Clinical Characterization of Patients with Syncope of Unclear Cause Using Unsupervised Machine-Learning Tools: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Conceptual Design of the Study
3. Results
3.1. Study Population
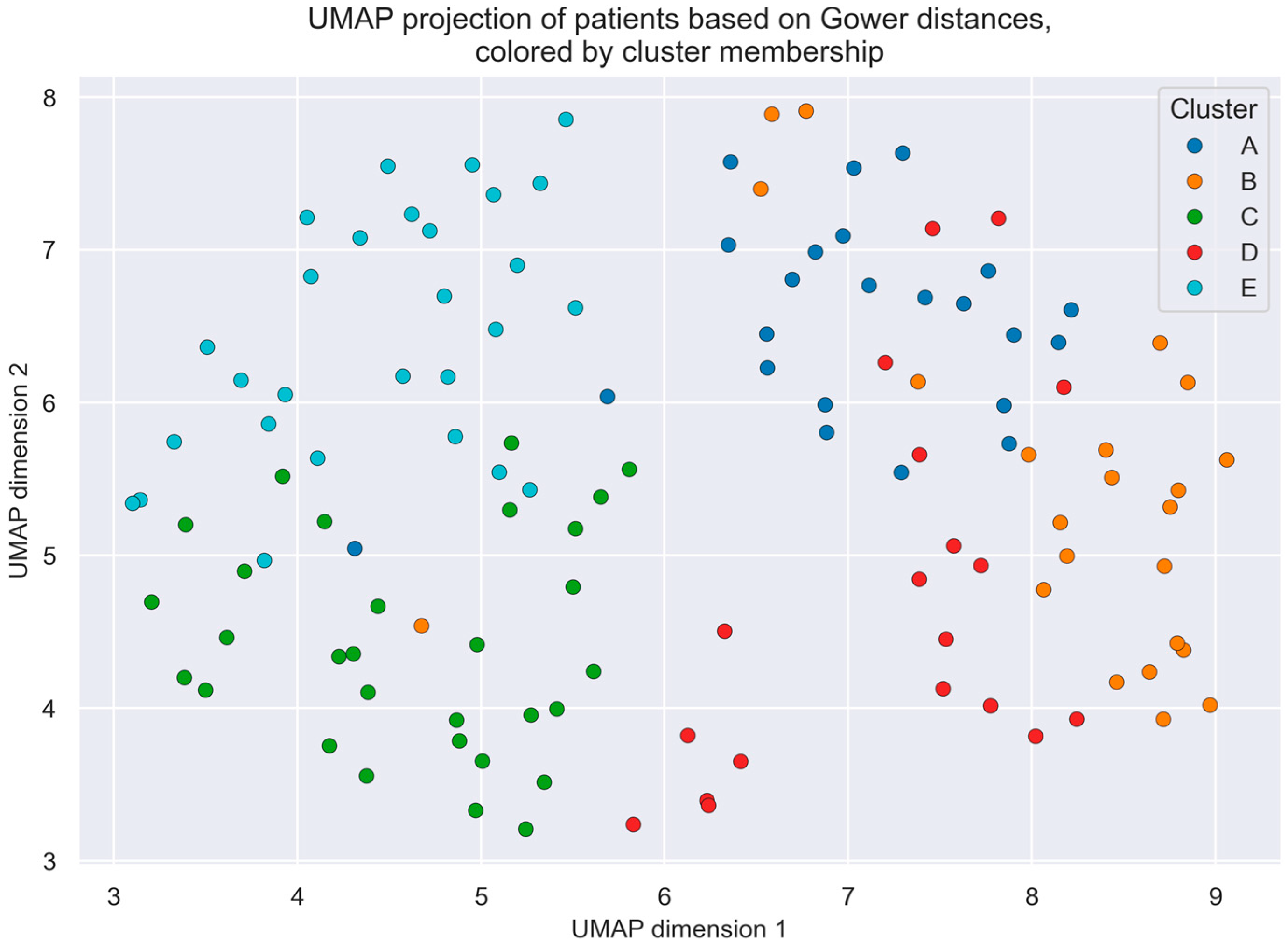
3.2. Cluster Analysis
- Cluster A—Young women with daytime fatigue and sleepiness, who are overweight, without cardiovascular risk factors: This group represents 18.7% of the sample and is composed predominantly of women (60.9%). It is the youngest cluster (51.5 ± 14.2 years), followed by Cluster E, with an intermediate BMI (28.2 ± 4.9 kg/m2), no history of diabetes, who are non-smokers and without cardiorespiratory disease. It presents the highest level of daytime sleepiness (Epworth score: 11.9 ± 6.2). Regarding HRV, this cluster shows low SDNN (100.57 ± 29.84 ms), RMSSD (58.22 ± 24.68 ms), and SDANN (78.0 ± 37.7 ms) values, indicating predominant nocturnal sympathetic activation.
- Cluster B—Elderly women with no daytime symptoms, who are overweight, with cardiovascular risk factors: This group also accounts for 18.7% of the patients and includes the oldest individuals (75.4 ± 5.8 years). It is characterized by a predominance of women (60.9%) with multiple cardiovascular risk factors, such as hypertension (91.6%) and dyslipidemia (66.6%), but low daytime sleepiness (Epworth score: 5.8 ± 5.4). Atrial fibrillation is present in 25% of patients. None are smokers. HRV is also diminished (SDNN: 80.52 ± 26.79 ms; RMSSD: 62.17 ± 32.23 ms), again suggesting enhanced nocturnal sympathetic activity.
- Cluster C—Older, but not elderly, asymptomatic men without cardiovascular risk factors: Representing 24.4% of the sample, this cluster consists mainly of men (73.3%) with intermediate age (62.0 ± 9.9 years) and the lowest BMI (24.2 kg/m2). Patients report no significant fatigue or excessive sleepiness (Epworth score: 4.2 ± 3.9) and have the lowest number of syncopal episodes in the past 12 months. HRV parameters (SDNN: 140.93 ± 166.27 ms; RMSSD: 88.17 ± 68.11 ms) are consistent with a preserved parasympathetic tone.
- Cluster D—Elderly obese men with cardiovascular risk factors: This group represents 15.4% of the sample, with a predominance of men (63.2%), and is the only cluster characterized by obesity (BMI: 30.0 ± 4.8 kg/m2). Patients present with hypertension (73.7%), dyslipidemia (68%), and the highest prevalence of diabetes (36.8%). Nearly half have atrial fibrillation (47.4%), and 26.3% have ischemic heart disease. While most are ex-smokers, they show the highest cumulative tobacco exposure. Together with Cluster B, they report the highest total number of syncopal episodes (10.7 ± 13.0). HRV parameters (SDNN: 266.37 ± 206.98 ms; RMSSD: 296.89 ± 70.73 ms) reflect parasympathetic predominance, though with wide variability.
- Cluster E—Young overweight male or female smokers with dyslipidemia, daytime fatigue, and frequent nocturnal awakenings: This is the largest cluster (28.8%) and the second youngest (52.6 ± 11.2 years), with a balanced male–female distribution. A high proportion are current smokers (67.9%), while 32.1% are former smokers. This group shows the highest prevalence of asthma (17.9%), and although they do not present with hypertension or diabetes, 39.3% have dyslipidemia. They report the highest number of syncopal episodes in the last 12 months (5.5 ± 7.5). Daytime sleepiness is comparable to Cluster A (Epworth score: 11.7 ± 6.1), and sleep quality is the poorest, with more awakenings and marked non-restorative sleep. HRV indicates marked nocturnal sympathetic activity (SDNN: 102.64 ± 50.21 ms; RMSSD: 90.11 ± 96.48 ms), with the lowest mean RR interval (893.89 ± 136.89 ms) and the highest LF/HF ratio (3.8 ± 3.0).
3.3. Association of Clusters with Respiratory Polygraphy Variables
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Cluster | Silhouette Score Per Cluster |
|---|---|
| A | 0.24 |
| B | 0.17 |
| C | 0.14 |
| D | 0.14 |
| E | 0.08 |
References
- Brignole, M.; Moya, A.; De Lange, F.J.; Deharo, J.C.; Elliott, P.M.; Fanciulli, A.; Fedorowski, A.; Furlan, R.; Kenny, R.A.; Martín, A.; et al. 2018 ESC Guidelines for the Diagnosis and Management of Syncope. Eur. Heart J. 2018, 39, 1883–1948. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, T.; du Fay de Lavallaz, J.; Nestelberger, T.; Gualandro, D.M.; Strebel, I.; Badertscher, P.; Lopez-Ayala, P.; Widmer, V.; Freese, M.; Miró, Ò.; et al. Incidence, Characteristics, Determinants, and Prognostic Impact of Recurrent Syncope. Europace 2020, 22, 1885–1895. [Google Scholar] [CrossRef] [PubMed]
- Barón-Esquivias, G.; Quintanilla, M.; Díaz-Martín, A.J.; Barón-Solís, C.; Almeida-González, C.V.; García-Romero, C.; Paneque, I.; Rubio-Guerrero, C.; Rodríguez-Corredor, R.; Valle-Racero, J.I.; et al. Long-Term Recurrences and Mortality in Patients with Noncardiac Syncope. Rev. Española Cardiol. 2022, 75, 568–575. [Google Scholar] [CrossRef]
- Galron, E.; Kehat, O.; Weiss-Meilik, A.; Furlan, R.; Jacob, G. Diagnostic Approaches to Syncope in Internal Medicine Departments and Their Effect on Mortality. Eur. J. Intern. Med. 2022, 102, 97–103. [Google Scholar] [CrossRef]
- Johansson, M.; Rogmark, C.; Sutton, R.; Fedorowski, A.; Hamrefors, V. Risk of Incident Fractures in Individuals Hospitalised Due to Unexplained Syncope and Orthostatic Hypotension. BMC Med. 2021, 19, 188. [Google Scholar] [CrossRef]
- Sun, B.C. Quality-of-Life, Health Service Use, and Costs Associated with Syncope. Prog. Cardiovasc. Dis. 2013, 55, 370–375. [Google Scholar] [CrossRef]
- Kenny, R.A.; Bhangu, J.; King-Kallimanis, B.L. Epidemiology of Syncope/Collapse in Younger and Older Western Patient Populations. Prog. Cardiovasc. Dis. 2013, 55, 357–363. [Google Scholar] [CrossRef]
- Bennett, M.T.; Leader, N.; Krahn, A.D. Recurrent Syncope: Differential Diagnosis and Management. Heart 2015, 101, 1591–1599. [Google Scholar] [CrossRef]
- Ruwald, M.H.; Hansen, M.L.; Lamberts, M.; Vinther, M.; Torp-Pedersen, C.; Hansen, J.; Gislason, G.H. Unexplained Syncope and Diagnostic Yield of Tests in Syncope According to the ICD-10 Discharge Diagnosis. J. Clin. Med. Res. 2013, 5, 441. [Google Scholar] [CrossRef][Green Version]
- Goldberger, Z.D.; Petek, B.J.; Brignole, M.; Shen, W.K.; Sheldon, R.S.; Solbiati, M.; Deharo, J.C.; Moya, A.; Hamdan, M.H. ACC/AHA/HRS Versus ESC Guidelines for the Diagnosis and Management of Syncope: JACC Guideline Comparison. J. Am. Coll. Cardiol. 2019, 74, 2410–2423. [Google Scholar] [CrossRef]
- Wakai, A.; Sinert, R.; Zehtabchi, S.; de Souza, I.S.; Benabbas, R.; Allen, R.; Dunne, E.; Richards, R.; Ardilouze, A.; Rovic, I. Risk-Stratification Tools for Emergency Department Patients with Syncope: A Systematic Review and Meta-Analysis of Direct Evidence for SAEM GRACE. Acad. Emerg. Med. 2024, 32, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, X.; Zhang, L.; Li, L.; Huang, Y.; Sun, Y.; Yuan, X. Artificial Intelligence in Clinical Decision Support Systems for Oncology. Int. J. Med. Sci. 2023, 20, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Ramgopal, S.; Sanchez-Pinto, L.N.; Horvat, C.M.; Carroll, M.S.; Luo, Y.; Florin, T.A. Artificial Intelligence-Based Clinical Decision Support in Pediatrics. Pediatr. Res. 2022, 93, 334–341. [Google Scholar] [CrossRef]
- Casal-Guisande, M.; Cerqueiro-Pequeño, J.; Comesaña-Campos, A.; Bouza-Rodríguez, J.B. Proposal of a Methodology Based on Expert Systems for the Treatment of Diabetic Foot Condition. In Proceedings of the Eighth International Conference on Technological Ecosystems for Enhancing Multiculturality, Association for Computing Machinery. Salamanca, Spain, 21 October 2020; pp. 491–495. [Google Scholar]
- Casal-Guisande, M.; Comesaña-Campos, A.; Núñez-Fernández, M.; Torres-Durán, M.; Fernández-Villar, A. Proposal and Definition of an Intelligent Clinical Decision Support System Applied to the Prediction of Dyspnea after 12 Months of an Acute Episode of COVID-19. Biomedicines 2024, 12, 854. [Google Scholar] [CrossRef]
- Corbacho-Abelaira, D.; Casal-Guisande, M.; Corbacho-Abelaira, F.; Arnaiz-Fernandez, M.; Trinidad-Lopez, C.; Delgado Sanchez-Gracian, C.; Sanchez-Montanes, M.; Ruano-Ravina, A.; Fernandez-Villar, A. Proposal and Definition of an Intelligent Decision- Support System Based on Deep Learning Techniques for the Management of Possible COVID-19 Cases in Patients Attending Emergency Departments. IEEE Access 2024, 12, 95035–95046. [Google Scholar] [CrossRef]
- López-Canay, J.; Casal-Guisande, M.; Pinheira, A.; Golpe, R.; Comesaña-Campos, A.; Fernández-García, A.; Represas-Represas, C.; Fernández-Villar, A. Predicting COPD Readmission: An Intelligent Clinical Decision Support System. Diagnostics 2025, 15, 318. [Google Scholar] [CrossRef]
- Casal-Guisande, M.; Fernández-Villar, A.; Mosteiro-Añón, M.; Comesaña-Campos, A.; Cerqueiro-Pequeño, J.; Torres-Durán, M. Integrating Tabular Data through Image Conversion for Enhanced Diagnosis: A Novel Intelligent Decision Support System for Stratifying Obstructive Sleep Apnoea Patients Using Convolutional Neural Networks. Digital Health 2024, 10, 20552076241272632. [Google Scholar] [CrossRef]
- Grant, L.; Joo, P.; Nemnom, M.J.; Thiruganasambandamoorthy, V. Machine Learning versus Traditional Methods for the Development of Risk Stratification Scores: A Case Study Using Original Canadian Syncope Risk Score Data. Intern. Emerg. Med. 2022, 17, 1145–1153. [Google Scholar] [CrossRef]
- Goh, C.H.; Ferdowsi, M.; Gan, M.H.; Kwan, B.H.; Lim, W.Y.; Tee, Y.K.; Rosli, R.; Tan, M.P. Assessing the Efficacy of Machine Learning Algorithms for Syncope Classification: A Systematic Review. MethodsX 2023, 12, 102508. [Google Scholar] [CrossRef]
- Casal-Guisande, M.; Represas-Represas, C.; Golpe, R.; Fernández-García, A.; González-Montaos, A.; Comesaña-Campos, A.; Ruano-Raviña, A.; Fernández-Villar, A. Clinical and Social Characterization of Patients Hospitalized for COPD Exacerbation Using Machine Learning Tools. Arch. Bronconeumol. 2024, 61, 264–273. [Google Scholar] [CrossRef]
- Puel, V.; Pepin, J.L.; Gosse, P. Sleep Related Breathing Disorders and Vasovagal Syncope, a Possible Causal Link? Int. J. Cardiol. 2013, 168, 1666–1667. [Google Scholar] [CrossRef] [PubMed]
- Puel, V.; Godard, I.; Papaioannou, G.; Gosse, P.; Pepin, J.L.; Thoin, F.; Deharo, J.C.; Roche, F.; Zarqane, N.; Gagnadoux, F.; et al. Management of Sleep Apnoea Syndrome (SAS) in Patients with Vasovagal Syncope (VVS): A Protocol for the VVS-SAS Cohort Study. BMJ Open 2020, 10, e038791. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Martínez, M.J.; Fernández-Villar, A.; Casal-Guisande, M.; García-Campo, E.; Corbacho-Abelaira, D.; Souto-Alonso, A.; Sopeña, B. Prevalence of Sleep Apnea in Patients with Syncope of Unclear Cause: SINCOSAS Study. Medicina 2025, 61, 887. [Google Scholar] [CrossRef]
- Mediano, O.; González Mangado, N.; Montserrat, J.M.; Alonso-Álvarez, M.L.; Almendros, I.; Alonso-Fernández, A.; Barbé, F.; Borsini, E.; Caballero-Eraso, C.; Cano-Pumarega, I.; et al. International Consensus Document on Obstructive Sleep Apnea. Arch. Bronconeumol. 2022, 58, 52–68. [Google Scholar] [CrossRef]
- Doane, D.P.; Seward, L.E. Measuring Skewness: A Forgotten Statistic? J. Stat. Educ. 2011, 19. [Google Scholar] [CrossRef]
- Osborne, J.W. Improving Your Data Transformations: Applying the Box-Cox Transformation. Pract. Assess. Res. Eval. 2010, 15, 12. [Google Scholar] [CrossRef]
- Agresti, A. Categorical Data Analysis; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2002; ISBN 0471360937. [Google Scholar]
- Powers, D.; Xie, Y. Statistical Methods for Categorical Data Analysis; Emerald Group Publishing: Bradford, UK, 2008. [Google Scholar]
- Huang, Z.X. Clustering Large Datasets with Mixed Numeric and Categorical Values. In Proceedings of the First Pacific-Asia Knowledge Discovery and Data Mining Conference, Singapore, 23–24 February 1997; pp. 21–34. [Google Scholar]
- Han, J.; Kamber, M.; Pei, J. Data Mining: Concepts and Techniques, 3rd ed.; Morgan Kaufmann: Waltham, MA, USA, 2012. [Google Scholar]
- Cao, F.; Liang, J.; Bai, L. A New Initialization Method for Categorical Data Clustering. Expert. Syst. Appl. 2009, 36, 10223–10228. [Google Scholar] [CrossRef]
- Shahapure, K.R.; Nicholas, C. Cluster Quality Analysis Using Silhouette Score. In Proceedings of the 2020 IEEE 7th International Conference on Data Science and Advanced Analytics, DSAA, Sydney, Australia, 6–9 October 2020; pp. 747–748. [Google Scholar] [CrossRef]
- Gower, J.C. A General Coefficient of Similarity and Some of Its Properties. Biometrics 1971, 27, 857. [Google Scholar] [CrossRef]
- McInnes, L.; Healy, J.; Melville, J. UMAP: Uniform Manifold Approximation and Projection for Dimension Reduction. arXiv 2018, arXiv:1802.03426. [Google Scholar]
- Samuels, M.L.; Witmer, J.A.; Schaffner, A.A. Statistics for the Life Sciences, 5th ed.; Pearson Education: London, UK, 2016. [Google Scholar]
- Statz, G.M.; Evans, A.Z.; Johnston, S.L.; Adhaduk, M.; Mudireddy, A.R.; Sonka, M.; Lee, S.; Barsotti, E.J.; Ricci, F.; Dipaola, F.; et al. Can Artificial Intelligence Enhance Syncope Management?: A JACC: Advances Multidisciplinary Collaborative Statement. JACC Adv. 2023, 2, 100323. [Google Scholar] [CrossRef]
- Aamir, A.; Jamil, Y.; Bilal, M.; Diwan, M.; Nashwan, A.J.; Ullah, I. Artificial Intelligence in Enhancing Syncope Management—An Update. Curr. Probl. Cardiol. 2024, 49, 102079. [Google Scholar] [CrossRef] [PubMed]
- Dipaola, F.; Gebska, M.A.; Gatti, M.; Levra, A.G.; Parker, W.H.; Menè, R.; Lee, S.; Costantino, G.; Barsotti, E.J.; Shiffer, D.; et al. Will Artificial Intelligence Be “Better” Than Humans in the Management of Syncope? JACC Adv. 2024, 3, 101072. [Google Scholar] [CrossRef] [PubMed]
- Muzammil, M.A.; Javid, S.; Afridi, A.K.; Siddineni, R.; Shahabi, M.; Haseeb, M.; Fariha, F.N.U.; Kumar, S.; Zaveri, S.; Nashwan, A.J. Artificial Intelligence-Enhanced Electrocardiography for Accurate Diagnosis and Management of Cardiovascular Diseases. J. Electrocardiol. 2024, 83, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Moloney, D.; O’Connor, J.; Newman, L.; Scarlett, S.; Hernandez, B.; Kenny, R.A.; Romero-Ortuno, R. Clinical Clustering of Eight Orthostatic Haemodynamic Patterns in The Irish Longitudinal Study on Ageing (TILDA). Age Ageing 2021, 50, 854–860. [Google Scholar] [CrossRef]
- Guftar, M.; Ali, S.H.; Raja, A.A.; Qamar, U. A Novel Framework for Classification of Syncope Disease Using K-Means Clustering Algorithm. In Proceedings of the 2015 SAI Intelligent Systems Conference (IntelliSys), London, UK, 10–11 November 2015; pp. 127–132. [Google Scholar] [CrossRef]
- Stein, P.K.; Pu, Y. Heart Rate Variability, Sleep and Sleep Disorders. Sleep. Med. Rev. 2012, 16, 47–66. [Google Scholar] [CrossRef]
- Baharav, A.; Kotagal, S.; Gibbons, V.; Rubin, B.K.; Pratt, G.; Karin, J.; Akselrod, S. Fluctuations in Autonomic Nervous Activity during Sleep Displayed by Power Spectrum Analysis of Heart Rate Variability. Neurology 1995, 45, 1183–1187. [Google Scholar] [CrossRef]
- Noda, A.; Yasuma, F.; Okada, T.; Yokota, M. Circadian Rhythm of Autonomic Activity in Patients with Obstructive Sleep Apnea Syndrome. Clin. Cardiol. 1998, 21, 271–276. [Google Scholar] [CrossRef]
- Lin, C.Y.; Tsai, S.J.; Peng, C.K.; Yang, A.C. Sleep State Instabilities in Patients with Periodic Limb Movements in Sleep—Detection and Quantification with Heart Rate Variability. Psychiatry Res. 2020, 293, 113454. [Google Scholar] [CrossRef]
- Zhao, W.; Jiang, B. Heart Rate Variability in Patients with Insomnia Disorder: A Systematic Review and Meta-Analysis. Sleep. Breath. 2023, 27, 1309–1313. [Google Scholar] [CrossRef]
- Weber, F.; Schneider, H.; Von Arnim, T.; Urbaszek, W. Heart Rate Variability and Ischaemia in Patients with Coronary Heart Disease and Stable Angina Pectoris; Influence of Drug Therapy and Prognostic Value. TIBBS Investigators Group. Total Ischemic Burden Bisoprolol Study. Eur. Heart J. 1999, 20, 38–50. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, F.; Xiao, J.; Chen, L.; Zhang, Y.; Li, J.; Yi, Y.; Min, W.; Su, L.; Liu, X.; et al. Heart Rate Variability Changes in Patients with Obstructive Sleep Apnea: A Systematic Review and Meta-Analysis. J. Sleep. Res. 2023, 32, e13708. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Lv, T.; She, F.; Miao, G.; Liu, Y.; He, R.; Xue, Y.; Nu, N.K.; Yang, J.; Li, K.; et al. The Impact of Continuous Positive Airway Pressure on Heart Rate Variability in Obstructive Sleep Apnea Patients during Sleep: A Meta-Analysis. Heart Lung 2018, 47, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.; Machado, C.C.V.; Burr, C.; Cowls, J.; Joshi, I.; Taddeo, M.; Floridi, L. The Ethics of AI in Health Care: A Mapping Review. Soc. Sci. Med. 2020, 260, 113172. [Google Scholar] [CrossRef] [PubMed]
- Grote, T.; Berens, P. On the Ethics of Algorithmic Decision-Making in Healthcare. J. Med. Ethics 2020, 46, 205–211. [Google Scholar] [CrossRef]
- Vayena, E.; Blasimme, A.; Cohen, I.G. Machine Learning in Medicine: Addressing Ethical Challenges. PLoS Med. 2018, 15, e1002689. [Google Scholar] [CrossRef]
- Sovrano, F.; Palmirani, M.; Vitali, F. Combining Shallow and Deep Learning Approaches against Data Scarcity in Legal Domains. Gov. Inf. Q. 2022, 39, 101715. [Google Scholar] [CrossRef]
- Juan, W.; Ahn, K.W.; Chen, Y.G.; Lin, C.W. CCI: A Consensus Clustering-Based Imputation Method for Addressing Dropout Events in ScRNA-Seq Data. Bioengineering 2025, 12, 31. [Google Scholar] [CrossRef]
- Harder, A.A.; Olbricht, G.R.; Ekuma, G.; Hier, D.B.; Obafemi-Ajayi, T. Multiple Imputation for Robust Cluster Analysis to Address Missingness in Medical Data. IEEE Access 2024, 12, 42974–42991. [Google Scholar] [CrossRef]
- Hao, K.A.; Vasilopoulos, T.; Elwell, J.; Roche, C.P.; Hones, K.M.; Wright, J.O.; King, J.J.; Wright, T.W.; Simovitch, R.W.; Schoch, B.S. Missing Data in Orthopaedic Clinical Outcomes Research: A Sensitivity Analysis of Imputation Techniques Utilizing a Large Multicenter Total Shoulder Arthroplasty Database. J. Clin. Med. 2025, 14, 3829. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, J.; Zhang, X. BMDD: A Probabilistic Framework for Accurate Imputation of Zero-Inflated Microbiome Sequencing Data. bioRxiv 2025. bioRxiv:2025.05.08.652808. [Google Scholar] [CrossRef]


| Variable | Population Summary | Cluster A (n = 23) | Cluster B (n = 23) | Cluster C (n = 30) | Cluster D (n = 19) | Cluster E (n = 28) | Significant Comparisons |
|---|---|---|---|---|---|---|---|
| Age | 62.0 ± 14.4 | 51.5 ± 14.2 | 75.4 ± 5.8 | 62.0 ± 9.9 | 72.5 ± 12.2 | 52.6 ± 11.2 | A ≠ B: p < 0.001, A ≠ C: p = 0.005, A ≠ D: p < 0.001, B ≠ C: p < 0.001, B ≠ E: p < 0.001, C ≠ D: p < 0.001, C ≠ E: p = 0.002, D ≠ E: p < 0.001 |
| Man (%) | 55.3% (43.5, 67.1) | 39.1% (19.2, 59.1) | 39.1% (19.2, 59.1) | 73.3% (57.5, 89.2) | 63.2% (41.5, 84.9) | 57.1% (38.8, 75.5) | A ≠ C: p = 0.026, B ≠ C: p = 0.026 |
| BMI (5) | 27.8 ± 5.3 | 28.2 ± 4.9 | 27.6 ± 3.2 | 26.9 ± 5.3 | 30.0 ± 4.8 | 27.1 ± 6.8 | C ≠ D: p = 0.024, D ≠ E: p = 0.035 |
| Diabetes: No (%) | 86.2% (79.6, 92.8) | 100.0% (100.0, 100.0) | 73.9% (56.0, 91.9) | 90.0% (79.3, 100.0) | 63.2% (41.5, 84.9) | 96.4% (89.6, 100.0) | A ≠ B: p = 0.029, A ≠ D: p = 0.006, D ≠ E: p = 0.010 |
| Diabetes: Yes, no insulin (%) | 11.4% (0.0, 28.0) | 0.0% (0.0, 0.0) | 26.1% (8.1, 44.0) | 10.0% (0.0, 20.7) | 26.3% (6.5, 46.1) | 0.0% (0.0, 0.0) | A ≠ B: p = 0.029, A ≠ D: p = 0.032, B ≠ E: p = 0.015, D ≠ E: p = 0.017 |
| Diabetes: Yes, with insulin (%) | 2.4% (0.0, 19.9) | 0.0% (0.0, 0.0) | 0.0% (0.0, 0.0) | 0.00% (0.00, 0.00) | 10.5% (0.0, 24.3) | 3.6% (0.0, 10.5) | - |
| Dyslipidemia (%) | 41.5% (27.9, 55.0) | 17.4% (1.9, 32.9) | 69.6% (50.8, 88.4) | 23.3% (8.2, 38.5) | 68.% (47.5, 89.3) | 39.3% (21.2, 57.4) | A ≠ B: p = 0.001, A ≠ D: p = 0.002, B ≠ C: p = 0.002, C ≠ D: p = 0.005 |
| Hypertension (%) | 43.1% (29.8, 56.4) | 13.0% (0.0, 26.8) | 87.0% (73.2, 100.0) | 30.0% (13.6, 46.4) | 73.7% (53.9, 93.5) | 25.0% (9.0, 41.0) | A ≠ B: p < 0.001, A ≠ D: p < 0.001, B ≠ C: p < 0.001, B ≠ E: p < 0.001, C ≠ D: p = 0.007, D ≠ E: p = 0.003 |
| Never smoker (%) | 45.5% (32.5, 58.6) | 91.3% (79.8, 100.0) | 95.7% (87.3, 100.0) | 0.0% (0.0, 0.0) | 68.4% (47.5, 89.3) | 0.0% (0.0, 0.0) | A ≠ C: p < 0.001, A ≠ E: p < 0.001, B ≠ C: p < 0.001, B ≠ E: p < 0.001, C ≠ D: p < 0.001, D ≠ E: p < 0.001 |
| Smoker (%) | 21.95% (6.3, 37.6) | 4.4% (0.0, 12.7) | 0.0% (0.0, 0.0) | 20.0% (5.7, 34.3) | 5.3% (0.0, 15.3) | 67.9% (50.6, 85.2) | A ≠ E: p < 0.001, B ≠ E: p < 0.001, C ≠ E: p < 0.001, D ≠ E: p < 0.001 |
| Former smoker (%) | 32.52% (18.0, 47.0) | 4.4% (0.0, 12.7) | 4.4% (0.0, 12.7) | 80.0% (65.7, 94.3) | 26.3% (6.5, 46.1) | 32.1% (14.8, 49.4) | A ≠ C: p < 0.001, A ≠ E: p = 0.033, B ≠ C: p < 0.001, B ≠ E: p = 0.033, C ≠ D: p < 0.001, C ≠ E: p < 0.001 |
| Pack-year | 16.5 ± 24.8 | 0.4 ± 1.2 | 0.3 ± 1.5 | 30.1 ± 26.5 | 18.0 ± 33.3 | 27.3 ± 22.5 | A ≠ C: p < 0.001, A ≠ D: p = 0.041, A ≠ E: p < 0.001, B ≠ C: p < 0.001, B ≠ D: p = 0.016, B ≠ E: p < 0.001, C ≠ D: p = 0.002, D ≠ E: p = 0.003 |
| Asthma (%) | 8.9% (0.0, 25.8) | 8.7% (0.0, 20.2) | 8.7% (0.0, 20.2) | 6.7% (0.0, 15.6) | 0.0% (0.0, 0.0) | 17.9% (3.7, 32.0) | - |
| COPD (%) | 5.7% (0.0, 22.9) | 4.4% (0.0, 12.7) | 0.0% (0.0, 0.0) | 10.0% (0.0, 20.7) | 10.5% (0.0, 24.3) | 3.6% (0.0, 10.5) | - |
| Ischemic heart disease (%) | 13.01% (0.0, 29.5) | 0.0% (0.0, 0.0) | 17.4% (1.9, 32.9) | 16.7% (3.3, 30.0) | 26.3% (6.5, 46.1) | 7.1% (0.0, 16.7) | A ≠ D: p = 0.032 |
| Atrial fibrillation (%) | 15.5% (0.0, 31.7) | 0.0% (0.0, 0.0) | 26.1% (8.1, 44.0) | 13.3% (1.2, 25.5) | 47.4% (24.9, 69.8) | 0.0% (0.0, 0.0) | A ≠ B: p = 0.029, A ≠ D: p < 0.001, B ≠ E: p = 0.015, C ≠ D: p = 0.022, D ≠ E: p < 0.001 |
| Stroke (%) | 1.6% (0.0, 19.2) | 0.0% (0.0, 0.0) | 4.4% (0.0, 12.7) | 0.0% (0.0, 0.0) | 0.0% (0.0, 0.0) | 3.6% (0.0, 10.5) | - |
| Syncope last 12 months (%) | 3.7 ± 5.3 | 4.1 ± 6.7 | 3.8 ± 4.5 | 1.7 ± 1.6 | 3.89 ± 3.16 | 5.5 ± 7.5 | B ≠ C: p = 0.045, C ≠ D: p = 0.006 |
| Total number of syncopes | 8.6 ± 11.7 | 9.7 ± 11.8 | 10.5 ± 15.7 | 4.6 ± 4.1 | 10.7 ± 13.0 | 8.9 ± 12.0 | - |
| Injuries last 12 months | 1.0 ± 1.7 | 0.5 ± 0.8 | 1.3 ± 2.2 | 0.9 ± 1.9 | 1.6 ± 2.5 | 0.9 ± 0.9 | - |
| Daytime Tiredness | 48.8% (36.1, 61.4) | 60.9% (40.9, 80.8) | 34.8% (15.3, 54.3) | 20.0% (5.7, 34.3) | 42.1% (19.9, 64.3) | 85.7% (72.8, 98.7) | A ≠ C: p = 0.006, B ≠ E: p < 0.001, C ≠ E: p < 0.001, D ≠ E: p = 0.005 |
| Nocturnal awakenings | 52.0% (39.8, 64.3) | 47.8% (27.4, 68.2) | 69.6% (50.8, 88.4) | 40.0% (22.5, 57.5) | 42.1% (19.9, 64.3) | 60.7% (42.6, 78.8) | - |
| Lack of concentration | 35.0 (20.7, 49.2) | 26.1% (8.1, 44.0) | 26.1% (8.1, 44.0) | 16.7% (3.3, 30.0) | 36.8% (15.2, 58.5) | 67.9% (50.6, 85.2) | A ≠ E: p = 0.007, B ≠ E: p = 0.007, C ≠ E: p < 0.001 |
| Witnessed apneas | 19.5% (3.7, 35.4) | 17.4% (1.9, 32.9) | 13.0% (0.0, 26.8) | 6.7% (0.0, 15.6) | 15.8% (0.0, 32.2) | 42.9% (24.5, 61.2) | B ≠ E: p = 0.044, C ≠ E: p = 0.004 |
| Asphyxia episodes | 8.9% (0.0, 25.8) | 21.7% (4.9, 38.6) | 8.7% (0.0, 20.2) | 3.3% (0.0, 9.8) | 0.0% (0.0, 0.0) | 10.7% (0.0, 22.2) | - |
| Non-restorative sleep | 50.4% (38.0, 62.9) | 69.6% (50.8, 88.4) | 30.4% (11.6, 49.2) | 33.3% (16.5, 50.2) | 36.8% (15.2, 58.5) | 78.6% (63.4, 93.8) | A ≠ B: p = 0.018, A ≠ C: p = 0.019, B ≠ E: p = 0.002, C ≠ E: p = 0.001, D ≠ E: p = 0.010 |
| Epworth | 8.5 ± 6.5 | 11.9 ± 6.2 | 5.8 ± 5.4 | 4.2 ± 3.9 | 9.5 ± 6.9 | 11.7 ± 6.1 | A ≠ B: p = 0.001, A ≠ C: p < 0.001, B ≠ E: p = 0.001, C ≠ D: p = 0.009, C ≠ E: p < 0.001 |
| Average RR | 967.44 ± 152.86 | 994.04 ± 150.97 | 953.13 ± 149.78 | 1034.73 ± 157.73 | 954.68 ± 132.73 | 893.89 ± 136.89 | A ≠ E: p = 0.017, C ≠ E: p < 0.001 |
| SDNN | 132.75 ± 132.12 | 100.57 ± 29.84 | 80.52 ± 26.79 | 140.93 ± 166.27 | 266.37 ± 206.98 | 102.64 ± 50.21 | A ≠ B: p = 0.011, A ≠ D: p < 0.001, B ≠ C: p < 0.001, B ≠ D: p < 0.001, C ≠ D: p < 0.001, D ≠ E: p < 0.001 |
| SDNN index | 93.44 ± 64.54 | 68.96 ± 22.39 | 54.87 ± 21.82 | 84.33 ± 40.26 | 214.37 ± 47.77 | 72.93 ± 46.53 | A ≠ B: p = 0.036, A ≠ D: p < 0.001, B ≠ C: p = 0.001, B ≠ D: p < 0.001, C ≠ D: p < 0.001, D ≠ E: p < 0.001 |
| RMSSD | 110.39 ± 103.84 | 58.22 ± 24.68 | 62.17 ± 32.23 | 88.17 ± 68.11 | 296.89 ± 70.73 | 90.11 ± 96.48 | A ≠ D: p < 0.001, B ≠ D: p < 0.001, C ≠ D: p < 0.001, D ≠ E: p < 0.001 |
| NN50 | 4774.1 ± 6303.0 | 4038.3 ± 3105.7 | 1787.2 ± 2229.5 | 3683.5 ± 3385.7 | 15,350.6 ± 8937.2 | 1823.7 ± 2216.5 | A ≠ B: p = 0.007, A ≠ D: p < 0.001, A ≠ E: p = 0.004, B ≠ C: p = 0.042, B ≠ D: p < 0.001, C ≠ D: p < 0.001, C ≠ E: p = 0.024, D ≠ E: p < 0.001 |
| pNN50 | 22.2 ± 34.6 | 16.5 ± 13.4 | 5.4 ± 5.8 | 16.7 ± 15.5 | 78.9 ± 56.4 | 8.1 ± 8.9 | A ≠ B: p = 0.003, A ≠ D: p < 0.001, A ≠ E: p = 0.017, B ≠ C: p = 0.009, B ≠ D: p < 0.001, C ≠ D: p < 0.001, C ≠ E: p = 0.042, D ≠ E: p < 0.001 |
| SDANN | 184.7 ± 531.4 | 78.0 ± 37.7 | 90.3 ± 122.4 | 131.3 ± 158.3 | 481.1 ± 1226.0 | 206.0 ± 388.6 | B ≠ C: p = 0.040 |
| Total power | 27,605.2 ± 18,804.3 | 38,752.5 ± 15,615.9 | 15,682.9 ± 10,137.8 | 36,500.8 ± 24,360.4 | 12,323.3 ± 4680.3 | 29,080.5 ± 13,485.7 | A ≠ B: p < 0.001, A ≠ D: p < 0.001, A ≠ E: p = 0.027, B ≠ C: p < 0.001, B ≠ E: p < 0.001, C ≠ D: p < 0.001, D ≠ E: p < 0.001 |
| VLF power | 12,664.6 ± 11,167.0 | 18,494.0 ± 9711.6 | 7203.7 ± 5403.4 | 18,113.1 ± 14,762.2 | 1471.6 ± 1724.4 | 14,119.4 ± 7006.8 | A ≠ B: p < 0.001, A ≠ D: p < 0.001, B ≠ C: p = 0.006, B ≠ D: p < 0.001, B ≠ E: p < 0.001, C ≠ D: p < 0.001, D ≠ E: p < 0.001 |
| LF | 10,033.1 ± 8326.8 | 13,331.7 ± 6290.2 | 5489.4 ± 4888.5 | 14,095.4 ± 11,352.6 | 3708.0 ± 2158.9 | 10,995.3 ± 6763.4 | A ≠ B: p < 0.001, A ≠ D: p < 0.001, B ≠ C: p < 0.001, B ≠ E: p < 0.001, C ≠ D: p < 0.001, D ≠ E: p < 0.001 |
| HF | 3814.0 ± 2249.0 | 6106.6 ± 2785.3 | 2267.0 ± 1491.9 | 4113.1 ± 1721.4 | 3568.3 ± 1728.8 | 3048.0 ± 1509.1 | A ≠ B: p < 0.001, A ≠ C: p = 0.007, A ≠ D: p = 0.006, A ≠ E: p < 0.001, B ≠ C: p < 0.001, B ≠ D: p = 0.029, C ≠ E: p = 0.016 |
| Triangular index | 17.0 ± 8.7 | 16.6 ± 4.7 | 10.3 ± 6. | 17.7 ± 5.9 | 26.8 ± 13.1 | 15.4 ± 5.6 | A ≠ B: p < 0.001, A ≠ D: p = 0.005, B ≠ C: p < 0.001, B ≠ D: p < 0.001, B ≠ E: p = 0.003, C ≠ D: p = 0.016, D ≠ E: p < 0.001 |
| LF/HF | 2.4 ± 2.5 | 2.2 ± 2.0 | 2.0 ± 1.6 | 2.8 ± 2.9 | 0.5 ± 0.7 | 3.8 ± 3.0 | A ≠ D: p < 0.001, A ≠ E: p = 0.028, B ≠ D: p = 0.001, B ≠ E: p = 0.027, C ≠ D: p < 0.001, D ≠ E: p < 0.001 |
| Variable | Population Summary | Cluster A (n = 23) | Cluster B (n = 23) | Cluster C (n = 30) | Cluster D (n = 19) | Cluster E (n = 28) | Significant Comparisons |
|---|---|---|---|---|---|---|---|
| AHI | 17.1 ± 16.2 | 9.4 ± 8.1 | 21.4 ± 19.4 | 13.2 ± 13.8 | 30.8 ± 13.7 | 14.8 ± 16.7 | A ≠ B: p = 0.041, A ≠ D: p < 0.001, C ≠ D: p < 0.001, D ≠ E: p < 0.001 |
| ID3 | 16.7 ± 16.1 | 8.8 ± 9.1 | 22.3 ± 19.3 | 14.0 ± 13.8 | 28.6 ± 14.5 | 13.5 ± 16.0 | A ≠ B: p = 0.007, A ≠ D: p < 0.001, C ≠ D: p < 0.001, D ≠ E: p < 0.001 |
| TC90 | 9.5 ± 17.7 | 0.9 ± 2.3 | 14.0 ± 22.3 | 9.5 ± 18.2 | 13.1 ± 23.1 | 10.6 ± 14.0 | A ≠ B: p = 0.001, A ≠ C: p = 0.004, A ≠ D: p < 0.001, A ≠ E: p < 0.001 |
| Number of obstructive apneas | 24.7 ± 52.0 | 8.5 ± 21.9 | 39.3 ± 79.5 | 16.7 ± 38.4 | 53.8 ± 63.4 | 15.0 ± 35.6 | A ≠ B: p = 0.011, A ≠ D: p = 0.008, C ≠ D: p = 0.033 |
| Number of central apneas | 6.9 ± 20.9 | 3.9 ± 7.7 | 4.2 ± 8.2 | 4.8 ± 12.8 | 24.2 ± 46.3 | 2.0 ± 3.8 | B ≠ D: p = 0.020, C ≠ D: p = 0.007, D ≠ E: p = 0.006 |
| Number of hypopneas | 79.5 ± 74.8 | 51.0 ± 42.7 | 95.4 ± 93.9 | 66.3 ± 57.9 | 120.1 ± 65.2 | 76.3 ± 88.8 | A ≠ D: p < 0.001, C ≠ D: p = 0.004, D ≠ E: p = 0.010 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Martínez, M.-J.; Casal-Guisande, M.; Torres-Durán, M.; Sopeña, B.; Fernández-Villar, A. Clinical Characterization of Patients with Syncope of Unclear Cause Using Unsupervised Machine-Learning Tools: A Pilot Study. Appl. Sci. 2025, 15, 7176. https://doi.org/10.3390/app15137176
Muñoz-Martínez M-J, Casal-Guisande M, Torres-Durán M, Sopeña B, Fernández-Villar A. Clinical Characterization of Patients with Syncope of Unclear Cause Using Unsupervised Machine-Learning Tools: A Pilot Study. Applied Sciences. 2025; 15(13):7176. https://doi.org/10.3390/app15137176
Chicago/Turabian StyleMuñoz-Martínez, María-José, Manuel Casal-Guisande, María Torres-Durán, Bernardo Sopeña, and Alberto Fernández-Villar. 2025. "Clinical Characterization of Patients with Syncope of Unclear Cause Using Unsupervised Machine-Learning Tools: A Pilot Study" Applied Sciences 15, no. 13: 7176. https://doi.org/10.3390/app15137176
APA StyleMuñoz-Martínez, M.-J., Casal-Guisande, M., Torres-Durán, M., Sopeña, B., & Fernández-Villar, A. (2025). Clinical Characterization of Patients with Syncope of Unclear Cause Using Unsupervised Machine-Learning Tools: A Pilot Study. Applied Sciences, 15(13), 7176. https://doi.org/10.3390/app15137176






