022 (Eureca 2022), Subang Jaya, Malaysia, 30 November 2022; p. 012020. [Google Scholar]
- Song, H.; Chen, Y.; Huang, Q.; Yang, L.; Wang, T. Physicochemical and Dielectric Properties of Natural Ester Retrofilled Mineral Oil Transformer Liquid. In Proceedings of the 2024 7th International Conference on Energy, Electrical and Power Engineering (CEEPE), Yangzhou, China, 26–28 April 2024; pp. 111–115. [Google Scholar]
- Liapis, I.D.; Kontargyri, V.T.; Christodoulou, C.A. Evaluation of oil aging for power transformers. In Proceedings of the 2024 IEEE International Conference on High Voltage Engineering and Applications (ICHVE), Berlin, Germany, 18–22 August 2024; pp. 1–4. [Google Scholar]
- Szcześniak, D.; Przybylek, P. Oxidation stability of natural ester modified by means of fullerene nanoparticles. Energies 2021, 14, 490. [Google Scholar] [CrossRef]
- Nguyen, T.T. Transfer and Characterisation of Graphene for Integration with Gaseous Electron Multipliers. Ph.D. Thesis, UCL University College London, London, UK, 2016. [Google Scholar]
- Borugadda, V.B.; Goud, V.V. Improved thermo-oxidative stability of structurally modified waste cooking oil methyl esters for bio-lubricant application. J. Clean. Prod. 2016, 112, 4515–4524. [Google Scholar] [CrossRef]
- Xu, Y.; Qian, S.; Liu, Q.; Wang, Z. Oxidation stability assessment of a vegetable transformer oil under thermal aging. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 683–692. [Google Scholar] [CrossRef]
- Uğuz, G.; Atabani, A.; Mohammed, M.; Shobana, S.; Uğuz, S.; Kumar, G.; Al-Muhtaseb, A.a.H. Fuel stability of biodiesel from waste cooking oil: A comparative evaluation with various antioxidants using FT-IR and DSC techniques. Biocatal. Agric. Biotechnol. 2019, 21, 101283. [Google Scholar] [CrossRef]
- Paul, A.K.; Borugadda, V.B.; Goud, V.V. In-situ epoxidation of waste cooking oil and its methyl esters for lubricant applications: Characterization and rheology. Lubricants 2021, 9, 27. [Google Scholar] [CrossRef]
- Borugadda, V.B.; Goud, V.V. Hydroxylation and hexanoylation of epoxidized waste cooking oil and epoxidized waste cooking oil methyl esters: Process optimization and physico-chemical characterization. Ind. Crops Prod. 2019, 133, 151–159. [Google Scholar] [CrossRef]
- Borugadda, V.B.; Goud, V.V. Long-term storage stability of epoxides derived from vegetable oils and their methyl esters. Energy Fuels 2018, 32, 3428–3435. [Google Scholar] [CrossRef]
- Kongyai, C.; Chalermsinsuwan, B.; Hunsom, M. Epoxidation of waste used-oil biodiesel: Effect of reaction factors and its impact on the oxidative stability. Korean J. Chem. Eng. 2013, 30, 327–336. [Google Scholar] [CrossRef]
- Bashiri, S.; Ghobadian, B.; Soufi, M.D.; Gorjian, S. Chemical modification of sunflower waste cooking oil for biolubricant production through epoxidation reaction. Mater. Sci. Energy Technol. 2021, 4, 119–127. [Google Scholar] [CrossRef]
- Zheng, T.; Wu, Z.; Xie, Q.; Fang, J.; Hu, Y.; Lu, M.; Xia, F.; Nie, Y.; Ji, J. Structural modification of waste cooking oil methyl esters as cleaner plasticizer to substitute toxic dioctyl phthalate. J. Clean. Prod. 2018, 186, 1021–1030. [Google Scholar] [CrossRef]
- Kaliappan, G.; Rengaraj, M. Aging assessment of transformer solid insulation: A review. Mater. Today Proc. 2021, 47, 272–277. [Google Scholar] [CrossRef]
- Oria, C.; Ferreño, D.; Carrascal, I.; Ortiz, A.; Fernández, I. Study on the mechanical failure of the cellulosic insulation of continuously transposed conductors in power transformers under the influence of short circuits and thermal ageing. Eng. Fail. Anal. 2021, 124, 105356. [Google Scholar] [CrossRef]
- Sanz, J.; Renedo, C.; Ortiz, A.; Quintanilla, P.; Ortiz, F.; García, D. A brief review of the impregnation process with dielectric fluids of cellulosic materials used in electric power transformers. Energies 2023, 16, 3673. [Google Scholar] [CrossRef]
- Awogbemi, O.; Kallon, D.V.V. Conversion of hazardous waste cooking oil into non-fuel value added products. Int. J. Ambient Energy 2024, 45, 2345253. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C.; Zhang, L.; Chang, Y.; Hao, Y. Converting waste cooking oil to biodiesel in China: Environmental impacts and economic feasibility. Renew. Sustain. Energy Rev. 2021, 140, 110661. [Google Scholar] [CrossRef]
- Peiró, L.T.; Lombardi, L.; Méndez, G.V.; i Durany, X.G. Life cycle assessment (LCA) and exergetic life cycle assessment (ELCA) of the production of biodiesel from used cooking oil (UCO). Energy 2010, 35, 889–893. [Google Scholar] [CrossRef]
- Hosseinzadeh-Bandbafha, H.; Rafiee, S.; Mohammadi, P.; Ghobadian, B.; Lam, S.S.; Tabatabaei, M.; Aghbashlo, M. Exergetic, economic, and environmental life cycle assessment analyses of a heavy-duty tractor diesel engine fueled with diesel–biodiesel-bioethanol blends. Energy Convers. Manag. 2021, 241, 114300. [Google Scholar] [CrossRef]
- Kralisch, D.; Staffel, C.; Ott, D.; Bensaid, S.; Saracco, G.; Bellantoni, P.; Loeb, P. Process design accompanying life cycle management and risk analysis as a decision support tool for sustainable biodiesel production. Green Chem. 2013, 15, 463–477. [Google Scholar] [CrossRef]
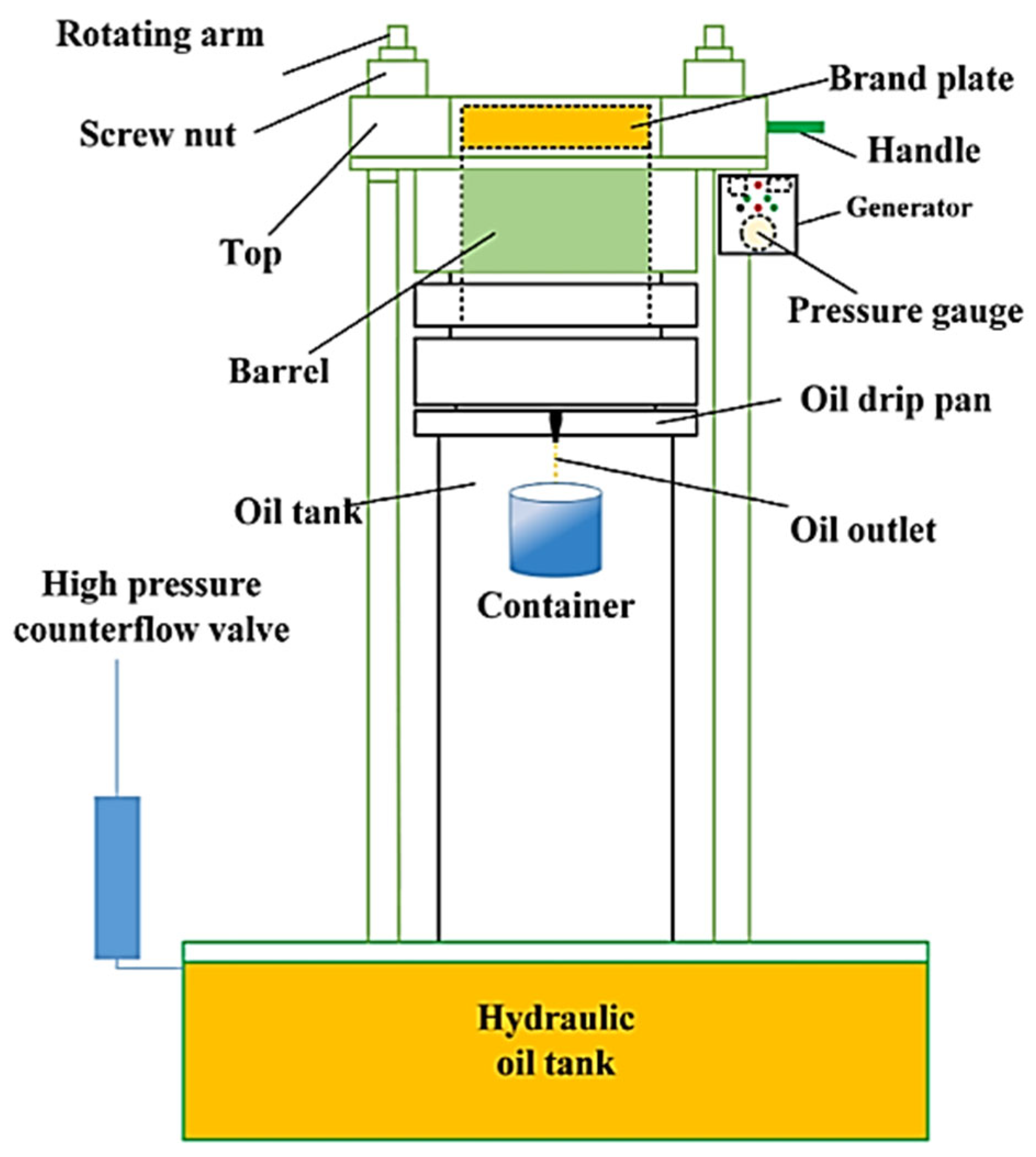


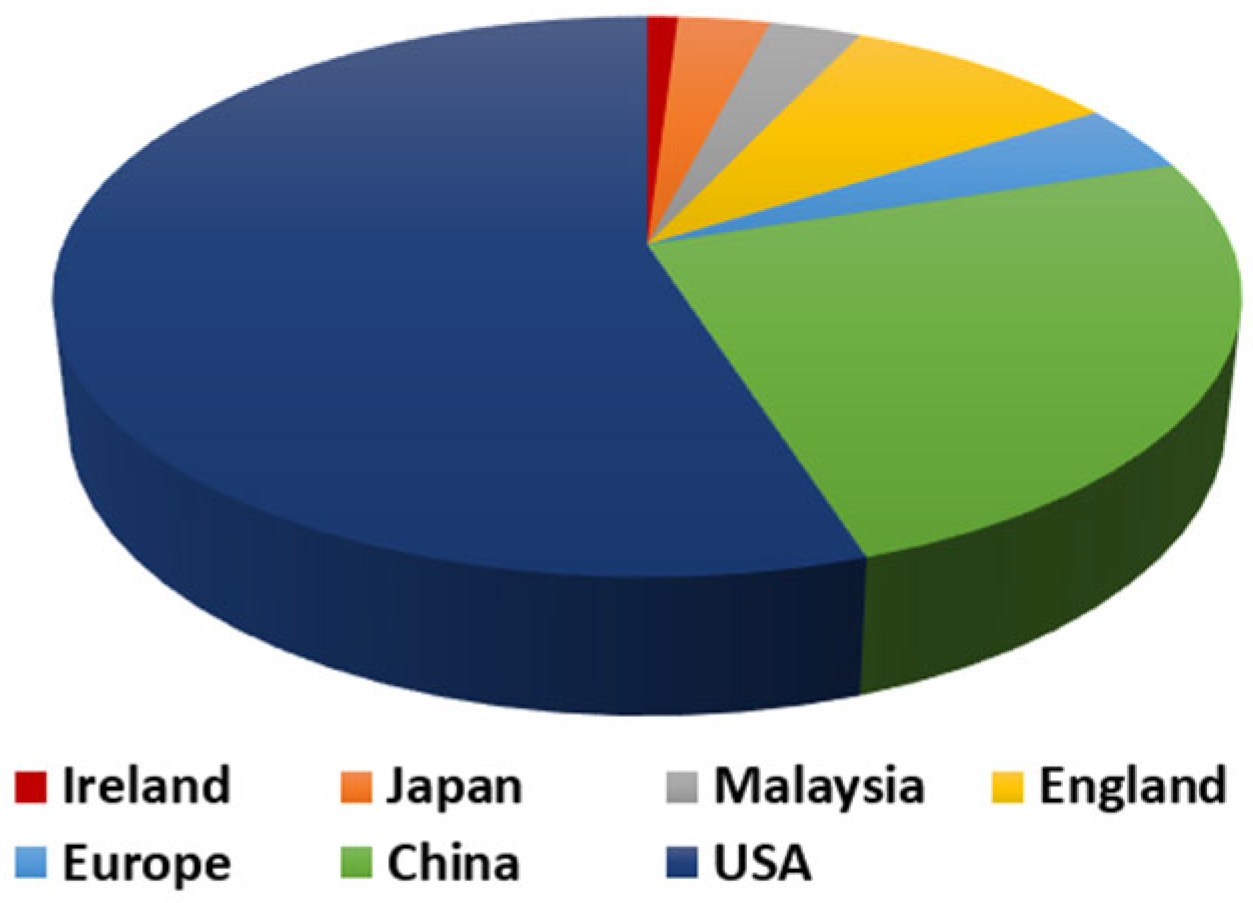

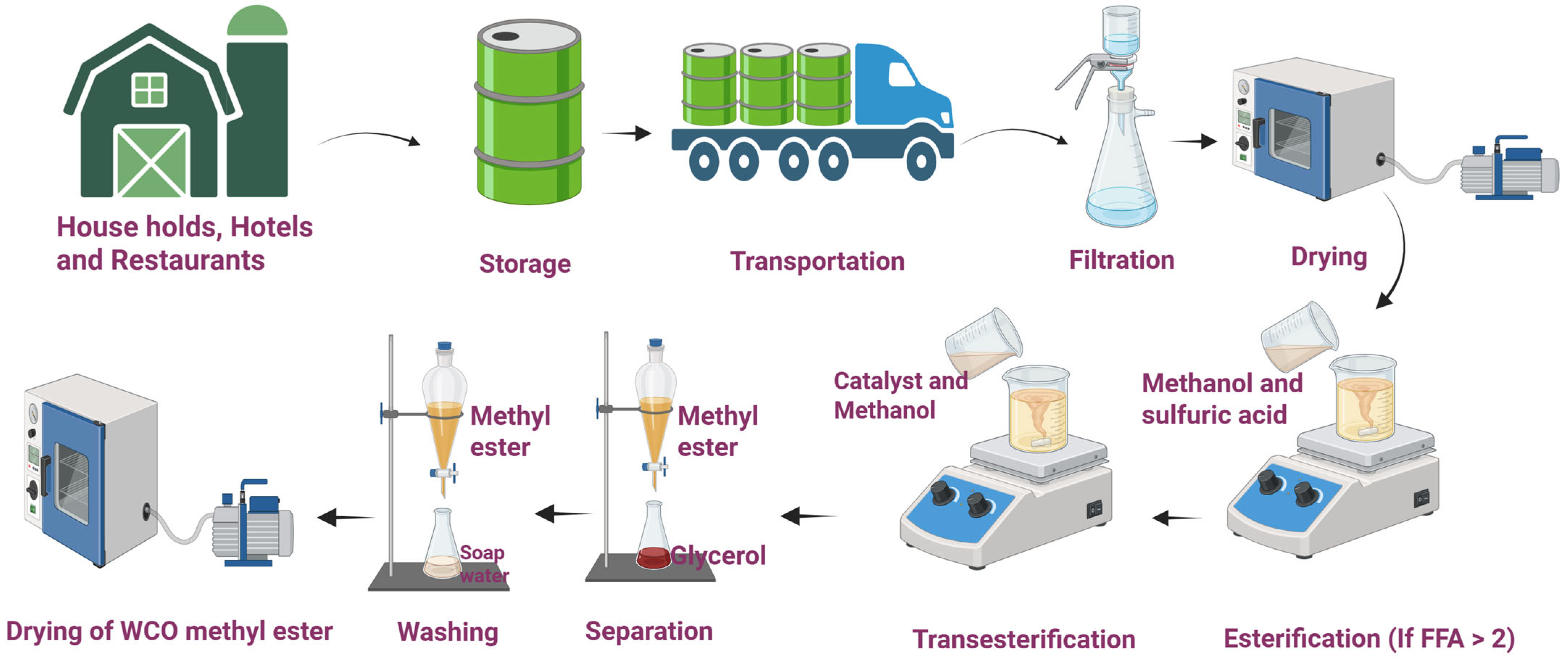
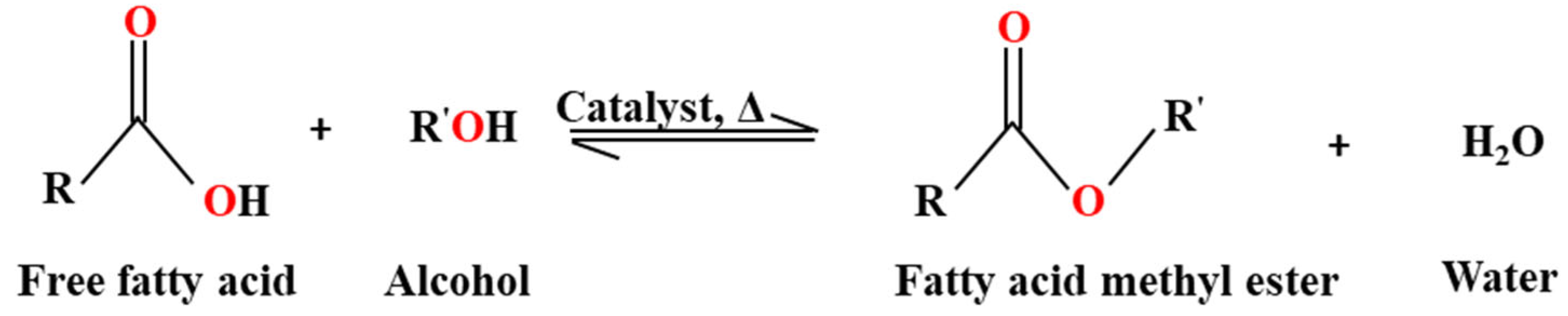
| Fatty Acid Type | Fatty Acid Name | Percentage of Waste Cooking Oil |
|---|---|---|
| Saturated Fatty Acids | Palmitic acid | 38.35 |
| Stearic acid | 4.33 | |
| Myristic acid | 1.03 | |
| Lauric acid | 0.34 | |
| Heneicosanoic acid | 0.07 | |
| Sub-Total | 44.12 | |
| Monounsaturated Fatty Acids | Oleic acid | 43.67 |
| Cis-11-Eicosenoic acid | 0.16 | |
| Sub-Total | 43.83 | |
| Polyunsaturated Fatty Acids | Linoleic acid | 11.39 |
| γ-Linolenic acid | 0.37 | |
| Linolenic | 0.29 | |
| Sub-Total | 12.05 | |
| Total | - | 100 |
| Properties | Waste Cooking Oil | Unused Vegetable Oil | Natural Ester Insulating Oils | Standard |
|---|---|---|---|---|
| Density (g/cm3) | 0.94 | 0.89 [66] | ASTM [84] | |
| Viscosity (mm2/s) @ 40 °C | 44.1 | 39.99 [66] | IEEE [85] | |
| Moisture (ppm) | 1130 | 105.70–125.8 [67] | ASTM [86] | |
| Acid value (mgKOH/g) | 4.03 | 0.3 [66] | Max. 0.6 | IEC [87] |
| Flash point (°C) | 269 | [43] | ASTM [86] | |
| Pour point (°C) | 9 | −18 [82] | IEC [87], ASTM [86] | |
| Breakdown voltage (kV) | 27 | 40 [82] | ASTM [88] | |
| Physical color rating | L5.0 | Clear and free from suspended particles [43] | Max. 1.0 | IEEE C57.147 [85] |
| S/n | Method of Purification | Free Fatty Acids Reduction | Reference |
|---|---|---|---|
| 1 | Preheated the oil at 50 °C, 1 atm, followed by filtration using cotton cloth. | [93] | |
| 2 | The oil samples were preheated at 100 °C and filtered. | Metal-catalyzed glycerolysis using zinc dust, ferric chloride, manganese chloride, and stannous chloride, along with supplementation of glycerol and acid treatment using conc. H2SO4, HCl and H3PO4. | [98] |
| 3 | The waste cooking oil was preheated and filtered using filter paper (110 mm). Oil degumming at 60 °C, for 30 min/800 rpm using concentrated H3PO4 (2% v/v). | Acid catalysis esterification using 2% (v/v) conc. H2SO4 and methanol at 60 °C with a stirring speed of 1000 rpm for 1 h. | [99] |
| 4 | The oil was heated at 60 °C and filtered under vacuum using a cellulose filter paper. | A total of 200 g of oil was heated to 65 °C with stirring, followed by the addition of methanol and catalyst (6:1 oil-to-methanol molar ratio, 5% v/v catalyst). | [92] |
| 5 | Vacuum filtration of oil followed by dehumidification at 105 °C. | Free fatty acid treatment using an adsorbent (coal ash). Used cooking oil with 2 g of activated coal and stirred for 60 min at 130 °C. | [76] |
| 6 | Waste cooking oil filtration was performed using a vacuum filtration system having a mesh size of 1–5 microns, followed by dehumidification at 120 °C. | The free fatty acids were removed using activated coal ash at a 1:10 ratio at 130 °C for 1 h. | [100] |
| References | Viscosity of Waste WCO (cSt) (40 °C) | Viscosity After Modification (cSt) (40 °C) | Catalyst | Enhancement (%) |
|---|---|---|---|---|
| [82] | 70 | 27 | NaOH | 61.43 |
| [76] | 42.8 | 6 | CaO | 85.98 |
| [46] | 40.84 | 14.19 | KOH | 65.25 |
| [140] | 41.85 | 15.23 | CaO | 63.61 |
| [100] | 42.41 | 4.52 | Coal ash | 89.34 |
| [141] | 24.3 | 14.76 | - | 39.26 |
| [142] | 32.85 | 4.2 | KOH | 87.21 |
| Reference | Pour Point of WCO (°C) | Pour Point After Modification (°C) | Catalyst | Percentage Decrease |
|---|---|---|---|---|
| [82] | 9 | −3 | NaOH | 133.33 |
| [76] | 0 | −6 | CaO | Absolute decrease of 6 units |
| [46] | −28 | −28 | KOH | No observable difference was reported |
| [140] | −15 | −21 | CaO | 40% |
| [100] | −30 | −39 | Coal ash | 30% |
| [142] | 10 | −6.7 | KOH | 167 |
| Reference | Acidity of Waste WCO | Acidity After Modification | Catalyst | Percentage Decrease |
|---|---|---|---|---|
| [82] | 0.13 | 0.06 | NaOH | 53.84 |
| [76] | 2.5 | 0.4 | CaO | 84 |
| [42] | 2.7972 | 0.2578 | NaOH | 90.78 |
| [46] | 2.7972 | 0.2561 | KOH | 90.84 |
| [140] | 2.793 | 0.144 | CaO | 94.84 |
| [100] | 2.82 | 0.03 | Coal ash | 98.94 |
| [161] | 1.82 | 0.13 | NaOH | 92.86 |
| [141] | 0.1 | 0.06 | - | |
| [142] | 1.122 | 0.16 | KOH | 85.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oparanti, S.O.; Obebe, E.O.; Fofana, I.; Jafari, R. A State-of-the-Art Review on the Potential of Waste Cooking Oil as a Sustainable Insulating Liquid for Green Transformers. Appl. Sci. 2025, 15, 7631. https://doi.org/10.3390/app15147631
Oparanti SO, Obebe EO, Fofana I, Jafari R. A State-of-the-Art Review on the Potential of Waste Cooking Oil as a Sustainable Insulating Liquid for Green Transformers. Applied Sciences. 2025; 15(14):7631. https://doi.org/10.3390/app15147631
Chicago/Turabian StyleOparanti, Samson Okikiola, Esther Ogwa Obebe, Issouf Fofana, and Reza Jafari. 2025. "A State-of-the-Art Review on the Potential of Waste Cooking Oil as a Sustainable Insulating Liquid for Green Transformers" Applied Sciences 15, no. 14: 7631. https://doi.org/10.3390/app15147631
APA StyleOparanti, S. O., Obebe, E. O., Fofana, I., & Jafari, R. (2025). A State-of-the-Art Review on the Potential of Waste Cooking Oil as a Sustainable Insulating Liquid for Green Transformers. Applied Sciences, 15(14), 7631. https://doi.org/10.3390/app15147631









