Natural Coagulants as an Efficient Alternative to Chemical Ones for Continuous Treatment of Aquaculture Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Coagulants
2.2. Water Samples
2.3. Continuous Flow Experimental Setup and Operational Conditions
2.4. Analytical Methods
3. Results
3.1. Coagulation–Flocculation in Continuous Mode
3.1.1. Color Removal
3.1.2. Organic Matter Removal
3.1.3. Nutrients
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lem, A.; Bjorndal, T.; Lappo, A. Economic Analysis of Supply and Demand for Food up to 2030—Special Focus on Fish and Fishery Products; Food and Agriculture Organization of the United Nations: Rome, Italy, 2014. [Google Scholar]
- United Nations. The State of World Fisheries and Aquaculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 2022. [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations). The State of World Fisheries and Aquaculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 2024. [Google Scholar]
- Chen, H.-C.; Xu, S.-Y.; Deng, K.-H. Water Color Identification System for Monitoring Aquaculture Farms. Sensors 2022, 22, 7131. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Khan, M.A.; Nielsen, R.; Islam, N. Total factor productivity and technical efficiency differences of aquaculture farmers in Bangladesh: Do environmental characteristics matter? J. World Aquac. Soc. 2019, 51, 918–930. [Google Scholar] [CrossRef]
- Fiorella, K.J.; Okronipa, H.; Baker, K.; Heilpern, S. Contemporary aquaculture: Implications for human nutrition. Curr. Opin. Biotechnol. 2021, 70, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Tom, A.P.; Jayakumar, J.S.; Biju, M.; Somarajan, J.; Ibrahim, M.A. Aquaculture wastewater treatment technologies and their sustainability: A review. Energy Nexus 2021, 4, 100022. [Google Scholar] [CrossRef]
- Clasen, B.; Storck, T.R.; Tiecher, T.L. Aquatic biomonitoring: Importance, challenges, and limitations. Integr. Environ. Assess. Manag. 2022, 18, 597–598. [Google Scholar] [CrossRef]
- Marcuello, C. Present and future opportunities in the use of atomic force microscopy to address the physico-chemical properties of aquatic ecosystems at the nanoscale level. Int. Aquat. Res. 2022, 14, 231–240. [Google Scholar] [CrossRef]
- Ahmad, A.; Abdullah, S.R.S.; Hasan, H.A.; Othman, A.R.; Ismail, N.I. Aquaculture industry: Supply and demand, best practices, effluent and its current issues and treatment technology. J. Environ. Manag. 2021, 287, 112271. [Google Scholar] [CrossRef]
- Letelier-Gordo, C.O.; Fernandes, P.M. Coagulation of phosphorous and organic matter from marine, land-based recirculating aquaculture system effluents. Aquac. Eng. 2021, 92, 102144102144. [Google Scholar] [CrossRef]
- Hizam, M.; Noor, M.; Ngadi, N. Global research landscape on coagulation-flocculation for wastewater treatment: A 2000–2023 bibliometric analysis. J. Water Process Eng. 2024, 64, 105696. [Google Scholar] [CrossRef]
- Gibson, T.F.; Watanabe, W.O.; Losordo, T.M.; Whitehead, R.F.; Carroll, P.M. Evaluation of chemical polymers as coagulation aids to remove suspended solids from marine fish recirculating aquaculture system discharge using a geotextile bag. Aquac. Eng. 2020, 90, 102065. [Google Scholar] [CrossRef]
- Tomasi, I.T.; Santos, I.; Gozubuyuk, E.; Santos, O.; Boaventura, R.A.R.; Botelho, C.M.S. A sustainable solution for aquaculture wastewater treatment: Evaluation of tannin-based and conventional coagulants. Chemosphere 2025, 377, 144320. [Google Scholar] [CrossRef] [PubMed]
- Hoyos-Martínez, P.L.d.; Merle, J.; Labidi, J.; Charrier, F.; Bouhtoury, E. Tannins extraction: A key point for their valorization and cleaner production. J. Clean. Prod. 2019, 206, 1138–1155. [Google Scholar] [CrossRef]
- Tomasi, I.T.; Machado, C.A.; Boaventura, R.A.R.; Botelho, C.M.S.; Santos, S.C.R. Tannin-based coagulants: Current development and prospects on synthesis and uses. Sci. Total Environ. 2022, 822, 153454. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, I.T.; Boaventura, R.A.R.; Botelho, C.M.S. Environmental impact assessment of tannin-based coagulants production from chestnut shells. J. Environ. Manag. 2025, 382, 125346. [Google Scholar] [CrossRef]
- Ahmad, A.; Abdullah, S.R.S.; Hasan, H.A.; Othman, A.R.; Kurniawan, S.B. Aquaculture wastewater treatment using plant-based coagulants: Evaluating removal efficiency through the coagulation-flocculation process. Results Chem. 2024, 7, 101390. [Google Scholar] [CrossRef]
- Karnena, M.K.; Konni, M.; Dwarapureddi, B.K.; Saritha, V. Blend of natural coagulants as a sustainable solution for challenges of pollution from aquaculture wastewater. Appl. Water Sci. 2022, 12, 47. [Google Scholar] [CrossRef]
- Igwegbe, C.A.; Ighalo, J.O.; Onukwuli, O.D.; Obiora-Okafo, I.A.; Anastopoulos, I. Coagulation-Flocculation of Aquaculture Wastewater Using Green Coagulant from Garcinia kola Seeds: Parametric Studies, Kinetic Modelling and Cost Analysis. Sustainability 2021, 13, 9177. [Google Scholar] [CrossRef]
- Kurniawan, S.B.; Imron, M.F.; Abdullah, S.R.S.; Othman, A.R.; Hasan, H.A. Coagulation–flocculation of aquaculture effluent using biobased flocculant: From artificial to real wastewater optimization by response surface methodology. J. Water Process Eng. 2023, 53, 103869. [Google Scholar] [CrossRef]
- Anyaene, I.H.; Onukwuli, O.D.; Babayemi, A.K.; Obiora-Okafo, I.A.; Ezeh, E.M. Application of Bio Coagulation–Flocculation and Soft Computing Aids for the Removal of Organic Pollutants in Aquaculture Effluent Discharge. Chem. Afr. 2024, 7, 455–478. [Google Scholar] [CrossRef]
- Tomasi, I.T.; Boaventura, R.A.R.; Botelho, C.M.S. Solid-Liquid Extraction of Polyphenols from Chestnut Shells: A Sustainable Approach for Coagulant Synthesis. Sustain. Chem. Pharm. 2024, 42, 101806. [Google Scholar] [CrossRef]
- Quamme, J.E.; Kemp, A.H. Stable Tannin Based Polymer Compound. U.S. Patent US4558080A, 1985. [Google Scholar]
- Tomasi, I.T.; Santos, S.C.R.; Ribeiro, A.; Homem, V.; Boaventura, R.A.R.; Botelho, C.M.S. Coagulants from chestnut shell tannins—Synthesis, characterization and performance on water treatment. J. Water Process Eng. 2025, 69, 106818. [Google Scholar] [CrossRef]
- Lamb, L.H.; Decusati, O.G. Manufacturing Process for Quaternary Ammonium Tannate, a Vegetable Coagulating/Flocculating Agent. Brazil Patent 6478986, 12 December 2002. [Google Scholar]
- Carlqvist, K.; Arshadi, M.; Mossing, T.; Östman, U.; Brännström, H.; Halmemies, E.; Nurmi, J.; Lidén, G.; Börjesson, P. Life-cycle assessment of the production of cationized tannins from Norway spruce bark as flocculants in wastewater treatment. Biofuels Bioprod. Biorefining 2020, 14, 1270–1285. [Google Scholar] [CrossRef]
- Pasch, J.; Palm, H.W. Economic Analysis and Improvement Opportunities of African Catfish (Clarias gariepinus) Aquaculture in Northern Germany. Sustainability 2021, 13, 13569. [Google Scholar] [CrossRef]
- Lisachov, A.; Nguyen, D.H.M.; Panthum, T.; Ahmad, S.F.; Singcha, W.; Ponjarat, J.; Jaisamut, K.; Srisapoome, P.; Duengkae, P.; Hatachote, S.; et al. Emerging importance of bighead catfish (Clarias macrocephalus) and north African catfish (C. gariepinus) as a bioresource and their genomic perspective. Aquaculture 2023, 573, 739585. [Google Scholar] [CrossRef]
- Somerville, C.; Cohen, M.; Pantanella, E.; Stankus, A.; Lovatelli, A. Food and Agriculture Organization of the United Nations. In Small-Scale Aquaponic Food Production—Integrated Fish and Plant Farming; 2070-7010; Food and Agriculture Organization of the United Nations: Rome, Italy, 2014. [Google Scholar]
- Yep, B.; Zheng, Y. Aquaponic trends and challenges—A review. J. Clean. Prod. 2019, 228, 1586–1599. [Google Scholar] [CrossRef]
- Edeline, F. Épuration Physico-Chimique des Eaux—Théorie et Technologie, 2nd ed.; INRAE: Paris, France, 1992. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater, 20th ed.; Association, A.P.H., Ed.; American Water Works Association and Water Environmental Federation: Denver, CO, USA, 1998. [Google Scholar]
- Okoro, B.U.; Sharifi, S.; Jesson, M.A.; Bridgeman, J. Natural organic matter (NOM) and turbidity removal by plant-based coagulants: A review. Environ. Chem. Eng. 2021, 9, 106588. [Google Scholar] [CrossRef]
- Dinis, M.T.; Rocha, R.M. Introdução à Aquacultura, 1st ed.; Lidel—Edições Técnicas, Ed.; Lidel—Edições Técnicas, Lda: Lisboa, Portugal, 2021; p. 263. [Google Scholar]
- Kurniawan, S.B.; Imron, M.F.; Abdullah, S.R.S.; Othman, A.R.; Purwanti, I.F.; Hasan, H.A. Treatment of real aquaculture effluent using bacteria-based bioflocculant produced by Serratia marcescens. J. Water Process Eng. 2022, 47, 102708. [Google Scholar] [CrossRef]
- Land, T.M.S.; Veit, M.T.; Gonçalves, G.d.C.; Palácio, S.M.; Nascimento, J.C.Z.B.C.d.O.C.; Campos, E.G.P. Evaluation of a Coagulation/Flocculation Process as the Primary Treatment of Fish Processing Industry Wastewater. Water Air Soil Pollut. 2020, 231, 452. [Google Scholar] [CrossRef]
- Aguilar-Alarcón, P.; Gonzalez, S.V.; Simonsen, M.A.; Borrero-Santiago, A.R.; Sanchís, J.; Meriac, A.; Kolarevic, J.; Asimakopoulos, A.G.; Mikkelsen, Ø. Characterizing changes of dissolved organic matter composition with the use of distinct feeds in recirculating aquaculture systems via high-resolution mass spectrometry. Sci. Total Environ. 2020, 749, 142326. [Google Scholar] [CrossRef]
- Matilainen, A.; Gjessing, E.T.; Lahtinen, T.; Hed, L.; Bhatnagar, A.; Sillanpää, M. An overview of the methods used in the characterization of natural organic matter (NOM) in relation to drinking water treatment. Chemosphere 2011, 83, 1431–1442. [Google Scholar] [CrossRef] [PubMed]
- Justina, M.D.; Muniz, B.R.B.; Bröring, M.M.; Costa, V.J.; Skoronski, E. Using vegetable tannin and polyaluminium chloride as coagulants for dairy wastewater treatment: A comparative study. J. Water Process Eng. 2018, 25, 173–181. [Google Scholar] [CrossRef]
- Lopes, E.C.; Santos, S.C.R.; Pintor, A.M.A.; Boaventura, R.A.R.; Botelho, C.M.S. Evaluation of a tannin-based coagulant on the decolorization of synthetic effluent. J. Environ. Chem. Eng. 2019, 7, 103125. [Google Scholar] [CrossRef]
- Polasek, P.; Wantenaar, C. The inability of organic coagulants to purify potable water to its best attainable quality. Water SA 2023, 43, 311–319. [Google Scholar] [CrossRef]
- Tomasi, I.T.; Ferreira, R.M.; Boaventura, R.A.R.; Botelho, C.M.S. Natural coagulants from chestnut shells: A sustainable approach for textile wastewater treatment. Chemosphere 2025, 376, 144286. [Google Scholar] [CrossRef]
- Beltrán-Heredia, J.; Sánchez-Martín, J.; Muñoz-Serrano, A.; Peres, J.A. Towards overcoming TOC increase in wastewater treated with Moringa oleifera seed extract. Chem. Eng. J. 2012, 188, 40–46. [Google Scholar] [CrossRef]
- Decree Law No. 236/98. Available online: https://diariodarepublica.pt/dr/detalhe/decreto-lei/236-1998-430457 (accessed on 1 May 2025).
- Neissi, A.; Rafiee, G.; Rahimi, S.; Farahmand, H.; Pandit, S.; Mijakovic, I. Enriched microbial communities for ammonium and nitrite removal from recirculating aquaculture systems. Chemosphere 2022, 295, 133811. [Google Scholar] [CrossRef]
- Molayemraftar, T.; Peyghan, R.; Jalali, M.R.; Shahriari, A. Single and combined effects of ammonia and nitrite on common carp, Cyprinus carpio: Toxicity, hematological parameters, antioxidant defenses, acetylcholinesterase, and acid phosphatase activities. Aquaculture 2022, 548, 737676. [Google Scholar] [CrossRef]
- Li, H.; Cui, Z.; Cui, H.; Bai, Y.; Yin, Z.; Qu, K. Hazardous substances and their removal in recirculating aquaculture systems: A review. Aquaculture 2023, 569, 739399. [Google Scholar] [CrossRef]
- Hart, K.A.; Trueman, B.; Halfyard, E.A.; Sterling, S.M. Detection and Prediction of Toxic Aluminum Concentrations in High-Priority Salmon Rivers in Nova Scotia. Environ. Toxicol Chem. 2024, 43, 2545–2556. [Google Scholar] [CrossRef]
- Farooq, A.; Verma, A.K.; Hittinahalli, C.M.; Varghese, T.; Pathak, M.S. Iron supplementation in aquaculture wastewater and its impact on osmoregulatory, haematological, blood biochemical, and stress responses of pangasius with spinach in nutrient film technique based aquaponics. Aquaculture 2023, 567, 739250. [Google Scholar] [CrossRef]
- Bai, D.; Li, X.; Liu, Z.; Wan, L.; Song, C.; Zhou, Y.; Cao, X. Nitrogen and phosphorus turnover and coupling in ponds with different aquaculture species. Aquaculture 2023, 563, 738997. [Google Scholar] [CrossRef]
- Getahun, M.; Asaithambi, P.; Befekadu, A.; Alemayehu, E. Optimization of indigenous natural coagulants process for nitrate and phosphate removal from wet coffee processing wastewater using response surface methodology: In the case of Jimma Zone Mana district. Case Stud. Chem. Environ. Eng. 2023, 8, 100370. [Google Scholar] [CrossRef]
- Klykken, C.; Reed, A.K.; Dalum, A.S.; Olsen, R.E.; Moe, M.K.; Attramadal, K.J.K.; Boissonnot, L. Physiological changes observed in farmed Atlantic salmon (Salmo salar L.) with nephrocalcinosis. Aquaculture 2022, 554, 738104. [Google Scholar] [CrossRef]
- Romano, N.; Egnew, N.; Quintero, H.; Kelly, A.; Sinha, A.K. The effects of water hardness on the growth, metabolic indicators and stress resistance of largemouth bass Micropterus salmoides. Aquaculture 2020, 527, 735469. [Google Scholar] [CrossRef]
- El-Shenawy, A.M.; Gad, D.M.; Yassin, S.A. Effect of Iron Nanoparticles on the Development of Fish Farm Feeds. Alex. J. Vet. Sci. 2019, 60, 102–115. [Google Scholar] [CrossRef]
- Singh, G.; Chauhan, R. Exploring the effects of iron nanoparticles in aquaculture: Review. Int. J. Adv. Biochem. Res. 2025, 9, 935–939. [Google Scholar] [CrossRef]
- Siqwepu, O.; Salie, K.; Goosen, N. Evaluation of chelated iron and iron sulfate in the diet of African catfish, Clarias gariepinus to enhance iron excretion for application in integrated aquaponics systems. J. World Aquac. Soc. 2020, 51, 1034–1053. [Google Scholar] [CrossRef]
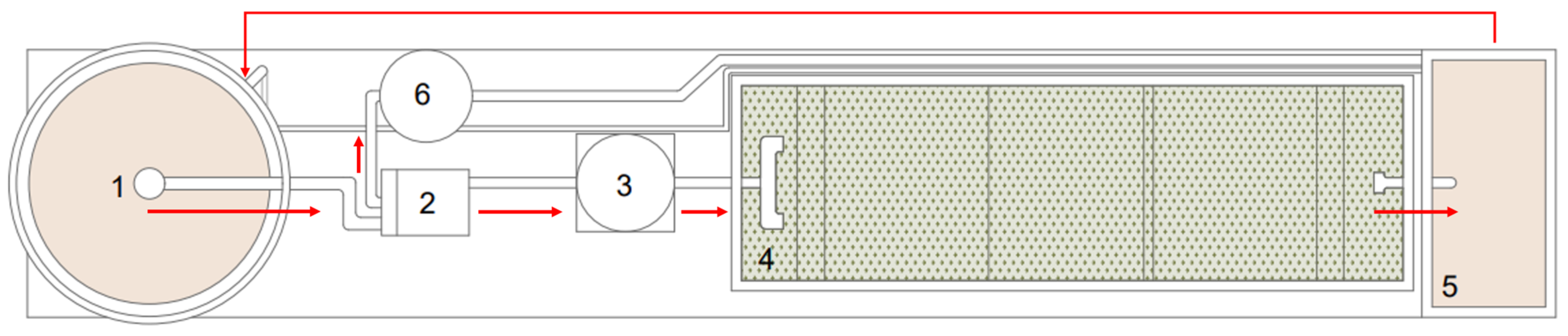
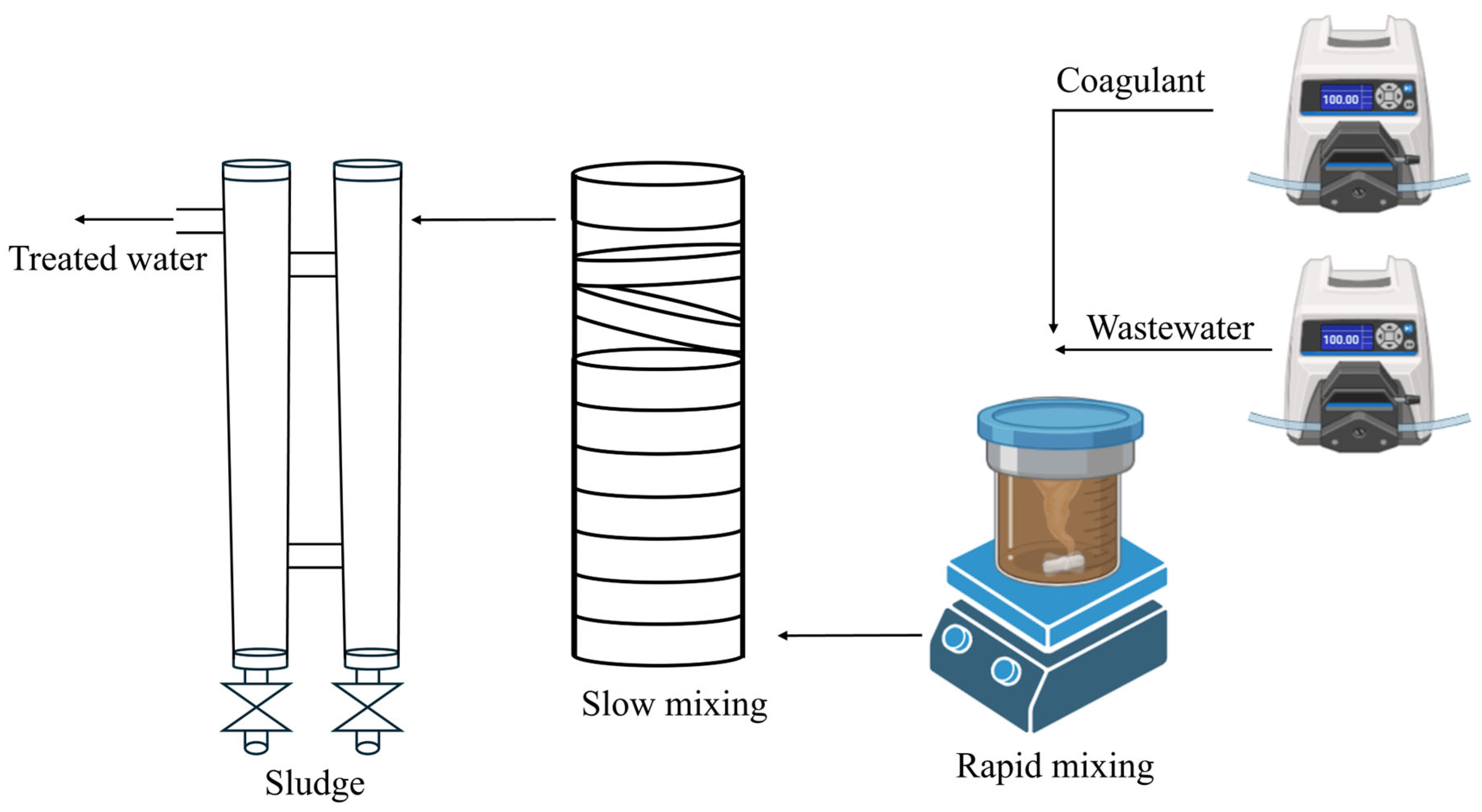
| Parameters | Units | Value |
|---|---|---|
| pH | 6.5 ± 0.3 | |
| Apparent color | Scale Pt-Co | 75 |
| DOC | mg·L−1 | 39.3 ± 0.3 |
| Absorbance (254 nm) | 0.8326 ± 0.0003 | |
| Total N | mg·L−1 | 151 ± 2 |
| N–NO2− | mg·L−1 | 0.065 ± 0.001 |
| BOD5 | mg·L−1 | <DL |
| Total P | mg·L−1 | 13.5 ± 0.2 |
| Coagulant | pH | Coagulant Concentration (mg·L−1) | DOC Removal (%) | UV254 Removal (%) | Color Removal (%) |
|---|---|---|---|---|---|
| Al2(SO4)3.14H2O | 6.5 | 20 | - | 12.06 ± 0.02 | 33.5 ± 0.2 |
| 50 | 45.4 ± 0.5 | 28.00 ± 0.01 | 48.8 ± 0.4 | ||
| 100 | - | 53.62 ± 0.01 | 62.4 ± 0.2 | ||
| 8 | 20 | 59.1 ± 0.4 | 30.64 ± 0.03 | 30.1 ± 0.3 | |
| FeCl3.6H2O | 6.5 | 20 | - | 16.00 ± 0.01 | 40.3 ± 0.2 |
| 50 | 44.4 ± 0.6 | 51.89 ± 0.01 | 47.8 ± 0.2 | ||
| 100 | - | 55.93 ± 0.01 | 70.9 ± 0.5 | ||
| 8 | 25 | 50.8 ± 0.4 | 26.82 ± 0.02 | 31.4 ± 0.2 | |
| Tanfloc | 6.5 | 20 | - | 38.16 ± 0.02 | 52.0 ± 0.2 |
| 50 | 52.8 ± 0.5 | 50.05 ± 0.01 | 65.5 ± 0.6 | ||
| 100 | - | 63.94 ± 0.03 | 75.4 ± 0.3 | ||
| 7 | 25 | 66.2 ± 0.4 | 29.87 ± 0.02 | 45.9 ± 0.5 | |
| CS-based | 6.5 | 20 | - | 20.90 ± 0.03 | 42.5 ± 0.4 |
| 50 | 31.7 ± 0.3 | 53.42 ± 0.01 | 61.0 ± 0.3 | ||
| 100 | - | 64.45 ± 0.01 | 62.3 ± 0.2 | ||
| 8 | 10 | 47.7 ± 0.5 | 20.33 ± 0.01 | 33.6 ± 0.3 |
| Coagulant Concentration (mg·L−1) | pH | P (mg·L−1) | N (mg·L−1) | Ca (mg·L−1) | Mg (mg·L−1) | |
|---|---|---|---|---|---|---|
| Raw effluent | - | 6.5 ± 0.3 | 13.5 ± 0.2 | 151 ± 2 | 71.5 ± 0.1 | 17.0 ± 0.5 |
| CS | 50 | 6.5 ± 0.3 | 1.1 ± 0.2 | 126 ± 5 | 44.2 ± 0.6 | 6.5 ± 0.3 |
| 10 | 8.0 ± 0.2 | 1.22 ± 0.04 | 97 ± 2 | 30.2 ± 0.4 | 5.7 ± 0.3 | |
| Tanfloc | 50 | 6.5 ± 0.3 | 1.0 ± 0.1 | 106 ± 3 | 34.7 ± 0.5 | 5.3 ± 0.3 |
| 25 | 7.0 ± 0.2 | 1.07 ± 0.02 | 93 ± 3 | 35.5 ± 0.5 | 5.6 ± 0.3 | |
| Al2(SO4)3 | 50 | 6.5 ± 0.3 | 1.0 ± 0.1 | 123 ± 4 | 47.9 ± 0.6 | 6.7 ± 0.3 |
| 20 | 8.0 ± 0.2 | 1.13 ± 0.01 | 89 ± 3 | 31.4 ± 0.4 | 5.3 ± 0.3 | |
| FeCl3 | 50 | 6.5 ± 0.3 | 1.052 ± 0.002 | 123 ± 3 | 40.6 ± 0.5 | 5.9 ± 0.3 |
| 25 | 8.0 ± 0.2 | 1.22 ± 0.02 | 106 ± 4 | 28.1 ± 0.4 | 4.1 ± 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomasi, I.T.; Boaventura, R.A.R.; Botelho, C.M.S. Natural Coagulants as an Efficient Alternative to Chemical Ones for Continuous Treatment of Aquaculture Wastewater. Appl. Sci. 2025, 15, 6908. https://doi.org/10.3390/app15126908
Tomasi IT, Boaventura RAR, Botelho CMS. Natural Coagulants as an Efficient Alternative to Chemical Ones for Continuous Treatment of Aquaculture Wastewater. Applied Sciences. 2025; 15(12):6908. https://doi.org/10.3390/app15126908
Chicago/Turabian StyleTomasi, Isabella T., Rui A. R. Boaventura, and Cidália M. S. Botelho. 2025. "Natural Coagulants as an Efficient Alternative to Chemical Ones for Continuous Treatment of Aquaculture Wastewater" Applied Sciences 15, no. 12: 6908. https://doi.org/10.3390/app15126908
APA StyleTomasi, I. T., Boaventura, R. A. R., & Botelho, C. M. S. (2025). Natural Coagulants as an Efficient Alternative to Chemical Ones for Continuous Treatment of Aquaculture Wastewater. Applied Sciences, 15(12), 6908. https://doi.org/10.3390/app15126908







