Honey as a Bioindicator: Pollution’s Effects on Its Quality in Mining vs. Protected Sites
Abstract
1. Introduction
2. Materials and Methods
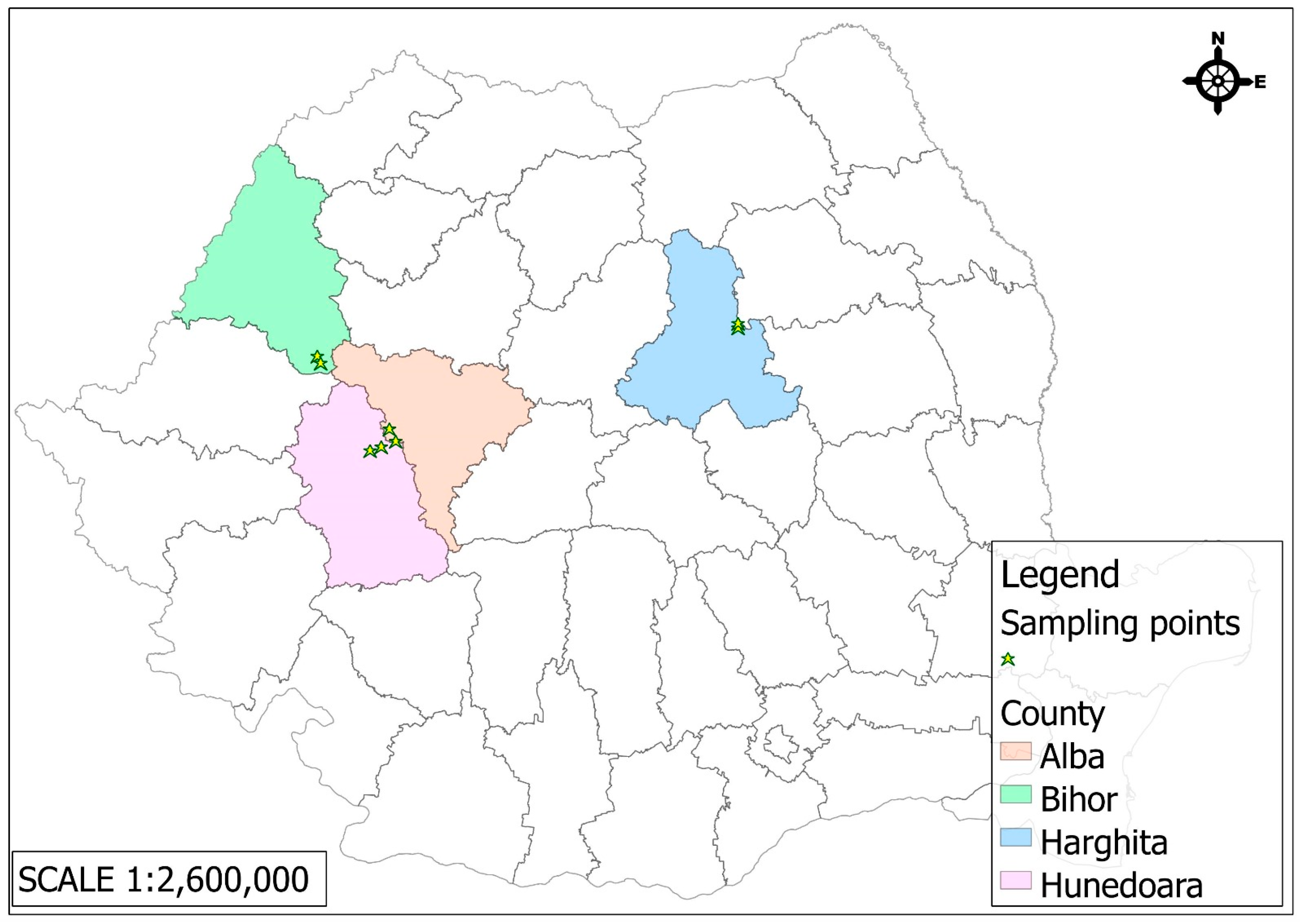
2.1. Honey Samples and the Description of the Area
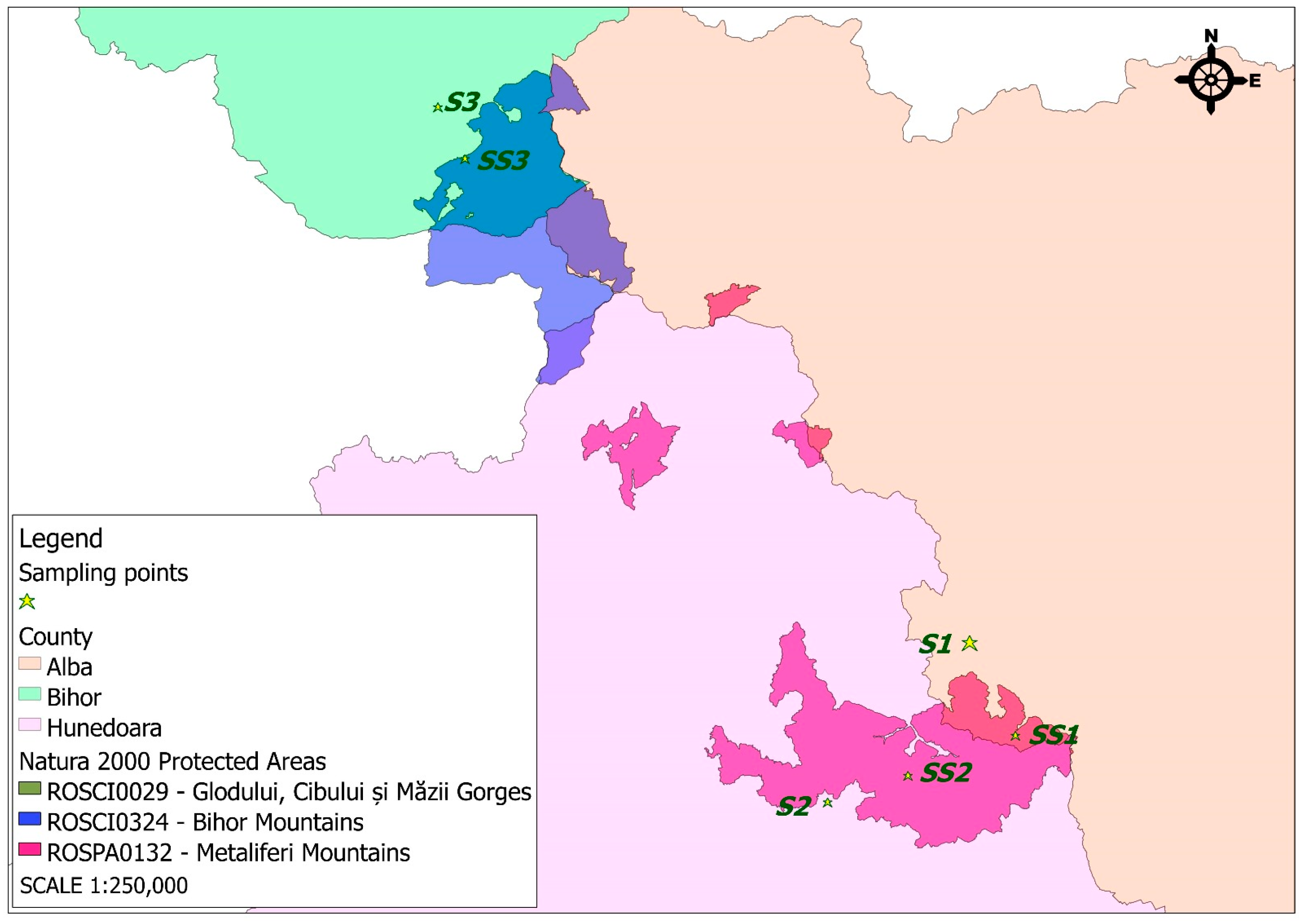
- The Zlatna Mining Operation—Haneș Mine (Alba County), located in the Apuseni Mountains, has a millennia-long mining tradition. Industrial exploitation began in the 18th century under Austrian administration, focusing on polymetallic ores—mainly copper, as well as gold, silver, lead, and zinc. The mine expanded significantly during the communist era, becoming part of the Zlatna Mining Complex after nationalization in 1948. Operations declined in the 1990s, due to declining resources, high operational costs, and environmental issues, and it was officially closed in 2003. The area has undergone ecological rehabilitation, through heavy metal pollution and abandoned infrastructure remain [22,23].
- The Certeju de Sus Mining Operation (Hunedoara County), part of the gold-rich Metaliferi Mountains, was industrially developed starting in the 19th century and intensified under communism. The mine produced complex ores rich in gold and silver, as well as copper, lead, and zinc. Its mineralization is characterized by the presence of auriferous pyrites, commonly associated with galena, sphalerite, chalcopyrite, and gold tellurides. It became a key site within the Deva Mining Complex, equipped with flotation plants and concentrating processing facilities. A major ecological disaster occurred in 1971 due to a tailings dam failure. Declining profitability led to its closure in 2006. Recent years have brought ecological efforts and controversial plans to restart mining using cyanide, which have faced strong public opposition [24,25].
- The Băița Mining Operation (Bihor County), a historically significant site, shifted from polymetallic mining to uranium extraction after World War II in collaboration with the Soviet Union (Sovrom-Kvarțit). Mining was conducted under hazardous, secretive conditions involving political prisoners. Post-Soviet operations continued under Romanian control but declined after 1990. In addition to uranium, the Băița area also produced smaller quantities of gold, silver, molybdenum, bismuth, and other rare metals. Mining activity was officially discontinued in the 2000s. Nevertheless, the former mining perimeter remains under environmental monitoring, as radioactive waste deposits and ongoing soil and water contamination continue to pose a significant ecological risk [26,27].
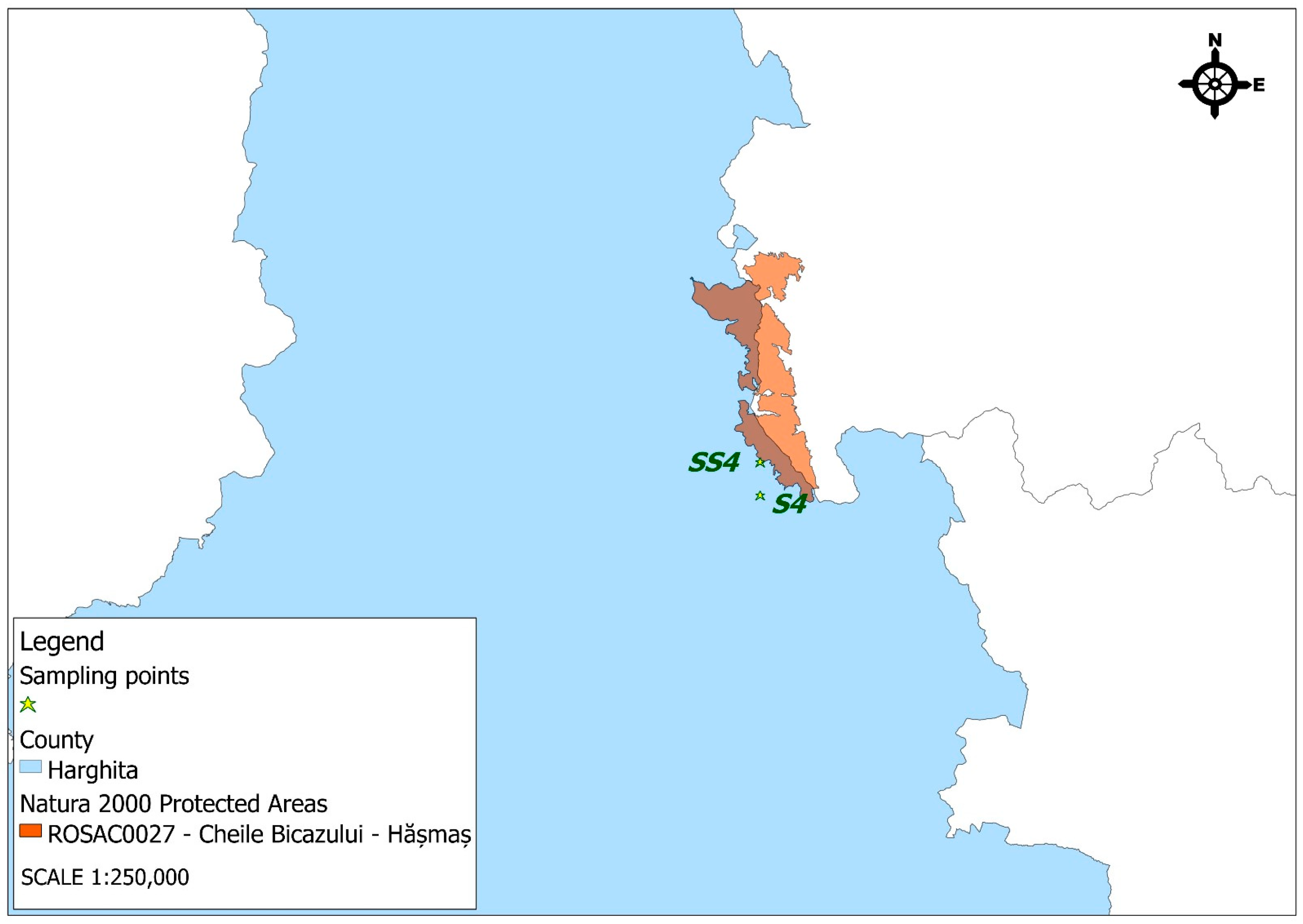
- The Bălan Mining Operation (Harghita County), active since the late 18th century, was a major copper extraction site. The primary mineral extracted was copper ore (chalcopyrite), although the deposit also contained variable amounts of gold, silver, pyrite, galena (lead sulfide), and sphalerite (zinc sulfide). The Bălan mine was officially closed in 2006. In recent years, the area around the former mine has been undergoing ecological rehabilitation, though issues related to heavy metal pollution and landscape degradation persist, caused by abandoned industrial buildings and abandoned mining galleries [28,29].
- The Natura 2000 Site ROSCI0029 Glodului, Cibului, and Măzii Gorges, is a 735 ha Site of Community Importance (SCI) located entirely in Alba and Hunedoara counties, within the Continental biogeographical region. The following protected habitat types and species of conservation interest have been identified within the site: 6110 *—Rupicolous calcareous or basophilic grasslands of the Alysso-Sedion albi; 6190—Rupicolous Pannonic grasslands (Stipo-Festucetalia pallentis); 6210 *—semi-natural dry grasslands and scrubland facies on calcareous substrates (Festuco-Brometalia); 8210—calcareous rocky slopes with chasmophytic vegetation; 8310—caves not open to the public; 9110—Luzulo-Fagetum beech forests; 9180 *—Tilio-Acerion forests of slopes, screes, and ravines (mixed broadleaf forests); 91E0 *—alluvial forests with Alnus glutinosa and Fraxinus excelsior (Alno-Padion, Alnion incanae, and Salicion albae), Iris aphylla ssp. hungarica (Hungarian leafless iris), Bombina variegata (yellow-bellied toad), Triturus vulgaris ampelensis (Danube crested newt), Barbastella barbastellus (Barbastelle bat), Miniopterus schreibersii (Schreiber’s bat), Myotis blythii (lesser mouse-eared bat), Myotis myotis (greater mouse-eared bat), Rhinolophus blasii (Blasius’s horseshoe bat), Rhinolophus euryale (Mediterranean horseshoe bat), Rhinolophus ferrumequinum (greater horseshoe bat), Rhinolophus hipposideros (lesser horseshoe bat), and Lutra lutra (Eurasian otter) [30].
- The Natura 2000 Site ROSPA0132 Metaliferi Mountains, is a 26,673.4 ha Special Protection Area (SPA) for birds in Alba and Hunedoara counties, spanning both the Alpine and Continental biogeographical regions. Within its territory, 15 bird species are considered species of conservation interest: Aquila chrysaetos (Golden Eagle), Bubo bubo (Eurasian Eagle-Owl), Caprimulgus europaeus (European Nightjar), Circaetus gallicus (Short-toed Snake Eagle), Dendrocopos leucotos (White-backed Woodpecker), Dendrocopos medius (Middle Spotted Woodpecker), Dryocopus martius (Black Woodpecker), Falco peregrinus (Peregrine Falcon), Ficedula albicollis (Collared Flycatcher), Ficedula parva (Red-breasted Flycatcher), Lanius collurio (Red-backed Shrike), Lullula arborea (Wood Lark), Milvus migrans (Black Kite), Pernis apivorus (European Honey Buzzard), and Picus canus (Grey-headed Woodpecker). These species have benefitted from special protection under the Natura 2000 network [31].
- The Natura 2000 Site ROSCI0324 Bihor Mountains, is a Site of Community Importance (SCI) of 20,932.2 ha located across Arad, Alba, Bihor, and Hunedoara counties, being in the Alpine biogeographical region. The following protected habitat types and species of conservation interest have been identified within the site: 4070 *—bushes of Pinus mugo and Rhododendron hirsutum (Mugo-Rhododendretum hirsuti); 9110—Luzulo-Fagetum beech forests; 9130—Asperulo-Fagetum beech forests; 9170—Galio-Carpinetum oak–hornbeam forests; 91V0—Dacian beech forests (Symphyto-Fagion); and 9410—acidophilous Picea abies forests in a mountain zone (Vaccinio-Piceetea); Rosalia alpina (Rosalia longhorn beetle), Carabus variolosus (varied ground beetle); Bombina variegata (yellow-bellied toad), Triturus vulgaris ampelensis (Danube crested newt), Canis lupus (grey wolf), Lynx lynx (Eurasian lynx), and Ursus arctos (brown bear) [32].
- The Natura 2000 site ROSAC0027 Bicaz Gorge-Hășmaș, is a 7642 ha Special Area of Conservation (SAC) in Harghita and Neamț counties, entirely within an Alpine biogeographic region. The following habitat types have been identified within the special conservation area: 150—natural eutrophic lakes with Magnopotamion- or Hydrocharition-type vegetation; 4060—alpine and boreal heaths; 6170—alpine and subalpine calcareous grasslands; 6190—Rupicolous Pannonic grasslands (Stipo-Festucetalia pallentis); 6210*—semi-natural dry grasslands on calcareous substrates (Festuco-Brometalia); 6430—hydrophilious, tall herb fringe communities of plains and montane to alpine levels; 6510—lowland hay meadows (Alopecurus pratensis, Sanguisorba officinalis); 6520—mountain hay meadows; 8120—calcareous and calcshist screes of montane to alpine levels (Thlaspietea rotundifolii); 8210—calcareous rocky slopes with chasmophytic vegetation; 8310—caves not open to the public; 91E0 *—alluvial forests with Alnus glutinosa and Fraxinus excelsior (Alno-Padion, Alnion incanae, and Salicion albae); 91Q0—calcicolous Pinus sylvestris forests; 91V0—Dacian beech forests (Symphyto-Fagion); 9110—Luzulo-Fagetum beech forests; and 9410—acidophilous Picea abies forests of the mountain zone (Vaccinio-Piceetea). The species of conservation interest identified within this site are as follows: Cypripedium calceolus (Lady’s Slipper Orchid), Asplenium adulterinum (Ladder Spleenwort), Campanula serrata (Serrated Bellflower), Iris aphylla ssp. hungarica (Hungarian Leafless Iris), Pholidoptera transsylvanica (Transylvanian Dark Bush-cricket), Euphydryas aurinia (Marsh Fritillary Butterfly), Barbus petenyi (Petényi’s Barbel), Cottus gobio (European Bullhead), Cottus poecilopus (Alpine Bullhead), Triturus cristatus (Great Crested Newt), Triturus montandoni (Carpathian Newt), Bombina variegata (Yellow-bellied Toad), Barbastella barbastellus (Western Barbastelle Bat), Miniopterus schreibersii (Common Bent-wing Bat), Myotis bechsteinii (Bechstein’s Bat), Myotis blythii (Lesser Mouse-eared Bat), Myotis myotis (Greater Mouse-eared Bat), Rhinolophus hipposideros (Lesser Horseshoe Bat), Canis lupus (Gray Wolf), Lynx lynx (Eurasian Lynx), and Ursus arctos (Brown Bear) [33].
2.2. Physico-Chemical Analyses
2.2.1. Water Activity (aw)
2.2.2. Moisture Content (H)
2.2.3. Phenolic Compounds (Phenols)
2.2.4. Flavonoid Compounds (Flavonoids)
2.2.5. Hydroxymethylfurfural Content—White Method (HMF)
2.2.6. Sugar [41]
- A.
- Determination of Easily Hydrolysable Sugar by the Elser Method—Honey
- B.
- Determination of Invert Sugar
2.2.7. Heavy Metals (Pb, Cd, Fe, Cu, and Zn)
2.2.8. Microbiological Analysis
2.3. Determination of Botanical Origin
2.4. Statistical Analysis
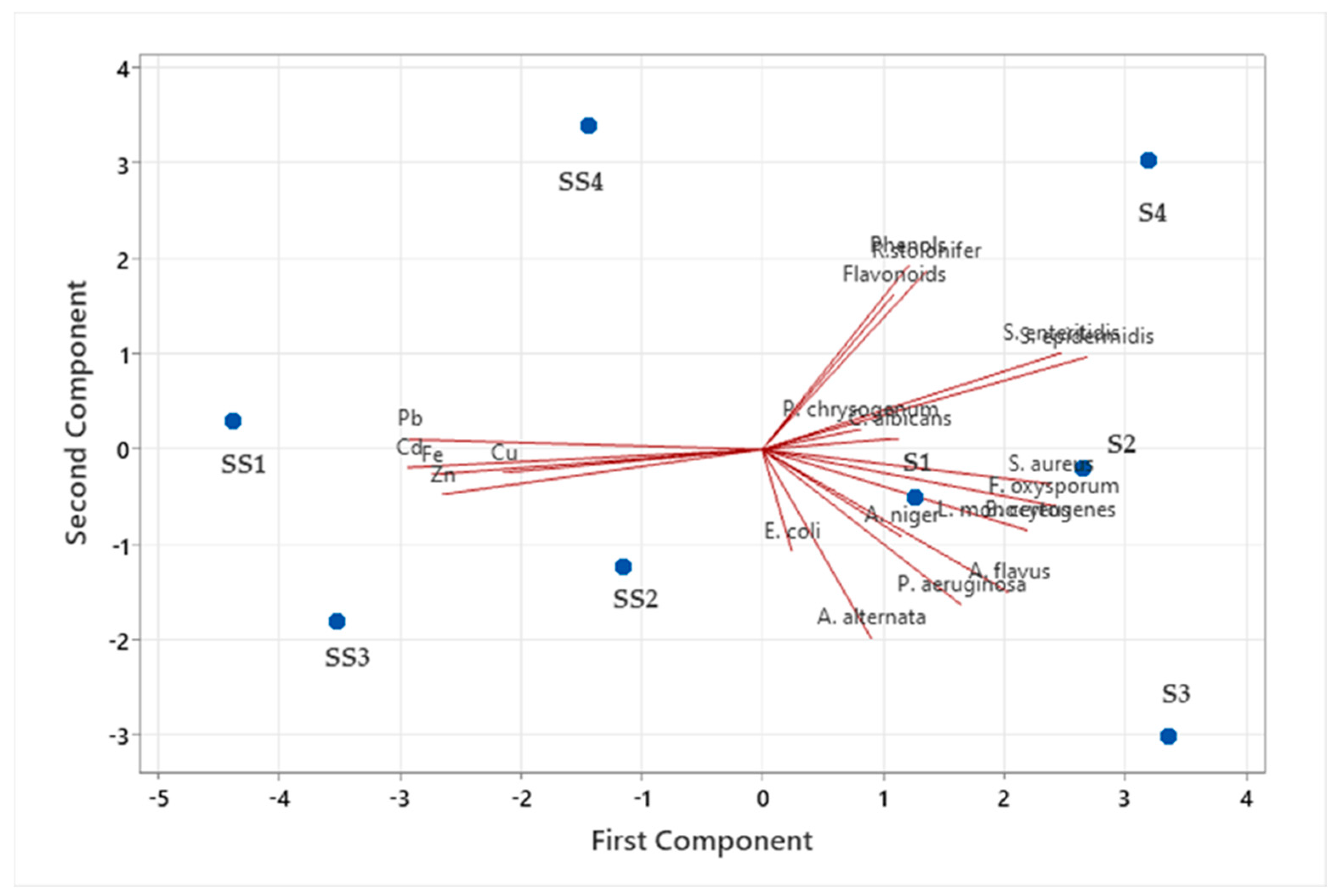
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bonsignore, G.; Martinotti, S.; Ranzato, E. Honey Bioactive Molecules: There Is a World Beyond the Sugars. BioTech 2024, 13, 47. [Google Scholar] [CrossRef]
- Tafere, D.A. Chemical composition and uses of Honey: A Review. J. Food Sci. Nutr. Res. 2021, 4, 194–201. [Google Scholar] [CrossRef]
- Celli, G.; Maccagnani, B. Honey bees as bioindicators of environmental pollution. Bull. Insectology 2003, 56, 137–139. [Google Scholar]
- Bora, F.D.; Andrecan, A.F.; Călugăr, A.; Bunea, C.I.; Popescu, M.; Petrescu-Mag, I.V.; Bunea, A. Comprehensive Elemental Profiling of Romanian Honey: Exploring Regional Variance, Honey Types, and Analyzed Metals for Sustainable Apicultural and Environmental Practices. Foods 2024, 13, 1253. [Google Scholar] [CrossRef] [PubMed]
- Tlak Gajger, I.; Pavliček, D.; Oreščanin, V.; Varenina, I.; Sedak, M.; Bilandžić, N. Mineral Concentrations in Different Types of Honey Originating from Three Regions of Continental Croatia. Foods 2024, 13, 2754. [Google Scholar] [CrossRef]
- Lin, Z.; Shen, S.; Wang, K.; Ji, T. Biotic and abiotic stresses on honeybee health. Integr. Zool. 2024, 19, 442–457. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; Ahmed, H.R.; El-Wahed, A.A.A.; Saeed, A.; Algethami, A.F.; Attia, N.F.; Guo, Z.; Musharraf, S.G.; Khatib, A.; Alsharif, S.M.; et al. Bee Stressors from an Immunological Perspective and Strategies to Improve Bee Health. Vet. Sci. 2022, 9, 199. [Google Scholar] [CrossRef] [PubMed]
- International Statistics. Bee colonies: Worldwide Population on the Rise. Available online: https://www.destatis.de/EN/Themes/Countries-Regions/International-Statistics/Data-Topic/AgricultureForestryFisheries/Bees.html (accessed on 10 April 2025).
- Margaoan, R.; Papa, G.; Nicolescu, A.; Cornea-Cipcigan, M.; Kösoğlu, M.; Topal, E.; Negri, I. Environmental pollution effect on honey bees and their derived products: A comprehensive analysis. Environ. Sci. Pollut. Res. Int. 2025, 32, 10370–10391. [Google Scholar] [CrossRef]
- Šerevičienė, V.; Zigmontienė, A.; Paliulis, D. Heavy Metals in Honey Collected from Contaminated Locations: A Case of Lithuania. Sustainability 2022, 14, 9196. [Google Scholar] [CrossRef]
- Tong, D.Q.; Gill, T.E.; Sprigg, W.A.; Van Pelt, R.S.; Baklanov, A.A.; Barker, B.M.; Bell, J.E.; Castillo, J.; Gassó, S.; Gaston, C.J.; et al. Health and Safety Effects of Airborne Soil Dust in the Americas and Beyond. Rev. Geophys. 2023, 61, e2021RG000763. [Google Scholar] [CrossRef]
- Borsuk, G.; Sulborska, A.; Stawiarz, E.; Olszewski, K.; Wiącek, D.; Ramzi, N.; Nawrocka, A.; Jędryczka, M. Capacity of honeybees to remove heavy metals from nectar and excrete the contaminants from their bodies. Apidologie 2021, 52, 1098–1111. [Google Scholar] [CrossRef]
- Demková, L.; Hauptvogl, M.; Oboňa, J.; Bobuľská, L.; Jančo, I.; Harangozo, Ľ.; Lakatošová, J.; Kowalski, S.; Árvay, J. Comprehensive assessment of mercury contamination in bees, bee products and moss and lichen bags. Ecotoxicol. Environ. Saf. 2024, 285, 117132. [Google Scholar] [CrossRef] [PubMed]
- Abd Wahid, D.N.; Wan Mohamad Nazarie, W.F.; Jawan, R.; Abdulla, R.; Gansau, J.A.; Sabullah, M.K. Potential Existence of Heavy Metal Pollution and Pesticide in Honey-Based Products. Preprints 2021, 2021120379. [Google Scholar] [CrossRef]
- Godebo, T.R.; Stoner, H.; Taylor, P.; Jeuland, M. Metals in honey from bees as a proxy for environmental contamination in the United States. Environ. Pollut. 2025, 364, 125221. [Google Scholar] [CrossRef]
- Moghimi Dehkordi, M.; Pournuroz Nodeh, Z.; Soleimani Dehkordi, K.; Salmanvandi, H.; Rasouli Khorjestan, R.; Ghaffarzadeh, M. Soil, air, and water pollution from mining and industrial activities: Sources of pollution, environmental impacts, and prevention and control methods. Results Eng. 2024, 23, 102729. [Google Scholar] [CrossRef]
- Angon, P.B.; Islam, M.S.; Kc, S.; Das, A.; Anjum, N.; Poudel, A.; Suchi, S.A. Sources, effects and present perspectives of heavy metals contamination: Soil, plants and human food chain. Heliyon 2024, 10, e28357. [Google Scholar] [CrossRef]
- Burden, C.M.; Morgan, M.O.; Hladun, K.R.; Amdam, G.V.; Trumble, J.J.; Smith, B.H. Acute sublethal exposure to toxic heavy metals alters honey bee (Apis mellifera) feeding behavior. Sci. Rep. 2019, 9, 4253. [Google Scholar] [CrossRef]
- Costa, A.; Veca, M.; Barberis, M.; Tosti, A.; Notaro, G.; Nava, S.; Lazzari, M.; Agazzi, A.; Tangorra, F.M. Heavy Metals on Honeybees Indicate Their Concentration in the Atmosphere. a Proof of Concept. Ital. J. Anim. Sci. 2018, 18, 309–315. [Google Scholar] [CrossRef]
- Vică, M.L.; Glevitzky, I.; Glevitzky, M.; Siserman, C.V.; Matei, H.V.; Teodoru, C.A. Antibacterial Activity of Propolis Extracts from the Central Region of Romania against Neisseria gonorrhoeae. Antibiotics 2021, 10, 689. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006. Available online: https://eur-lex.europa.eu/eli/reg/2023/915/oj (accessed on 10 April 2024).
- Ispas, G.; Roba, C.; Bălc, R.; Coadă, M.; Gligor, D. The Quality of Water and Soil in Haneș Mining Area, Alba County, Romania. Carpathian J. Earth Environ. Sci. 2018, 13, 505–514. [Google Scholar] [CrossRef]
- Iatan, E.-L. Environmental Problems Associated with the Mining Activities in the Apuseni Mountains, Romania Conference. In Proceedings of the The Annual Scientific Session Of The Institute Of Geodynamics, Bucharest, Romania, 27–28 March 2018; pp. 82–86. Available online: https://www.geodin.ro/CUTE/wp-content/uploads/2019/11/Iatan-2019.pdf (accessed on 24 March 2025).
- Certej—Mining Watch Romania. Available online: https://miningwatch.ro/en/certej-2/ (accessed on 24 March 2025).
- 42 Years Since the Accident at the GOLD mine in Certej, Romania. Available online: https://2celsius.org/media/42-years-since-the-accident-at-the-gold-mine-in-certej-romania/ (accessed on 25 March 2025).
- Zajzon, N.; Szentpéteri, K.; Szakáll, S.; Kristály, F. The origin of the Avram Iancu U–Ni–Co–Bi–As mineralization, Băiţa (Bihor) metallogenic district, Bihor Mts., Romania. Int. J. Earth Sci. (Geol. Rundsch.) 2015, 104, 1865–1887. [Google Scholar] [CrossRef]
- Environmental Restoration Plans and Activities in the Zones of Uranium Ore Extraction and Milling in Romania: 1995–1996 Progress Report, Planning for Environmental Restoration of Uranium Mining and Milling Sites in Central and Eastern Europe. Proceedings of a Workshop Held Under the Technical Co-Operation Project RER/9/022 on Environmental Restoration in Central and Eastern Europe, Workshop on Planning for Environmental Restoration of Uranium Mining and Milling Sites in Central and Eastern Europe. 4–8 Nov 1996, Felix (Romania). pp. 135–152. International Atomic Energy AgENcY. 1997. Available online: https://inis.iaea.org/records/3yfjf-9h824 (accessed on 1 March 2025).
- Bara, N.; Stefanescu, L. Water Quality Assessment in Balan, Romania—Case Study. 2018. Available online: https://www.researchgate.net/publication/327499313_WATER_QUALITY_ASSESSMENT_IN_BALAN_ROMANIA_-_CASE_STUDY?channel=doi&linkId=5b923f95a6fdccfd541faafd&showFulltext=true (accessed on 3 March 2025). [CrossRef]
- Bara, N.; Gati, G.; Pop, C.; Gurzău, E. The Pollution Effects of Mining Industry on Small Rivers—A Case Study. 2016. Available online: https://www.researchgate.net/publication/327499303_The_pollution_effects_of_mining_industry_on_small_rivers_-_A_Case_Study?channel=doi&linkId=5b923f954585153a5300465a&showFulltext=true (accessed on 7 March 2025). [CrossRef]
- National Agency for Protected Natural Areas—Note No. 2415 Dated 19.04.2021, Regarding the Approval of the Minimum Set of Special Protection and Conservation Measures for Biological Diversity, as Well as the Conservation of Natural Habitats, Wild Flora and Fauna, Public Safety, and Investments in ROSCI0029 Glodului, Cibului, and Măzii Gorges. Available online: https://ananp.gov.ro/wp-content/uploads/ROSCI0029-SKM_28722030210380.V.pdf (accessed on 4 March 2025).
- National Agency for Protected Natural Areas—Note No. 4647 Dated 26.07.2021, Regarding the Approval of the Minimum Set of Special Protection and Conservation Measures for Biological Diversity, as Well as the Conservation of Natural Habitats, Wild Flora and Fauna, Public Safety, and Investments in ROSPA0132 Metaliferi Mountains. Available online: https://ananp.gov.ro/wp-content/uploads/ROSPA0132-SKM_28721080508200.pdf (accessed on 4 March 2025).
- National Agency for Protected Natural Areas—Note No. 1787 Dated 24.03.2022, Regarding the Approval of the Minimum Set of Special Protection and Conservation Measures for Biological Diversity, as Well as the Conservation of Natural Habitats, Wild Flora and Fauna, Public Safety, and Investments in ROSCI0324 Bihor Mountains. Available online: https://ananp.gov.ro/wp-content/uploads/ROSCI0324-SKM_28722033010380.pdf (accessed on 4 March 2025).
- National Agency for Protected Natural Areas—Decision No. 145 Dated 08.04.2021, Regarding the Approval of the Methodological Rules for Implementing the Conservation Objectives from the Annex to Order No. 1523/2016 of the Ministry of Environment, Waters and Forests, Concerning the Approval of the Management Plan and the Regulation of the Bicazului-Hășmaș Gorges National Park and the Natura 2000 sites ROSCI0027 and ROSPA0018 Bicazului-Hășmaș Gorges (Excluding the Overlapping Area with ROSCI0033 Șugăului-Munticelu Gorges). Available online: https://ananp.gov.ro/wp-content/uploads/ROSCI0027-SKM_28722030210320.V.pdf (accessed on 4 March 2025).
- AOAC. 978.18 Water Activity. In Official Methods of Analysis of the Association of Official Analytical Chemist, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1995. [Google Scholar]
- Ebrahimi, Y.; Ramírez-Coronel, A.A.; Al-Dhalimy, A.M.B.; Alfilm, R.H.C.; Al-Hassan, M.; Obaid, R.F.; Alameri, A.A.; Rastiani, F.; Yousef, K.; Shokri, S. Contamination of honey products by Clostridium botulinum spores and fungi along with their effects on human health. Casp. J. Environ. Sci. 2023, 20, 1143–1148. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 29, 10–18. [Google Scholar]
- Vica, M.L.; Glevitzky, M.; Dumitrel, G.A.; Junie, L.M.; Popa, M. Antibacterial activity of different natural honeys from Transylvania, Romania. J. Environ. Sci. Health—B Pestic. Food Contam. Agric. Wastes. 2014, 49, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Sultana, S.; Lawag, I.L.; Lim, L.Y.; Foster, K.J.; Locher, C. A Critical Exploration of the Total Flavonoid Content Assay for Honey. Methods Protoc. 2024, 7, 95. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, S.; Harmonized Methods of the European Honey Commission. International Honey Commission. 2009. Available online: www.bee-hexagon.net (accessed on 5 September 2024).
- Vică, M.L.; Glevitzky, M.; Dumitrel, G.-A.; Bostan, R.; Matei, H.V.; Kartalska, Y.; Popa, M. Qualitative Characterization and Antifungal Activity of Romanian Honey and Propolis. Antibiotics 2022, 11, 1552. [Google Scholar] [CrossRef]
- SR 784/3:2009/C(91:2012); Honey Part 3: Analysis Methods. ASRO: Bucharest, Romania, 1984. Available online: https://e-standard.eu/en/standard/174480 (accessed on 7 January 2025). (In Romanian)
- Glevitzky, M.; Corcheş, M.T.; Popa, M.; Glevitzky, I.; Vică, M.L. Propolis: Biological Activity and Its Role as a Natural Indicator of Pollution in Mining Areas. Environments 2025, 12, 105. [Google Scholar] [CrossRef]
- CLSI M100; Performance Standards for Antimicrobial Susceptibility Testing. Clinical and Laboratory Standard Institute: Wayne, PA, USA, 2020. Available online: https://clsi.org/shop/standards/m100/ (accessed on 22 January 2025).
- Vică, M.L.; Glevitzky, M.; Heghedűş-Mîndru, R.C.; Glevitzky, I.; Matei, H.V.; Balici, S.; Popa, M.; Teodoru, C.A. Potential Effects of Romanian Propolis Extracts against Pathogen Strains. Int. J. Environ. Res. Public Health 2022, 19, 2640. [Google Scholar] [CrossRef]
- Louveaux, J.; Maurizio, A.; Vorwohl, G. Methods of Melissopalynology. Bee World. 1978, 59, 139–157. [Google Scholar] [CrossRef]
- Marghitaș, L.A. Bees and Their Products; Ceres Press: Bucharest, Romania, 2008; p. 390. (In Romanian) [Google Scholar]
- 2001/110/EC; Council Directive of 20 December 2001 Relating to Honey. European Parliament: Luxembourg, 2001.
- Sharaf El-Din, M.G.; Farrag, A.F.S.; Wu, L.; Huang, Y.; Wang, K. Health benefits of honey: A critical review on the homology of medicine and food in traditional and modern contexts. J. Tradit. Chin. Med. Sci. 2025, 12, 147–164. [Google Scholar] [CrossRef]
- Derrar, S.; Nava, V.; Ayad, M.A.; Saim, M.S.; Aggad, H.; Spanò, I.M.; Litrenta, F.; Leonardi, M.; Albergamo, A.; Lo Turco, V.; et al. Safety Assessment of Honeys from Northern and Southern Algerian Regions. Agriculture 2024, 14, 1503. [Google Scholar] [CrossRef]
- Perna, A.; Intaglietta, I.; Simonetti, A.; Gambacorta, E. Metals in Honeys from Different Areas of Southern Italy. Bull. Environ. Contam. Toxicol. 2014, 92, 253–258. [Google Scholar] [CrossRef]
- Aghamirlou, H.M.; Khadem, M.; Rahmani, A.; Sadeghian, M.; Mahvi, A.H.; Akbarzadeh, A.; Nazmara, S. Heavy Metals Determination in Honey Samples Using Inductively Coupled Plasma-Optical Emission Spectrometry. J. Environ. Health Sci. Eng. 2015, 13, 39. [Google Scholar] [CrossRef]
- Dag, A.; Afik, O.; Yeselson, Y.; Schaffer, A.; Shafir, S. Physical, chemical and palynological characterization of avocado (Persea americana Mill.) honey in Israel. Int. J. Food Sci. Technol. 2006, 41, 387–394. [Google Scholar] [CrossRef]
- Tutun, H.; Kahraman, H.A.; Aluc, Y.; Avci, T.; Ekici, H. Investigation of some metals in honey samples from West Mediterranean region of Turkey. Vet. Res. Forum 2019, 10, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Chudzinska, M.; Baralkiewicz, D. Estimation of honey authenticity by multielements characteristics using inductively coupled plasma-mass spectrometry (ICP-MS) combined with chemometrics. Food Chem. Toxicol. 2010, 48, 284–290. [Google Scholar] [CrossRef]
- Pisani, A.; Protano, G.; Riccobono, F. Minor and trace elements in different honey types produced in Siena County (Italy). Food Chem. 2008, 107, 1553–1560. [Google Scholar] [CrossRef]
- Czipa, N.; Andrasi, D.; Kovacs, B. Determination of essential and toxic elements in Hungarian honeys. Food Chem. 2015, 175, 536–542. [Google Scholar] [CrossRef]
- Vanhanen, L.P.; Emmertz, A.; Savage, G.P. Mineral analysis of mono-floral New Zealand honey. Food Chem. 2011, 128, 236–240. [Google Scholar] [CrossRef]
- Bazeyad, A.Y.; Al-Sarar, A.S.; Rushdi, A.I.; Hassanin, A.S.; Abobakr, Y. Levels of heavy metals in a multifloral Saudi honey. Environ. Sci. Pollut. Control Ser. 2019, 26, 3946–3953. [Google Scholar]
- Shaw, J.; Cunningham, C.; Harper, S.; Ragazzon-Smith, A.; Lythgoe, P.R.; Walker, T.R. Biomonitoring of honey metal(loid) pollution in Northwest England by citizen scientists. Environ. Adv. 2023, 13, 100406. [Google Scholar] [CrossRef]
- Karabagias, I.; Louppis, A.; Kontakos, S.; Papastephanou, C.; Kontominas, M. Characterization and geographical discrimination of Greek pine and thyme honeys based on their mineral content, using chemometrics. Eur. Food Res. Technol. Z. Lebensm. Unters. Forsch. 2016, 243, 101–113. [Google Scholar] [CrossRef]
- Bartha, S.; Taut, I.; Goji, G.; Vlad, I.A.; Dinulică, F. Heavy Metal Content in Polyfloral Honey and Potential Health Risk. A Case Study of Copșa Mică, Romania. Int. J. Environ. Res. Public Health 2020, 17, 1507. [Google Scholar] [CrossRef]
- Bora, F.D.; Babeș, A.C.; Călugăr, A.; Jitea, M.I.; Hoble, A.; Filimon, R.V.; Bunea, A.; Nicolescu, A.; Bunea, C.I. Unravelling Heavy Metal Dynamics in Soil and Honey: A Case Study from Maramureș Region, Romania. Foods 2023, 12, 3577. [Google Scholar] [CrossRef] [PubMed]
- The Food Safety and Standards Authority of India, 2.8.3 Honey and It’s by Products. Available online: https://fpas.fssai.gov.in/getSubCategoryList?exportProduct=false&isNewProduct=1&productid=470&productname=1&utm (accessed on 17 March 2025).
- Regulation of the Minister of Health of January 13, 2003 on the Maximum Levels of Chemical and Biological Contaminants That May Be Present in Food, Food Ingredients, Permitted Food Additives, Processing Aids, or on the Surface of Food the Official Journal of 2003, No. 37, Item 326, Annex 1, pp. 2411–2439. Available online: https://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20030370326/O/D20030326.pdf (accessed on 15 June 2025).
- National Committee of Standardization. GB2762-2022; Maximum Levels of Contaminants in Foods. State Administration for Market Regulation: Beijing, China; Standardization Administration of PRC: Beijing, China, 2022.
- GB14963-2011; National Food Safety Standard Honey. National Standards of People’s Republic of China, National Food Safety Standard Honey People’s Republic of China Ministry of Health: Beijing China, 2011.
- Mititelu, M.; Udeanu, D.I.; Nedelescu, M.; Neacsu, S.M.; Nicoara, A.C.; Oprea, E.; Ghica, M. Quality Control of Different Types of Honey and Propolis Collected from Romanian Accredited Beekeepers and Consumer’s Risk Assessment. Crystals 2022, 12, 87. [Google Scholar] [CrossRef]
- Yılmaz Aksu, F.; Sandikci Altunatmaz, S. Risks related to heavy metal contamination in foods. J. Food Hyg. Technol.-Spec. Top. 2017, 3, 218–230. [Google Scholar]
- Junie, L.M.; Vică, M.L.; Glevitzky, M.; Matei, H.V. Physico-chemical Characterization and Antibacterial Activity of Different Types of Honey Tested on Strains Isolated from Hospitalized Patients. J. Apic. Sci. 2016, 60, 5–18. [Google Scholar] [CrossRef]
- Tajik, H.; Shokouhi, F. In vitro evaluation of antimicrobial efficacy of natural honey in comparison with sulfonamide derivatives. J. Anim. Vet. Adv. 2009, 8, 23–25. [Google Scholar]
- Tumin, N.; Arsyiah, N.; Halim, A.; Shahjahan, M.; Izani, N.; Sattar, M. Antibacterial activity of local Malaysian honey. Malay. J. Pharm. Sci. 2005, 13, 129. [Google Scholar]
- Bastías, J.M.; Jambon, P.; Muñoz, O.; Manquián, N.; Bahamonde, P.; Neira, M. Honey as a bioindicator of arsenic contamination due to volcanic and mining activities in Chile. Chil. J. Agric. Res. 2013, 73, 147–153. [Google Scholar] [CrossRef]
- Herrero-Latorre, C.; Barciela-García, J.; García-Martín, S.; Peña-Crecente, R.M. The use of honeybees and honey as environmental bioindicators for metals and radionuclides: A review. Environ. Rev. 2017, 25, 463–480. [Google Scholar] [CrossRef]
| Sample No. | County | Area | Mineral Resource Type |
|---|---|---|---|
| S1 | Alba | Zlatna Mining Operation, Haneș Mine | Gold, silver, copper, lead, and zinc. |
| S2 | Hunedoara | Certeju de Sus Mining Operation | Gold, silver, copper, lead, and zinc. |
| S3 | Bihor | Băița Mining Operation | Gold, silver, copper, lead, zinc, and uranium. |
| S4 | Harghita | Bălan Mining Operation | Copper, gold, silver, lead, and zinc. |
| Sample No. | County | Area | Biodiversity Protection Site |
|---|---|---|---|
| SS1 | Alba | Almașu Mare Commune | ROSCI0029 Glodului, Cibului, and Măzii Gorges |
| SS2 | Hunedoara | Balșa Commune | ROSPA0132 Metaliferi Mountains |
| SS3 | Bihor | Criștioru de Jos Commune | ROSCI0324 Bihor Mountains |
| SS4 | Harghita | Bălan Town | ROSAC0027 Bicaz–Hășmaș Gorges |
| Sample No. | H, % | aw | Phenols, mg GAE/100 g | Flavonoids, mg QE/100 g | Sugar, % | Inverted Sugar, % | HMF, mg/100 g |
|---|---|---|---|---|---|---|---|
| S1 | 16.53 ± 0.52 | 0.583 ± 0.011 | 70.39 ± 0.47 | 4.11 ± 0.20 | 2.29 ± 0.33 | 72.23 ± 1.39 | 0.59 ± 0.10 |
| SS1 | 17.18 ± 0.18 | 0.547 ± 0.013 | 82.14 ± 0.61 | 4.26 ± 0.18 | 2.84 ± 0.30 | 79.99 ± 2.80 | 0.76 ± 0.14 |
| S2 | 16.42 ± 0.39 | 0.528 ± 0.026 | 63.84 ± 0.30 | 2.30 ± 0.11 | 2.88 ± 0.28 | 78.96 ± 2.44 | 1.10 ± 0.23 |
| SS2 | 17.09 ± 0.31 | 0.569 ± 0.018 | 72.67 ± 0.52 | 2.67 ± 0.04 | 2.68 ± 0.36 | 78.44 ± 1.05 | 0.92 ± 0.20 |
| S3 | 16.74 ± 0.20 | 0.571 ± 0.023 | 84.91 ± 0.86 | 4.08 ± 0.23 | 3.68 ± 0.41 | 79.11 ± 2.67 | 0.80 ± 0.16 |
| SS3 | 16.90 ± 0.44 | 0.535 ± 0.009 | 96.49 ± 1.03 | 5.45 ± 0.17 | 3.65 ± 0.77 | 78.47 ± 2.53 | 1.03 ± 0.12 |
| S4 | 18.03 ± 0.57 | 0.592 ± 0.014 | 59.73 ± 0.26 | 3.08 ± 0.10 | 1.89 ± 0.20 | 80.97 ± 3.31 | 0.68 ± 0.10 |
| SS4 | 17.25 ± 0.26 | 0.560 ± 0.015 | 63.20 ± 0.44 | 3.31 ± 0.15 | 3.16 ± 0.63 | 79.15 ± 2.02 | 0.49 ± 0.15 |
| Sample No. | Pb, mg/kg | Cd, mg/kg | Fe, mg/kg | Cu, mg/kg | Zn, mg/kg |
|---|---|---|---|---|---|
| S1 | 0.607 ± 0.061 | 0.018 ± 0.001 | 12.131 ± 0.885 | 0.428 ± 0.047 | 5.250 ± 0.278 |
| SS1 | 0.072 ± 0.005 | <0.001 | 1.068 ± 0.070 | 0.073 ± 0.003 | 0.091 ± 0.016 |
| S2 | 0.451 ± 0.043 | 0.020 ± 0.004 | 9.260 ± 0.651 | 0.211 ± 0.051 | 6.170 ± 0.235 |
| SS2 | 0.058 ± 0.004 | 0.013 ± 0.001 | 0.472 ± 0.032 | 0.031 ± 0.002 | 0.114 ± 0.023 |
| S3 | 0.320 ± 0.039 | 0.014 ± 0.002 | 2.210 ± 0.093 | 0.185 ± 0.037 | 0.822 ± 0.057 |
| SS3 | 0.056 ± 0.003 | <0.001 | 0.623 ± 0.081 | 0.060 ± 0.008 | 0.060 ± 0.019 |
| S4 | 0.218 ± 0.025 | <0.001 | 3.432 ± 0.126 | 0.545 ± 0.064 | 1.202 ± 0.162 |
| SS4 | 0.055 ± 0.002 | <0.001 | 0.067 ± 0.019 | 0.063 ± 0.005 | 0.075 ± 0.024 |
| Bacterial Strain | Sample | Synthetic Antibiotic | |||||||
|---|---|---|---|---|---|---|---|---|---|
| S1 | SS1 | S2 | SS2 | S3 | SS3 | S4 | SS4 | Ciprofloxacin, 5 µg | |
| E. coli | 9 | 11 | 12 | 14 | 8 | 8 | 8 | 10 | 29 |
| S. enteritidis | 7 | 8 | 7 | 8 | 8 | 9 | 8 | 8 | 27 |
| P. aeruginosa | 14 | 17 | 13 | 15 | 11 | 13 | 15 | 18 | 25 |
| S. aureus | 18 | 21 | 19 | 19 | 18 | 20 | 18 | 20 | 30 |
| S. epidermidis | 16 | 18 | 16 | 18 | 18 | 19 | 17 | 18 | 29 |
| B. cereus | 8 | 8 | 8 | 9 | 9 | 10 | 7 | 8 | 30 |
| L. monocytogenes | 8 | 9 | 8 | 8 | 8 | 9 | 9 | 10 | 24 |
| Fungal Strain | Sample | Synthetic Antibiotic | |||||||
|---|---|---|---|---|---|---|---|---|---|
| S1 | SS1 | S2 | SS2 | S3 | SS3 | S4 | SS4 | Voriconazole, 1 µg | |
| C. albicans | 9 | 10 | 9 | 10 | 9 | 9 | 8 | 9 | 37 |
| A. flavus | 8 | 9 | 9 | 9 | 8 | 9 | 9 | 10 | 43 |
| A. niger | 9 | 10 | 9 | 10 | 7 | 9 | 8 | 9 | 45 |
| R.stolonifer | 10 | 11 | 8 | 10 | 11 | 12 | 9 | 9 | 16 |
| F. oxysporum | 8 | 9 | 9 | 10 | 9 | 10 | 9 | 11 | 29 |
| P. chrysogenum | 10 | 11 | 11 | 11 | 8 | 12 | 8 | 9 | 18 |
| A. alternata | 9 | 10 | 9 | 9 | 7 | 8 | 10 | 11 | 16 |
| Correlation IZD Versus | Phenols | Flavonoids | HMF | Pb, mg/kg | Cd, mg/kg | Fe, mg/kg | Cu, mg/kg | Zn, mg/kg |
|---|---|---|---|---|---|---|---|---|
| E. coli | −0.36 *9 | −0.74 *11 | 0.81 *3 | 0.44 *5 | 0.64 *4 | 0.57 *4 | −0.45 *9 | 0.84 *3 |
| S. enteritidis | 0.27 *8 | 0.25 *6 | −0.27 *8 | −0.89 *11 | −0.77 *11 | −0.96 *12 | 0.15 *6 | −0.99 *12 |
| P. aeruginosa | −0.88 *11 | −0.29 *8 | −0.39 *9 | 0.01 *6 | −0.50 *10 | 0.32 *5 | 0.91 *2 | 0.19 *6 |
| S. aureus | −0.36 *9 | −0.84 *11 | 0.92 *2 | 0.21 *5 | 0.52 *4 | 0.35 *5 | −0.50 *10 | 0.68 *4 |
| S. epidermidis | 0.64 *4 | 0.42 *5 | −0.15 *8 | −0.70 *10 | −0.43 *9 | −0.92 *12 | −0.22 *8 | −0.92 *12 |
| B. cereus | −0.60 *10 | −0.24 *8 | −0.34 *9 | −0.72 *11 | −0.96 *12 | −0.47 *9 | 0.78 *3 | −0.53 *10 |
| L. monocytogenes | −0.60 *10 | −0.24 *8 | −0.34 *9 | −0.72 *11 | −0.96 *12 | −0.47 *9 | 0.78 *3 | −0.53 *10 |
| Correlation IZD Versus | Phenols | Flavonoids | HMF | Pb, mg/kg | Cd, mg/kg | Fe, mg/kg | Cu, mg/kg | Zn, mg/kg |
|---|---|---|---|---|---|---|---|---|
| C. albicans | 0.60 *10 | 0.24 *6 | 0.34 *5 | 0.72 *3 | 0.69 *4 | 0.47 *5 | −0.78 *11 | 0.53 *4 |
| A. flavus | −0.83 *11 | −0.93 *12 | 0.51 *4 | −0.44 *9 | 0.01 *6 | −0.10 *8 | 0.24 *6 | 0.14 *6 |
| A. niger | −0.64 *10 | −0.42 *9 | 0.15 *6 | 0.70 *11 | 0.82 *3 | 0.92 *2 | 0.22 *6 | 0.92 *2 |
| R. stolonifer | 0.86 *3 | 0.94 *2 | −0.57 *10 | −0.01 *8 | −0.44 *9 | −0.34 *9 | −0.15 *8 | −0.55 *10 |
| F. oxysporum | −0.04 *8 | −0.55 *10 | 0.61 *4 | −0.82 *11 | −0.44 *9 | −0.76 *11 | −0.33 *9 | −0.46 *9 |
| P. chrysogenum | −0.33 *9 | −0.47 *9 | 0.52 *4 | 0.76 *3 | 0.98 *2 | 0.86 *3 | −0.28 *8 | 0.99 *2 |
| A. alternata | −0.97 *12 | −0.51 *10 | −0.15 *8 | −0.04 *3 | 0.08 *6 | 0.32 *5 | 0.79 *3 | 0.28 *6 |
| Correlation IZD Versus | Phenols | Flavonoids | HMF | Pb, mg/kg | Cd, mg/kg | Fe, mg/kg | Cu, mg/kg | Zn, mg/kg |
|---|---|---|---|---|---|---|---|---|
| E. coli | −0.53 *10 | −0.85 *11 | −0.01 *8 | 0.19 *6 | 0.87 *3 | 0.01 *6 | −0.69 *10 | 0.99 *2 |
| S. enteritidis | 0.84 *3 | 0.84 *3 | 0.65 *4 | −0.36 *9 | −0.33 *9 | 0.11 *6 | 0.12 *6 | −0.72 *11 |
| P. aeruginosa | −0.79 *11 | −0.52 *10 | −0.94 *12 | 0.31 *5 | −0.23 *8 | −0.21 *8 | 0.37 *5 | 0.21 *6 |
| S. aureus | 0.27 *6 | 0.54 *4 | −0.28 *8 | 0.72 *3 | −0.82 *11 | 0.59 *4 | 0.95 *2 | −0.41 *9 |
| S. epidermidis | 0.84 *3 | 0.84 *3 | 0.65 *4 | −0.36 *9 | −0.33 *9 | 0.11 *6 | 0.12 *6 | −0.72 *11 |
| B. cereus | −0.27 *8 | 0.22 *6 | −0.75 *11 | −0.15 *8 | −0.82 *11 | −0.40 *9 | 0.72 *3 | −0.69 *10 |
| L. monocytogenes | −0.27 *8 | 0.22 *6 | −0.75 *11 | −0.15 *8 | −0.82 *11 | −0.40 *9 | 0.72 *3 | −0.60 *10 |
| Correlation IZD Versus | Phenols | Flavonoids | HMF | Pb, mg/kg | Cd, mg/kg | Fe, mg/kg | Cu, mg/kg | Zn, mg/kg |
|---|---|---|---|---|---|---|---|---|
| C. albicans | −0.10 *8 | −0.44 *9 | 0.20 *6 | 0.69 *4 | 0.58 *4 | 0.59 *4 | −0.30 *9 | 0.87 *3 |
| A. flavus | −0.72 *11 | −0.34 *9 | −0.88 *11 | −0.44 *9 | −0.33 *9 | −0.80 *11 | 0.23 *6 | −0.29 *8 |
| A. niger | −0.10 *8 | −0.44 *9 | 0.20 *6 | 0.69 *4 | 0.58 *4 | 0.59 *4 | −0.30 *9 | 0.87 *3 |
| R. stolonifer | 0.99 *2 | 0.86 *3 | 0.80 *3 | 0.28 *6 | −0.26 *8 | 0.71 *3 | 0.24 *6 | −0.38 *9 |
| F. oxysporum | −0.54 *10 | −0.32 *9 | −0.47 *9 | −0.88 *11 | 0.00 *7 | −0.99 *12 | −0.23 *8 | −0.28 *8 |
| P. chrysogenum | 0.91 *2 | 0.60 *4 | 0.96 *2 | 0.21 *6 | 0.13 *6 | 0.67 *4 | −0.14 *8 | −0.06 *8 |
| A. alternata | −0.82 *11 | −0.52 *10 | −0.98 *12 | 0.18 *6 | −0.26 *8 | −0.33 *9 | 0.37 *5 | 0.12 *6 |
| Country/Organization | Lead, mg/kg | Cadmium, mg/kg | Mercury, mg/kg | Methyl-mercury, mg/kg | Arsenic, mg/kg | Copper, mg/kg | Tin, mg/kg |
|---|---|---|---|---|---|---|---|
| UE (EFSA) | 0.10 | – | – | – | – | – | – |
| Codex Alimentarius | 0.10 | – | – | – | – | – | – |
| Poland (RMH) | 0.30 | 0.03 | 0.01 | - | 0.20 | - | - |
| India (FSSAI) | 2.50 | 1.50 | 1.00 | 0.25 | 1.10 | 30.00 | 250.00 |
| China (CFSA) | 0.05 | 1.00 | 0.01 | – | 0.05 | – | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glevitzky, M.; Corcheş, M.-T.; Popa, M.; Vică, M.L. Honey as a Bioindicator: Pollution’s Effects on Its Quality in Mining vs. Protected Sites. Appl. Sci. 2025, 15, 7297. https://doi.org/10.3390/app15137297
Glevitzky M, Corcheş M-T, Popa M, Vică ML. Honey as a Bioindicator: Pollution’s Effects on Its Quality in Mining vs. Protected Sites. Applied Sciences. 2025; 15(13):7297. https://doi.org/10.3390/app15137297
Chicago/Turabian StyleGlevitzky, Mirel, Mihai-Teopent Corcheş, Maria Popa, and Mihaela Laura Vică. 2025. "Honey as a Bioindicator: Pollution’s Effects on Its Quality in Mining vs. Protected Sites" Applied Sciences 15, no. 13: 7297. https://doi.org/10.3390/app15137297
APA StyleGlevitzky, M., Corcheş, M.-T., Popa, M., & Vică, M. L. (2025). Honey as a Bioindicator: Pollution’s Effects on Its Quality in Mining vs. Protected Sites. Applied Sciences, 15(13), 7297. https://doi.org/10.3390/app15137297







