Featured Application
The results of the work can be used in technologies of alkali-activated cement materials.
Abstract
The paper is devoted to the plasticization mechanisms of alkali-activated cement system Na2O-CaO-Al2O3-SiO2-H2O. The fundamentals and basic factors determining the effectiveness of plasticizing surfactants for alkali-activated cement materials are discussed. The factors under consideration in the study were alkali-activated cement basicity (the content of granulated blast furnace slag), the anion of the alkaline component or activator, and the degree of dispersing of the cement particles in the system. The action effect of plasticizers was determined by finding the interrelation between the stability of its molecular structure, degree of adsorption, and molecular weight depending on mentioned basic factors. A systematic approach to the systematization of surfactants and their choice to be taken into consideration to control technology-related and physico-mechanical properties of alkali-activated cement-based heavyweight concretes, building mortars, and lightened grouts has been proposed.
1. Introduction
Current trends in construction prescribe the wider use of concretes with enhanced performance and durability, prioritizing cements alternative to ordinary Portland cement (OPC) [1]. The production of 1 ton of OPC requires an energy consumption of 3000–6000 kJ/kg and 1.5 tons of natural resources [2]. The requirement to reduce energy consumption and limit CO2 emissions through the substitution of OPC clinker for wastes and industrial by-products [3,4,5,6,7] has been prescribed by the UN recommendations (Sustainable Development Goals) and the EU Green Deal [8,9].
Thus, the use of alkali-activated cements (further, AAC), including the compositions without clinker constituent, is becoming more important in light of the above [10]. AACs are presented mainly by the system R2O-CaO-Al2O3-SiO2-H2O [11,12], where R—Li, Na, and K. The system R2O-Al2O3-SiO2-H2O (geopolymers) is a particular case of AAC and is used in materials for special uses, such as coatings (sulphate-resistant, fire-resistant, decorative), adhesives, bonds, low-temperature ceramics, etc. [13,14,15].
The requirement for the concrete mix design with high consistency is determined by practical application [16,17]. Concretes with controlled rheological properties are in great demand today [18,19].
Expanding the AAC scope needs a one-part technology, using an alkaline component (i.e., precursor, alkaline activator) in a solid form [20,21] and further mixing with water as it occurs in the case of OPC. The AAC production requires the use of plasticizing admixtures [22,23]. This is necessary to achieve the required content of the alkaline component in the process of AAC hydration and control technology-related and physico-mechanical properties [24,25,26].
The action mechanism of traditional plasticizing admixtures in AAC differs from that in OPC [27,28,29,30]. The effectiveness of plasticizing surfactant is connected with such characteristic features related to a chemical nature as the degree of adsorption on the surface of cement mineral particles and the stability of their molecular structure.
Adsorption of plasticizing surfactants (polymers) on the surface of particles in AAC systems is lower than in OPC systems. This can be attributed to the following: an electrokinetic (zeta) potential on the surface of the AAC mineral particles differs greatly from that of OPC—that is, less positive or negative values. The value of the zeta potential depends upon a cement compositional build-up and characteristics of the hydration medium or aqueous pore solution [31,32,33]. Charges of the functional groups of the polymer and zeta potential on the surface of the AAC mineral particles are in contradiction, negatively affecting the adsorption of plasticizing surfactants and, respectively, their effectiveness. Another reason is that in the systems with a high ionic force, the worsening of the adsorption of ionic surfactants is connected with “competition” in the adsorption action that takes place between the cement itself and functional groups of admixtures, for example, hydroxyl [33], sulphate ions [34], and anion of citric acid [35]. There is also competition between superplasticizers and alkaline activators in their interaction with the precursors (whether blast furnace slags or fly ashes). An assumption was put forward that in aqueous solutions of sodium silicates, a possibility of adsorption of the functional groups of anionic surfactants by the cement mineral particles is restricted by the competition not only with hydroxyls (OH−) but anions of silicic acid (SiO32−) as well [36].
The adsorption ability of anionic surfactants, like melamine- or naphthalene-based surfactants, as well as vinyl copolymer in AAC, almost does not depend upon pH values; however, it is considerably lower compared to that of OPC [31,37].
The lower degree of adsorption of PCE in AAC is a result of a competitive adsorption action of the functional groups of polymer and hydroxides [38,39]. Thus, a certain molecular structure of PCE is to be synthesized to provide the required degree of adsorption.
The adsorption of polymer increases with the increase in charge of its main chain and molecular weight, as well as the decrease of the surface area of the cement mineral particles and surface covered by polymer [40,41]. In AAC, the required adsorption of the polymer can be reached through an increase in charge, as well as the adjustment of its molecular weight and length of side chains [39].
A variety of high- and low-molecular-weight compounds or their combination can be used with high effectiveness as plasticizing surfactants in AAC [36]. High molecular surfactants form, first of all, structured adsorption layers on the surface of the interphase, whereas low molecular surfactants decrease surface tension, thus promoting the processes of dispergation and plasticization.
The use of ionic surfactants in combination with nonionic surfactants is effective in the plasticization of AAC [42,43,44]. Ionic surfactants are adsorbed due to hydrophilic groups; nonionic surfactants increase surface activity due to the covalent bonds of hydrophobic (lipophilic) groups.
The stability of a molecular structure is another important factor that determines the action effect of plasticizing admixtures. The results of infrared spectroscopy confirmed the degradation of the molecular structure of admixtures in the hydration medium of AAC. Naphthalene-based admixtures are characterized by a relatively stable molecular structure in NaOH, but they undergo alterations in the SO3 groups when waterglass is used as an alkaline component [29,45,46]. Melamine-based admixtures undergo the same alterations in their formulation both NaOH and waterglass. PCE undergoes alkaline hydrolysis of the ester groups in the mentioned high alkaline mediums [46].
Furthermore, it can be concluded that a decrease in the action effect of plasticizers in the AAC water suspensions is caused not only by high pH values but also by another independent factor—the nature of the alkaline component (its anion). The highest degree of degradation, accompanied by cleaving of side chains due to alkaline hydrolysis, was found in sodium and potassium silicate solutions compared with sodium and potassium hydroxides [47]. Soluble sodium silicates also lead to structural changes in melamine- and naphthalene-based admixtures [48,49].
A molecular structure degradation of traditionally used plasticizing admixtures, when used in the AAC concretes, results in a higher water content. With the increase in water requirement, the strength decreases [50].
When the alkaline component is represented by sodium hydroxide (NaOH) of a low concentration (0.3%), the plasticizing effect is achieved through the use of sodium salts as admixtures: sodium phosphate (Na5P3O10), sodium gluconate (NaC6H11O7), sodium citrate (Na3C6H5O7), and sodium tartrate (Na2C4H4O6) [51].
Polyols (ethylene glycol C2H4(OH)2, glycerol C3H5(OH)3, sorbitol C6H8(OH)6) are effective admixtures for plasticizing the NaOH-activated AAC [52]. The influence of polyols on the consistency of the AAC concrete mix and its retention time, as well as on the concrete strength, depends upon the quantity of hydroxyl groups in a molecule and its molecular weight [36].
Sodium lignosulphonate (NaLST) exhibits an independent plasticizing effect on AAC with different activators (NaOH, Na2CO3, Na2SiO3) [53,54,55]. However, the action effect of NaLST as a plasticizing admixture is restricted by its high dosage [56].
A highly alkaline medium, like in the case of sodium hydroxide and sodium silicates, does not change the structure of the admixture, such as polypropylene glycol (HO-[CH2-CH(CH3)-O]n-H) [46,57].
The results of infrared spectroscopy confirmed the high effectiveness of the plasticizers without ester bonds, represented by NaLST, polyethers, ethoxylated fatty alcohol, the salt of carboxylic acid, carboxylic acids, and acyclic polyamine [58]. The silicate component (sodium metasilicate) caused a higher degradation of polycarboxylate esters than sodium carbonate (soda-ash) due to the higher pH value, i.e., 12.6 and 11.3, respectively.
These results concerning the stability of plasticizing admixtures with various molecular structures in the AAC hydration medium correlate well with the influence on the AAC structure formation observed by changes in rheological properties of the AAC pastes [36], as well as in the heat evolution using thermo-kinetic criteria [59,60].
The use of PCEs in AAC. Conventional methacrylate ester-based (MPEG) PCEs are the most effective plasticizing admixtures for Portland cement-based materials due to the steric mechanism of plasticization [61,62,63,64]. However, they are ineffective in alkali-activated cement materials. As shown above, this phenomenon is primarily caused by the instability of the molecular structure in the hydration environment of AAC and by the low adsorption of PCEs on the surface of AAC particles due to competitive adsorption between PCEs and the other anions present in the cement pore solution, e.g., anion (SiO32−) in the case of silicate-based activators [36]. The inefficiency of conventional PCEs is also due to their lack of solubility in alkaline salt solutions [65,66], as well as the conformational compression of PCE molecules, which reduces their steric repulsion [67]. High ionic force in pore solution of AAC, activated by 0.25–0.5 M Na2SiO3, resulted in coiling of the PCE molecules, hampering their steric hinderance [68]. At very high ionic strengths (e.g., 3 M NaOH or 1 M Na2SiO3), PCE molecules self-assemble into large aggregates (>1 µm), which ultimately leads to flocculation and insolubility.
It was demonstrated that MPEG-based PCE with the co-monomer acrylic acid was more effective than methacrylic acid [69]. This difference was attributed to the acrylic acid-based MPEG PCE having a higher hydrophilic–lipophilic balance value, resulting in higher adsorption and solubility in the highly alkaline medium of AAC.
The compositions of ether-based PCE were synthesized to be effective in AAC [40]. Thus, ether-based polymers with a cationic charge possessing a side chain of 43 ethylene oxide units and a quaternary ammonium to side chain ratio of 5:1 were synthesized as effective superplasticizers for a sodium silicate-activated ground granulated blast-furnace slag (GBFS) system.
Furthermore, α-allyl-ω-hydroxy poly (ethylene glycol)-based PCE (APEG), suitable for the NaOH-activated AAC, was synthesized from allyl ether macromonomer and maleic anhydride. The proposed polymer exhibited a short side chain made of seven ethylene oxide units and a higher molecular weight (Mw > 30,000 Da) [65]. The superior dispersing effect of the polymer was attributed to its good solubility in NaOH solution and the enhanced chelating ability of maleic anhydride for Ca2+ cations, promoting the adsorption of these PCE molecules on ground granulated blast-furnace slag.
PCE, synthesized via free-radical copolymerization from acrylic acid as backbone monomer and α-methallyl-ω-hydroxy poly (ethylene glycol) ether (HPEG) as macromonomer, was proposed as well. It was concluded that such PCE of high anionicity, high molecular weight, and side chains of short length adsorbed particularly high amounts on AAC particles (NaOH—4% by mass of ground granulated blast-furnace slag), thus providing the enhanced dispersion capacity as compared to other polymers [66].
Another type of ether-based PCE is isoprenyl oxy poly(ethylene glycol) ether (IPEG) PCEs [70]. The dispersing ability of such superplasticizer in AAC and degree of adsorption depends on the molecular weight and anionicity of the polymer.
A novel polycarboxylate amine superplasticizer with high adsorption capacity was obtained by replacing the polyethylene oxide side chain in the classical PCE with a bulky pendant group, sterically hindered amine (2,2,6,6-tetramethyl-4-piperidyl methacrylate), employing the Reversible-Addition Fragmentation Chain Transfer polymerization [71]. This polycarboxylate amine copolymer was found to significantly reduce the yield stress of the AAC pastes at low dosages (i.e., 0.1%) due to pendant groups, below the values that were previously obtained for PCE with varying polyethylene oxide side-chain lengths [72].
However, PCEs of the proposed molecular structures, that are specifically synthesized for the AAC materials, are rarely produced by the industry. On the other side, conventional ester-based (MPEG)PCEs, widely used in the manufacturing of OPC materials, are ineffective due to the reduced degree of adsorption on the AAC mineral particles.
Thus, the action effect of plasticizing admixtures in AAC depends upon the degree of adsorption and stability of the molecular structure, as well as the molecular weight. The AAC basicity, which depends upon the content and composition of the aluminosilicate component, as well the anion of the alkaline component, are the main factors determining the action of plasticizing admixtures. The degree of dispersing of the AAC suspension in a material is one more important factor [40]. So, plasticizing admixtures decrease the yield stress value at a low and high degree of dispersing [73].
However, with the addition of plasticizing surfactant, the plastic viscosity of a concrete mix remains practically without changes, whereas in a cement paste, this admixture can change a plastic viscosity similar to water. Changes in the cement suspension structure and thixotropic properties are clearly expressed in the suspensions with the higher degree of dispersing [74].
Rheology of the AAC materials differs from that of the OPC-based materials and depends upon the AAC composition, as well as the type and concentration of the alkaline component and technological parameters [22,23]. Furthermore, the rheological properties of the AAC suspensions are more sensitive to changes in solid-phase concentration compared to those of OPC suspensions [75].
For all of this, the purpose of the present study is to establish an interrelation between the molecular structure stability, degree of adsorption, and molecular weight of a surfactant and its plasticizing effect depending upon the AAC basicity, anion of the alkaline component, and degree of dispersing of AAC in the materials for a wide range of AAC-based materials, from lightened grouts to building mortars and heavyweight concretes.
2. Materials and Testing Methods
2.1. Raw Materials
The following were as components of AAC:
- ground granulated blast-furnace slag (further, GBFS) (oxides, % by mass: CaO—47.30; SiO2—39.00; Al2O3—5.90; Fe2O3—0.30; MgO—5.82; SO3—1.50; LOI—0.18), specific surface area (S) = 450 m2/kg (by Blaine), modulus of basicity Mb = 1.2, glassy phase content—75.0%;
- Portland cement clinker (clinker) (oxides, % by mass: CaO—64.93; SiO2—22.60; Al2O3—5.29; Fe2O3—3.93; MgO—0.84; SO3—0.50; K2O + Na2O—0.70; LOI—0.90), specific surface area (S) = 450 m2/kg (by Blaine), modulus of basicity Mb = 2.4.
The following were used as alkaline components of AAC:
- calcined soda-ash of technical grade (Na2CO3) (further, soda-ash) per CAS 497-19-8, pH (1.0% aqueous solution, 25 °C) = 11.4;
- sodium metasilicate pentahydrate Na2SiO3∙5H2O (sodium metasilicate) per CAS 497-19-8, pH (1.0% aqueous solution, 25 °C) = 12.7.
To evaluate the basicity of the cement systems under study, a modulus of basicity, similar to that used in the evaluation of basicity of GBFS, namely Mb = (CaO + MgO)/(SiO2 + Al2O3)—a ratio of masses of oxides, was applied [36].
NaLST (sodium lignosulphonate) per CAS 8061-51-6 (pH ≥ 8.5) was used as a component in AAC to ensure satisfactory setting time and strength.
A waterproofing agent based on polyhydrosiloxane (0.06% by mass of grinding material) per CAS 63148-57-2 was used to intensify grinding, prevent sorption of water from air, and retain the properties of AAC over time.
Calcium sulphate hemihydrate (CaSO4∙0.5H2O), density 2.7 g/cm3, was used in AAC to retard the initial setting and enhance the physico-mechanical properties as recommended in [76].
Bentonite clay powder (bentonite) (oxides, % by mass: CaO—2.27; SiO2—66.17; Al2O3—16.51; FeO + Fe2O3—4.21; MgO—1.61; MnO—0.33; K2O + Na2O—8.90), output of clay solution 18 m3/t, density 2.64 g/cm3, specific surface area (S) = 450 m2/kg (by Blaine). Bentonite was used in AAC lightened grouts to decrease of density and water separation, prolong the setting times, and increase of water-retaining capacity [77,78].
Preparation of the one-part AAC was done by dry mixing the above-listed components.
In total, the mixture compositions of AAC mortars and concretes are presented in Table 1 and Table 2. A mortar mix design of the reference compositions of the AAC building mortars (Mix 1–Mix 4) was used according to [36].

Table 1.
Mix design of the AAC mortars.

Table 2.
Mix design of the AAC concretes.
A concrete mix design of the reference compositions of the AAC heavyweight concretes (Mix 5–Mix 10) was used according to [26].
A reference composition of the AAC lightened grout (Mix 11) was used according to [78]: AAC—100%, W/C—1.25. Its characteristics were: grout density—1.40 ± 0.04 g/cm3, consistency (flow) > 250 mm.
Milled silica sand fr. < 0.16 mm (sieve residue, %: <0.05 mm—80.0, 0.05 mm—9.5, 0.063 mm—7.0, 0.10 mm—2.5, 0.16 mm—1.0) was used as filler in the preparation of the building mortars.
River silica sand (sieve residue, %: <0.16 mm—10.3, 0.16 mm—14.75, 0.315 mm—33.05, 0.63 mm—41.1, 1.25 mm—0.8) per the National Standard of Ukraine DSTU B V.2.7-232:2010 [79] (Sand for construction work. Testing methods) was used as fine aggregate in heavyweight concretes.
Mix 4 contained NaLST—0.2% by mass, and carboxymethyl cellulose “Gabrosa HV” (Nouryon, Amsterdam, The Netherlands)—0.1 by mass.
Consistency (flow) of reference composition for anchoring application: Mix 1—7.0 cm. Consistency (flow) of reference compositions for floor application: Mix 2—17.3 cm, Mix 3—8.0 cm, Mix 4—17.1 cm.
Silica sand with grain sizes between 0.16 and 0.63 mm (fr. 0.16/0.63) and between 0.63 and 1.25 mm (fr. 0.63/1.25), and washed granite screening with grain sizes between fr. = 1.25 and 2.5 mm (fr. 1.25/2.5) was used as fine aggregate in preparation of building mortars.
Granite gravel with grain sizes between 2.5 and 5.0 mm (fr. 1.25/2.5), between 5.0 and 10.0 mm (fr. 5/10), and between 10.0 and 20.0 mm (fr. 10/20) was used as coarse aggregate in the heavyweight concretes.
The plasticizing surfactants, used in research, are presented in Table 3.

Table 3.
The plasticizing surfactants.
The defoaming agent was based on silicon “XIAMETER AFE-0310 Antifoam Emulsion” (Dow Corning, Midland, MI, USA).
The components of complex additives in mortar for anchoring application were presented by:
- aluminum powder according to CAS No. 7429-90-5 (content, %: active aluminum—85.00–93.00, Fe—0.50, Si—0.40, Cu—0.05, Mn—0.05, moisture—0.20, fatty admixtures—3.80), water covering ≥ 7000 cm2/g;
- sodium nitrate (NaNO3) according to CAS No. 7631-99-4.
Carboxymethyl cellulose “Gabrosa HV” (Nouryon, Amsterdam, The Netherlands) (Na-CMC) was used as a water-retaining admixture in the building mortar compositions for floor application.
2.2. Testing Methods
2.2.1. Tests on Mortars and Concrete in the Fresh State
A slump of fresh concrete was determined per EN 12350-2:2019 [80].
Consistency (flow), consistency retention time, and water retention capacity of building mortars were determined per the National Standard of Ukraine DSTU B V.2.7-126:2011 [81] (Modified dry building mixtures. General specifications).
Density, consistency (flow), and water separation in lightened grouts were determined per the National Standard of Ukraine DSTU B V.2.7-86-99 [82].
Plastic viscosity, static shear stress, and dynamic shear stress of lightened grouts at variable temperatures were determined with the help of a rotary viscometer “Fann 35 SA” (Houston, TX, USA) and atmospheric consistometer “Chandler 1200” (Tulsa, OK, USA) under a procedure per ISO 10426-2:2003 [83].
2.2.2. Tests on Mortars and Concrete in the Hardened State
The concrete compressive strength at 3, 7, and 28 days was determined per EN 12390-3:2019 [84].
Freeze–thaw resistance of concrete specimens was studied in accordance with the third test method prescribed by the national standard of Ukraine DSTU B V.2.7-49-96 (Concretes. Rapid methods for determination of frost resistance by repeated alternated freezing and thawing) [85]. According to this accelerated method, the cube specimens (100 mm), after 28 days of storage under normal conditions (t = 20 ± 2 °C and RH = 95 ± 5%), were saturated with 5% solution of NaCl at t = 18 ± 2 °C (48 h), and after that, they were subjected to freezing at t = −50 ± 5 °C. Thawing was done in 5% solution of NaCl. A grade of concrete in freeze–thaw resistance (F) is a number of alternate freezing and thawing at which a mean compressive strength decreased by no more than 5%. The freeze–thaw resistance of the concrete was assessed by the correspondence between permissible number of freezing–thawing cycles by the accelerated method and by the first (basic) method prescribed in the national standard of Ukraine DSTU B V.2.7-47 (Concretes. Methods for determination of frost resistance. General requirements) [86].
The flexural and compressive strength of building mortars at 1, 3, 7, and 28 days were determined per EN 196-1:2005 [87].
The bond strength of building mortars with the substrate was determined per EN 1542:1999 [88].
The pull-out strength of the anchor was determined per EN 1881:2006 [89].
The flexural strength of lightened grouts at 2 days at temperatures t = 22 °C and t = 75 °C were determined per the National Standard of Ukraine DSTU B V.2.7-86-99.
3. Fundamentals
The main results about the influence of the AAC basicity, anion of the alkaline component, and degree of dispersing of AAC in the materials on the effect of different plasticizing surfactants (factor #1—molecular structure, factor #2—charge of polar groups, factor #3—molecular weight) are presented in the following.
3.1. The Influence of the AAC Basicity
3.1.1. Factor #1: Molecular Structure of a Surfactant
The subject of research was a building mortar based on an AAC with Mb = 2.4 (Mix 1, see Table 1) (reference composition). The criteria for evaluating the effectiveness of a surfactant were the consistency (flow) of fresh mortar and the compressive strength of mortar.
The effectiveness of plasticizing surfactants based on PCE—PCE1 (“JK-04PP”), PCE2 (“Vinavil fluxe”), and modified polyethylene glycol—PEG-M (“Melflux PP100F”) was evaluated. These PCE-based anionic surfactants were chosen as the most effective for use in AAC with a minimal required content of the alkaline component [58]. The surfactant content was 0.5% by mass.
The action effect of a surfactant was evaluated by the changes in consistency of the mortar mix within the range of (150 ± 15)–(200 ± 15) mm as W/C decreased from 0.65 to 0.45–0.47 (Figure 1), as well as by the changes in strength of the AAC building mortar at 1 day and 28 days (Figure 2).
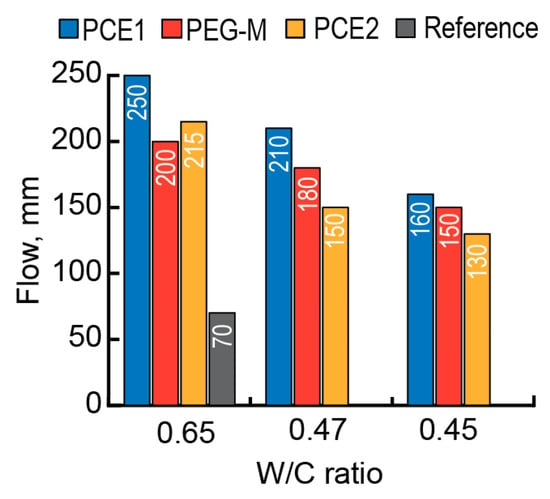
Figure 1.
Consistency (flow) of the fresh mortar based on the AAC with Mb = 2.4 (Mix 1) vs. the admixture type (PCE1—blue, PCE2—red, PEG-M—yellow, and without admixture (reference)—black), and W/C (0.45, 0.47, and 0.65).
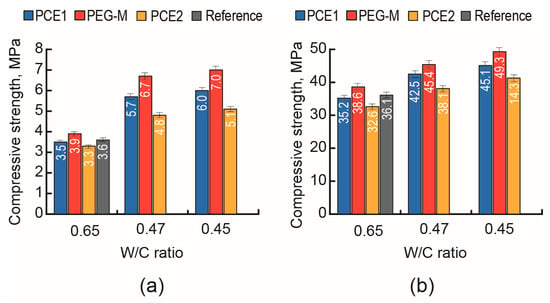
Figure 2.
Compressive strength of the mortar based on the AAC with Mb = 2.4 (Mix 1) at 1 day (a) and 28 days (b) vs. the surfactant type (PCE1—blue, PCE2—red, PEG-M—yellow, and without admixture (reference)—black) and W/C (0.45, 0.47, and 0.65).
Among three tested admixtures, the addition of PEG-M produced the greatest effect on the compressive strength values at 1 day (Figure 2a) and 28 days (Figure 2b) at the W/C values from 0.65 to 0.47 and lower to 0.45.
A conclusion can be drawn that the addition of a copolymer of polyethylene glycol and acrylic acid (PEG-M) exhibits the highest effectiveness in the materials based on AAC with Mb = 2.4 (Mix 1) compared to those of PCE1 and PCE2.
3.1.2. Factor #2: Charge of Polar Groups of a Surfactant
The subject of research was a concrete based on an AAC with Mb = 1.2–1.6 (Mixes 5–9) (see Table 2). The criteria for evaluating the effectiveness of a surfactant was the consistency (slump) and its retention time of fresh concrete, as well as thecompressive strength of concrete.
The effectiveness of the combined action of anionic, nonionic, and cationic surfactants (1.0% by mass), each taken alone and in conjunction with NaLST, was evaluated.
With the AAC basicity decrease from Mb = 2.4 to Mb = 1.6 (Mixes 5 and 6) (Figure 3a,b) and further to Mb = 1.3–1.2 (Mixes 7–9) (Figure 3c–e), higher contents of the alkaline component were required and, as a result, the effectiveness of anionic surfactants (PCE) decreased, resulting in a decrease of the plasticizing effect and consistency retention time. This effect can be attributed to a lower adsorption ability of surfactants due to the change of the mineral particles from positive to negative with the increase in GBFS content in AAC. Additionally, a shorter consistency retention time can be attributed to PCE destruction (hydrolysis) in a highly alkaline medium [63].
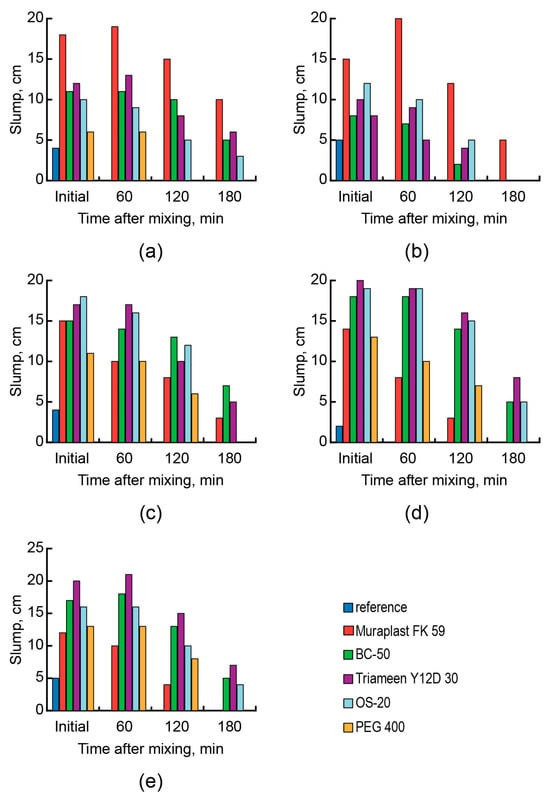
Figure 3.
The consistency (slump) of fresh concrete vs. the type of surfactant (reference—blue, Muraplast FK 59—red, BC-50—green, Triameen Y12D 30—violet, OS-20—light blue, PEG 400—orange), AAC composition, and time after mixing with water: AAC (soda-ash, Mb = 1.6) (Mix 6) (a); AAC (sodium metasilicate, Mb = 1.6) (Mix 5) (b); AAC (soda-ash, Mb = 1.3) (Mix 7) (c); AAC (soda-ash, Mb = 1.2) (Mix 9) (d); AAC (sodium metasilicate, Mb = 1.2) (Mix 8) (e).
On the contrary to anionic surfactants, the effectiveness of cationic surfactants increased proportionally with the decrease in the AAC basicity and increased in the alkaline component content.
The changes in the AAC basicity from Mb = 1.6 (Mixes 5 and 6) (Figure 3a,b) to Mb = 1.2 (Mixes 7 and 8) (Figure 3d,e), regardless of the anion of the alkaline component, were accompanied by the higher consistency and the longer consistency retention time. The higher adsorption ability of cationic surfactants can be attributed to the higher values of negative charge (zeta-potential) on the surface of mineral particles.
With low AAC basicity, nonionic surfactants like polyethylene glycol exhibited a similar higher effect. These plasticizers were ineffective for the AAC with Mb = 1.6 (Mixes 5 and 6) (Figure 3a,b), even used in conjunction with NaLST, which should have provided the adsorption (Figure 3a,b). However, with the decrease in basicity (Mb) from 1.3 to 1.2 (Mixes 7–9) (Figure 3c–e), the consistency of fresh concrete during the first 60 min remained within one class in consistency. The consistency retention of AAC concrete mixes was attributed to the stability of the molecular structure of cationic and nonionic surfactants at the early stages of hydration.
Also, the influence of the AAC basicity on the action effect of surfactants under a criterion of strength of concrete produced with a constant W/C (Figure 4) was studied.
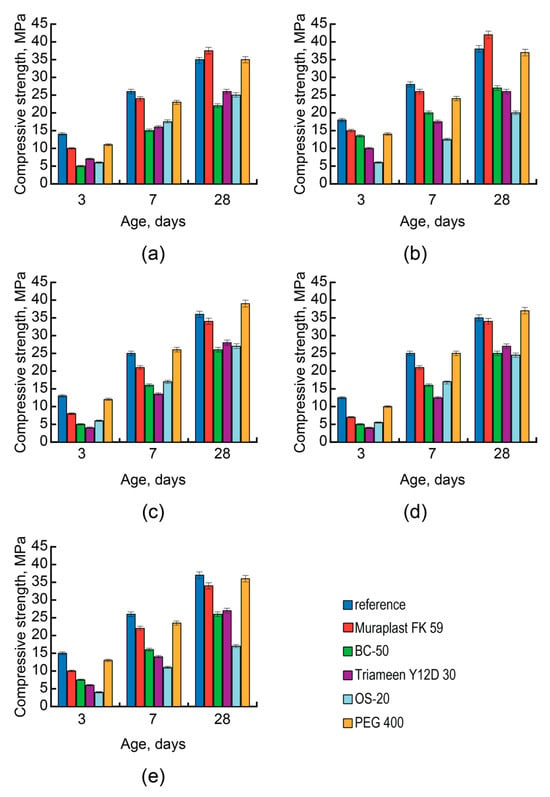
Figure 4.
The compressive strength of concrete vs. thetype of surfactant (reference—blue, Muraplast FK 59—red, BC-50—green, Triameen Y12D 30—violet, OS-20—light blue, and PEG 400—orange), AAC composition, and age (3, 7, and 28 days): AAC with Mb = 1.6 and soda-ash (Mix 6) (a); AAC with Mb = 1.6 and sodium metasilicate (Mix 5) (b); AAC with Mb = 1.3 and soda-ash (Mix 7) (c); AAC with Mb = 1.2 and soda-ash (Mix 9) (d); AAC with Mb = 1.2 and sodium metasilicate (Mix 8) (e).
The use of anionic surfactants in the AAC with Mb = 1.6 (Mixes 5 and 6) resulted in some retarded hardening of the AAC concretes at early ages (Figure 4a,b). However, after 28 days, the strength of modified concrete exceeded that of the reference concrete.
The effectiveness of anionic surfactants decreased with the decrease in the AAC basicity and increased in the content of the alkaline component. This effect was accompanied by the retarded hardening of AAC concrete and can be attributed to the increase in the concentration of the alkaline component due to the hydrolysis of polyesters.
On the contrary, the effectiveness of nonionic surfactants increased with the decrease in the AAC basicity and, respectively, the increase of the content of the alkaline component. So, PEG 400 decreased the strength gain in the AAC with Mb = 1.6 (Mixes 5 and 6)—based concrete both at the early stages of hardening and at 28 days (Figure 4a,b).
With the AAC basicity decreasing from 1.6 to 1.3 (Mix 7), the early strength values of the plasticized concrete and reference concrete were close to each other (Figure 4c). With the AAC basicity ≤1.3 (Mixes 7–9), the strength at 28 days of the plasticized AAC concrete exceeded that of the reference concrete (Figure 4c–e). A conclusion can be made about the higher effectiveness of nonionic surfactants in conjunction with NaLST with the decrease in the AAC basicity.
The use of nonionic surfactants like oxyethylated fatty alcohols in the ACC concretes decreased strength independent of the AAC basicity (Figure 4).
With modification using cationic surfactants, the strength decreased, independent of the AAC basicity, as well as type and content of the alkaline component (Figure 4). This effect was attributed to the foam formation in a concrete mix, which negatively affected the pore structure and strength of the AAC concrete. To escape this effect, these surfactants are to be used in conjunction with a defoaming agent [90].
3.1.3. Factor #3: Molecular Weight of a Surfactant
The subject of research was an AAC with Mb = 1.1 (Mix 3, see Table 1) building mortar (reference composition). The criteria for evaluating the effectiveness of a surfactant were the consistency (flow) and consistency retention time of fresh mortar, as well as the compressive strength of mortar.
Low-molecular-weight anionic aliphatic compounds (polyols) were used in building mortars, and their high effectiveness in the case of AAC with low basicity was discovered.
Ethylene glycol (2-atomic polyol) increased the consistency of the AAC mortar mix by 57% (Figure 5a), glycerol (3-atomic polyol) by 26% (Figure 5b), and sorbitol (6-atomic polyol) by 40% (Figure 5c) compared to that of the reference composition.
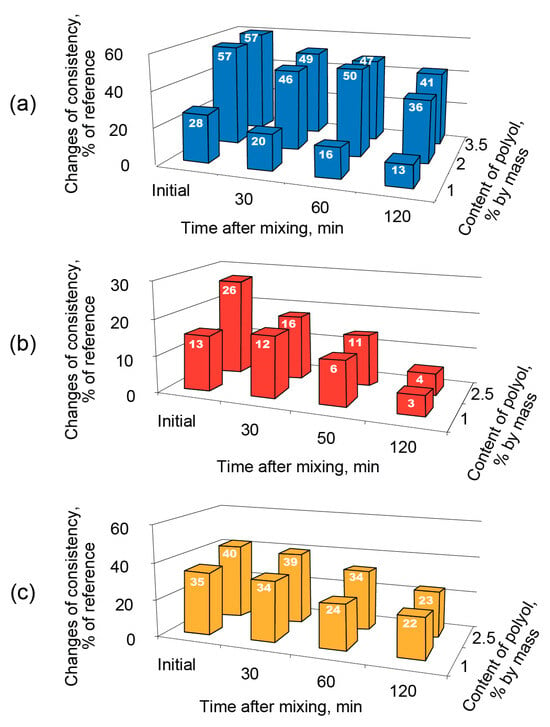
Figure 5.
Changes in the consistency (flow) of fresh mortar based on the AAC with Mb = 1.1 (Mix 3) vs. the type of admixture (ethylene glycol—blue (a), glycerol—red (b), and sorbitol—yellow (c) and the time after mixing.
Thus, no linear interrelation between the quantity of the OH−-groups in a molecule of polyol (molecular weight) and its (polyol) influence on the consistency of the AAC mortar mix could be discovered. Apart from a plasticizing action, the consistency of the plasticized mortar remained constant over time.
With the dosages of polyols, exceeding the optimal ones (1% by mass of AAC), the strength gain decelerated, though a high plasticizing effect remained (Figure 6).
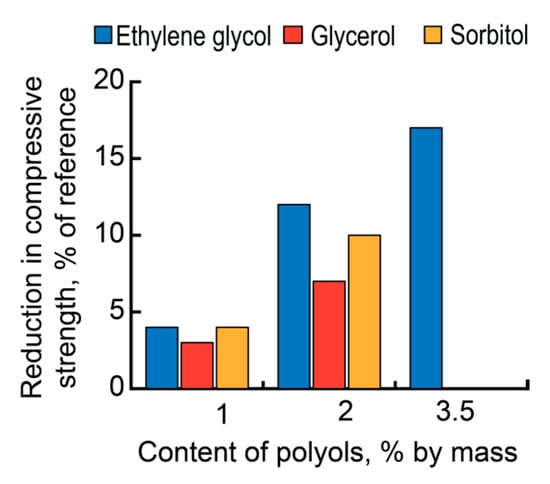
Figure 6.
Reduction in the compressive strength of mortar based on the AAC with Mb = 1.1 (Mix 3) vs. the type of admixture (ethylene glycol—blue, glycerol—red, and sorbitol—yellow).
3.2. The Influence of the Anion of the Alkaline Component
3.2.1. Factors #1 and #2: Molecular Structure and Charge of Polar Groups of a Surfactant
The subject of research was a concrete based on an AAC (Mb = 1.2–1.6) (Mixes 5, 6, 8, and 9) (reference compositions, see Table 2). The criteria for evaluating the effectiveness of a surfactant were the consistency (slump) and the consistency retention time of fresh concrete, as well as the compressive strength of concrete.
The substitution in AAC of carbonates (Figure 4a,d) for silicates (Figure 4b,e) resulted in the lower effectiveness of the PCE concerning the plasticizing effect and consistency retention.
On the contrary, no dependence was discovered between the consistency of the AAC concrete mix and the anion of the alkaline component in the case of surfactants without ester bonds. This was valid for the initial consistency and its retention in the case of cationic and nonionic surfactants. Thus, the surface activity of a surfactant can be regulated by a proper choice of types of functional groups and changing a molecular weight to ensure a rather high level of adsorption.
As to the effect of the anion of the alkaline component on the effectiveness of various types of surfactants under a criterion of the strength of the AAC concrete, the following regularities listed below can be observed.
With the substitution in AAC with Mb = 1.6 of soda-ash (Mix 6) (Figure 4a) for sodium metasilicate (Mix 5) (Figure 4b), the early strength of the PCE-modified AAC concrete increased.
However, with the higher concentrations of the alkaline component in the AAC with Mb = 1.2 (Figure 4d,e) the anion of the alkaline component was no longer a determining factor of influence on the compressive strength of the AAC concrete in the case of nonionic surfactants like polyethers. When using oxyethylated fatty alcohols as nonionic surfactants, an essential decrease of the AAC concrete strength took place, especially in the case of silicates (Figure 4b,e). Thus, nonionic surfactants should be used exclusively in conjunction with a defoaming agent to escape the air-entraining effect without control (Figure 4b,e).
3.2.2. Factor #3: Molecular Weight of a Surfactant
The subject of research was a mortar based on an AAC with Mb = 1.2 (Mixes 2 and 3) (reference compositions, see Table 1). The criteria for evaluating the effectiveness of a surfactant was the consistency (flow) of fresh mortar and the compressive strength of mortar.
The substitution of carbonates (Mix 3) for silicates (Mix 2) initiated a necessity to decrease the molecular weight of a surfactant. A comparative consistency (flow) of fresh mortar based on the AAC with Mb = 1.2 (Mixes 2 and 3) depending upon the type of surfactant (0.5% by mass) is shown (Figure 7).
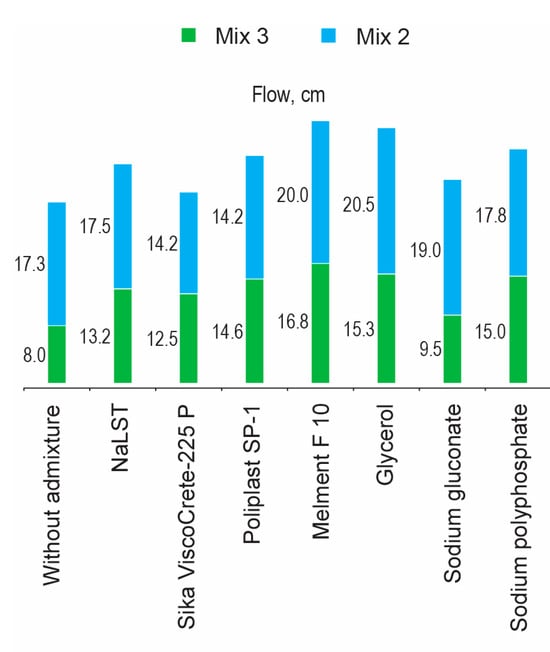
Figure 7.
The consistency (flow) of fresh mortar based on the AAC with Mb = 1.2 (Mix 2 (green) and Mix 3 (light blue)) vs. the type of surfactant and the anion of the alkaline component.
High-molecular-weight surfactants based on sulphonated naphthalene formaldehyde condensates and low-molecular-weight surfactants (polyols, sodium salts of carbonic acids, sodium polyphosphates) were the most effective admixtures for use in the AAC with Mb = 1.2 and soda-ash (Mix 2) and sodium metasilicate (Mix 3) with consideration of the consistency. These admixtures provided compressive strength at a level of the reference compositions and, above all, exhibited two times longer retention time.
The substitution of carbonates for silicates and with the decrease in the AAC basicity decreased the effectiveness of PCE. On the contrary, the effectiveness of surfactants like polyols and polyethers, including those used in conjunction with NaLST, increased.
3.3. The Influence of the Degree of Dispersing of the AAC in the Materials
3.3.1. Factors #1 and #2: Molecular Structure and Charge of Polar Groups of a Surfactant
The subject of research was a concrete based on an AAC with Mb = 1.2 (Mix 10) (reference composition, see Table 2). The criteria for evaluating the effectiveness of a surfactant was the consistency (slump) and consistency retention time of fresh concrete, as well as the compressive strength of concrete.
The rheological properties of the fresh AAC concrete (Figure 8b) were significantly more influenced by the molecular structure of the surfactant (1% by mass) compared to those of the analog, which was based on OPC cement (Figure 8a). In the presence of an anionic high-molecular-weight plasticizer (NaLST), the action effect of cationic and nonionic surfactants (BC-50, Triameen Y12D 30, OS-20, PEG 400) increased. On the contrary, the effectiveness of anionic surfactants (PCE) decreased.
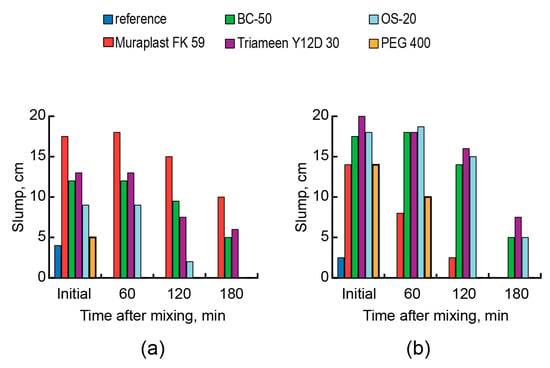
Figure 8.
The consistency of fresh concrete vs. the type of surfactant (without admixture (reference)—blue, Muraplast FK 59—red, BC-50—green, Triameen Y12D 30—violet, OS-20—light blue, and PEG 400—orange), the type of cement (strength class 32.5 N): CEM II/A-S (a) and AAC with Mb = 1.2 (Mix 9) (b), and the time after mixing.
At low values of the AAC basicity resulting from high contents of GBFS, cationic surfactants exhibited a higher adsorption ability compared to that of anionic surfactants.
The decreased effectiveness of polyesters (Figure 9b) compared to that of the OPC-based analog (Figure 9a) resulted in low strength gains at different stages of hardening. A conclusion about the high effectiveness of nonionic surfactants like, for example, PEG, used in AAC in conjunction with NaLST, is supported by the absence of the early-age strength decrease and the higher strength of concrete at an age of 28 days compared to those of the reference compositions.
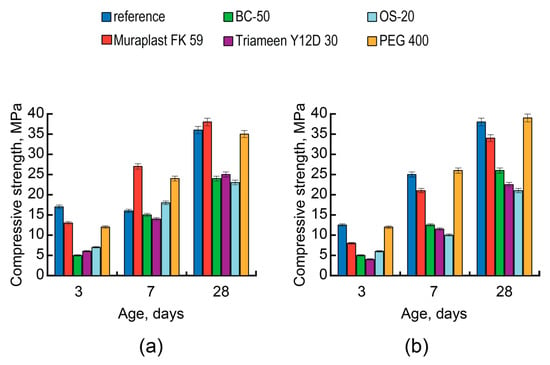
Figure 9.
The compressive strength of concretes vs. the type of surfactant (without admixture (reference)—blue, Muraplast FK 59—red, BC-50—green, Triameen Y12D 30—violet, OS-20—light blue, and PEG 400—orange), the type of cement (strength class 32.5 N): CEM II/A-S (a) and AAC with Mb = 1.2 (Mix 9) (b), and the time after mixing.
With the modification of the AAC concrete by cationic surfactants, the early strength and strength at 28 days of the AAC concrete, not depending upon the AAC composition, decreased as a result of foam formation (Figure 9). The addition of a defoaming agent increased the consistency of fresh concrete and prevented the strength from decreasing [90].
A conclusion can be drawn that the use of cationic surfactants in the concrete mixes with low AAC basicity values is more effective compared to nonionic and anionic surfactants.
3.3.2. Factor #3: Molecular Weight of a Surfactant
The subject of research was a building mortar based on a hybrid AAC with Mb = 1.2 (Mix 4) (reference composition, see Table 1). The criteria for evaluating the effectiveness of a surfactant was the consistency (flow) of fresh mortar and the compressive strength of mortar.
The higher degree of dispersing of the AAC suspensions (higher content of water) in the transition from heavyweight concretes to building mortars was accompanied by the lesser role played by molecular weight in ensuring the surface activity of a surfactant. For example, a plasticizing action of a surfactant (Figure 10) in the mortar mixes for floor application based on AAC with Mb = 1.2 (Mix 10) increased with the decrease in molecular weight. As a result, there was no need to use NaLST in conjunction with high- or low-molecular-weight surfactants.
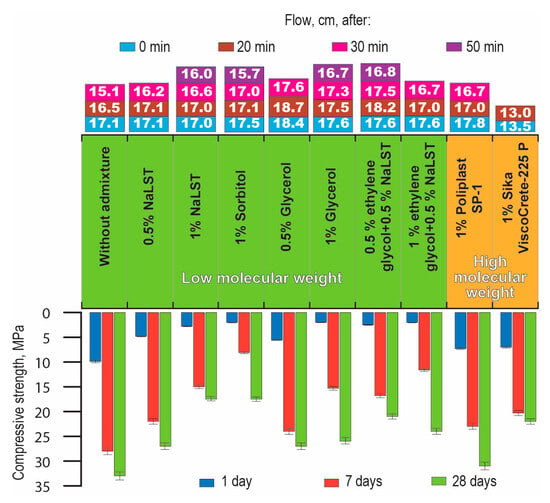
Figure 10.
The consistency (flow) of mortar mixes based on the AAC with Mb = 1.2 (Mix 4) during the time after mixing (0 min—light blue, 20 min—brown, 30 min—pink, 50 min—violet), and the compressive strength of mortars after hardening for 1 (blue), 7 (red), and 28 days (green) vs. the type of surfactant.
The subject of research was a lightened grout based on a hybrid AAC with Mb = 1.1 (Mix 11) (reference composition). The criteria for evaluating the effectiveness of a surfactant were the plastic viscosity, as well as the dynamic and static shear stresses.
Low molecular weight aliphatic surfactants are the most effective for lightened grouts which are rich-in-water AAC (Mb = 1.1) suspensions with a maximal degree of dispersing.
The addition of polyol with a maximum number of hydroxyl groups in its molecule (sorbitol) had the greatest effect on plastic viscosity (Figure 11a), dynamic shear stress (Figure 11b), and static shear stress (Figure 12). The higher action effect of sorbitol on rheological characteristics, compared to those of glycerol and ethylene glycol, is attributed to its action, which is not dependent upon temperature.
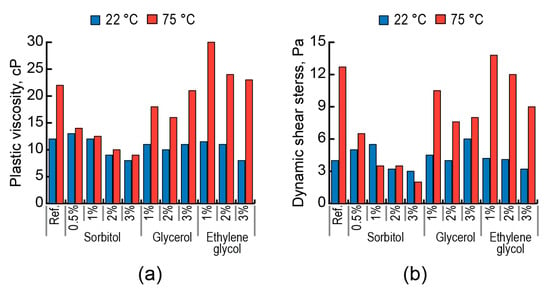
Figure 11.
Effect of polyols (sorbitol, glycerol, ethylene glycol) on the plastic viscosity (a) and dynamic shear stress (b) of lightened grouts based on AAC with Mb = 1.1 (Mix 11) at t = 22 °C (blue) and t = 75 °C (red).
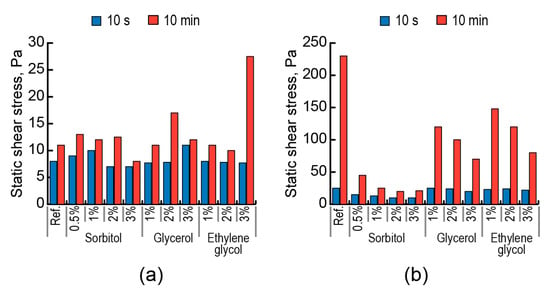
Figure 12.
Effect of polyols (sorbitol, glycerol, ethylene glycol) on the static shear stress of lightened grouts based on AAC with Mb = 1.1 (Mix 11) at t = 22 °C (a) and t = 75 °C (b).
The action effect of low-molecular-weight aliphatic surfactants to control rheological properties of the AAC suspensions with high degrees of dispersing, for example, in the case of lightened grouts, within a wide temperature range of 22–75 °C, is shown. These surfactants act like stabilizing, plasticizing, and structure retarding admixtures. The action effect of polyols becomes greater with the increase in the quantity of hydroxyl groups in a molecule, indicating better hydrophilic properties as a factor of surface activity.
4. Discussion
The principles for choosing plasticizing surfactants for use in the AAC materials based on the systematization of plasticizing surfactants that are known in the art were developed and proposed. The AAC materials were studied in terms of the basicity of AAC based on GBFS (Mb = 1.6–2.4), the content of alkaline components, and three degrees of dispersing of the AAC suspensions (low—heavyweight concretes, medium—building mortars, high—lightened grouts). Plasticizing surfactants were divided into high- (Figure 13) and low-molecular-weight surfactants (Figure 14). Depending on the type of hydrophilic (functional) groups, the above-mentioned surfactants were divided into anionic, cationic, and nonionic, and depending upon the structure of the carbon chain, they were divided into cyclic and acyclic (aliphatic) surfactants.
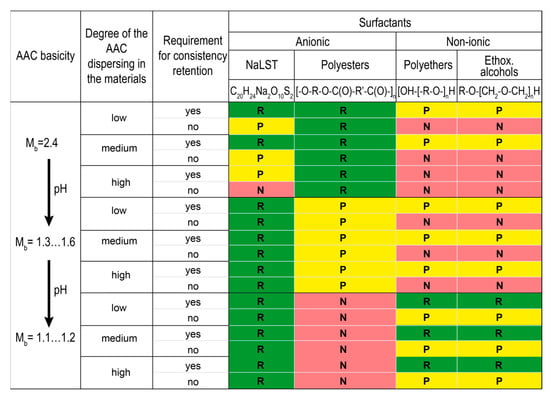
Figure 13.
Systematization of high-molecular-weight plasticizing surfactants classified in the action effect for the AAC materials. Designation: R—recommended (green), P—permissible (yellow), N—not recommended (red).
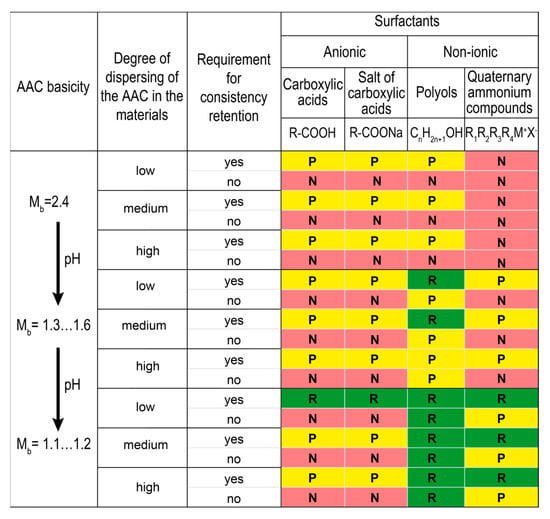
Figure 14.
Systematization of low-molecular-weight plasticizing surfactants in the action effect for the AAC materials. Designation: R—recommended (green), P—permissible (yellow), N—not recommended (red).
A combined action of NaLST and polyhydrosiloxane in the AAC materials was studied, and a conclusion was made about the importance of a surfactant molecular weight as a factor determining its surface activity.
The main criteria to be considered for choosing an appropriate surfactant were the following: the type of hydrophilic (functional) groups, structure of the carbon chain, and molecular weight.
Polyesters were discovered to be the most effective for the materials based on AAC with high basicity (Mb = 2.4) like heavyweight concretes, building mortars, and lightened grouts, regardless of the degree of dispersing. These surfactants are still good for use in the suspensions based on AAC with Mb = 1.3–1.6. However, they are not recommended for the materials based on AAC with Mb = 1.1–1.2 due to a loss of plasticizing effect and uncontrolled changes of the consistency.
High-molecular-weight surfactants like polyethers and ethoxylated fatty alcohols are –required for AAC with Mb = 1.1–1.2 to stabilize consistency, whereas low-molecular-weight surfactants like carboxylic acids and their alkaline salts are required for plasticizing the materials with low degrees of dispersing.
Polyols are recommended for the materials based on AAC with Mb = 1.1–1.6 for a wider range of applications. These surfactants are effective independent of the degree of dispersing and are especially recommended for lightened grouts based on AAC with Mb = 1.1–1.2.
Quaternary ammonium compounds are recommended to preserve the consistency retention of the suspensions based on AAC with Mb = 1.3.
5. Technology and Case Studies
The above-described principles were used in designing the following AAC materials: lightened grouts, building mortars, and heavyweight concretes.
5.1. Lightened AAC Grouts
The AAC with Mb = 1.1 was chosen for lightened grouts for various work conditions (temperatures of 22–75 °C and pressures of 0.1–35.0 MPa) for gas–oil well cementing due to its high corrosion and temperature resistance [10,78]. The use of admixtures is intended to regulate their viscosity and reduce shear stress [91,92,93].
The compositions of the lightened grouts (Table 4) were designed based on the specific features of gas–oil well cementation of a casing pipe in two steps (two sections) (Figure 15). The first step required that Grout #1 would flow through the lowest point (well bottom) (t = 75 °C, P = 35 MPa) and rise to the upper point (head) of the first section (work conditions: temperature range of 60–50 °C and pressures of 20–25 MPa). The second step included the cementation of the second section, where Grout #2 flowed through its bottom (t = 50 °C, P = 20 MPa) with the following rise to the well heel (t = 35–22 °C and P = 0.1–10 MPa).

Table 4.
Compositions of the lightened AAC grouts.
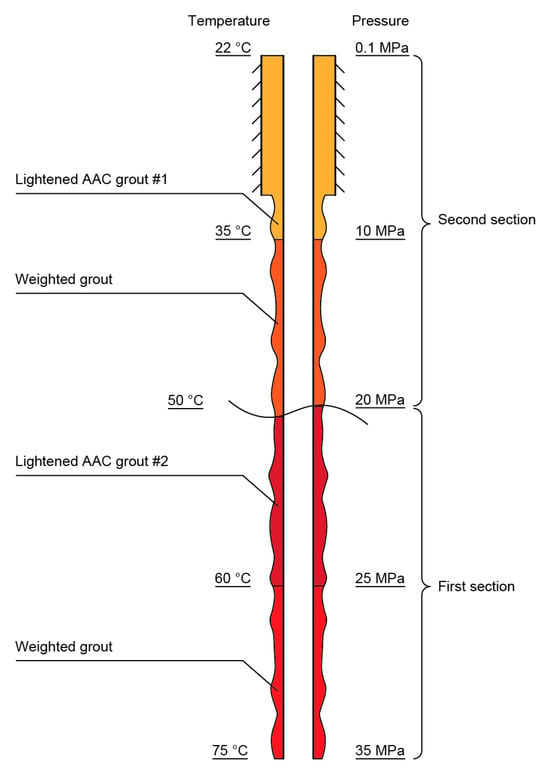
Figure 15.
A scheme of the cementation of a well casing pipe in two stages using lightened AAC grouts.
The rheological properties of the lightened grouts can be regulated by polyols. The values of plastic viscosity and dynamic shear stress comply with the recommended values for effective use in oil and gas well cementation. These characteristics do not change with the temperature increase, testifying to the long-term retention of the action effect of sorbitol. Sorbitol ensures stabilization of plastic viscosity at various rates of deformation, similar to the rheological properties of Bingham fluid, within the use temperature range of 22–75 °C.
The formulated mixes of lightened AAC grouts had properties that met the requirements for oil–gas well cementation of a well casing pipe in two steps (two sections).
The properties of the designed lightened AAC grouts:
Grout #1: density—1.42 g/cm3; consistency (flow)—250 mm; water separation—5.2 mL; plastic viscosity—8.5 mPa∙s (22 °C) and 12.3 (75 °C); dynamic shear stress—2.0 Pa (22 °C) and 1.6 (75 °C); flexural strength at 2 days—0.7 MPa (22 °C) and 1.7 MPa (75 °C);
Grout #2: density—1.43 g/cm3; consistency (flow)—250 mm; water separation—4.6 mL; plastic viscosity—12.6 mPa∙s (22 °C) and 11.8 mPa∙s (50 °C); dynamic shear stress—2.7 Pa (22 °C) and 2.9 Pa (50 °C); flexural strength at 2 days—0.8 MPa (22 °C) and 2.1 MPa (75 °C).
5.2. Building AAC Mortars
The following specific conditions are to be met in the production of dry mixes for the modified AAC building mortars:
- to escape intermixing of AAC components;
- to store the components in separate tanks.
Two examples of dry mixes based on AAC with Mb = 2.4 for anchoring application and Mb = 1.2 for floor application were studied. These Mb covered the whole range of basicities under study.
5.2.1. Dry Mixes for Anchoring Application
Dry mixes should meet requirements that are difficult to combine: the high consistency and required consistency retention time of fresh mortar, high strength, adhesion, and the required expansion of mortar [94,95,96]. A mix design, which is characteristic of rapid hardening and high strength [97], was as follows: % by mass: AAC with Mb = 2.4–38.9, granite screening (fr. 1.25/2.5)—14.6, sand (fr. 0.63/1.25)—17.5, sand (fr. 0.16/0.63)—11.7, milled sand fr. < 0.16–14.6, PEG-M—0.4, NaNO3—2.4, aluminum powder—0.01.
The properties of the resulting mortar were consistency (flow)—190 mm; consistency retention time—15 min, flexural/compressive strength of mortar—6.1/25.7 MPa and 12.9/68.5 MPa at 1 day and 28 days, respectively; bond strength to the substrate—0.9 MPa; pull-out strength—110 MPa; linear elongation—0.37 mm/m.
The composition of such dry mixes additionally included aluminum powder, salt-electrolyte (NaNO3), and calcium sulfate hemihydrate (CaSO4∙0.5H2O). The admixtures were represented by modified polyester and polyhydrosiloxane.
The expansion of the AAC mortar in combination with the increased strength was achieved by the decrease in water content and the formation of an effective pore structure. The proper adjustment of the duration of the processes of gas release and structure formation resulted in an optimal structure with additional stresses due to ettringite formation.
5.2.2. Dry Mixes for Floor Application
The AAC composition with Mb = 1.2 was chosen for use in such mortars due to its quick strength gain and corrosion resistance. The required properties include high bond strength and low shrinkage and taking into account its work in thin layers [98,99,100]. A dry mix design was, % by mass: AAC with Mb = 2.4–20.0, milled sand fr. <0.16–29.9, sand (fr. 0.16/0.63)—49.8, NaLST—0.2, Na-CMC—0.1.
The properties of the resulting mortar were consistency (flow)—20.4 cm; water retention capacity—98.8%; consistency retention time—45 min; bond strength to the substrate—0.6 MPa; flexural/compressive strength—2.1/7.4, 2.9/13.1, 3.2/ 18.1, 5.1/21.4 after 1, 3, 7, and 28 days, respectively; shrinkage—1.9 mm/m (28 days).
Shrinkage of the AAC mortar was regulated through its modification by admixtures of polyols and re-dispersible polymer powders without ester bonds. These admixtures, used in conjunction with polyhydrosiloxane, NaLST, and cellulose ether, reduced the shrinkage of the AAC mortar to norm-specified values (≤2 mm/m).
5.3. Heavyweight AAC Concretes
5.3.1. Concrete for Pavement Application
The AAC composition with Mb = 1.2 was used in the production of a concrete due to its high strength gain to ensure the required elasticity and durability (corrosion resistance, freeze–thaw resistance, etc.) [90,101]. The use of properly chosen admixtures ensures the effective modification of a pore structure [102].
The concrete mix design used in the study was, % by mass: AAC with Mb = 1.2–380, silica sand (fineness modulus 1.94)—710, granite gravel (fr. 5/10)—330, granite gravel (fr. 10/20)—780, triamine + silicone-based defoaming agent—3, water—125.
Properties of the resulting concrete were compressive strength class C12/15–C40/50, 28 days tensile strength in bending—7.6–7.8 MPa (28 days), modulus of elasticity—28.2·103–29.9·103 MPa, grade in water impermeability—W8, grade in freeze–thaw resistance—F200, coefficient of corrosion resistance in the solutions of sodium sulphate and magnesium—0.90–1.03, and abrasion—0.53–0.54 g/cm2.
The use in the concrete mix of triamine in conjunction with polyhydrosiloxane and silicone-based defoaming agent decreased the open porosity and increased the volume of closed capillary pores. The proposed modification made the concrete based on AAC with Mb = 1.2 suitable for making pavements with high traffic intensity (road and airport pavement slabs, sidewalk slabs for main streets, etc.).
5.3.2. Concrete for Monolithic-Framed Construction
The concrete mix design (in total, eight formulations) for frame-monolithic construction based on AAC with Mb = 1.3 and Mb = 1.6 is given in Table 5.

Table 5.
The AAC concrete mix design for monolithic-framed construction.
As it follows from the obtained data (Table 5), with the decrease of the AAC basicity from 1.6 to 1.3, polyesters should be substituted for polyethers and should be used in conjunction with polyhydrosiloxane and NaLST.
Properties of the designed AAC concretes were consistency classes—S4 and S5, consistency retention—2 h, grade in compressive strength—C12/15–C40/50, and grade in freeze–thaw resistance—F200.
The designed AAC concrete mixes (Table 5) were used in the erection of residential buildings under the technology of monolithic-framed construction.
The effectiveness of the plasticized AAC concretes intended for service in aggressive mediums, i.e., seawater, or even the use of seawater as mixing water, has also been confirmed [103,104].
6. Conclusions
A compilation of existing approaches to the plasticization of building materials (lightened grouts, building mortars, and heavyweight concretes) from the alkali-activated cements of the system Na2O-CaO-Al2O3-SiO2-H2O was performed, and these approaches were properly analyzed. The action effect of plasticizers was studied in the interrelation between the stability of the molecular structure, degree of adsorption, and molecular weight depending on the alkali-activated cement basicity, anion of the alkaline component, and degree of dispersing of the alkali-activated cement in the resulting material. Systematization of plasticizers to be applied to their choice to control technology-related and physico-mechanical properties of the alkali-activated cement materials was developed and proposed.
- A conclusion was made that with the decrease of the alkali-activated cement basicity from Mb = 2.4 to Mb ≤ 1.3, the content of the alkaline component should be increased. As a result, the action effect of admixtures with ester bonds (PCE) in the molecular structure due to alkaline hydrolysis decreased. The substitution of anionic for cationic or nonionic surfactants increased the action effect, with its dependence from the surfactant molecular weight decreasing. The use of polyhydrosiloxane in conjunction with NaLST was effective for obtaining the desirable effect from plasticization, regardless of the alkali-activated cement basicity, especially with regard to the surface activity of nonionic surfactants.
- The substitution of carbonates for silicates in the alkaline component required substituting PCE for polyols or polyethers, taken alone or in conjunction with NaLST. The effectiveness of plasticization increased with the decrease of the surfactant molecular weight. With the increase of the content of the alkaline component as a result of the decrease of the alkali-activated cement basicity, the importance of the anion of the alkaline component decreased, and anionic surfactants were to be substituted for nonionic surfactants.
- With the increase of the degree of dispersing of the alkali-activated cement suspensions in the transition from heavyweight concretes to building mortars and further to lightened grouts, polyester-based plasticizers were to be substituted for polyols, that is, the role of the molecular weight decreased. This could be attributed to the higher contents of the liquid phase, which added a plasticizing effect.
- The developed and proposed principles which take into account the technology-related features of AAC materials like lightened grouts, building mortars, and heavyweight concretes were explored in a pilot-scale production. The lightened grouts were obtained using polyols, building mortars, and heavyweight concretes—polyethers and carboxylic acid salts in conjunction with NaLST.
Author Contributions
Project administration, funding acquisition, conceptualization, supervision, validation, writing—review and editing, P.K.; methodology, resources, data curation, software, investigation, formal analysis, visualization, writing—original draft preparation, I.R. and O.K. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to express their gratitude to the Ministry of Education and Science of Ukraine for financial support of this research that is being carried out within the budgetary financing of research topics with the registration No. 0123U101831 and 0123U101832 with the implementation period of 2023–2026.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are not publicly available, as this work was carried out within the framework of the mentioned projects, and its results can be made available on request after registration of a report in the state scientific institution of the Ukrainian Institute of Scientific and Technical Expertise and Information (http://www.uintei.kiev.ua/en) at https://nddkr.ukrintei.ua/ (accessed on 1 January 2025).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Scrivener, K.L.; John, V.M.; Gartner, E.M. Eco-Efficient Cements: Potential Economically Viable Solutions for a Low-CO2 Cement-Based Materials Industry. Cem. Concr. Res. 2018, 114, 2–26. [Google Scholar] [CrossRef]
- Naqi, A.; Jang, J. Recent Progress in Green Cement Technology Utilizing Low-Carbon Emission Fuels and Raw Materials: A Review. Sustainability 2019, 11, 537. [Google Scholar] [CrossRef]
- Raghav, M.; Park, T.; Yang, H.-M.; Lee, S.-Y.; Karthick, S.; Lee, H.-S. Review of the Effects of Supplementary Cementitious Materials and Chemical Additives on the Physical, Mechanical and Durability Properties of Hydraulic Concrete. Materials 2021, 14, 7270. [Google Scholar] [CrossRef] [PubMed]
- Provis, J.L.; Winnefeld, F. Outcomes of the Round Robin Tests of RILEM TC 247-DTA on the Durability of Alkali-Activated Concrete. MATEC Web Conf. 2018, 199, 02024. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, L.; Li, J.; De Belie, N.; Shi, X.; Van Den Heede, P.; Zhang, C.; Liu, Z.; Talakokula, V.; Jin, Z.; et al. Report of RILEM TC 281-CCC: Effect of Loading on the Carbonation Performance of Concrete with Supplementary Cementitious Materials—An Interlaboratory Comparison of Different Test Methods and Related Observations. Mater. Struct. 2023, 56, 110. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Garcia-Lodeiro, I.; Maltseva, O.; Palomo, A. Sustainable Cements: Hybrid Alkaline Cements Overview. In Proceedings of the 75th RILEM Annual Week 2021; Escalante-Garcia, J.I., Castro Borges, P., Duran-Herrera, A., Eds.; RILEM Bookseries; Springer International Publishing: Cham, Switzerland, 2023; Volume 40, pp. 626–639. ISBN 978-3-031-21734-0. [Google Scholar]
- Von Greve-Dierfeld, S.; Lothenbach, B.; Vollpracht, A.; Wu, B.; Huet, B.; Andrade, C.; Medina, C.; Thiel, C.; Gruyaert, E.; Vanoutrive, H.; et al. Understanding the Carbonation of Concrete with Supplementary Cementitious Materials: A Critical Review by RILEM TC 281-CCC. Mater. Struct. 2020, 53, 136. [Google Scholar] [CrossRef]
- THE 17 GOALS. Available online: https://sdgs.un.org/goals (accessed on 8 September 2023).
- A European Green Deal. Available online: https://commission.europa.eu/strategy-and-policy/priorities-2019-2024/european-green-deal_en (accessed on 15 June 2025).
- Krivenko, P. Why Alkaline Activation—60 Years of the Theory and Practice of Alkali-Activated Materials. J. Ceram. Sci. Technol. 2017, 8, 323–334. [Google Scholar] [CrossRef]
- Fyfe, W.S.; Turner, F.J.; Verhoogen, J. Metamorphic Reactions and Metamorphic Facies Geological Society of America Memoirs; Geological Society of America: Ithaca, NY, USA, 1958. [Google Scholar]
- Palomo, A.; Maltseva, O.; Garcia-Lodeiro, I.; Fernández-Jiménez, A. Portland Versus Alkaline Cement: Continuity or Clean Break: “A Key Decision for Global Sustainability. Front. Chem. 2021, 9, 705475. [Google Scholar] [CrossRef]
- Krivenko, P.; Rudenko, I.; Konstantynovskyi, O.; Vaičiukynienė, D. Feasibility of Incorporating SO42−-Ions in Zeolite-like Matrices Based on Alkaline Aluminosilicate Binders. Constr. Build. Mater. 2023, 391, 131878. [Google Scholar] [CrossRef]
- Kryvenko, P.; Rudenko, I.; Konstantynovskyi, O.; Gelevera, O. Design, Characterization, and Incorporation of the Alkaline Aluminosilicate Binder in Temperature-Insulating Composites. Materials 2024, 17, 664. [Google Scholar] [CrossRef]
- Krivenko, P.; Puertas, F.; Gots, V.; Helevera, O.; Rogozina, N. Influence of Whitening Additives on the Properties of Decorative Slag-Alkali Cements and Mortars. Mater. Constr. 2023, 73, e311. [Google Scholar] [CrossRef]
- Guvalov, A.; Abbasova, S. Influence of Rheological Active Additives on the Properties of Self-Compacting Concrete. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2021, 36, 381–386. [Google Scholar] [CrossRef]
- Jiao, D.; Shi, C.; Yuan, Q.; An, X.; Liu, Y.; Li, H. Effect of Constituents on Rheological Properties of Fresh Concrete-A Review. Cem. Concr. Compos. 2017, 83, 146–159. [Google Scholar] [CrossRef]
- Bispo, R.A.; Vicente, G.O.; Silva Júnior, G.P.D.; Benjamim, D.U.; Alcântara, M.A.D.M. Investigation of Rheological Behavior of Self-Compacting and High Performance Composite Concretes. Mater. Res. 2021, 24, e20210264. [Google Scholar] [CrossRef]
- Revilla-Cuesta, V.; Skaf, M.; Santamaría, A.; Hernández-Bagaces, J.J.; Ortega-López, V. Temporal Flowability Evolution of Slag-Based Self-Compacting Concrete with Recycled Concrete Aggregate. J. Clean. Prod. 2021, 299, 126890. [Google Scholar] [CrossRef]
- Qin, Y.; Qu, C.; Ma, C.; Zhou, L. One-Part Alkali-Activated Materials: State of the Art and Perspectives. Polymers 2022, 14, 5046. [Google Scholar] [CrossRef] [PubMed]
- Elzeadani, M.; Bompa, D.V.; Elghazouli, A.Y. One Part Alkali Activated Materials: A State-of-the-Art Review. J. Build. Eng. 2022, 57, 104871. [Google Scholar] [CrossRef]
- Lei, L.; Hirata, T.; Plank, J. 40 Years of PCE Superplasticizers—History, Current State-of-the-Art and an Outlook. Cem. Concr. Res. 2022, 157, 106826. [Google Scholar] [CrossRef]
- Runova, R.F.; Kochevyh, M.O.; Rudenko, I.I. On the Slump Loss Problem of Superplasticized Concrete Mixes. In Proceedings of the Admixtures—Enhancing Concrete Performance, Scotland, UK, 6 July 2005; pp. 149–156. [Google Scholar]
- Puertas, F.; Varga, C.; Alonso, M.M. Rheology of Alkali-Activated Slag Pastes. Effect of the Nature and Concentration of the Activating Solution. Cem. Concr. Compos. 2014, 53, 279–288. [Google Scholar] [CrossRef]
- Puertas, F.; Alonso, M.M.; Gismera, S.; Lanzón, M.; Blanco-Varela, M.T. Rheology of Cementitious Materials: Alkali-Activated Materials or Geopolymers. MATEC Web Conf. 2018, 149, 01002. [Google Scholar] [CrossRef]
- DSTU B V.2.7-171:2008 (EN 934-2:2001, NEQ); Building Materials. Admixtures for Concretes and Building Mortars. General Specifications. National Standard of Ukraine: Kyiv, Ukraine, 2008.
- Palacios, M.; Puertas, F. Estabilidad de Aditivos Superplastificantes y Reductores de La Retracción En Medios Fuertemente Básicos. Mater. Constr. 2004, 54, 65–86. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.G.; Cheng, Y.-B. Effect of Admixtures on Properties of Alkali-Activated Slag Concrete. Cem. Concr. Res. 2000, 30, 1367–1374. [Google Scholar] [CrossRef]
- Palacios, M.; Puertas, F. Effect of Superplasticizer and Shrinkage-Reducing Admixtures on Alkali-Activated Slag Pastes and Mortars. Cem. Concr. Res. 2005, 35, 1358–1367. [Google Scholar] [CrossRef]
- Bessaies-Bey, H.; Khayat, K.H.; Palacios, M.; Schmidt, W.; Roussel, N. Viscosity Modifying Agents: Key Components of Advanced Cement-Based Materials with Adapted Rheology. Cem. Concr. Res. 2022, 152, 106646. [Google Scholar] [CrossRef]
- Palacios, M.; Houst, Y.F.; Bowen, P.; Puertas, F. Adsorption of Superplasticizer Admixtures on Alkali-Activated Slag Pastes. Cem. Concr. Res. 2009, 39, 670–677. [Google Scholar] [CrossRef]
- Plank, J.; Schroefl, C.; Gruber, M.; Lesti, M.; Sieber, R. Effectiveness of Polycarboxylate Superplasticizers in Ultra-High Strength Concrete: The Importance of PCE Compatibility with Silica Fume. J. Adv. Concr. Technol. 2009, 7, 5–12. [Google Scholar] [CrossRef]
- Flatt, R.J.; Houst, Y.F.; Bowen, P.; Hofmann, H.; Widmer, J.; Sulser, U.; Maeder, U.; Bürge, T.A. Interaction of Superplasticizers with Model Powders in Highly Alkaline Médium. In Superplasticizers and Other Chemical Admixtures in Concrete; American Concrete Institute: Detroit, MI, USA, 1997; pp. 743–762. [Google Scholar]
- Yamada, K.; Ogawa, S.; Hanehara, S. Controlling of the Adsorption and Dispersing Force of Polycarboxylate-Type Superplasticizer by Sulfate Ion Concentration in Aqueous Phase. Cem. Concr. Res. 2001, 31, 375–383. [Google Scholar] [CrossRef]
- Plank, J.; Winter, C. Competitive Adsorption between Superplasticizer and Retarder Molecules on Mineral Binder Surface. Cem. Concr. Res. 2008, 38, 599–605. [Google Scholar] [CrossRef]
- Krivenko, P.V.; Runova, R.F.; Rudenko, I.I. Plasticized Concrete and Mortar Based on Cements System Na2O–CaO–Al2O3–SiO2–H2O: Monography; Lira-K: Kyiv, Ukraine, 2022. [Google Scholar]
- Rashad, A.M. A Comprehensive Overview about the Influence of Different Additives on the Properties of Alkali-Activated Slag—A Guide for Civil Engineer. Constr. Build. Mater. 2013, 47, 29–55. [Google Scholar] [CrossRef]
- Conte, T.; Plank, J. Influence of Polycarboxylate Ether on the Rheological Behavior of Alkali Activated Slag Paste; F. A. Finger Institut für Baustoffkunde Bauhaus-Universität Weimar: Weimar, Germany, 2018; Volume 1, pp. 803–811. [Google Scholar]
- Marchon, D.; Sulser, U.; Eberhardt, A.; Flatt, R.J. Molecular Design of Comb-Shaped Polycarboxylate Dispersants for Environmentally Friendly Concrete. Soft Matter 2013, 9, 10719. [Google Scholar] [CrossRef]
- Kashani, A.; Provis, J.L.; Xu, J.; Kilcullen, A.R.; Qiao, G.G.; Van Deventer, J.S.J. Effect of Molecular Architecture of Polycarboxylate Ethers on Plasticizing Performance in Alkali-Activated Slag Paste. J. Mater. Sci. 2014, 49, 2761–2772. [Google Scholar] [CrossRef]
- Flatt, R.J.; Bowen, P. Electrostatic Repulsion between Particles in Cement Suspensions: Domain of Validity of Linearized Poisson–Boltzmann Equation for Nonideal Electrolytes. Cem. Concr. Res. 2003, 33, 781–791. [Google Scholar] [CrossRef]
- Rosen, M.J.; Kunjappu, J.T. Surfactants and Interfacial Phenomena: Monography; Willey: New York, NY, USA, 2012. [Google Scholar]
- Williams, J.M. High Internal Phase Water-in-Oil Emulsions: Influence of Surfactants and Cosurfactants on Emulsion Stability and Foam Quality. Langmuir 1991, 7, 1370–1377. [Google Scholar] [CrossRef]
- Holmberg, K.; Lindman, B.; Kronberg, B. Surface Chemistry of Surfactants and Polymers; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Tian, Y.; Yuan, Q.; Yang, C.; Yang, K.; Yu, L.; Zhang, M.; Zhu, X. Insights into the Efficiency Loss of Naphthalene Superplasticizer in Alkali-Activated Slag Pastes. J. Build. Eng. 2023, 68, 106176. [Google Scholar] [CrossRef]
- Palacios, M.; Puertas, F. Effect of Shrinkage-Reducing Admixtures on the Properties of Alkali-Activated Slag Mortars and Pastes. Cem. Concr. Res. 2007, 37, 691–702. [Google Scholar] [CrossRef]
- Partschefeld, S.; Tutal, A.; Halmanseder, T.; Schneider, J.; Osburg, A. Investigations on Stability of Polycarboxylate Superplasticizers in Alkaline Activators for Geopolymer Binders. Materials 2023, 16, 5369. [Google Scholar] [CrossRef] [PubMed]
- Alrefaei, Y.; Wang, Y.-S.; Dai, J.-G. The Effectiveness of Different Superplasticizers in Ambient Cured One-Part Alkali Activated Pastes. Cem. Concr. Compos. 2019, 97, 166–174. [Google Scholar] [CrossRef]
- Chang, J.J. A Study on the Setting Characteristics of Sodium Silicate-Activated Slag Pastes. Cem. Concr. Res. 2003, 33, 1005–1011. [Google Scholar] [CrossRef]
- Keser, H.E.; Ramyar, K.; Gultekin, A. Effect of Water-reducing Admixtures Water Content on Rheology, Workability, and Mechanical Properties of Fly Ash-based Geopolymer and Slag-based Alkali-activated Mixtures. Struct. Concr. 2023, suco.202300113. [Google Scholar] [CrossRef]
- Jolicoeur, C.; Simard, M.A.; Sharman, J.; Zamojska, R.; Dupuis, M.; Spiratos, E.; Doglas, E. Chemical Activation of Blast-Furnace Slag: An Overview and Systematic Experimental Investigations; Energy, Mines and Resources: Ottawa, ON, Canada, 1992. [Google Scholar]
- Collepardi, M.; Grossi, G.; Pellizon Birelli, M.; Ventura, G. Influence of D-Sorbitol on the Properties of Binders to Immobilize Acid Nuclear Wastes. In Proceedings of the SP-239: 8th CanMET/ACI International Conference on Superplasticizers and Other Chemical Admixtures in Concrete, Sorrento, Italy, 29 October–1 November 2006; American Concrete Institute: Detroit, MI, USA, 2006. [Google Scholar]
- Tong, S.; Yuqi, Z.; Qiang, W. Recent Advances in Chemical Admixtures for Improving the Workability of Alkali-Activated Slag-Based Material Systems. Constr. Build. Mater. 2021, 272, 121647. [Google Scholar] [CrossRef]
- Luukkonen, T.; Abdollahnejad, Z.; Ohenoja, K.; Kinnunen, P.; Illikainen, M. Suitability of Commercial Superplasticizers for One-Part Alkali-Activated Blast-Furnace Slag Mortar. J. Sustain. Cem.-Based Mater. 2019, 8, 244–257. [Google Scholar] [CrossRef]
- Kalina, L.; Bílek, V.; Hrubý, P.; Iliushchenko, V.; Kalina, M.; Smilek, J. On the Action Mechanism of Lignosulfonate Plasticizer in Alkali-Activated Slag-Based System. Cem. Concr. Res. 2022, 157, 106822. [Google Scholar] [CrossRef]
- Kalina, L.; Hajdúchová, M.; Langová, M.; Enev, V. Possibilities of Using Plasticizers in Alkali-Activated Systems. Mater. Sci. Forum 2016, 851, 57–62. [Google Scholar] [CrossRef]
- Mikhailova, O.; Rovnanik, P. Effect of Polyethylene Glycol on the Rheological Properties and Heat of Hydration of Alkali Activated Slag Pastes. IOP Conf. Ser. Mater. Sci. Eng. 2018, 385, 012037. [Google Scholar] [CrossRef]
- Runova, R.; Gots, V.; Rudenko, I.; Konstantynovskyi, O.; Lastivka, O. The Efficiency of Plasticizing Surfactants in Alkali-Activated Cement Mortars and Concretes. MATEC Web Conf. 2018, 230, 03016. [Google Scholar] [CrossRef]
- Krivenko, P.; Rudenko, I.; Konstantynovskyi, O. Comparison of Influence of Surfactants on Thermokinetic Characteristics of Alkali-Activated Slag Cement. East.-Eur. J. Enterp. Technol. 2021, 6, 39–48. [Google Scholar] [CrossRef]
- Usherov-Marshak, A.; Sopov, V.; Kabus, A. Calorimetry in Chemistry and Technology of Cement and Concrete. In Proceedings of the Thermal Analysis and Calorimetry, Chisinau, Moldova, 28–31 August 2017; p. 353. [Google Scholar]
- Performance of an Advanced Polycarboxylate-Based Powder Superplasticizer. In Proceedings of the SP-217: Seventh CANMET/ACI International Conference on Superplasticizers and Other Chemical Admixtures in Concrete, Berlin, Germany, 20–23 October 2003; American Concrete Institute: Detroit, MI, USA, 2003.
- Development of Slump-Loss Controlling Agent with Minimal Setting Retardation. In Proceedings of the SP-217: Seventh CANMET/ACI International Conference on Superplasticizers and Other Chemical Admixtures in Concrete, Berlin, Germany, 20–23 October 2003; American Concrete Institute: Detroit, MI, USA, 2003.
- Alonso, A.A. Comportamiento y Compatibilidad de Cementos y Aditivos Superplastificantes Basados En Policarboxilatos. Efectos de La Naturaleza de Los Cementos y Estructura de Los Aditivos. Ph.D. Thesis, Univesrsidad Autonoma de Madrid, Madrid, Spain, 2011. [Google Scholar]
- Burgos-Montes, O.; Palacios, M.; Rivilla, P.; Puertas, F. Compatibility between Superplasticizer Admixtures and Cements with Mineral Additions. Constr. Build. Mater. 2012, 31, 300–309. [Google Scholar] [CrossRef]
- Conte, T.; Plank, J. Impact of Molecular Structure and Composition of Polycarboxylate Comb Polymers on the Flow Properties of Alkali-Activated Slag. Cem. Concr. Res. 2019, 116, 95–101. [Google Scholar] [CrossRef]
- Lei, L.; Chan, H.-K. Investigation into the Molecular Design and Plasticizing Effectiveness of HPEG-Based Polycarboxylate Superplasticizers in Alkali-Activated Slag. Cem. Concr. Res. 2020, 136, 106150. [Google Scholar] [CrossRef]
- Su, T.; Wang, Q.; Lu, J. Effect of NaOH content on the fluidizing effect of PCEs with different structures in NaOH-activated slag. Cem. Concr. Res. 2023, 166, 107112. [Google Scholar] [CrossRef]
- Chen, J.; Plank, J. Which factors impact the effectiveness of PCEs in alkali-activated slag cements? Cem. Concr. Res. 2025, 190, 107807. [Google Scholar] [CrossRef]
- Zhang, Y.; Chan, H.-K.; Han, Z.; Lei, L. Why do conventional MAA-MPEG PCEs not work in alkali-activated slag systems? Cem. Concr. Res. 2024, 184, 107599. [Google Scholar] [CrossRef]
- Lei, L.; Zhang, Y. Preparation of isoprenol ether-based polycarboxylate superplasticizers with exceptional dispersing power in alkali-activated slag: Comparison with ordinary Portland cement. Compos. Part B Eng. 2021, 223, 109077. [Google Scholar] [CrossRef]
- Ezzat, M.; Latsrisaeng, P.; Lesage, K.; Yardimci, M.Y.; Hoogenboom, R.; De Schutter, G. Novel Concrete Superplasticizers with Sterically Hindered Amines as Stabilizing Pendant Side Chains. Eur. Polym. J. 2023, 196, 112301. [Google Scholar] [CrossRef]
- Ezzat, M.; Xu, X.; El Cheikh, K.; Lesage, K.; Hoogenboom, R.; De Schutter, G. Structure-Property Relationships for Polycarboxylate Ether Superplasticizers by Means of RAFT Polymerization. J. Colloid Interface Sci. 2019, 553, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Wallevik, O.H.; Wallevik, J.E. Rheology as a Tool in Concrete Science: The Use of Rheographs and Workability Boxes. Cem. Concr. Res. 2011, 41, 1279–1288. [Google Scholar] [CrossRef]
- Wallevik, J.E. Rheological Properties of Cement Paste: Thixotropic Behavior and Structural Breakdown. Cem. Concr. Res. 2009, 39, 14–29. [Google Scholar] [CrossRef]
- Alonso, M.M.; Gismera, S.; Blanco, M.T.; Lanzón, M.; Puertas, F. Alkali-Activated Mortars: Workability and Rheological Behaviour. Constr. Build. Mater. 2017, 145, 576–587. [Google Scholar] [CrossRef]
- Lastivka, O.V. Modified Concrete Based on Alkaline Slag Portland Cement for In-Situ Construction. Ph.D. Thesis, Kyiv National University of Construction and Architecture, Kyiv, Ukraine, 2015. [Google Scholar]
- Mamatkulov, A.M. Lightweight Slag Alkaline Tamping Mortats. Ph.D. Thesis, Kyiv National University of Construction and Architecture, Kyiv, Ukraine, 2000. [Google Scholar]
- Skoryk, V.V. Backfill Mortars Based on Alkaline Cement for Variable Cure Conditions in the Well. Ph.D. Thesis, Kyiv National University of Construction and Architecture, Kyiv, Ukraine, 2018. [Google Scholar]
- DSTU B V.2.7-232:2010; Sand for Construction Work. Testing Methods. National Standard of Ukraine: Kyiv, Ukraine, 2010.
- EN 12350-2:2019; Testing Fresh Concrete—Part 2: Slump Test. European Committee for Standardization: Brussels, Belgium, 2019.
- DSTU B V.2.7-126:2011; Modified Dry Building Mixtures. General Specifications. National Standard of Ukraine: Kyiv, Ukraine, 2011.
- DSTU B V.2.7-86-99; Oil-Well Cements. Test Methods. National Standard of Ukraine: Kyiv, Ukraine, 2000.
- ISO 10426-2:2003; Petroleum and Natural Gas Industries—Cements and Materials for Well Cementing. Part 2: Testing of Well Cements. International Organization for Standardization: Geneva, Switzerland, 2003.
- EN 12390-3:2019; Testing Hardened Concrete—Part 3: Compressive Strength of Test Specimens. European Committee for Standardization: Brussels, Belgium, 2019.
- DSTU B V.2.7-49-96; Concretes. Rapid Methods for Determination of Frost Resistance by Repeated Alternated Freezing and Thawing. National Standard of Ukraine: Kyiv, Ukraine, 1996.
- DSTU B V.2.7-47; Concretes. Methods for Determination of Frost Resistance. General Requirements. National standard of Ukraine: Kyiv, Ukraine, 1996.
- EN 196-1:2005; Methods of Testing Cement—Part 1: Determination of Strength. European Committee for Standardization: Brussels, Belgium, 2005.
- EN 1542:1999; Products and Systems for the Protection and Repair of Concrete Structures—Test Methods—Measurement of Bond Strength by Pull-Off. European Committee for Standardization: Brussels, Belgium, 1999.
- EN 1881:2006; Products and Systems for the Protection and Repair of Concrete Structures—Test Methods—Testing of Anchoring Products by the Pull-Out Method. European Committee for Standardization: Brussels, Belgium, 2006.
- Volynska, I.V. Eco-Concrete on the Basis of Slag-Cement on the Use of Mica-Based by-Products of the Mining Industry. Ph.D. Thesis, Kyiv National University of Construction and Architecture, Kyiv, Ukraine, 2018. [Google Scholar]
- Terlyha, V.; Sobol, K. Tamping Mortars with Stabilizing and Plasticizing Admixtures. SSP J. Civ. Eng. 2012, 7, 87–94. [Google Scholar] [CrossRef]
- Tao, C.; Kutchko, B.G.; Rosenbaum, E.; Massoudi, M. A Review of Rheological Modeling of Cement Slurry in Oil Well Applications. Energies 2020, 13, 570. [Google Scholar] [CrossRef]
- Munjal, P.; Hau, K.K.; Prabhakar, A.; Arthur, C.C.H. Oil Well Cement for High Temperature-A Review. IOP Conf. Ser. Mater. Sci. Eng. 2019, 652, 012055. [Google Scholar] [CrossRef]
- Ma, C.; Tan, Y.; Li, E.; Dai, Y.; Yang, M. High-Performance Grouting Mortar Based on Mineral Admixtures. Adv. Mater. Sci. Eng. 2015, 2015, 425456. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, A.; Wang, Y.; He, J.; Chen, Z. Investigations on Corrosion and Mechanical Properties of a 20 Year Old Ground Anchor Exhumed at a Power Station Site. Can. Geotech. J. 2016, 53, 589–602. [Google Scholar] [CrossRef]
- Kryvenko, P.V.; Gots, V.I.; Petropavlovskyi, O.; Rudenko, I.; Konstantynovskyi, O.P. Complex Shrinkage-Reducing Additives for Alkali Activated Slag Cement Fine Concrete. Solid State Phenom. 2021, 321, 165–170. [Google Scholar] [CrossRef]
- Krivenko, P.V.; Petropavlovskyi, O.M.; Rudenko, I.I.; Konstantynovskyi, O.P.; Kovalchuk, A.V. Complex Multifunctional Additive for Anchoring Grout Based on Alkali-Activated Portland Cement. IOP Conf. Ser. Mater. Sci. Eng. 2020, 907, 012055. [Google Scholar] [CrossRef]
- Hoła, J.; Sadowski, Ł.; Hoła, A. The Effect of Failure to Comply with Technological and Technical Requirements on the Condition of Newly Built Cement Mortar Floors. Proc. Inst. Mech. Eng. Part J. Mater. Des. Appl. 2018, 233, 268–275. [Google Scholar] [CrossRef]
- Troyan, V.V. Dry Mixes and Mortars on Their Basis for the Device of Floors of Industrial Buildings. Ph.D. Thesis, Kyiv National University of Construction and Architecture, Kyiv, Ukraine, 2007. [Google Scholar]
- Nosovskyi, Y.L. Mortars Based on Composite Binder for Flow Floors. Ph.D. Thesis, Kyiv National University of Construction and Architecture, Kyiv, Ukraine, 2004. [Google Scholar]
- Wasim, M.; Ngo, T.D.; Law, D. A State-of-the-Art Review on the Durability of Geopolymer Concrete for Sustainable Structures and Infrastructure. Constr. Build. Mater. 2021, 291, 123381. [Google Scholar] [CrossRef]
- Krivenko, P.; Rudenko, I.; Konstantynovskyi, O. Effect of Technological Factors on Freeze-Thaw Resistance of Alkali-Activated Slag Cement Concrete in NaCl Solution. AIP Conf. Proc. 2023, 2684, 040011. [Google Scholar]
- Krivenko, P.; Rudenko, I.; Konstantynovskyi, O.; Boiko, O. Prevention of Steel Reinforcement Corrosion in Alkali-Activated Slag Cement Concrete Mixed with Seawater. E3S Web Conf. 2021, 280, 07004. [Google Scholar] [CrossRef]
- Krivenko, P.; Rudenko, I.; Konstantynovskyi, O.; Vaičiukynienė, D. Mitigation of Corrosion Initiated by Cl− and SO42−-Ions in Blast Furnace Cement Concrete Mixed with Sea Water. Materials 2022, 15, 3003. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).