Bioactive Compounds in Breast Meat of Broiler Chickens Fed with Black Soldier Fly Wholemeal
Abstract
1. Introduction
2. Materials and Methods
2.1. Birds, Diets, and Experimental Design
2.2. Meat Quality Measurements
2.3. Quantification of Fatty Acids and CLA by Gas Chromatography
2.4. Preparation of Meat for Antioxidant Activity
2.5. Ferric-Reducing Antioxidant Power (FRAP)
2.6. 2-2′-Azino-di-[3-Ethylbenzthiazoline Sulfonate] (ABTS) Radical Scavenging Activity
2.7. Thiols Group
2.8. Creatine and Carnosine Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition of Breast Meat
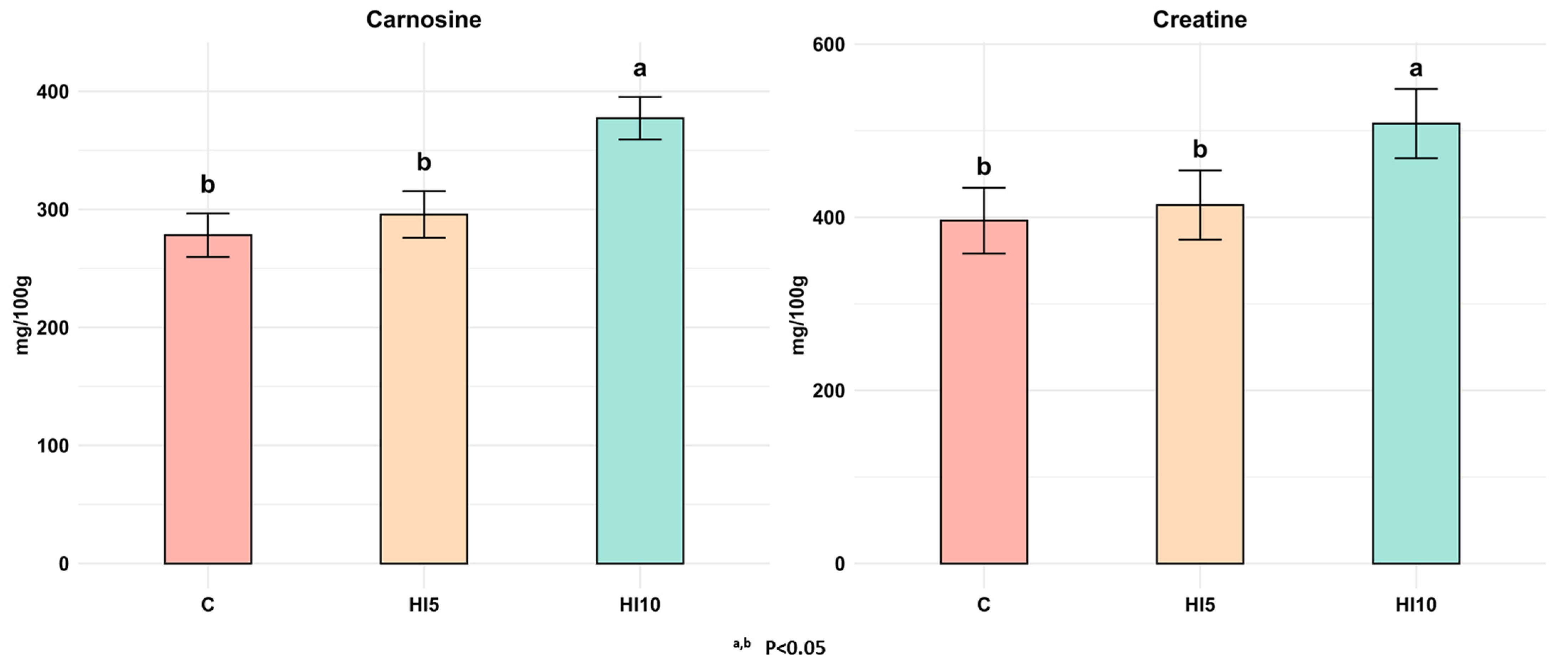
3.2. Bioactive Peptides: Carnosine e Carnitine
3.3. Functional Fatty Acids Content in Breast Meat
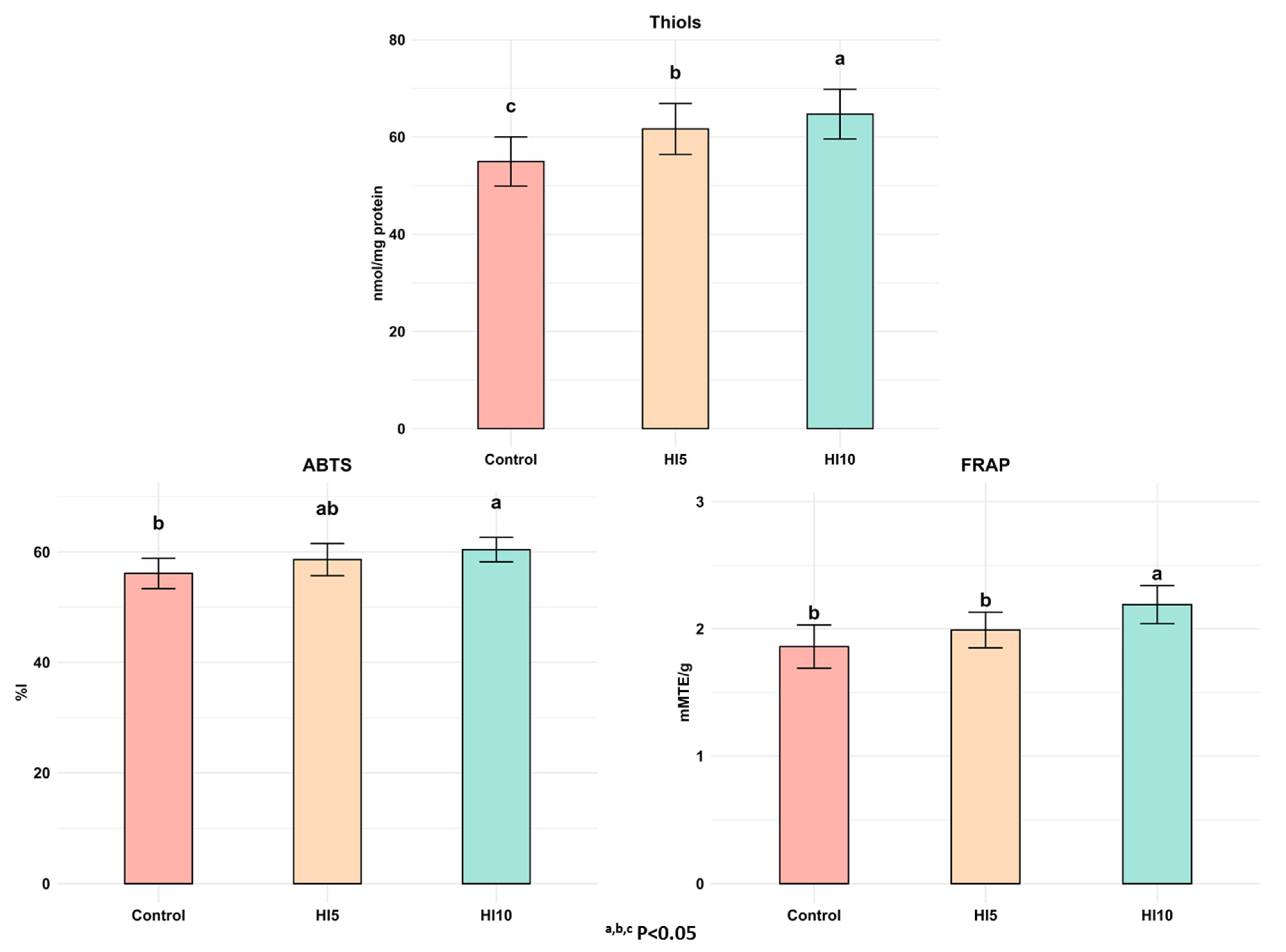
3.4. Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scieuzo, C.; Franco, A.; Salvia, R.; Triunfo, M.; Addeo, N.F.; Vozzo, S.; Piccolo, G.; Bovera, F.; Ritieni, A.; Francia, A.D.; et al. Enhancement of Fruit Byproducts through Bioconversion by Hermetia illucens (Diptera: Stratiomyidae). Insect Sci. 2023, 30, 991–1010. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.; Scieuzo, C.; Salvia, R.; Pucciarelli, V.; Borrelli, L.; Addeo, N.F.; Bovera, F.; Laginestra, A.; Schmitt, E.; Falabella, P. Antimicrobial Activity of Lipids Extracted from Hermetia illucens Reared on Different Substrates. Appl. Microbiol. Biotechnol. 2024, 108, 167. [Google Scholar] [CrossRef] [PubMed]
- English, G.; Wanger, G.; Colombo, S.M. A Review of Advancements in Black Soldier Fly (Hermetia Illucens) Production for Dietary Inclusion in Salmonid Feeds. J. Agric. Food Res. 2021, 5, 100164. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Ristow, B.; Rahayu, T.; Putra, N.S.; Widya Yuwono, N.; Nisa’, K.; Mategeko, B.; Smetana, S.; Saki, M.; Nawaz, A.; et al. Black Soldier Fly Larvae (BSFL) and Their Affinity for Organic Waste Processing. Waste Manag. 2022, 140, 1–13. [Google Scholar] [CrossRef]
- Lu, S.; Taethaisong, N.; Meethip, W.; Surakhunthod, J.; Sinpru, B.; Sroichak, T.; Archa, P.; Thongpea, S.; Paengkoum, S.; Purba, R.A.P.; et al. Nutritional Composition of Black Soldier Fly Larvae (Hermetia illucens L.) and Its Potential Uses as Alternative Protein Sources in Animal Diets: A Review. Insects 2022, 13, 831. [Google Scholar] [CrossRef]
- Spranghers, T.; Ottoboni, M.; Klootwijk, C.; Ovyn, A.; Deboosere, S.; De Meulenaer, B.; Michiels, J.; Eeckhout, M.; De Clercq, P.; De Smet, S. Nutritional Composition of Black Soldier Fly (Hermetia illucens) Prepupae Reared on Different Organic Waste Substrates. J. Sci. Food Agric. 2017, 97, 2594–2600. [Google Scholar] [CrossRef]
- De Souza Vilela, J.; Alvarenga, T.I.R.C.; Andrew, N.R.; McPhee, M.; Kolakshyapati, M.; Hopkins, D.L.; Ruhnke, I. Technological Quality, Amino Acid and Fatty Acid Profile of Broiler Meat Enhanced by Dietary Inclusion of Black Soldier Fly Larvae. Foods 2021, 10, 297. [Google Scholar] [CrossRef]
- Ullah, H. Feed Sustainability and Efficiency. In Poultry Science; IntechOpen: London, UK, 2023; ISBN 978-1-80356-155-4. [Google Scholar]
- Kulczyński, B.; Sidor, A.; Gramza-Michałowska, A. Characteristics of Selected Antioxidative and Bioactive Compounds in Meat and Animal Origin Products. Antioxidants 2019, 8, 335. [Google Scholar] [CrossRef]
- Cruz-Casas, D.E.; Aguilar, C.N.; Ascacio-Valdés, J.A.; Rodríguez-Herrera, R.; Chávez-González, M.L.; Flores-Gallegos, A.C. Enzymatic Hydrolysis and Microbial Fermentation: The Most Favorable Biotechnological Methods for the Release of Bioactive Peptides. Food Chem. Mol. Sci. 2021, 3, 100047. [Google Scholar] [CrossRef]
- Jukić, I.; Kolobarić, N.; Stupin, A.; Matić, A.; Kozina, N.; Mihaljević, Z.; Mihalj, M.; Šušnjara, P.; Stupin, M.; Ćurić, Ž.B.; et al. Carnosine, Small but Mighty—Prospect of Use as Functional Ingredient for Functional Food Formulation. Antioxidants 2021, 10, 1037. [Google Scholar] [CrossRef]
- Xing, L.; Li, G.; Toldrá, F.; Zhang, W. The Physiological Activity of Bioactive Peptides Obtained from Meat and Meat By-Products. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2021; Volume 97, pp. 147–185. ISBN 978-0-12-824580-4. [Google Scholar]
- Gulcin, İ. Antioxidants and Antioxidant Methods: An Updated Overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Thakur, M.; Gupta, N.; Sharma, H.K.; Devi, S. Physicochemical Characteristics and Mineral Status of Honey from Different Agro-Climatic Zones of Himachal Pradesh, India. BFJ 2021, 123, 3789–3804. [Google Scholar] [CrossRef]
- Virmani, M.A.; Cirulli, M. The Role of L-Carnitine in Mitochondria, Prevention of Metabolic Inflexibility and Disease Initiation. Int. J. Mol. Sci. 2022, 23, 2717. [Google Scholar] [CrossRef]
- Borsini, A.; Nicolaou, A.; Camacho-Muñoz, D.; Kendall, A.C.; Di Benedetto, M.G.; Giacobbe, J.; Su, K.-P.; Pariante, C.M. Omega-3 Polyunsaturated Fatty Acids Protect against Inflammation through Production of LOX and CYP450 Lipid Mediators: Relevance for Major Depression and for Human Hippocampal Neurogenesis. Mol. Psychiatry 2021, 26, 6773–6788. [Google Scholar] [CrossRef]
- Hennessy, A.A.; Ross, P.R.; Fitzgerald, G.F.; Stanton, C. Sources and Bioactive Properties of Conjugated Dietary Fatty Acids. Lipids 2016, 51, 377–397. [Google Scholar] [CrossRef] [PubMed]
- Dal Bosco, A.; Cavallo, M.; Menchetti, L.; Angelucci, E.; Cartoni Mancinelli, A.; Vaudo, G.; Marconi, S.; Camilli, E.; Galli, F.; Castellini, C.; et al. The Healthy Fatty Index Allows for Deeper Insights into the Lipid Composition of Foods of Animal Origin When Compared with the Atherogenic and Thrombogenicity Indexes. Foods 2024, 13, 1568. [Google Scholar] [CrossRef] [PubMed]
- Lackner, J.; Hess, V.; Marx, A.; Hosseini-Ghaffari, M.; Sauerwein, H. Effects of Dietary Supplementation with Histidine and β-Alanine on Blood Plasma Metabolome of Broiler Chickens at Different Ages. PLoS ONE 2022, 17, e0277476. [Google Scholar] [CrossRef]
- Zampiga, M.; Calini, F.; Sirri, F. Importance of Feed Efficiency for Sustainable Intensification of Chicken Meat Production: Implications and Role for Amino Acids, Feed Enzymes and Organic Trace Minerals. World’s Poult. Sci. J. 2021, 77, 1–21. [Google Scholar] [CrossRef]
- Scala, A.; Cammack, J.A.; Salvia, R.; Scieuzo, C.; Franco, A.; Bufo, S.A.; Tomberlin, J.K.; Falabella, P. Rearing Substrate Impacts Growth and Macronutrient Composition of Hermetia illucens (L.) (Diptera: Stratiomyidae) Larvae Produced at an Industrial Scale. Sci. Rep. 2020, 10, 19448. [Google Scholar] [CrossRef]
- Official Methods of Analysis Program—AOAC International. Available online: https://www.aoac.org/scientific-solutions/standards-and-official-methods/ (accessed on 23 February 2023).
- Khan, I.A.; Parker, N.B.; Löhr, C.V.; Cherian, G. Docosahexaenoic Acid (22:6 n-3)-Rich Microalgae along with Methionine Supplementation in Broiler Chickens: Effects on Production Performance, Breast Muscle Quality Attributes, Lipid Profile, and Incidence of White Striping and Myopathy. Poult. Sci. 2021, 100, 865–874. [Google Scholar] [CrossRef]
- Perna, A.; Gambacorta, E.; Simonetti, A.; Grassi, G.; Scopa, A. Effect of Ozone Treatment Exposure Time on Oxidative Stability of Cream Milk. Eur. J. Lipid Sci. Tech. 2022, 124, 2100238. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Perna, A.; Simonetti, A.; Grassi, G.; Gambacorta, E. Effect of a Cauliflower (Brassica oleraceae Var. Botrytis) Leaf Powder-Enriched Diet on Performance, Carcass and Meat Characteristics of Growing Rabbit. Meat Sci. 2019, 149, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Capasso, G.; Rando, A.; Perna, A.M. Antioxidant Activity of Beef, Pork and Chicken Burgers before and after Cooking and after In Vitro Intestinal Digestion. Foods 2023, 12, 4100. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Di Gregorio, P.; Rando, A.; Perna, A.M. Quality and Sensorial Evaluation of Beef Burgers Added with Sicilian Sumac (Rhus coriaria L.). Heliyon 2024, 10, e26848. [Google Scholar] [CrossRef]
- Simonetti, A.; Gambacorta, E.; Perna, A. Antioxidative and Antihypertensive Activities of Pig Meat before and after Cooking and in Vitro Gastrointestinal Digestion: Comparison between Italian Autochthonous Pig Suino Nero Lucano and a Modern Crossbred Pig. Food Chem. 2016, 212, 590–595. [Google Scholar] [CrossRef]
- Popova, T.L.; Petkov, E.; Ignatova, M. Effect of Black Soldier Fly (Hermetia illucens) Meals on the Meat Quality in Broilers. Agric. Food Sci. 2020, 29, 177–188. [Google Scholar] [CrossRef]
- Londok, J.; Sumiati, S.; Wiryawan, K.; Manalu, W. Antioxidant Enzyme Activity and Malondialdehyde Concentration on Broiler Fed Contain Lauric Acid and Areca Vestiaria Giseke. Bul. Peternak. 2018, 42. [Google Scholar] [CrossRef]
- Skřivan, M.; Marounek, M.; Englmaierová, M.; Čermák, L.; Vlčková, J.; Skřivanová, E. Effect of Dietary Fat Type on Intestinal Digestibility of Fatty Acids, Fatty Acid Profiles of Breast Meat and Abdominal Fat, and mRNA Expression of Lipid-Related Genes in Broiler Chickens. PLoS ONE 2018, 13, e0196035. [Google Scholar] [CrossRef]
- Schiavone, A.; Dabbou, S.; Petracci, M.; Zampiga, M.; Sirri, F.; Biasato, I.; Gai, F.; Gasco, L. Black Soldier Fly Defatted Meal as a Dietary Protein Source for Broiler Chickens: Effects on Carcass Traits, Breast Meat Quality and Safety. Animal 2019, 13, 2397–2405. [Google Scholar] [CrossRef] [PubMed]
- Hankel, J.; Popp, J.; Meemken, D.; Zeiger, K.; Beyerbach, M.; Taube, V.; Klein, G.; Visscher, C. Influence of Lauric Acid on the Susceptibility of Chickens to an Experimental Campylobacter Jejuni Colonisation. PLoS ONE 2018, 13, e0204483. [Google Scholar] [CrossRef] [PubMed]
- Wessels, K.; Rip, D.; Gouws, P. Salmonella in Chicken Meat: Consumption, Outbreaks, Characteristics, Current Control Methods and the Potential of Bacteriophage Use. Foods 2021, 10, 1742. [Google Scholar] [CrossRef]
- Cui, X.Y.; Gou, Z.Y.; Abouelezz, K.F.M.; Li, L.; Lin, X.J.; Fan, Q.L.; Wang, Y.B.; Cheng, Z.G.; Ding, F.Y.; Jiang, S.Q. Alterations of the Fatty Acid Composition and Lipid Metabolome of Breast Muscle in Chickens Exposed to Dietary Mixed Edible Oils. Animal 2020, 14, 1322–1332. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Sun, X.; Udenigwe, C.C. Role of Structural Properties of Bioactive Peptides in Their Stability during Simulated Gastrointestinal Digestion: A Systematic Review. Trends Food Sci. Technol. 2022, 120, 265–273. [Google Scholar] [CrossRef]
- Jung, S.; Bae, Y.S.; Kim, H.J.; Jayasena, D.D.; Lee, J.H.; Park, H.B.; Heo, K.N.; Jo, C. Carnosine, Anserine, Creatine, and Inosine 5′-Monophosphate Contents in Breast and Thigh Meats from 5 Lines of Korean Native Chicken. Poult. Sci. 2013, 92, 3275–3282. [Google Scholar] [CrossRef]
- Kopec, W.; Jamroz, D.; Wiliczkiewicz, A.; Biazik, E.; Pudlo, A.; Korzeniowska, M.; Hikawczuk, T.; Skiba, T. Antioxidative Characteristics of Chicken Breast Meat and Blood after Diet Supplementation with Carnosine, L-Histidine, and β-Alanine. Antioxidants 2020, 9, 1093. [Google Scholar] [CrossRef]
- Intarapichet, K.-O.; Maikhunthod, B. Genotype and Gender Differences in Carnosine Extracts and Antioxidant Activities of Chicken Breast and Thigh Meats. Meat Sci. 2005, 71, 634–642. [Google Scholar] [CrossRef]
- Juniper, D.; Rymer, C. The Effect of Rearing System and Cooking Method on the Carnosine and Anserine Content of Poultry and Game Meat. J. Food Nut. Agric. 2018, 1, 35–39. [Google Scholar] [CrossRef]
- Gkarane, V.; Ciulu, M.; Altmann, B.; Mörlein, D. Effect of Alternative Protein Feeds on the Content of Selected Endogenous Bioactive and Flavour-Related Compounds in Chicken Breast Meat. Foods 2020, 9, 392. [Google Scholar] [CrossRef]
- Ohata, M.; Uchida, S.; Zhou, L.; Arihara, K. Antioxidant Activity of Fermented Meat Sauce and Isolation of an Associated Antioxidant Peptide. Food Chem. 2016, 194, 1034–1039. [Google Scholar] [CrossRef]
- Chang, H.; Leem, Y.-H. The Potential Role of Creatine Supplementation in Neurodegenerative Diseases. Phys. Act. Nutr. 2023, 27, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Piccinin, E.; Cariello, M.; De Santis, S.; Ducheix, S.; Sabbà, C.; Ntambi, J.M.; Moschetta, A. Role of Oleic Acid in the Gut-Liver Axis: From Diet to the Regulation of Its Synthesis via Stearoyl-CoA Desaturase 1 (SCD1). Nutrients 2019, 11, 2283. [Google Scholar] [CrossRef]
- Skeaff, C.M.; Miller, J. Dietary Fat and Coronary Heart Disease: Summary of Evidence from Prospective Cohort and Randomised Controlled Trials. Ann. Nutr. Metab. 2009, 55, 173–201. [Google Scholar] [CrossRef] [PubMed]
- Daszkiewicz, T.; Murawska, D.; Kubiak, D.; Han, J. Chemical Composition and Fatty Acid Profile of the Pectoralis Major Muscle in Broiler Chickens Fed Diets with Full-Fat Black Soldier Fly (Hermetia illucens) Larvae Meal. Animals 2022, 12, 464. [Google Scholar] [CrossRef]
- Poureslami, R.; Turchini, G.M.; Raes, K.; Huyghebaert, G.; De Smet, S. Effect of Diet, Sex and Age on Fatty Acid Metabolism in Broiler Chickens: SFA and MUFA. Br. J. Nutr. 2010, 104, 204–213. [Google Scholar] [CrossRef]
- Smink, W.; Gerrits, W.J.J.; Hovenier, R.; Geelen, M.J.H.; Verstegen, M.W.A.; Beynen, A.C. Effect of Dietary Fat Sources on Fatty Acid Deposition and Lipid Metabolism in Broiler Chickens. Poult. Sci. 2010, 89, 2432–2440. [Google Scholar] [CrossRef] [PubMed]
- Cullere, M.; Tasoniero, G.; Giaccone, V.; Miotti-Scapin, R.; Claeys, E.; De Smet, S.; Dalle Zotte, A. Black Soldier Fly as Dietary Protein Source for Broiler Quails: Apparent Digestibility, Excreta Microbial Load, Feed Choice, Performance, Carcass and Meat Traits. Animal 2016, 10, 1923–1930. [Google Scholar] [CrossRef]
- Hwangbo, J.; Hong, E.C.; Jang, A.; Kang, H.K.; Oh, J.S.; Kim, B.W.; Park, B.S. Utilization of House Fly-Maggots, a Feed Supplement in the Production of Broiler Chickens. J. Environ. Biol. 2009, 30, 609–614. [Google Scholar]
- Isenmann, E.; Trittel, L.; Diel, P. The Effects of Alpha Lipoic Acid on Muscle Strength Recovery after a Single and a Short-Term Chronic Supplementation—A Study in Healthy Well-Trained Individuals after Intensive Resistance and Endurance Training. J. Int. Soc. Sports Nutr. 2020, 17, 61. [Google Scholar] [CrossRef]
- Salinthone, S.; Yadav, V.; Bourdette, D.N.; Carr, D.W. Lipoic Acid: A Novel Therapeutic Approach for Multiple Sclerosis and Other Chronic Inflammatory Diseases of the CNS. Available online: https://www.ingentaconnect.com/content/ben/emiddt/2008/00000008/00000002/art00008 (accessed on 2 May 2024).
- Cullere, M.; Schiavone, A.; Dabbou, S.; Gasco, L.; Dalle Zotte, A. Meat Quality and Sensory Traits of Finisher Broiler Chickens Fed with Black Soldier Fly (Hermetia illucens L.) Larvae Fat as Alternative Fat Source. Animals 2019, 9, 140. [Google Scholar] [CrossRef]
- Secci, G.; Moniello, G.; Gasco, L.; Bovera, F.; Parisi, G. Barbary Partridge Meat Quality as Affected by Hermetia Illucens and Tenebrio Molitor Larva Meals in Feeds. Food Res. Int. 2018, 112, 291–298. [Google Scholar] [CrossRef]
- Ian Givens, D.; Gibbs, R.A. Current Intakes of EPA and DHA in European Populations and the Potential of Animal-Derived Foods to Increase Them: Symposium on ‘How Can the n-3 Content of the Diet Be Improved? Proc. Nutr. Soc. 2008, 67, 273–280. [Google Scholar] [CrossRef] [PubMed]
- von Schacky, C. Importance of EPA and DHA Blood Levels in Brain Structure and Function. Nutrients 2021, 13, 1074. [Google Scholar] [CrossRef] [PubMed]
- Cunnane, S.C.; Ho, S.Y.; Dore-Duffy, P.; Ells, K.R.; Horrobin, D.F. Essential Fatty Acid and Lipid Profiles in Plasma and Erythrocytes in Patients with Multiple Sclerosis. Am. J. Clin. Nutr. 1989, 50, 801–806. [Google Scholar] [CrossRef]
- Cullere, M.; Singh, Y.; Pellattiero, E.; Berzuini, S.; Galasso, I.; Clemente, C.; Dalle Zotte, A. Effect of the Dietary Inclusion of Camelina Sativa Cake into Quail Diet on Live Performance, Carcass Traits, and Meat Quality. Poult. Sci. 2023, 102, 102650. [Google Scholar] [CrossRef] [PubMed]
- Renna, M.; Schiavone, A.; Gai, F.; Dabbou, S.; Lussiana, C.; Malfatto, V.; Prearo, M.; Capucchio, M.T.; Biasato, I.; Biasibetti, E.; et al. Evaluation of the Suitability of a Partially Defatted Black Soldier Fly (Hermetia illucens L.) Larvae Meal as Ingredient for Rainbow Trout (Oncorhynchus Mykiss Walbaum) Diets. J. Animal Sci. Biotechnol. 2017, 8, 57. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Banu, J.; Rahman, M.; Causey, J.; Fernandes, G. Biological Effects of Conjugated Linoleic Acids in Health and Disease. J. Nutr. Biochem. 2006, 17, 789–810. [Google Scholar] [CrossRef]
- Li, S.; Xu, L.; Qing, J.; Wu, X.; Li, H.; Chen, H.; Liu, X. Multiple Biological Activities and Biosynthesis Mechanisms of Specific Conjugated Linoleic Acid Isomers and Analytical Methods for Prospective Application. Food Chem. 2023, 409, 135257. [Google Scholar] [CrossRef]
- Seyedalmoosavi, M.M.; Mielenz, M.; Görs, S.; Wolf, P.; Daş, G.; Metges, C.C. Effects of Increasing Levels of Whole Black Soldier Fly (Hermetia illucens) Larvae in Broiler Rations on Acceptance, Nutrient and Energy Intakes and Utilization, and Growth Performance of Broilers. Poult. Sci. 2022, 101, 102202. [Google Scholar] [CrossRef]
- Polidori, P.; Vincenzetti, S.; Pucciarelli, S.; Polzonetti, V. CLAs in Animal Source Foods: Healthy Benefits for Consumers. In Bioactive Molecules in Food; Mérillon, J.-M., Ramawat, K.G., Eds.; Reference Series in Phytochemistry; Springer International Publishing: Cham, Switzerland, 2019; pp. 667–698. ISBN 978-3-319-78029-0. [Google Scholar]
- Liu, K.; Luo, M.; Wei, S. The Bioprotective Effects of Polyphenols on Metabolic Syndrome against Oxidative Stress: Evidences and Perspectives. Oxidative Med. Cell. Longev. 2019, 2019, e6713194. [Google Scholar] [CrossRef] [PubMed]
- Sehrawat, R.; Sharma, R.; Ahlawat, S.; Sharma, V.; Thakur, M.S.; Kaur, M.; Tantia, M.S. First Report on Better Functional Property of Black Chicken Meat from India. Indian J. Anim. Res. 2021, 55, 727–733. [Google Scholar] [CrossRef]
- Sultana, K.; Jayathilakan, K.; Pandey, M.C. Evaluation of Antioxidant Activity, Radical Scavenging, and Reducing Power of Clove Oil and Clove Oleoresin in Comparison with Natural and Synthetic Antioxidants in Chevon (Capra Aegagrus Hircus) and Chicken Meat. Def. Life Sci. J. 2017, 3, 51. [Google Scholar] [CrossRef]
- Monaghan, P.; Metcalfe, N.B.; Torres, R. Oxidative Stress as a Mediator of Life History Trade-offs: Mechanisms, Measurements and Interpretation. Ecol. Lett. 2009, 12, 75–92. [Google Scholar] [CrossRef]
- Torres-Castillo, J.A.; Olazarán-Santibáñez, F.E. Insects as Source of Phenolic and Antioxidant Entomochemicals in the Food Industry. Front. Nutr. 2023, 10, 1133342. [Google Scholar] [CrossRef] [PubMed]
- Rumpold, B.A.; Schlüter, O.K. Potential and Challenges of Insects as an Innovative Source for Food and Feed Production. Innov. Food Sci. Emerg. Technol. 2013, 17, 1–11. [Google Scholar] [CrossRef]
- Park, S.; Chang, B.S.; Yoe, S.M. Detection of Antimicrobial Substances from Larvae of the Black Soldier Fly, Hermetia illucens (Diptera: S Tratiomyidae). Entomol. Res. 2014, 44, 58–64. [Google Scholar] [CrossRef]
- Mouithys-Mickalad, A.; Schmitt, E.; Dalim, M.; Franck, T.; Tome, N.M.; Van Spankeren, M.; Serteyn, D.; Paul, A. Black Soldier Fly (Hermetia illucens) Larvae Protein Derivatives: Potential to Promote Animal Health. Animals 2020, 10, 941. [Google Scholar] [CrossRef]
- Zielińska, E.; Karaś, M.; Jakubczyk, A. Antioxidant Activity of Predigested Protein Obtained from a Range of Farmed Edible Insects. Int. J. Food Sci. Tech. 2017, 52, 306–312. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.R.; Abdel-Tawwab, M.; Khalil, R.H.; Metwally, A.A.; Shakweer, M.S.; Ghetas, H.A.; Khallaf, M.A. Black Soldier Fly (Hermetia illucens) Larvae Meal in Diets of European Seabass: Effects on Antioxidative Capacity, Non-Specific Immunity, Transcriptomic Responses, and Resistance to the Challenge with Vibrio Alginolyticus. Fish Shellfish Immunol. 2021, 111, 111–118. [Google Scholar] [CrossRef]
- Eggink, K.M.; Dalsgaard, J. Chitin Contents in Different Black Soldier Fly (Hermetia illucens) Life Stages. J. Insects Food Feed 2023, 9, 855–864. [Google Scholar] [CrossRef]
- Ngo, D.-N.; Lee, S.-H.; Kim, M.-M.; Kim, S.-K. Production of Chitin Oligosaccharides with Different Molecular Weights and Their Antioxidant Effect in RAW 264.7 Cells. J. Funct. Foods 2009, 1, 188–198. [Google Scholar] [CrossRef]
- Ulrich, K.; Jakob, U. The Role of Thiols in Antioxidant Systems. Free Radic. Biol. Med. 2019, 140, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Khatun, J.; Loh, T.C.; Foo, H.L.; Akit, H.; Mohamad, R.; Shazali, N. Effects of Vitamin E, an Oil Blend and L-Arginine on Breast Meat from Broiler Chickens. S. Afr. J. Anim. Sci. 2021, 50, 745–757. [Google Scholar] [CrossRef]
- Terevinto, A.; Del Puerto, M.; Da Silva, A.; Cabrera, M.C.; Saadoun, A. Effect of Chia Seeds (Salvia hispanica L.) Inclusion in Poultry Diet on n-3 Enrichment and Oxidative Status of Meat during Retail Display. CyTA—J. Food 2023, 21, 93–100. [Google Scholar] [CrossRef]
- Jayasena, D.D.; Jung, S.; Bae, Y.S.; Kim, S.H.; Lee, S.K.; Lee, J.H.; Jo, C. Changes in Endogenous Bioactive Compounds of Korean Native Chicken Meat at Different Ages and during Cooking. Poult. Sci. 2014, 93, 1842–1849. [Google Scholar] [CrossRef]
- Kar, S.K.; Schokker, D.; Harms, A.C.; Kruijt, L.; Smits, M.A.; Jansman, A.J.M. Local Intestinal Microbiota Response and Systemic Effects of Feeding Black Soldier Fly Larvae to Replace Soybean Meal in Growing Pigs. Sci. Rep. 2021, 11, 15088. [Google Scholar] [CrossRef]
| Ingredients (%) | Grower Ration | Finisher Ration | BSFL |
|---|---|---|---|
| (0–21 Days) | (22–35 Days) | (22–35 Days) | |
| Corn | 61.00 | 65.45 | |
| Soybean meal | 26.89 | 29.95 | |
| Wheat bran | 10.05 | 2.75 | |
| Dicalcium phosphate | 1.45 | 1.30 | |
| Salt | 0.30 | 0.30 | |
| DL-methionine | 0.11 | 0.05 | |
| Vitamin Premix * | 0.10 | 0.10 | |
| Mineral Premix ** | 0.10 | 0.10 | |
| Minerals, % | |||
| Calcium | 1.60 | 0.85 | 1.47 |
| Phosphorus | 0.60 | 0.48 | 0.95 |
| Magnesium | 0.25 | 0.20 | 0.54 |
| Chemical composition, %DM | |||
| Crude Protein | 21.20 | 18.89 | 44.74 |
| Crude Fat | 5.60 | 4.5 | 19.80 |
| Crude Fiber | 2.85 | 3.06 | 22.70 |
| Crude ash | 5.5 | 5.0 | 12.76 |
| Fatty Acids | Standard Diet |
|---|---|
| C12:0 | 37.63 |
| C14:0 | 8.42 |
| C14:1 | 0.29 |
| C15:0 | 0.21 |
| C16:0 | 16.74 |
| C16:1 | 0.40 |
| C17:0 | 3.64 |
| C17:1 | 0.30 |
| C18:1 cis 6 | 0.23 |
| C18:0 | 3.02 |
| C18:1 trans 9 | - |
| C18:1 trans 11 | 0.44 |
| C18:1 cis 9 | 12.31 |
| C18:1 cis 10 | - |
| C18:1 cis 11 | 0.05 |
| C18:2 cis n6 | 8.38 |
| C20:0 | 0.14 |
| C20:1 | 0.11 |
| C18:3 n3 | 1.86 |
| C22:0 | 0.64 |
| C22:1 | 0.15 |
| ∑ SFA 1 | 70.53 |
| ∑ MUFA 2 | 14.28 |
| ∑ PUFA 3 | 10.24 |
| ∑ n-6 | 9.01 |
| ∑ n-3 | 1.86 |
| Parameters | C 1 µ ± SD | HI5 µ ± SD | HI10 µ ± SD |
|---|---|---|---|
| pH | 5.80 ± 0.03 | 5.81 ± 0.04 | 5.81 ± 0.09 |
| Share force(kg/g) | 2.67 ± 0.16 | 2.51 ± 0.14 | 2.59 ± 0.26 |
| L* | 52.03 ± 1.42 | 52.48 ± 1.76 | 52.85 ± 1.02 |
| a* | 2.87 ± 0.26 | 2.81 ± 0.14 | 2.82 ± 0.25 |
| b* | 3.46 ± 0.36 | 3.62 ± 0.36 | 3.75 ± 0.28 |
| Total protein(%) | 21.35 ± 0.61 | 20.99 ± 0.83 | 21.67 ± 0.53 |
| Total lipid(%) | 1.59 ± 0.10 | 1.49 ± 0.07 | 1.44 ± 0.14 |
| % of Total FAME | |||
|---|---|---|---|
| Fatty Acids | C 1 µ ± SD | HI5 µ ± SD | HI10 µ ± SD |
| C12:0 | 0.23 ± 0.05 b | 3.11 ± 0.58 a | 4.54 ± 0.87 a |
| C13:0 | 0 b | 0.02 ± 0.01 a | 0.03 ± 0.001 a |
| C14:0 | 0.67 ± 0.02 c | 1.91 ± 0.27 b | 2.60 ± 0.20 a |
| C14:1 | 0.10 ± 0.02 a | 0.05 ± 0.03 b | 0.06 ± 0.02 b |
| C15:0 | 0.01 ± 0.01 b | 0.12 ± 0.01 a | 0.11 ± 0.02 a |
| C16:0 | 21.66 ± 1.32 | 20.84 ± 0.77 | 21.03 ± 1.50 |
| C16:1 | 1.10 ± 0.14 | 1.34 ± 0.43 | 1.56 ± 0.67 |
| C17:0 | 0.18 ± 0.01 b | 0.23 ± 0.02 a | 0.23 ± 0.03 a |
| C18:1 cis 6 | 0.03 ± 0.03 b | 0.08 ± 0.03 b | 0.09 ± 0.01 a |
| C18:0 | 11.01 ± 0.287 | 10.90 ± 0.48 | 10.84 ± 0.43 |
| C18:1 trans 9 | 0.22 ± 0.08 | 0.14 ± 0.01 | 0.15 ± 0.05 |
| C18:1 cis 9 | 28.15 ± 0.87 a | 24.27 ± 1.97 b | 21.55 ± 0.28 c |
| C18:1 cis 10 | 1.51 ± 0.16 | 1.55 ± 0.35 | 1.57 ± 0.27 |
| C18:1 cis 11 | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.07 ± 0.01 |
| C18:2 cis n6 | 22.92 ± 0.79 a | 21.82 ± 2.27 a | 18.94 ± 1.39 b |
| C20:0 | 0.13 ± 0.01 | 0.13 ± 0.02 | 0.11 ± 0.01 |
| C18:3 n6 | 0.07 ± 0.03 a | 0.08 ± 0.02 a | 0.06 ± 0.01 b |
| C20:1 | 0.22 ± 0.04 a | 0.16 ± 0.08 ab | 0.17 ± 0.02 a |
| C18:3 n3 | 1.24 ± 0.15 a | 1.18 ± 0.48 a | 0.71 ± 0.07 b |
| C18:2 cis9 trans11 | 0.06 ± 0.02 b | 0.51 ± 0.05 a | 0.56 ± 0.04 a |
| C20:2 n6 | 0.26 ± 0.03 b | 0.38 ± 0.08 a | 0.36 ± 0.01 a |
| C22:0 | 0.04 ± 0.01 b | 0.08 ± 0.01 a | 0.06 ± 0.01 ab |
| C20:3 n6 | 0.04 ± 0.01 b | 0.09 ± 0.05 ab | 0.07 ± 0.01 a |
| C22:1 | 0.32 ± 0.04 | 0.45 ± 0.17 | 0.37 ± 0.08 |
| C20:4 n6 | 3.01 ± 0.34 b | 4.76 ± 0.73 a | 4.45 ± 0.12 a |
| C23:0 | 0.02 ± 0.001 | 0.02 ± 0.01 | 0.03 ± 0.002 |
| C22:2 n6 | 0.08 ± 0.02 | 0.09 ± 0.03 | 0.08 ± 0.01 |
| C24:0 | 0.62 ± 0.12 b | 0.83 ± 0.38 ab | 0.89 ± 0.10 a |
| C20:5 n3 | 0.14 ± 0.01 b | 0.19 ± 0.10 ab | 0.20 ± 0.02 a |
| C22:5 n3 | 0.33 ± 0.06 b | 0.60 ± 0.36 ab | 0.54 ± 0.02 a |
| C22:6 n3 | 0.19 ± 0.01 b | 0.37 ± 0.22 ab | 0.33 ± 0.06 a |
| ∑ SFA 2 | 34.58 ± 1.65 c | 38.18 ± 0.75 b | 40.43 ± 0.35 a |
| ∑ MUFA 3 | 31.72 ± 0.85 a | 28.10 ± 1.86 b | 25.59 ± 0.82 b |
| ∑ PUFA 4 | 28.35 ± 0.44 b | 30.07 ± 0.80 a | 26.30 ± 1.61 b |
| ∑ n-6 | 26.39 ± 0.40 a | 27.22 ± 0.66 a | 23.96 ± 1.52 b |
| ∑ n-3 | 1.97 ± 0.17 a | 2.34 ± 0.21 a | 1.78 ± 0.13 b |
| CLA | 0.06 ± 0.02 b | 0.51 ± 0.05 a | 0.56 ± 0.04 a |
| PUFA/SFA | 0.82 ± 0.05 a | 0.79 ± 0.02 a | 0.65 ± 0.04 b |
| n6/n3 | 13.94 ± 1.17 a | 11.70 ± 0.78 b | 13.47 ± 0.40 a |
| LA/ALA | 18.67 ± 1.99 b | 20.54 ± 6.47 ab | 26.69 ± 1.16 a |
| AA/EPA | 20.98 ± 0.70 b | 24.51 ± 1.31 a | 22.54 ± 1.79 ab |
| % of Total FAME | ||||||
|---|---|---|---|---|---|---|
| Parameter | C | HI5 | HI10 | |||
| µ | SD | µ | SD | µ | SD | |
| PUFA | 28.39 a | 0.93 | 27.41 b | 1.73 | 25.89 c | 1.40 |
| C18:1 n-9 | 28.04 a | 0.87 | 24.27 b | 1.79 | 21.58 c | 0.22 |
| C18:3 n-3 (ALA) | 1.26 a | 0.15 | 1.18 a | 0.11 | 0.69 b | 0.06 |
| C20:5 n-3 (EPA) | 0.14 b | 0.01 | 0.19 a | 0.01 | 0.19 a | 0.02 |
| C22:6 n-3 (DHA) | 0.19 c | 0.02 | 0.37 a | 0.04 | 0.31 b | 0.04 |
| CLA | 0.06 c | 0.01 | 0.51 b | 0.05 | 0.57 a | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grassi, G.; Franco, A.; Scieuzo, C.; Capasso, G.; Lomonaco, G.; Salvia, R.; Perna, A.M.; Falabella, P. Bioactive Compounds in Breast Meat of Broiler Chickens Fed with Black Soldier Fly Wholemeal. Appl. Sci. 2025, 15, 7132. https://doi.org/10.3390/app15137132
Grassi G, Franco A, Scieuzo C, Capasso G, Lomonaco G, Salvia R, Perna AM, Falabella P. Bioactive Compounds in Breast Meat of Broiler Chickens Fed with Black Soldier Fly Wholemeal. Applied Sciences. 2025; 15(13):7132. https://doi.org/10.3390/app15137132
Chicago/Turabian StyleGrassi, Giulia, Antonio Franco, Carmen Scieuzo, Giambattista Capasso, Giovanni Lomonaco, Rosanna Salvia, Anna Maria Perna, and Patrizia Falabella. 2025. "Bioactive Compounds in Breast Meat of Broiler Chickens Fed with Black Soldier Fly Wholemeal" Applied Sciences 15, no. 13: 7132. https://doi.org/10.3390/app15137132
APA StyleGrassi, G., Franco, A., Scieuzo, C., Capasso, G., Lomonaco, G., Salvia, R., Perna, A. M., & Falabella, P. (2025). Bioactive Compounds in Breast Meat of Broiler Chickens Fed with Black Soldier Fly Wholemeal. Applied Sciences, 15(13), 7132. https://doi.org/10.3390/app15137132











