Experimental Study of Two-Stage Anaerobic Co-Digestion of Corn Steep Liquor and Agricultural Wastes for Hydrogen and Methane Production Including Metagenomics
Abstract
1. Introduction
2. Materials and Methods
2.1. Preliminary Batch Experiments Set-Up
2.2. Two-Stage Bioreactor System
2.3. Substrates
2.4. Inoculum
2.5. Analytical Methods
2.6. DNA Extraction
2.7. Metagenome Sequencing and Bioinformatics Analysis
2.8. Data Preprocessing and ASV Generation
2.9. Taxonomy Analysis and Community Diversity
3. Results and Discussion
3.1. Preliminary Batch Anaerobic Co-Digestion of CSL
3.2. Two-Stage Anaerobic Co-Digestion of CSL
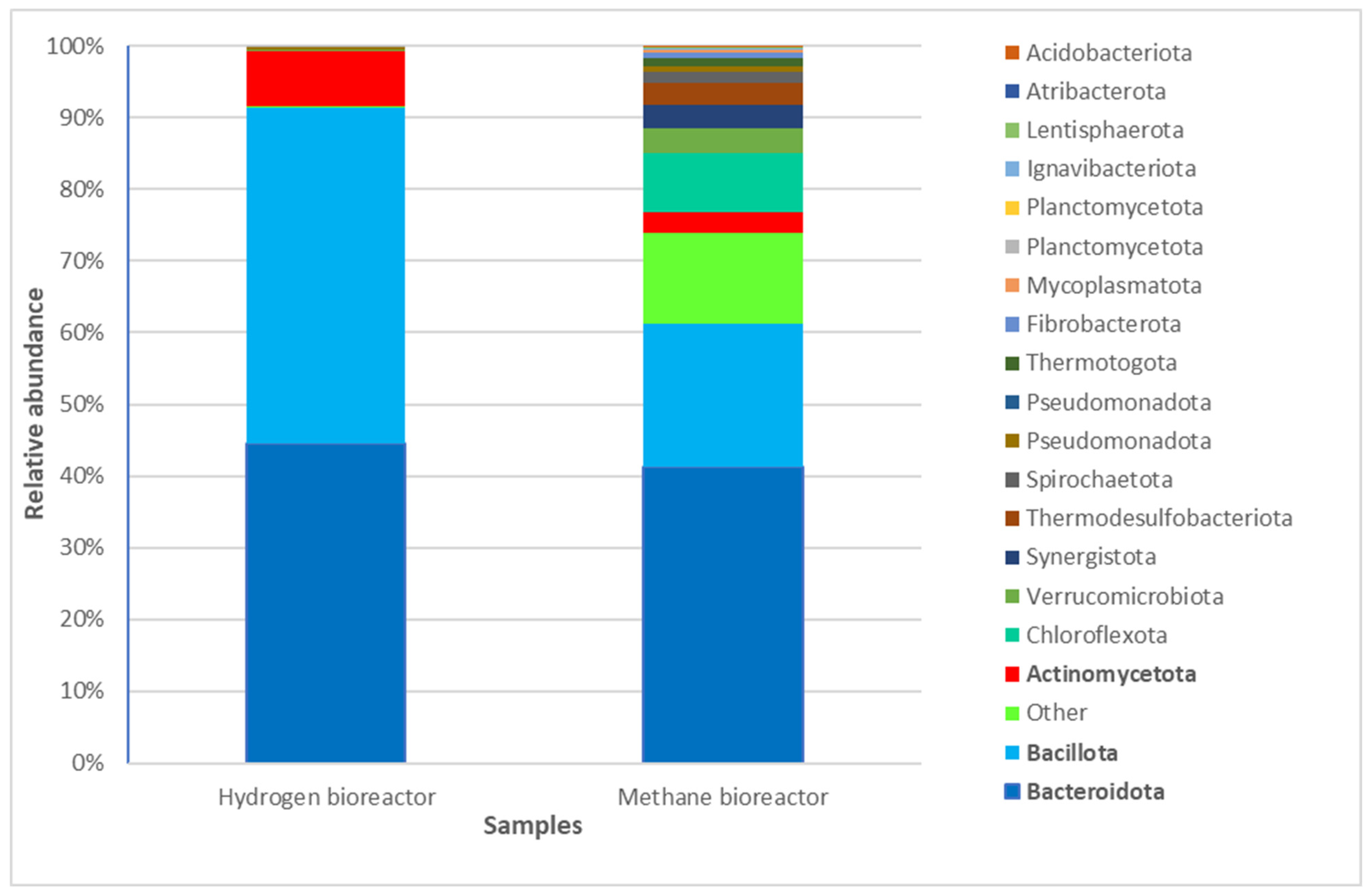
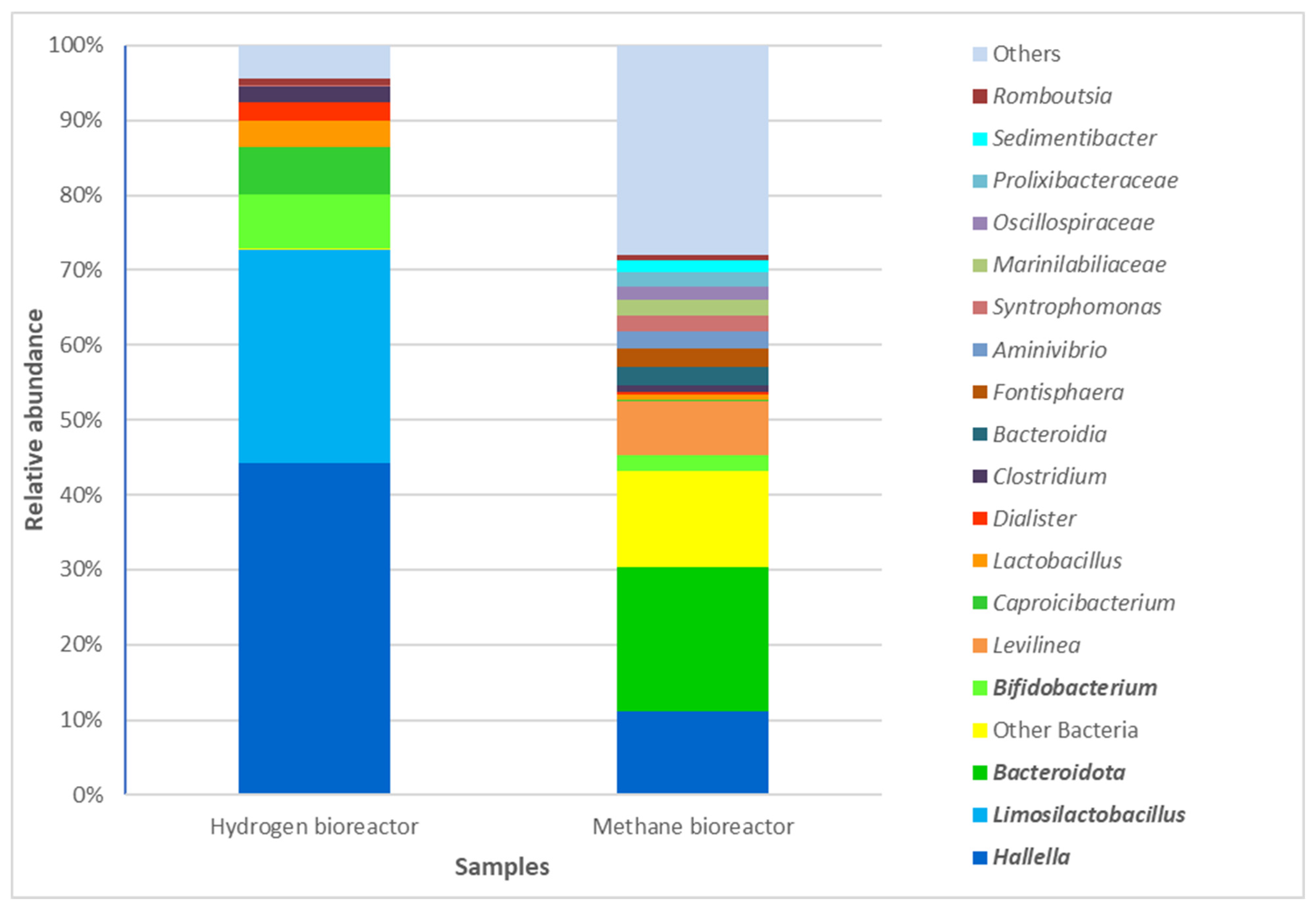
3.3. Comparative Analysis of the Taxonomic Composition at a Phylum Level in the Two Bioreactors for Anaerobic Waste Degradation with Hydrogen and Methane Production
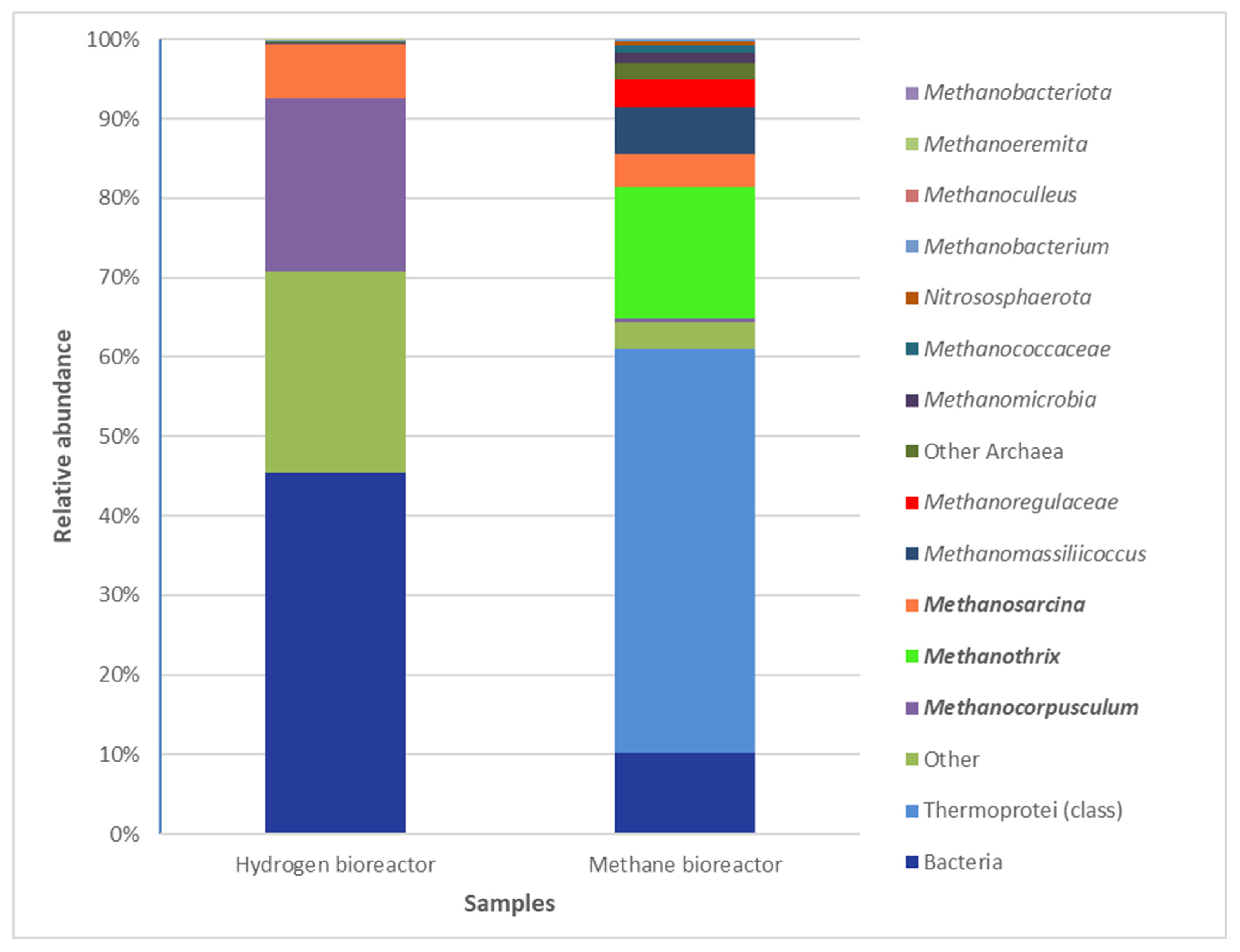
3.4. Archaeal Community in Both Bioreactors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Silva, F.M.; Mahler, C.F.; Oliveira, L.B.; Bassin, J.P. Hydrogen and methane production in a two-stage anaerobic digestion system by co-digestion of food waste, sewage sludge and glycerol. Waste Manag. 2018, 76, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Piadeh, F.; Offie, I.; Behzadian, K.; Rizzuto, J.P.; Bywater, A.; Córdoba-Pachón, J.-R.; Walker, M. A critical review for the impact of anaerobic digestion on the sustainable development goals. J. Environ. Manag. 2024, 349, 119458. [Google Scholar] [CrossRef] [PubMed]
- Chojnacka, K.; Moustakas, K. Anaerobic digestate management for carbon neutrality and fertilizer use: A review of current practices and future opportunities. Biomass Bioenergy 2024, 180, 106991. [Google Scholar] [CrossRef]
- Srisowmeya, G.M.; Chakravarthy, G.; Devi, N. Critical considerations in two-stage anaerobic digestion of food waste—A review. Renew. Sustain. Energy Rev. 2020, 119, 109587. [Google Scholar] [CrossRef]
- Tuan Le, T.; Sharma, P.; Jyoti Bora, B.; Tran, D.; Hai Truong, T.; Cuong Le, H.; Phong Nguyen, P.Q. Fueling the future: A comprehensive review of hydrogen energy systems and their challenges. Int. J. Hydrogen Energy 2024, 54, 791–816. [Google Scholar] [CrossRef]
- Franz, R.; Uslamin, E.A.; Pidko, E.A. Challenges for the utilization of methane as a chemical feedstock. Mendeleev Commun. 2021, 31, 584–592. [Google Scholar] [CrossRef]
- Ferdeș, M.; Paraschiv, G.; Ionescu, M.; Dincă, M.N.; Moiceanu, G.; Zăbavă, B.Ș. Anaerobic Co-Digestion: A Way to Potentiate the Synergistic Effect of Multiple Substrates and Microbial Diversity. Energies 2023, 16, 2116. [Google Scholar] [CrossRef]
- Zhou, K.; Yu, J.; Ma, Y.; Cai, L.; Zheng, L.; Gong, W.; Liu, Q. Corn Steep Liquor: Green Biological Resources for Bioindustry. Appl. Biochem. Biotechnol. 2022, 194, 3280–3295. [Google Scholar] [CrossRef]
- Martinez-Burgos, W.J.; Sydney, E.B.; Rodrigues de Paula, D.; Medeirosa, A.B.P.; Cesar de Carvalho, J.; Molina, D.; Soccol, C.R. Hydrogen production by dark fermentation using a new low-cost culture medium composed of corn steep liquor and cassava processing water: Process optimization and scale-up. Bioresour. Technol. 2021, 320, 124370. [Google Scholar] [CrossRef]
- Bertasini, D.; Battista, F.; Mancini, R.; Frison, N.; Bolzonella, D. Hydrogen and methane production through two stage anaerobic digestion of straw residues. Environ. Res. 2024, 247, 118101. [Google Scholar] [CrossRef]
- Chang, Y.; Liu, X.; Jiao, Y.; Zheng, X. Improved Cordycepin Production by Cordyceps Militaris Using Corn Steep Liquor Hydrolysate as an Alternative Protein Nitrogen Source. Foods 2024, 13, 813. [Google Scholar] [CrossRef] [PubMed]
- Hofer, A.; Hauer, S.; Kroll, P.; Fricke, J.; Herwig, C. In-depth characterization of the raw material corn steep liquor and its bioavailability in bioprocesses of Penicillium chrysogenum. Process Biochem. 2018, 70, 20–28. [Google Scholar] [CrossRef]
- Guilbert, D.; Vitale, G. Hydrogen as a Clean and Sustainable Energy Vector for Global Transition from Fossil-Based to Zero-Carbon. Clean Technol. 2021, 3, 881–909. [Google Scholar] [CrossRef]
- Rashidi, M.; Alavi, N.; Amereh, F.; Rafiee, M.; Amanidaz, N.; Partovi, K.; Mosanefi, S.; Bakhshoodeh, R. Biohydrogen production from co-digestion of sugarcane vinasse and bagasse using anaerobic dark fermentation. Bioresour. Technol. Rep. 2024, 25, 101793. [Google Scholar] [CrossRef]
- Simeonov, I.; Chorukova, E.; Kabaivanova, L. Two-Stage Anaerobic Digestion for Green Energy Production: A Review. Processes 2025, 13, 294. [Google Scholar] [CrossRef]
- Chorukova, E.; Hubenov, V.; Gocheva, Y.; Simeonov, I. Two-Phase Anaerobic Digestion of Corn Steep Liquor in Pilot Scale Biogas Plant with Automatic Control System with Simultaneous Hydrogen and Methane Production. Appl. Sci. 2022, 12, 6274. [Google Scholar] [CrossRef]
- Hubenov, V.; Miteva-Staleva, J.; Eneva, R.; Boteva, N.; Kabaivanova, L. Two-stage anaerobic digestion of wheat straw using immobilized microbial consortia. Ecol. Eng. Environ. Prot. 2021, 3, 35–44. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Updegraff, D.M. Semimicro determination of cellulose in biological materials. Anal. Biochem. 1969, 32, 420–424. [Google Scholar] [CrossRef] [PubMed]
- American Public Health Association (APHA). Standard Methods for Examination of Water and Waste Water, 23rd ed.; American Public Health Association (APHA): Washington, DC, USA, 2003; Available online: https://www.scirp.org/reference/referencespapers?referenceid=2459667 (accessed on 20 April 2025).
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Knight, R. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Seglah, P.A.; Wang, Y.; Wang, H.; Neglo, K.A.W.; Zhou, K.; Sun, N.; Gao, C. Utilization of food waste for hydrogen-based power generation: Evidence from four cities in Ghana. Heliyon 2023, 9, e14373. [Google Scholar] [CrossRef]
- Balcioglu, G.; Jeswani, H.K.; Azapagic, A. Evaluating the environmental and economic sustainability of energy from anaerobic digestion of different feedstocks in Turkey. Sustain. Prod. Consum. 2022, 32, 924–941. [Google Scholar] [CrossRef]
- Guo, X.M.; Trably, E.; Latrille, E.; Carrère, H.; Steyer, J.P. Hydrogen production from agricultural waste by dark fermentation: A review. Int. J. Hydrogen Energy 2010, 35, 10660–10673. [Google Scholar] [CrossRef]
- Font-Palma, C. Methods for the Treatment of Cattle Manure—A Review. C J. Carbon Res. 2019, 5, 27. [Google Scholar] [CrossRef]
- Chukwudi, O.O.; Chigbogu, G.O.; Tochukwu, N.N.; Tonia, N.N.; Ifeanyichukwu, E.E.; Victor, C.I.; Emmanuel, T.U.; Chika, J.U. Cattle manure as a sustainable bioenergy source: Prospects and environmental impacts of its utilization as a major feedstock in Nigeria. Bioresour. Technol. Rep. 2022, 19, 101151. [Google Scholar] [CrossRef]
- El Mekawy, A.; Srikanth, S.; Bajracharya, S.; Hegab, H.M.; Nigam, P.S.; Singh, A.; Mohan, S.V.; Pant, D. Food and agricultural wastes as substrates for bioelectrochemical system (BES): The synchronized recovery of sustainable energy and waste treatment. Food Res. Int. 2015, 73, 213–225. [Google Scholar] [CrossRef]
- Nindhia, T.S.; Nyoman Gde Bidura, I.G.; Sampurna, P.; Nindhia, T.G.T. Utilization of Continuous Anaerobic Digesters for Processing Cattle Dung and Cabbage (Brassica oleracea) Waste. Fermentation 2025, 11, 50. [Google Scholar] [CrossRef]
- Mata-Alvarez, J.; Macé, S.; Llabrés, P. Anaerobic digestion of organic solid wastes. An overview of research achievements and perspectives. Bioresour. Technol. 2000, 74, 3–16. [Google Scholar] [CrossRef]
- Sakamoto, M.; Umeda, M.; Ishikawa, I.; Benno, Y. Prevotella multisaccharivorax sp. nov., isolated from human subgingival plaque. Int. J. Syst. Evol. Microbiol. 2005, 55, 1839–1843. [Google Scholar] [CrossRef]
- Hitch, T.C.A.; Bisdorf, K.; Afrizal, A.; Riedel, T.; Overmann, J.; Strowig, T.; Clavel, T. A taxonomic note on the genus Prevotella: Description of four novel genera and emended description of the genera Hallella and Xylanibacter. Syst. Appl. Microbiol. 2022, 45, 126354. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Kumar, G.; Sivagurunathan, P. Microbiome involved in anaerobic hydrogen producing granules: A mini review. Biotechnol. Rep. 2019, 21, e00301. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, H.; Yan, R.; Li, S.; Guo, X.; Qiu, L.; Yao, Y. Limosilactobacillus regulating microbial communities to overcome the hydrolysis bottleneck with efficient one-step co-production of H2 and CH4. Adv. Sci. 2024, 11, 2406119. [Google Scholar] [CrossRef]
- Hou, W.; Wang, X.; Tian, T.; Wang, J.; Xiao, B.; Li, L. Effects of lactic acid fermentation on the biomethanation of food waste in two-stage anaerobic digestion. Fuel 2025, 400, 135785. [Google Scholar] [CrossRef]
- Li, W.; Siddhu, M.A.H.; Amin, F.R.; He, Y.; Zhang, R.; Liu, G.; Chen, C. Methane production through anaerobic co-digestion of sheep dung and waste paper. Energy Convers. Manag. 2018, 156, 279–287. [Google Scholar] [CrossRef]
- Zhang, L.; Loh, K.C.; Lim, J.W.; Zhang, J. Bioinformatics analysis of metagenomics data of biogas-producing microbial communities in anaerobic digesters: A review. Renew. Sustain. Energy Rev. 2019, 100, 110–126. [Google Scholar] [CrossRef]
- Stoyancheva, G.; Kabaivanova, L.; Hubenov, V.; Chorukova, E. Metagenomic analysis of bacterial, archaeal and fungal diversity in two-stage anaerobic biodegradation for production of hydrogen and methane from corn steep liquor. Microorganisms 2023, 11, 1263. [Google Scholar] [CrossRef] [PubMed]
- Bassani, I.; Kougias, P.G.; Treu, L.; Angelidaki, I. Biogas Upgrading via Hydrogenotrophic Methanogenesis in Two-Stage Continuous Stirred Tank Reactors at Mesophilic and Thermophilic Conditions. Environ. Sci. Technol. 2015, 49, 12585–12593. [Google Scholar] [CrossRef]
- Regueiro, L.; Veiga, P.; Figueroa, M.; Alonso-Gutierrez, J.; Stams, A.J.M.; Lema, J.M.; Carballa, M. Relationship between microbial activity and microbial community structure in six full-scale anaerobic digesters. Microbiol. Res. 2012, 167, 581–589. [Google Scholar] [CrossRef]
- Wang, W.; Chang, J.-S.; Lee, D.-J. Integrating anaerobic digestion with bioelectrochemical system for performance enhancement: A mini review. Bioresour. Technol. 2022, 345, 126519. [Google Scholar] [CrossRef]
- Rahman, M.H.; Bhoi, P.R.; Menezes, P.L. Pyrolysis of waste plastics into fuels and chemicals: A review. Renew. Sustain. Energy Rev. 2023, 188, 113799. [Google Scholar] [CrossRef]
- Patlolla, S.R.; Katsu, K.; Sharafian, A.; Wei, K.; Herrera, O.E.; Mérida, W. A review of methane pyrolysis technologies for hydrogen production. Renew. Sustain. Energy Rev. 2023, 181, 113323. [Google Scholar] [CrossRef]
- Makaryan, I.A.; Sedov, I.V.; Salgansky, E.A.; Arutyunov, A.V.; Arutyunov, V.S. A Comprehensive Review on the Prospects of Using Hydrogen–Methane Blends. Energies 2022, 15, 2265. [Google Scholar] [CrossRef]

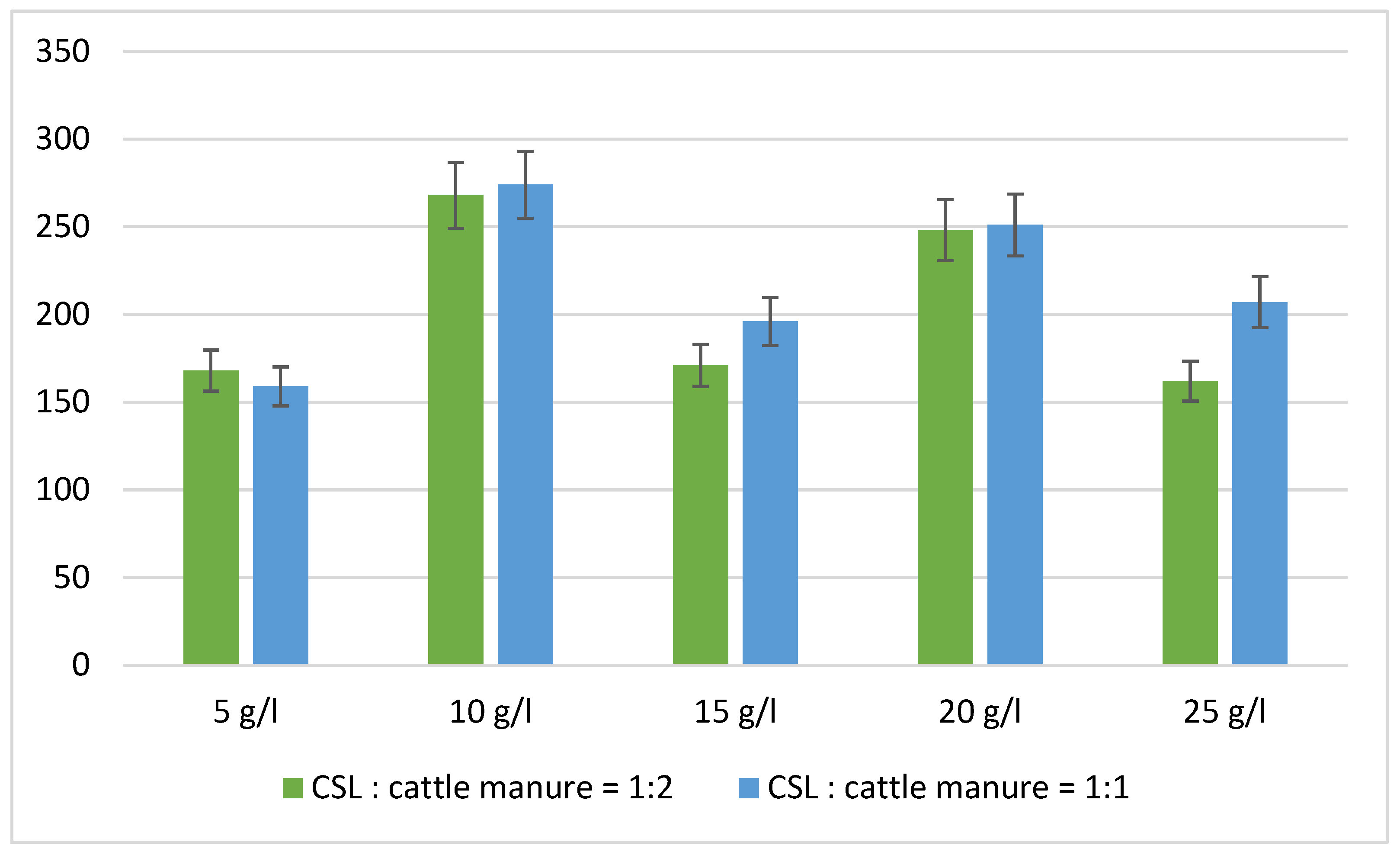

| Co-Substrates, Ratio | H2 [mL] 24 h | H2 [mL] 48 h | H2 [mL] 72 h |
|---|---|---|---|
| CSL | 67 ± 5.3 | 59 ± 4.5 | 10 ± 0.5 |
| CSL:potatoes = 1:1 | 155 ± 9.0 | 24 ± 1.6 | 5 ± 0.5 |
| CSL:tomatoes and cucumbers = 1:1 | 124 ± 8.2 | 29 ± 2.4 | 8 ± 1.2 |
| CSL:cattle manure = 1:1 | 168 ± 8.6 | 37 ± 2.9 | 10 ± 2.2 |
| Co-Substrates, Ratio | H2 [mL] 24 h | H2 [mL] 48 h | H2 [mL] 72 h |
|---|---|---|---|
| CSL | 72 ± 5.3 | 56 ± 4.5 | 12 ± 1.2 |
| CSL:cattle manure = 1:1 | 160 ± 9.4 | 29 ± 2.4 | 10 ± 2.6 |
| CSL:cattle manure = 1:2 | 153 ± 10.2 | 12 ± 0.8 | 5 ± 0.8 |
| CSL:cattle manure = 2:1 | 128 ± 6.5 | 42 ± 3.3 | 16 ± 4.8 |
| Organic Load | H2 [mL] 24 h | H2 [mL] 48 h | H2 [mL] 72 h |
|---|---|---|---|
| 5 g/L | 98 ± 7.8 | 63 ± 4.9 | 7 ± 1.2 |
| 10 g/L | 152 ± 9.4 | 90 ± 6.9 | 26 ± 9.7 |
| 15 g/L | 145 ± 10.6 | 21 ± 1.6 | 5 ± 0.8 |
| 20 g/L | 130 ± 8.2 | 86 ± 6.9 | 32 ± 2.4 |
| 25 g/L | 149 ± 9.8 | 8 ± 0.8 | 5 ± 1.2 |
| Organic Load | H2 [mL] 24 h | H2 [mL] 48 h | H2 [mL] 72 h |
|---|---|---|---|
| 5 g/L | 103 ± 7.8 | 48 ± 3.7 | 8 ± 0.8 |
| 10 g/L | 190 ± 9.0 | 67 ± 4.9 | 17 ± 5.7 |
| 15 g/L | 130 ± 9.8 | 44 ± 3.3 | 22 ± 7.3 |
| 20 g/L | 188 ± 8.2 | 43 ± 2.9 | 20 ± 1.6 |
| 25 g/L | 128 ± 8.6 | 69 ± 4.5 | 10 ± 2.4 |
| Day | Biogas Yield, L/day | Hydrogen Yield, % | Total Solids, % | Volatile Solids, % | Glucose, g/L | Cellulose, g/L | Protein, g/L |
|---|---|---|---|---|---|---|---|
| 1 | 0.00 | 0.00 | 46.1 | 75.4 | 0.27 | 0.19 | 0.12 |
| 2 | 4.90 | 25.45 | 43.7 | 74.2 | 0.36 | 0.16 | 0.14 |
| 3 | 5.28 | 19.29 | 43.7 | 75.0 | 0.31 | 0.16 | 0.11 |
| 4 | 5.28 | 18.59 | 34.3 | 73.6 | 0.30 | 0.21 | 0.14 |
| 5 | 5.59 | 16.80 | 38.2 | 73.2 | 0.25 | 0.19 | 0.15 |
| 6 | 5.59 | 12.16 | 40.3 | 72.7 | 0.24 | 0.20 | 0.17 |
| 7 | 4.43 | 16.60 | 42.9 | 73.5 | 0.23 | 0.18 | 0.18 |
| 8 | 4.64 | 13.64 | 35.0 | 73.0 | 0.19 | 0.18 | 0.24 |
| 9 | 4.95 | 12.60 | 35.1 | 72.9 | 0.18 | 0.17 | 0.23 |
| Day | Biogas Yield, L/day | Methane Yield, % | Total Solids, % | Volatile Solids, % | Glucose, g/L | Cellulose, g/L | Protein, g/L |
|---|---|---|---|---|---|---|---|
| 1 | 27.96 | 69.00 | 27.4 | 72.4 | 0.18 | 0.11 | 0.33 |
| 2 | 25.56 | 73.23 | 30.2 | 73.0 | 0.12 | 0.15 | 0.31 |
| 3 | 22.44 | 72.47 | 29.5 | 74.0 | 0.13 | 0.12 | 0.34 |
| 4 | 25.24 | 71.30 | 31.5 | 74.6 | 0.20 | 0.16 | 0.36 |
| 5 | 23.28 | 72.80 | 32.7 | 73.1 | 0.14 | 0.15 | 0.33 |
| 6 | 34.70 | 69.45 | 31.3 | 72.7 | 0.12 | 0.16 | 0.27 |
| 7 | 20.01 | 77.52 | 34.3 | 73.0 | 0.15 | 0.11 | 0.39 |
| 8 | 21.32 | 71.19 | 39.4 | 74.0 | 0.14 | 0.11 | 0.37 |
| 9 | 20.86 | 77.37 | 38.5 | 74.0 | 0.12 | 0.10 | 0.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chorukova, E.; Stoyancheva, G.; Kabaivanova, L. Experimental Study of Two-Stage Anaerobic Co-Digestion of Corn Steep Liquor and Agricultural Wastes for Hydrogen and Methane Production Including Metagenomics. Appl. Sci. 2025, 15, 7076. https://doi.org/10.3390/app15137076
Chorukova E, Stoyancheva G, Kabaivanova L. Experimental Study of Two-Stage Anaerobic Co-Digestion of Corn Steep Liquor and Agricultural Wastes for Hydrogen and Methane Production Including Metagenomics. Applied Sciences. 2025; 15(13):7076. https://doi.org/10.3390/app15137076
Chicago/Turabian StyleChorukova, Elena, Galina Stoyancheva, and Lyudmila Kabaivanova. 2025. "Experimental Study of Two-Stage Anaerobic Co-Digestion of Corn Steep Liquor and Agricultural Wastes for Hydrogen and Methane Production Including Metagenomics" Applied Sciences 15, no. 13: 7076. https://doi.org/10.3390/app15137076
APA StyleChorukova, E., Stoyancheva, G., & Kabaivanova, L. (2025). Experimental Study of Two-Stage Anaerobic Co-Digestion of Corn Steep Liquor and Agricultural Wastes for Hydrogen and Methane Production Including Metagenomics. Applied Sciences, 15(13), 7076. https://doi.org/10.3390/app15137076






