Occurrence Modes of Arsenic in Coal: A Case Study from the Hanshuiquan Coal Mine, Santanghu Coalfield, Xinjiang Province, China
Abstract
1. Introduction
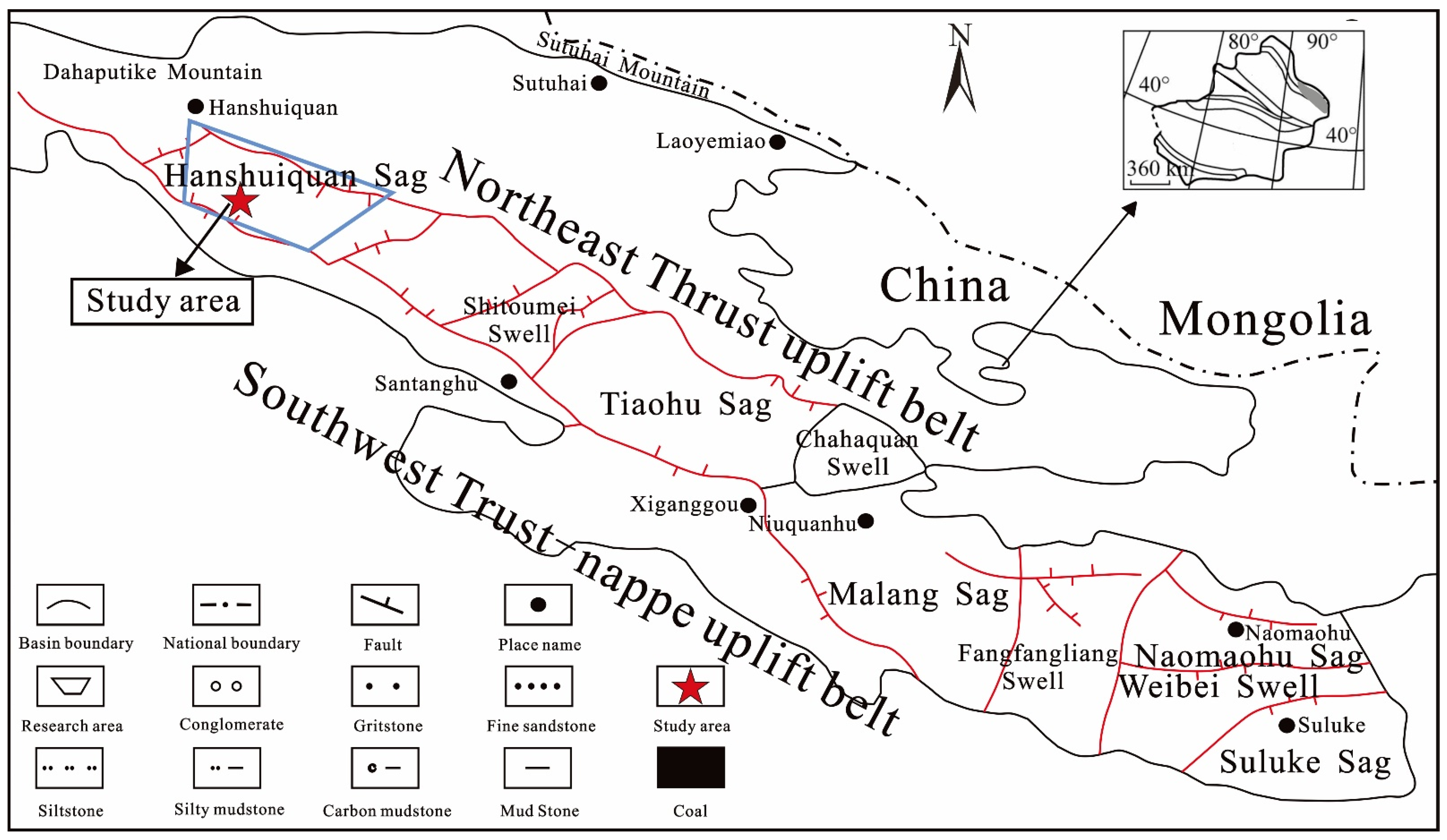
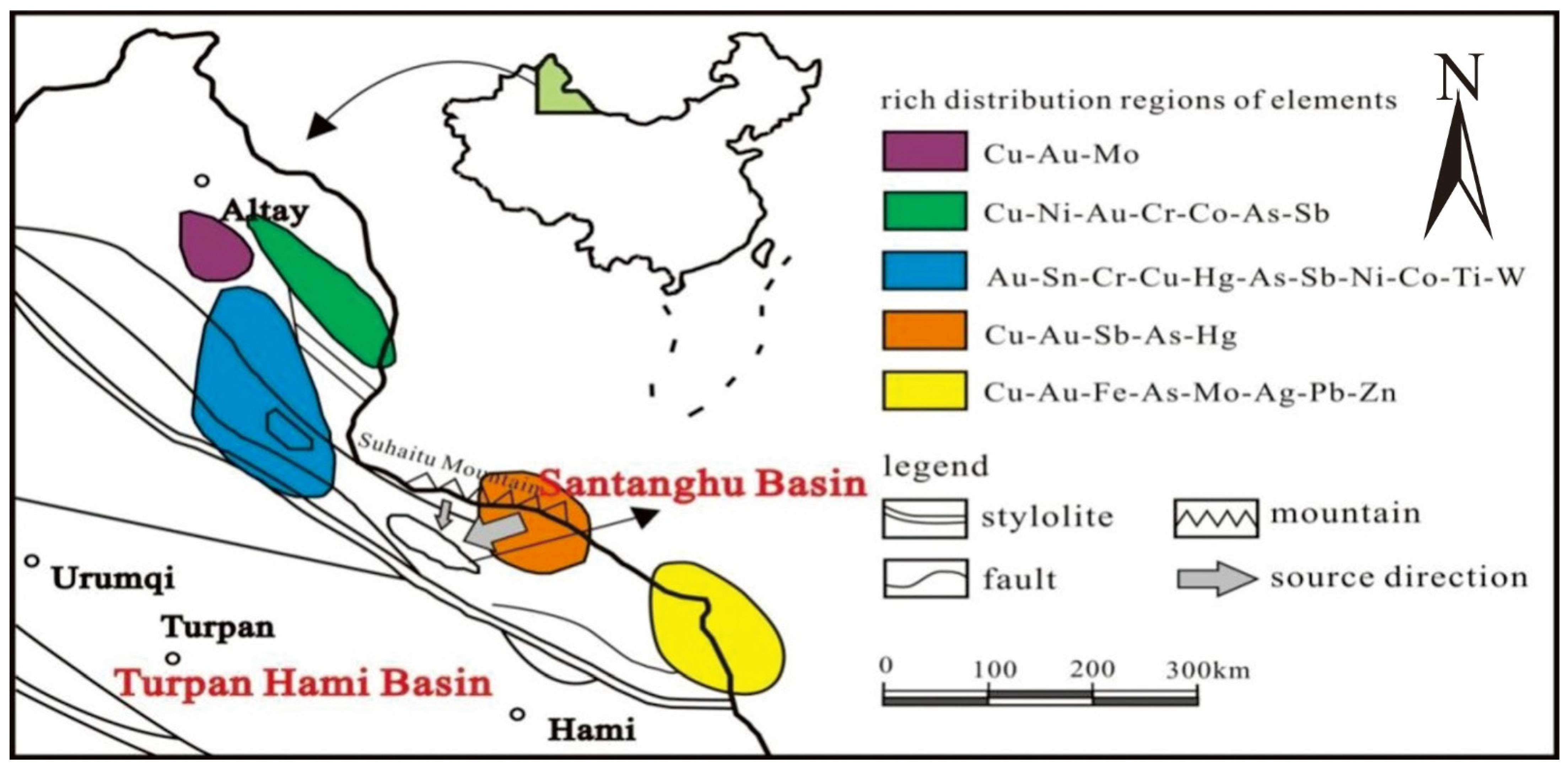
2. Geological Setting
3. Samples and Methods
4. Results
5. Discussion
5.1. Enrichment Characteristics of As in the Coal
5.2. Occurrence Modes of As in the Coal
5.3. Origin of As in the Coal
5.4. Occurrence Modes for Arsenic in Coal and Its Influence on the Environment
5.4.1. Occurrence Modes for Arsenic in Coal
5.4.2. The Impact of Arsenic in Coal on the Environment
6. Conclusions
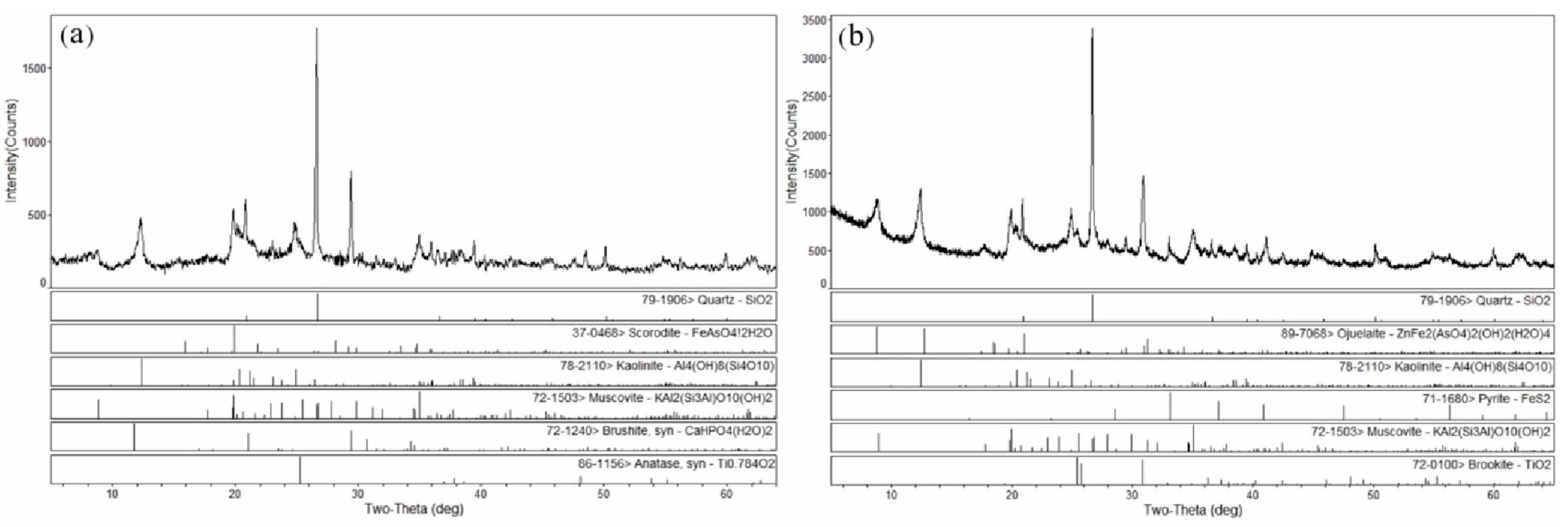
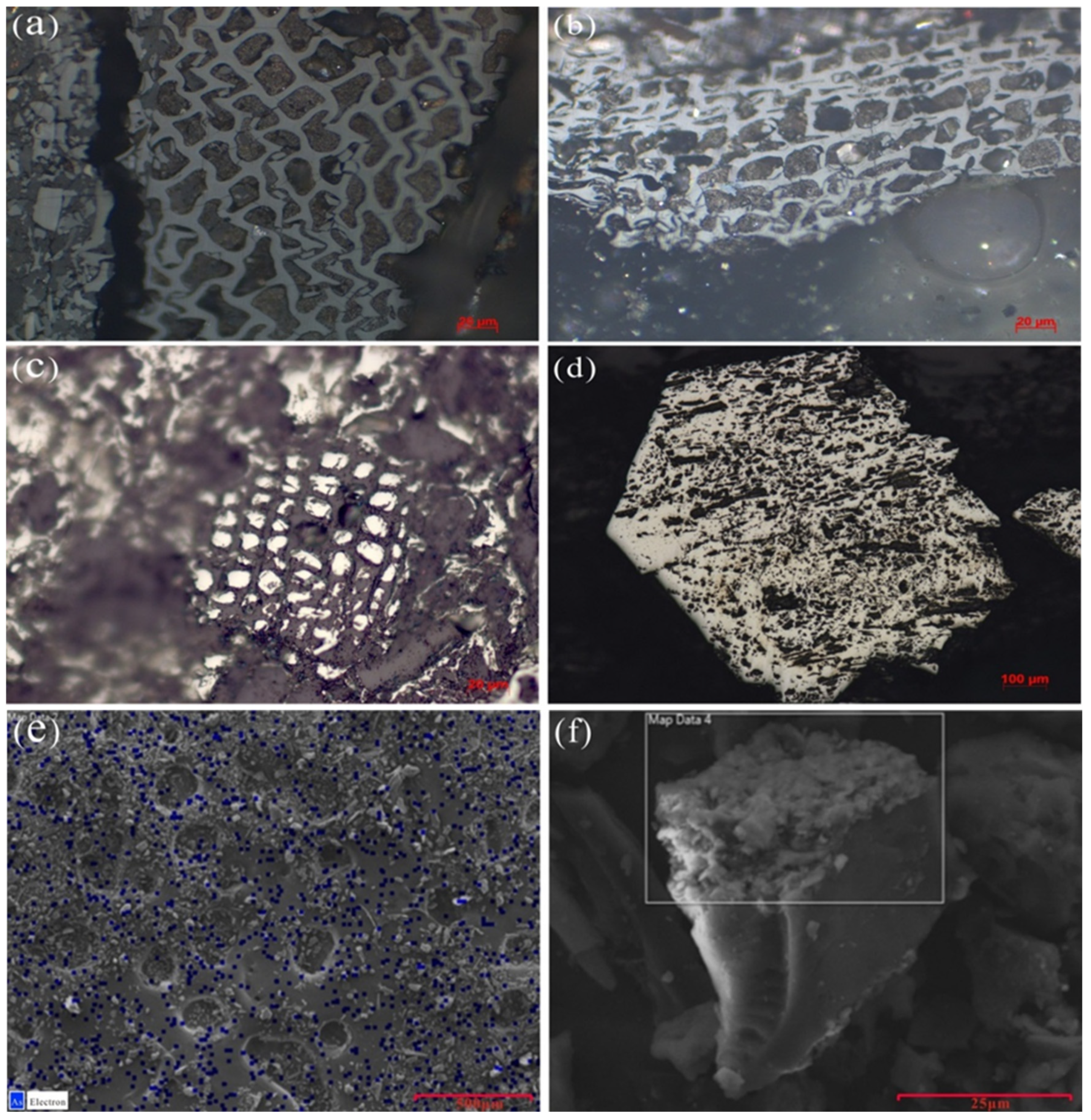
- Analysis of coal samples from the Hanshuiquan mine in the Santanghu Coalfield shows that the main minerals are quartz, kaolinite, and dolomite, with a certain amount of secondary minerals such as brookite and anatase; there is also a small amount of pyrite. Some samples (from 9# coal seam) were also found to contain arsenate minerals. The kaolinite filled the plant tissue pores in the fusinite, indicating an early diagenesis. The subhedral and filled plant tissue pores of the pyrite denotes an early diagenesis.
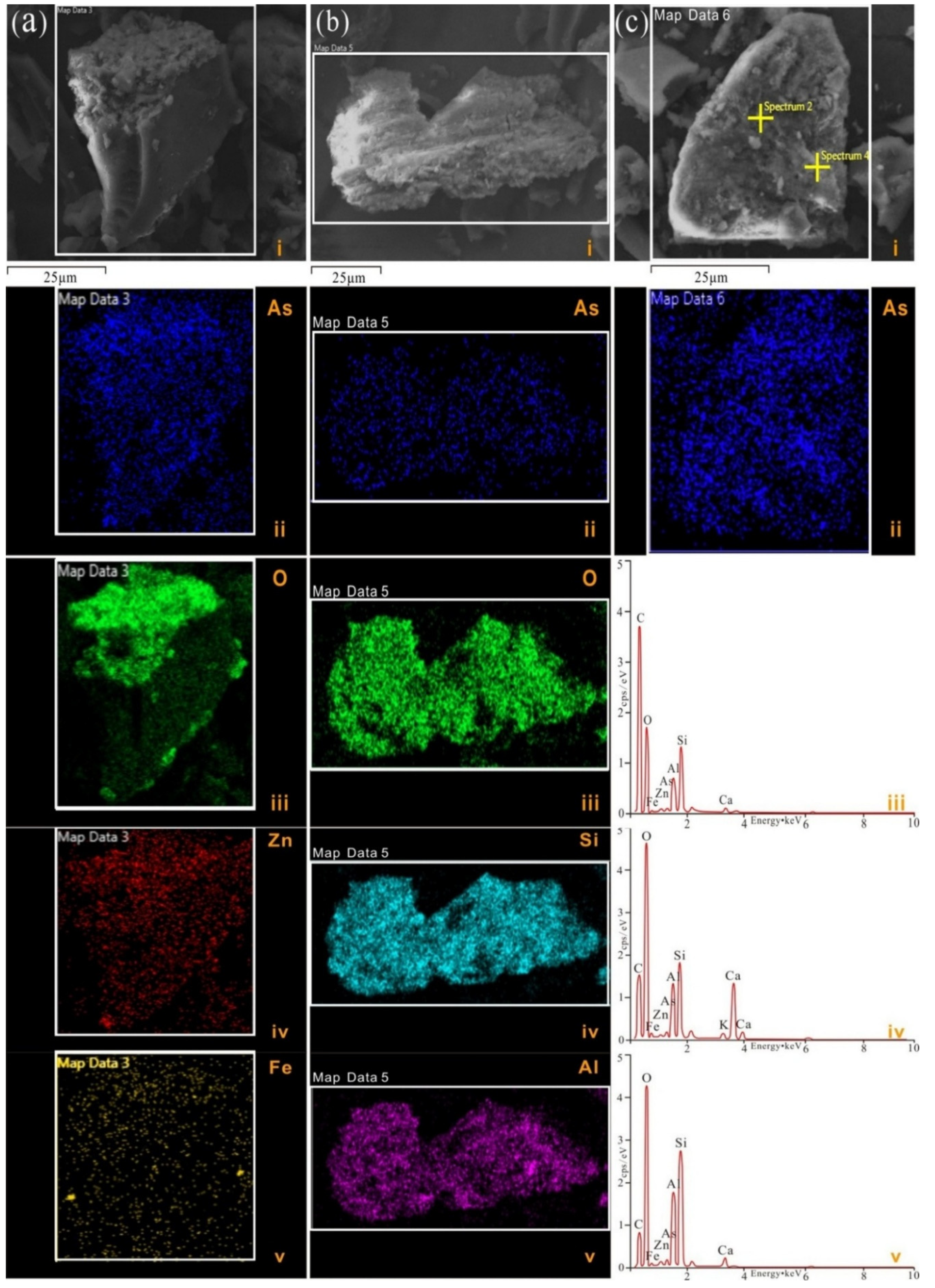
- The occurrence mode of the arsenic in the coal from the Hanshuiquan mine in the Santanghu Coalfield is dominated by the arsenate form state. The results of XRD and SEM-EDS show the existence of ojuelaite, and the presence of scorodite may come from the provenance.
- The pyrite and arsenopyrite, as the dominant gold-bearing minerals, provide the most abundant originate of arsenic for the coal of the Xishanyao Formation in the Santanghu Coalfield, and these gold-bearing minerals are formed to be produced from the epithermal Au mineral deposit at both the Qiongheba and the Kelameili ore-concentrated areas.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dai, S.F.; Hower, J.C.; Finkelman, R.B.; Graham, I.T.; French, D.; Ward, C.R.; Eskenazy, G.; Wei, Q.; Zhao, L. Organic associations of non-mineral elements in coal: A review. Int. J. Coal Geol. 2020, 218, 103347. [Google Scholar] [CrossRef]
- Dai, S.F.; Bechtel, A.; Eble, C.F.; Flores, R.M.; French, D.; Graham, I.T.; Hood, M.M.; Hower, J.C.; Korasidis, V.A.; Moore, T.A.; et al. Recognition of peat depositional environments in coal: A review. Int. J. Coal Geol. 2020, 219, 103383. [Google Scholar]
- Dai, S.F.; Finkelman, R.B.; French, D.; Hower, J.C.; Graham, I.T.; Zhao, F.H. Modes of occurrence of elements in coal: A critical evaluation. Earth Sci. Rev. 2021, 222, 103815. [Google Scholar] [CrossRef]
- Liu, J.J.; Ward, C.R.; Graham, I.T.; French, D.; Dai, S.F.; Song, X.L. Modes of occurrence of non-mineral inorganic elements in lignites from the Mile Basin, Yunnan Province, China. Fuel 2018, 222, 146–155. [Google Scholar] [CrossRef]
- Dai, S.F.; Ren, D.Y.; Chou, C.L.; Finkelman, R.B.; Seredin, V.V.; Zhou, Y.P. Geochemistry of trace elements in Chinese coals: A review of abundance, genetic types, impacts on human health, and industrial utilization. Int. J. Coal Geol. 2012, 94, 3–21. [Google Scholar] [CrossRef]
- Jia, R.K.; Liu, J.J.; Han, Q.C.; Zhao, S.M.; Shang, N.D.; Tang, P.Q.; Zhang, Y.Q. Mineral matter transition in lignite during ashing process: A case study of Early Cretaceous lignite from the Hailar Basin, Inner Mongolia, China. Fuel 2022, 328, 125252. [Google Scholar] [CrossRef]
- Che, K.X.; Li, J.X.; Lu, Q.F.; Shao, F.J.; Wang, W.L.; Wang, W.F.; He, X. Occurrence of Differences between Alkali and Alkaline Earth Metals (AAEMs), Including Sodium, Potassium, Calcium, and Magnesium, in the Maceral Groups of the Xiheishan Coal, Zhundong Coalfield, Xinjiang, China. Minerals 2024, 14, 525. [Google Scholar] [CrossRef]
- Swaine, D.J. Trace Elements in Coal; Butterworth-Heinemann: London, UK, 1990; 278p. [Google Scholar]
- Jin, Y.L.; Liang, C.K.; He, G.L.; Cao, J.X. Study on distribution of endemic arsenism in China. J. Hyg. Res. 2003, 32, 519–540, (In Chinese with English Abstract). [Google Scholar]
- Kang, Y.; Liu, G.J.; Chou, C.L.; Wong, M.H.; Zheng, L.G.; Ding, R. Arsenic in Chinese coals, Distribution, modes of occurrence, and environmental effects. Sci. Total Environ. 2011, 412, 1–13. [Google Scholar] [CrossRef]
- Goodarzi, F.; Huggins, F.E.; Sanei, H. Assessment of elements, speciation of As, Cr, Ni and emitted Hg for a Canadian power plant burning bituminous coal. Int. J. Coal Geol. 2008, 74, 1–12. [Google Scholar] [CrossRef]
- Van Aken, B.; Quaranta, J.D.; Mack, B. Environmental Contaminants in Coal Slurry Intended for Underground Injection in the State of West Virginia. J. Environ. Eng. 2015, 141, 13–25. [Google Scholar] [CrossRef]
- Finkelman, R.B. Modes of Occurrence of Trace Elements in Coal; U. S. Geological Survey Open-File Report No. OFR-81-99; U. S. Geological Survey: Reston, VA, USA, 1981; p. 301. [Google Scholar]
- Finkelman, R.B.; William, O.; Vincent, C. Health impacts of coal and coal use, possible solutions. Int. J. Coal Geol. 2002, 50, 425–443. [Google Scholar] [CrossRef]
- Dai, S.F.; Zhang, W.W.; Colin, R. Mineralogical and geochemical anomalies of Late Permian coals from the Fusui coalfield, Guangxi Province, southern China, Influences of Terrigenous materials and hydrothermal fluids. Int. J. Coal Geol. 2013, 105, 60–84. [Google Scholar] [CrossRef]
- Fu, C.; Bai, X.F.; Jiang, Y. Discussion on the relationship between the content of Arsenic and the coal quality characteristic and the Arsenic modes of occurrence in Chinese high Arsenic coal. J. China Coal Soc. 2012, 37, 96–102. (In Chinese) [Google Scholar]
- Huang, C.; Tian, J.J. Research progress on the occurrence of arsenic in coal in China. West-China Explor. Eng. 2013, 7, 100–103. (In Chinese) [Google Scholar]
- Chapman, A.C. Arsenic in coal and coke. Analyst 1901, 26, 252–256. [Google Scholar] [CrossRef]
- Huggins, F.E.; Shah, N.; Zhao, J.; Huffman, G.P. Nondestructive determination of trace element speciation in coal and coal ash by XAFS spectroscopy. Energy Fuel 1993, 7, 482–489. [Google Scholar] [CrossRef]
- Kolker, A. Minor element distribution in iron disulfides in coal: A geochemical review. Int. J. Coal Geol. 2012, 94, 32–43. [Google Scholar] [CrossRef]
- Zheng, L.; Liu, G.; Chou, C.; Gao, L.; Peng, Z. Arsenic in Chinese Coals, Its abundance, distribution, modes of occurrence, enrichment processes, and environmental significance. Acta Geosci. Sin. 2006, 27, 355–366. (In Chinese) [Google Scholar]
- Xie, H.; Nie, A.G. The modes of occurrence and washing floatation characteristic of arsenic in coal from Western Guizhou. J. China Coal Soc. 2010, 35, 117–121. (In Chinese) [Google Scholar]
- Huggins, F.E.; Goodarzi, F.; Lafferty, C.J. Mode of occurrence of arsenic in subbituminous Coals. Energy Fuels 1996, 10, 1001–1004. [Google Scholar] [CrossRef]
- Zhao, F.H.; Zheng, B.S. Research on arsenic modes of occurrence of high-arsenic coal by X-ray abosorption fine structure. Chin. Sci. Bull. 1998, 14, 1549–1551. (In Chinese) [Google Scholar]
- Zhang, Y.Y.; Tian, J.J.; Feng, S.; Yang, F.; Lu, X.Y. The occurrence modes and geologic origins of arsenic in coal from Santanghu Coalfield, Xinjiang. J. Geochem. Explor. 2018, 186, 225–234. [Google Scholar] [CrossRef]
- Feng, S.; Tian, J.J.; Zhang, Y.Y.; Yang, F.; Lu, X.Y. Modes of arsenic occurrence in coal from the Santanghu Coalfield, Xinjiang, Northwest China, evidence from coal petrography, coal facies, mineralogy and geochemistry. Geochem. Explor. Environ. Anal. 2018, 18, 294–302. [Google Scholar] [CrossRef]
- Liu, G.J.; Peng, Z.C.; Wang, G.L. Study on trace elements in coal. Adv. Earth Sci. 2002, 17, 53–62. (In Chinese) [Google Scholar]
- Zhao, F.H.; Ren, D.Y.; Peng, S.P. The modes of occurrence of arsenic in coal. Adv. Earth Sci. 2003, 18, 214–220. (In Chinese) [Google Scholar]
- Wang, M.S.; Zheng, B.S.; Hu, J. Distribution of arsenic in southwest coals. J. China Coal Soc. 2005, 30, 344–348. (In Chinese) [Google Scholar]
- Wang, M.S.; Zheng, B.S.; Liu, X.J.; Wang, Y.; Hu, J. Estimation of Arsenic Contents in Chinese Coals. Environ. Sci. 2006, 27, 420–423. (In Chinese) [Google Scholar]
- Ding, Z.H.; Zheng, B.S.; Finkelmam, R.B.; Hu, T.D.; Zhou, Y.S. Subsequent Leaching Study of Typical High-As Coal Samples from Southwest Guizhou Province. Earth Sci. 2003, 28, 209–213. (In Chinese) [Google Scholar]
- Ren, D.Y.; Zhao, F.H.; Zhang, J.Y.; Xu, D.W. A Preliminary Study on Genetic Type of Enrichment for Hazardous Minor and Trace Elements in coal. Earth Sci. Front. 1999, 6, 7–22. (In Chinese) [Google Scholar]
- Ren, D.Y.; Xu, D.W.; Zhang, J.Y.; Zhao, F.H. Distribution of Associated Elements in Coals from Shenbei Coalfield. J. China Univ. Min. Technol. 1999, 28, 5–8. [Google Scholar]
- Ketris, M.P.; Yudovich, Y.E. Estimations of clarkes for carbonaceous biolithes, World average for trace element concentrations in black shales and coals. Int. J. Coal Geol. 2009, 78, 135–148. [Google Scholar] [CrossRef]
- Dai, S.F.; Seredin, V.V.; Ward, C.R. Enrichment of U–Se–Mo–Re–V in coals preserved within marine carbonate successions, geochemical and mineralogical data from the Late Permian Guiding Coalfield, Guizhou, China. Miner. Depos. 2015, 50, 159–186. [Google Scholar] [CrossRef]
- Huang, L.B.; Wu, X.; Tan, J.Y.; Tan, H.M. The analysis on quality characteristics of coal from the west of Santanghu coalfield, Xinjiang. Sci. Technol. West China 2010, 26, 53–54. (In Chinese) [Google Scholar]
- An, Q.; Ma, F.Z. Analysis and evaluation on coal characteristics of 9-1 coal seam in Hanshuiquan exploration district of Santanghu coalfield in Xinjiang. West-China Explor. Eng. 2010, 9, 53–54. (In Chinese) [Google Scholar]
- Rieder, M.; Crelling, J.C.; Šustai, O.; Drábek, M.; Weiss, Z.; Klementová, M. Arsenic in iron disulfides in a brown coal from the North Bohemian Basinm, Czech Republic. Int. J. Coal Geol. 2007, 71, 115–121. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, G.; Qin, L.B.; Han, Y.X.; Zhang, Q.; Chen, W.S.; Han, J. Effect of coal blending on arsenic and fine particles emission during coal combustion. J. Clean. Prod. 2021, 311, 127645. [Google Scholar] [CrossRef]
- Dai, S.F.; Wang, X.B.; Seredin, V.V.; Hower, J.C.; Ward, C.R.; O’Keefe, J.M.K.; Huang, W.; Li, T.; Li, X.; Liu, H.; et al. Petrology, mineralogy, and geochemistry of the Ge-rich coal from the Wulantuga Ge ore deposit, Inner Mongolia, China, new data and genetic implications. Int. J. Coal Geol. 2012, 90–91, 72–99. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, Y.C.; Teng, Y.; Zhang, K. Migration behaviors of arsenic and sulfur from coal during washing process and their release characteristics during combustion process. J. Fuel Chem. Technol. 2022, 50, 787–797, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Sia, S.G.; Abdullah, W.H. Enrichment of arsenic, lead, and antimony in Balingian coal from Sarawak, Malaysia, Modes of occurrence, origin, and partitioning behavior during coal combustion. Int. J. Coal Geol. 2012, 101, 1–15. [Google Scholar] [CrossRef]
- Dai, S.; Zeng, R.; Sun, Y. Enrichment of arsenic, antimony, mercury, and thallium in a Late Permian anthracite from Xingren, Guizhou, Southwest China. Int. J. Coal Geol. 2006, 66, 217–226. [Google Scholar] [CrossRef]
- Tian, C.; Hu, Y.Q.; Tian, X.; Huang, Z.F. Deep insights on arsenic speciation and partition in coal-fired particles from micro to nano size. Fuel 2022, 332, 126159. [Google Scholar] [CrossRef]
- Wang, X.Y.; Garrabrants, A.C.; Chen, Z.L.; Sloot, H.A.V.D.; Brown, K.G.; Qiu, Q.L.; Delapp, R.C.; Hensel, B.; Kosson, D.S. The influence of redox conditions on aqueous-solid partitioning of arsenic and selenium in a closed coal ash impoundment. J. Hazard. Mater. 2022, 428, 128255. [Google Scholar] [CrossRef]
- Huang, W.H.; Tang, Q.; Yang, D.Z.; Tang, X.Y.; Zhao, Z.G. The Affinity of Trace Elements of Permian Coal from Panji Mine on Huainan Coal Field. Earth Sci. Front. 2000, 7, 263–270. [Google Scholar]
- Li, X.Y.; Chen, J.; Yu, Y.; Zhao, R.H.; Lu, C.M. Comprehensive insight into the role of HCl on arsenic capture by CaO during coal combustion: A combined experimental and theoretical study. Fuel 2022, 310, 122474. [Google Scholar] [CrossRef]
- Xu, F.; Chu, M.; Hao, C.L.; Zhou, L.M.; Sun, X.B.; Gu, Z.Y. Volatilization characteristics and relationship of arsenic and sulfur during coal pyrolysis. Fuel 2022, 315, 123223. [Google Scholar] [CrossRef]
- Sun, Z.M.; Xiong, B.X.; Li, Y.L.; He, H.Q. Structural Characteristics and Favorable Beltfor Hydrocarbon Exploration in Santanghu Basin. Pet. Geol. Exp. 2001, 23, 23–26+37. [Google Scholar]
- Zhang, H.; Kuang, H.W.; Liu, Y.Q.; Peng, N. The sedimentary differences of the Middle Jurassic Xishanyao Formation between Santanghu area and Turpan-Hami area and their constraint on the uplifting of the Bogda Mountain. Geol. Bull. China 2013, 32, 443–455. [Google Scholar]
- Song, G.C.; Xu, W.T.; Yang, X.Y.; Song, Q. Coupling effects of mineral components on arsenic transformation during coal combustion. J. Hazard. Mater. 2022, 435, 129040. [Google Scholar] [CrossRef]
- Wang, Y.W.; Wang, J.B.; Long, L.L.; Zou, T.; Wang, L.J. Tectonic evolution stages of northern Xinjiang and tectonic types of porphyry epithermal deposits. Geol. China 2012, 39, 695–716. [Google Scholar]
- Li, Y.; Lu, Z.; Yang, H.; Jin, L.; Hu, H. Release characteristics of arsenic, selenium, lead and transformation of minerals during ashing process of coal. J. Fuel Chem. Technol. 2022, 50, 11–18. [Google Scholar] [CrossRef]
- Wiśniewska, F.W.; Makowska, D.; Strugała, A. Arsenic in polish coals: Content, mode of occurrence, and distribution during coal combustion process. Fuel 2022, 312, 122992. [Google Scholar] [CrossRef]
- White, R.N.; Smith, J.V.; Spears, D.A.; Rivers, M.L.; Sutton, S.R. Analysis of iron sulphides from UK coal by synchrotron radiation X-ray fluorescence. Fuel 1989, 68, 1480–1486. [Google Scholar] [CrossRef]
- Mastalerz, M.; Drobniak, A. Arsenic, cadmium, lead, and zinc in the Danville and Springfield coal members (Pennsylvanian) from Indiana. Int. J. Coal Geol. 2007, 71, 37–53. [Google Scholar] [CrossRef]
- Diehl, S.F.; Goldhaber, M.B.; Koenig, A.E.; Lowers, H.A.; Ruppert, L.F. Distribution of arsenic, selenium, and other trace elements in high pyrite Appalachian coals: Evidence for multiple episodes of pyrite formation. Int. J. Coal Geol. 2012, 94, 238–249. [Google Scholar] [CrossRef]

| Rank | Peat | Lignite | Bitumite | Anthracite | Bone Coal |
|---|---|---|---|---|---|
| As(μg/g) | 3–32.3 | 0.3–670 | 0.5–176 | 0.7–31 | 15–2820 |
| Period | Carboniferous | Permian | Triassic | Jurassic | Tertiary |
| As(μg/g) | 3.7 | 4.2 | 11.1 | 3.5 | 10.5 |
| Sample | STH7-1 | STH7-2 | STH7-3 | STH8-1 | STH9-1 | STH9-2 |
|---|---|---|---|---|---|---|
| As | 61.13 | 9.67 | 14.37 | 35.34 | 108.37 | 9.71 |
| CC1 | 7.37 | 1.17 | 1.73 | 4.26 | 13.06 | 1.17 |
| CC2 | 21.99 | 3.48 | 5.17 | 12.71 | 38.98 | 3.49 |
| Sample | STH9-3 | STH9-4 | STH9-5 | STH9-6 | STH9-7 | Average |
| As | 34.63 | 24.84 | 26.25 | 56.45 | 72.65 | 41.22 |
| CC1 | 4.17 | 2.99 | 3.16 | 6.80 | 8.75 | 4.97 |
| CC2 | 12.46 | 8.94 | 9.44 | 20.31 | 26.13 | 10.87 |
| Sample Number | Adsorption Arsenic | Organic Bound Arsenic | Residual Form of Arsenic | Sulfide Bound Arsenic | The Result of Reductive Difference Method | Percentage of As5+ | Sum of All Forms | The Total Arsenic Content (by AFS) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | As(III) | As(III) + As(V) | As(V) | (%) | (µg/g) | ||
| µg/g | µg/g | µg/g | µg/g | ug/g | ug/g | ug/g | ||||
| 3# | 0.55 | 3.12 | 6.45 | 60.54 | 8.14 | 46 | 37.9 | 52.64 | 70.66 | 72 |
| 5# | 0 | 2.4 | 6.4 | 40.8 | 15.6 | 60.54 | 45 | 88.24 | 49.6 | 51 |
| 6# | 0 | 23 | 35 | 46 | 12.5 | 40.8 | 28.3 | 26.20 | 104 | 108 |
| 7# | 0 | 11 | 6 | 63 | 32.2 | 63 | 30.8 | 38.02 | 80 | 81 |
| NW | + | + | + | |||||||
| GZ | + | + | + | |||||||
| WLTG | + | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, B.; Wang, W.; Tian, J.; Wang, W.; Feng, S.; Wang, M. Occurrence Modes of Arsenic in Coal: A Case Study from the Hanshuiquan Coal Mine, Santanghu Coalfield, Xinjiang Province, China. Appl. Sci. 2025, 15, 7092. https://doi.org/10.3390/app15137092
Zhu B, Wang W, Tian J, Wang W, Feng S, Wang M. Occurrence Modes of Arsenic in Coal: A Case Study from the Hanshuiquan Coal Mine, Santanghu Coalfield, Xinjiang Province, China. Applied Sciences. 2025; 15(13):7092. https://doi.org/10.3390/app15137092
Chicago/Turabian StyleZhu, Bo, Wenfeng Wang, Jijun Tian, Wenlong Wang, Shuo Feng, and Meng Wang. 2025. "Occurrence Modes of Arsenic in Coal: A Case Study from the Hanshuiquan Coal Mine, Santanghu Coalfield, Xinjiang Province, China" Applied Sciences 15, no. 13: 7092. https://doi.org/10.3390/app15137092
APA StyleZhu, B., Wang, W., Tian, J., Wang, W., Feng, S., & Wang, M. (2025). Occurrence Modes of Arsenic in Coal: A Case Study from the Hanshuiquan Coal Mine, Santanghu Coalfield, Xinjiang Province, China. Applied Sciences, 15(13), 7092. https://doi.org/10.3390/app15137092







