How Does Ceramic-Based Scaffold Microarchitecture Impact Maxillofacial Bone Regeneration? A Systematic Review of Large Animal Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol
2.2. Focused Question
2.3. Search Strategy (Supplementary Materials)
2.4. Eligibility Criteria
2.5. Study Selection
2.6. Data Extraction
2.7. Quality and Risk-of-Bias Assessments
2.8. Effect Measures
3. Results
3.1. Article Selection
3.2. Animal Model’s Characteristics
3.3. Outcomes Analysis
3.4. Scaffold Fabrication
3.5. Scaffold’s Geometrical Parameters and Main Findings
3.5.1. Scaffold Size
3.5.2. Porosity
3.5.3. Pore Size
3.5.4. Scaffold’s Macroscopy
3.5.5. Pore Shape
3.5.6. Interconnectivity
3.5.7. Biomechanical Properties
3.6. Compliance with the ARRIVE Guidelines
3.7. SYRCLE Risk-of-Bias Tool
- Six studies (Cao et al. 2021 [26], Henkel et al. 2006 [28], Wu et al. 2006 [29], Zhang et al. 2019 [30], Zeng et al. 2017 [31], Bachtiar et al. 2016 [34]) were rated as having a high risk of bias, with multiple domains marked as high or unclear, particularly regarding allocation concealment, random housing, and the blinding of outcome assessors.
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CSBDs | Critical-sized bone defects |
| 3D | Three-dimensional |
| μm | Micrometer |
| OMS | Oral and Maxillofacial Surgery |
| HA | Hydroxyapatite |
| TCP | Tricalcium Phosphate |
| β-TCP | Beta-Tricalcium Phosphate |
| AM | Additive Manufacturing |
| mm3 | Cubic millimeters |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| ARRIVE | Animal Research: Reporting of In Vivo Experiments |
| SYRCLE | Systematic Review Centre for Laboratory Animal Experimentation |
| Micro-CT | Micro-Computed Tomography |
| PET-CT | Positron Emission Tomography–Computed Tomography |
| CT | Computed Tomography |
| 99mTc-MDP SPECT/CT | Technetium-99m Methyl Diphosphonate Single-Photon Emission Computed Tomography/Computed Tomography |
| H&E | Hematoxylin–Eosin Saffron |
| SPECT | Single-Photon Emission Computed Tomography |
References
- Huang, X.; Lou, Y.; Duan, Y.; Liu, H.; Tian, J.; Shen, Y.; Wei, X. Biomaterial scaffolds in maxillofacial bone tissue engineering: A review of recent advances. Bioact. Mater. 2024, 33, 129–156. [Google Scholar] [CrossRef] [PubMed]
- Pillia, M.; Guda, T.; Appleford, M. Development of Composite Scaffolds for Load-Bearing Segmental Bone Defects. Biomed. Res. Int. 2013, 2013, 458253. [Google Scholar] [CrossRef]
- Dussault, A.; Pitaru, A.A.; Weber, M.H.; Haglund, L.; Rosenzweig, D.H.; Villemure, I. Optimizing Design Parameters of PLA 3D-Printed Scaffolds for Bone Defect Repair. Surgeries 2022, 3, 162–174. [Google Scholar] [CrossRef]
- Liu, W.; Liu, S.; Li, Y.; Zhou, P.; Ma, Q. Biomimetic Design of 3D Printed Tissue-Engineered Bone Constructs. Curr. Nanosci. 2021, 17, 223–240. [Google Scholar] [CrossRef]
- Basyuni, S.; Ferro, A.; Santhanam, V.; Birch, M.; McCaskie, A. Systematic scoping review of mandibular bone tissue engineering. Br. J. Oral. Maxillofac. Surg. 2020, 58, 632–642. [Google Scholar] [CrossRef]
- Bahraminasab, M. Challenges on optimization of 3D-printed bone scaffolds. BioMed. Eng. OnLine 2020, 19, 69. [Google Scholar] [CrossRef]
- Schemitsch, E.H. Size Matters: Defining Critical in Bone Defect Size! J. Orthop. Trauma 2017, 31 (Suppl. 5), S20–S22. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Chen, X.; Xu, Y.; Shi, L.; Zhang, M.; Nie, M.; Liu, X. Significance and considerations of establishing standardized critical values for critical size defects in animal models of bone tissue regeneration. Heliyon 2024, 10, e33768. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dalfino, S.; Savadori, P.; Piazzoni, M.; Connelly, S.T.; Giannì, A.B.; Del Fabbro, M.; Tartaglia, G.M.; Moroni, L. Regeneration of Critical-Sized Mandibular Defects Using 3D-Printed Composite Scaffolds: A Quantitative Evaluation of New Bone Formation in In Vivo Studies. Adv. Healthc. Mater. 2023, 12, e2300128. [Google Scholar] [CrossRef]
- Döbelin, N.; Luginbühl, R.; Bohner, M. Synthetic Calcium Phosphate Ceramics for Treatment of Bone Fractures. Chimia 2010, 64, 723. [Google Scholar] [CrossRef]
- Valtanen, R.S.; Yang, Y.P.; Gurtner, G.C.; Maloney, W.J.; Lowenberg, D.W. Synthetic and Bone Tissue Engineering Graft Substitutes: What Is the Future? Injury 2021, 52 (Suppl. 2), S72–S77. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.H.; Ghayor, C.; Siegenthaler, B.; Schuler, F.; Rüegg, J.; De Wild, M.; Weber, F.E. Lattice Microarchitecture for Bone Tissue Engineering from Calcium Phosphate Compared to Titanium. Tissue Eng. Part A 2018, 24, 1554–1561. [Google Scholar] [CrossRef] [PubMed]
- Busch, A.; Jäger, M.; Mayer, C.; Sowislok, A. Functionalization of Synthetic Bone Substitutes. Int. J. Mol. Sci. 2021, 22, 4412. [Google Scholar] [CrossRef] [PubMed]
- Ryan, E.; Yin, S. Compressive Strength of β-TCP Scaffolds Fabricated via Lithography-Based Manufacturing for Bone Tissue Engineering. Ceram. Int. 2022, 48, 15516–15524. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Z.; Li, J.; Liu, C.; Lao, C.; Fu, Y.; Liu, C.; Li, Y.; Wang, P.; He, Y. 3D Printing of Ceramics: A Review. J. Eur. Ceram. Soc. 2019, 39, 661–687. [Google Scholar] [CrossRef]
- Zocca, A.; Colombo, P.; Gomes, C.M.; Günster, J. Additive Manufacturing of Ceramics: Issues, Potentialities, and Opportunities. J. Am. Ceram. Soc. 2015, 98, 1983–2001. [Google Scholar] [CrossRef]
- Shuai, C.; Feng, P.; Zhang, L.; Gao, C.; Hu, H.; Peng, S.; Min, A. Correlation between Properties and Microstructure of Laser Sintered Porous β-Tricalcium Phosphate Bone Scaffolds. Sci. Technol. Adv. Mater. 2013, 14, 055002. [Google Scholar] [CrossRef]
- Baino, F.; Novajra, G.; Vitale-Brovarone, C. Bioceramics and Scaffolds: A Winning Combination for Tissue Engineering. Front. Bioeng. Biotechnol. 2015, 3, 202. [Google Scholar] [CrossRef]
- De Carvalho, A.B.G.; Rahimnejad, M.; Oliveira, R.L.M.S.; Sikder, P.; Saavedra, G.S.F.A.; Bhaduri, S.B.; Gawlitta, D.; Malda, J.; Kaigler, D.; Trichês, E.S.; et al. Personalized bioceramic grafts for craniomaxillofacial bone regeneration. Int. J. Oral. Sci. 2024, 16, 62. [Google Scholar] [CrossRef]
- Cancedda, R.; Giannoni, P.; Mastrogiacomo, M.A. Tissue Engineering Approach to Bone Repair in Large Animal Models and in Clinical Practice. Biomaterials 2007, 28, 4240–4250. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Iglhaut, G.; Becker, J. Quality assessment of reporting of animal studies on pathogenesis and treatment of peri-implant mucositis and peri-implantitis. A systematic review using the ARRIVE guidelines. J. Clin. Periodontol. 2012, 39 (Suppl. 12), 63–72. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, J.L.G.C.; Guastaldi, F.P.; Troulis, M.J.; McCain, J.P.; do Egito Vasconcelos, B.C. Induction, Treatment, and Prevention of Temporomandibular Joint Ankylosis-A Systematic Review of Comparative Animal Studies. J. Oral. Maxillofac. Surg. 2021, 79, 109–132.e6. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Cao, S.; Li, S.; Geng, Y.; Kapat, K.; Liu, S.; Perera, F.H.; Li, Q.; Terheyden, H.; Wu, G.; Che, Y.; et al. Prefabricated 3D-Printed Tissue-Engineered Bone for Mandibular Reconstruction: A Preclinical Translational Study in Primate. ACS Biomater. Sci. Eng. 2021, 7, 5727–5738. [Google Scholar] [CrossRef]
- Johnson, Z.M.; Yuan, Y.; Li, X.; Jashashvili, T.; Jamieson, M.; Urata, M.; Chen, Y.; Chai, Y. Mesenchymal Stem Cells and Three-Dimensional-Osteoconductive Scaffold Regenerate Calvarial Bone in Critical Size Defects in Swine. Stem Cells Transl. Med. 2021, 10, 1170–1183. [Google Scholar] [CrossRef]
- Henkel, K.O.; Gerber, T.; Lenz, S.; Gundlach, K.K.H.; Bienengräber, V. Macroscopical, Histological, and Morphometric Studies of Porous Bone-Replacement Materials in Minipigs 8 Months after Implantation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2006, 102, 606–613. [Google Scholar] [CrossRef]
- Wu, W.; Chen, X.; Mao, T.; Chen, F.; Feng, X. Bone Marrow-Derived Osteoblasts Seeded into Porous Beta-Tricalcium Phosphate to Repair Segmental Defect in Canine’s Mandibula. Ulus. Travma Acil Cerrahi Derg. 2006, 12, 268–276. [Google Scholar]
- Zhang, Z.; Wang, P.; Li, X.; Wang, Y.; Qin, Z.; Zhang, C.; Li, J. Reconstruction of Mandibular Bone Defects Using Biphasic Calcium Phosphate Bone Substitutes with Simultaneous Implant Placement in Mini-Swine: A Pilot In Vivo Study. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 2071–2079. [Google Scholar] [CrossRef]
- Zeng, N.; van Leeuwen, A.C.; Grijpma, D.W.; Bos, R.R.M.; Kuijer, R. Poly(Trimethylene Carbonate)-Based Composite Materials for Reconstruction of Critical-Sized Cranial Bone Defects in Sheep. J. Cranio-Maxillofac. Surg. 2017, 45, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Paré, A.; Charbonnier, B.; Veziers, J.; Vignes, C.; Dutilleul, M.; De Pinieux, G.; Laure, B.; Bossard, A.; Saucet-Zerbib, A.; Touzot-Jourde, G.; et al. Standardized and Axially Vascularized Calcium Phosphate-Based Implants for Segmental Mandibular Defects: A Promising Proof of Concept. Acta Biomater. 2022, 154, 626–640. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Li, X.; Bao, C.; Fan, H.; Zhang, X.; Chen, Z. A Novel Technique to Reconstruct a Boxlike Bone Defect in the Mandible and Support Dental Implants with In Vivo Tissue-Engineered Bone. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 91B, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Bachtiar, E.W.; Amir, L.R.; Suhardi, P.; Abas, B. Scaffold Degradation During Bone Tissue Reconstruction in Macaca Nemestrina Mandible. Interv. Med. Appl. Sci. 2016, 8, 77–81. [Google Scholar] [CrossRef]
- Yuan, J.; Cui, L.; Zhang, W.J.; Liu, W.; Cao, Y. Repair of canine mandibular bone defects with bone marrow stromal cells and porous β-tricalcium phosphate. Biomaterials 2007, 28, 1005–1013. [Google Scholar] [CrossRef]
- Nokhbatolfoghahaei, H.; Bastami, F.; Farzad-Mohajeri, S.; Rezai Rad, M.; Dehghan, M.M.; Bohlouli, M.; Farajpour, H.; Nadjmi, N.; Khojasteh, A. Prefabrication technique by preserving a muscular pedicle from masseter muscle as an in vivo bioreactor for reconstruction of mandibular critical-sized bone defects in canine models. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 1675–1686. [Google Scholar] [CrossRef]
- Sillmann, Y.M.; Eber, P.; Orbeta, E.; Wilde, F.; Gross, A.J.; Guastaldi, F.P.S. Milestones in Mandibular Bone Tissue Engineering: A Systematic Review of Large Animal Models and Critical-Sized Defects. J. Clin. Med. 2025, 14, 2717. [Google Scholar] [CrossRef]
- Li, Y.; Chen, S.-K.; Li, L.; Qin, L.; Wang, X.-L.; Lai, Y.-X. Bone defect animal models for testing efficacy of bone substitute biomaterials. J. Orthop. Transl. 2015, 3, 95–104. [Google Scholar] [CrossRef]
- Roohani-Esfahani, S.I.; Dunstan, C.R.; Davies, B.; Pearce, S.; Williams, R.; Zreiqat, H. Repairing a critical-sized bone defect with highly porous modified and unmodified baghdadite scaffolds. Acta Biomater. 2012, 8, 4162–4172. [Google Scholar] [CrossRef]
- Pérez-Sánchez, L.; Ortiz de la O, M.A.; Álvarez-Pérez, M.A.; Llaguno-Munive, M.; Chanes-Cuevas, O.A.; Serrano-Bello, J. Standardization of 3D printing parameters to control the size and shape of pores in polylactic acid scaffolds. MedComm 2024, 3, e74. [Google Scholar] [CrossRef]
- Velasco, M.A.; Lancheros, Y.; Garzón-Alvarado, D.A. Geometric and mechanical properties evaluation of scaffolds for bone tissue applications designing by a reaction-diffusion models and manufactured with a material jetting system. J. Comput. Des. Eng. 2016, 3, 385–397. [Google Scholar] [CrossRef]
- Feng, Y.; Zhu, S.; Mei, D.; Li, J.; Zhang, J.; Yang, S.; Guan, S. Application of 3D printing technology in bone tissue engineering: A review. Curr. Drug Deliv. 2021, 18, 847–861. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zhang, L.; Zhou, Z.; Luo, X.; Wang, T.; Zhao, X.; Lu, B.; Chen, F.; Zheng, L. Calcium phosphate-based biomaterials for bone repair. J. Funct. Biomater. 2022, 13, 187. [Google Scholar] [CrossRef] [PubMed]
- Pires, L.C.A.; da Silva, R.C.; Poli, P.P.; Ruas Esgalha, F.; Hadad, H.; Palin, L.P.; Piquera Santos, A.F.; Teixiera Colombo, L.; Kawamata de Jesus, L.; Bassi, A.P.F.; et al. Evaluation of osteoconduction of a synthetic hydroxyapatite/β-tricalcium phosphate block fixed in rabbit mandibles. Materials 2020, 13, 4902. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, Y.; Wang, A.; Zhu, Z.; Li, Y.; Zhu, C.; Che, Z.; Mon, T.; Liu, H.; Huang, L. Application of BMP in bone tissue engineering. Front. Bioeng. Biotechnol. 2022, 10, 810880. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Farjaminejad, R.; Farjaminejad, S.; Hasani, M.; Garcia-Godoy, F.; Sayahpour, B.; Marya, A.; Jamilian, A. The Role of Tissue Engineering in Orthodontic and Orthognathic Treatment: A Narrative Review. Oral 2025, 5, 21. [Google Scholar] [CrossRef]

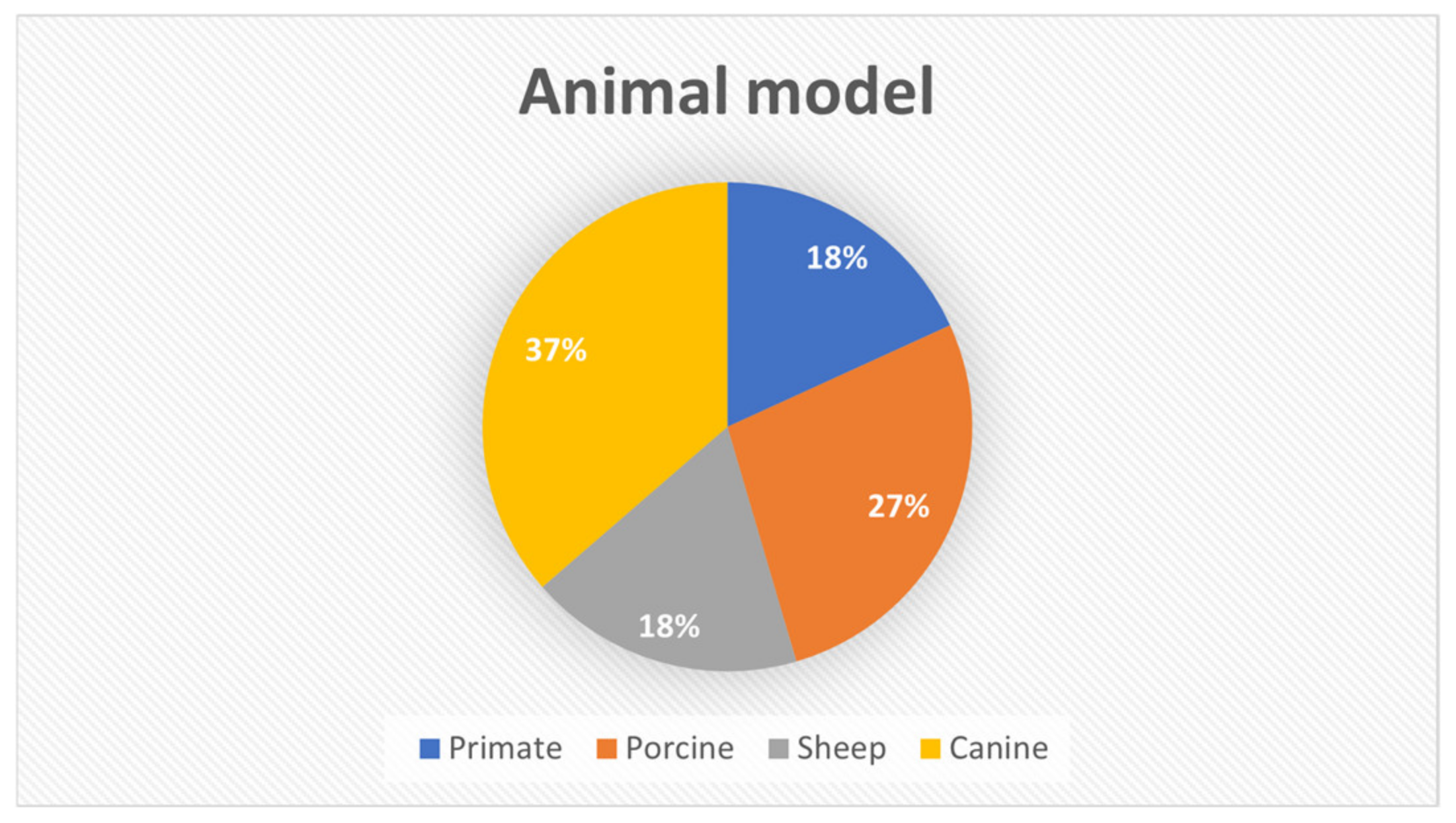

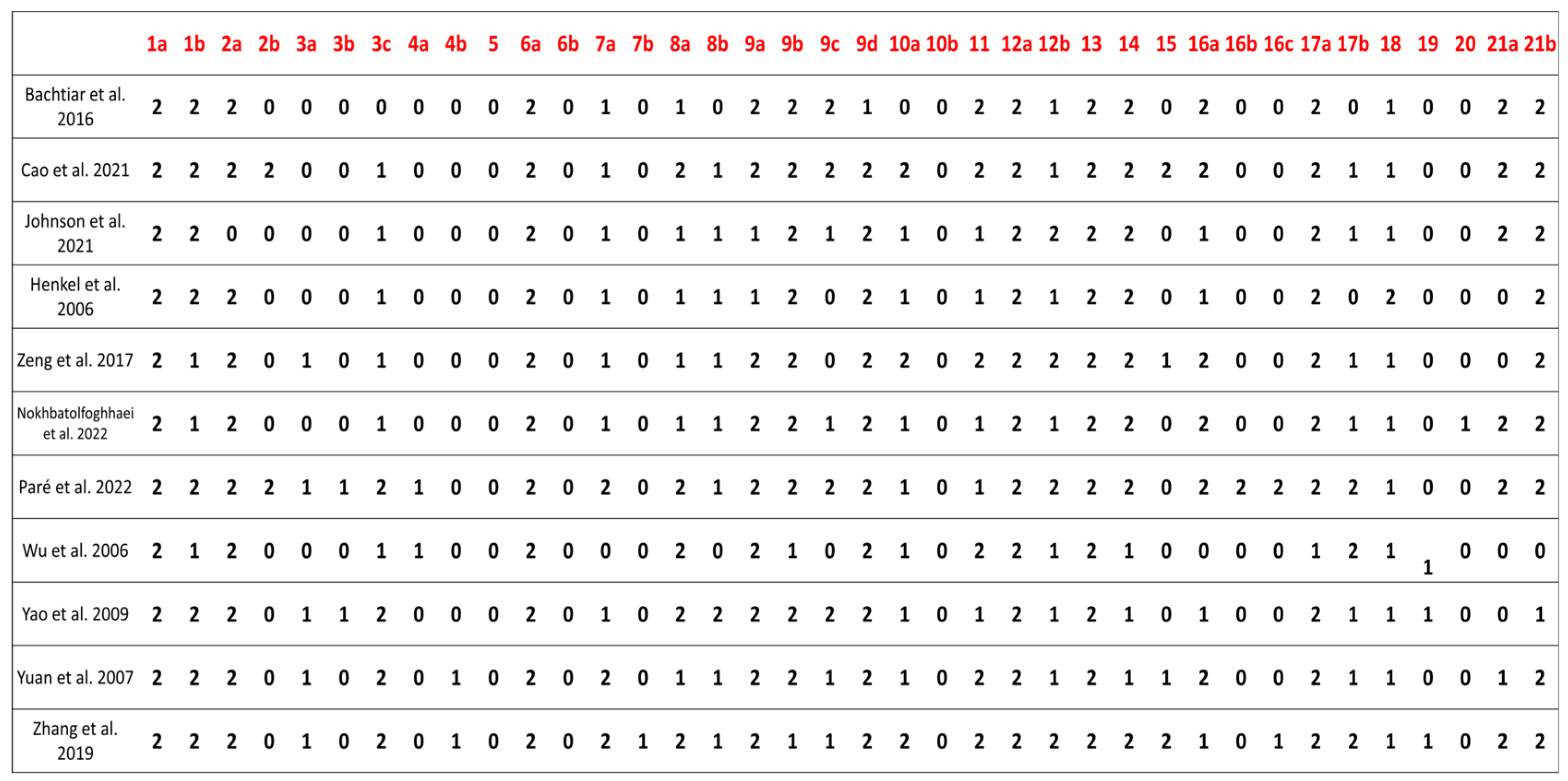
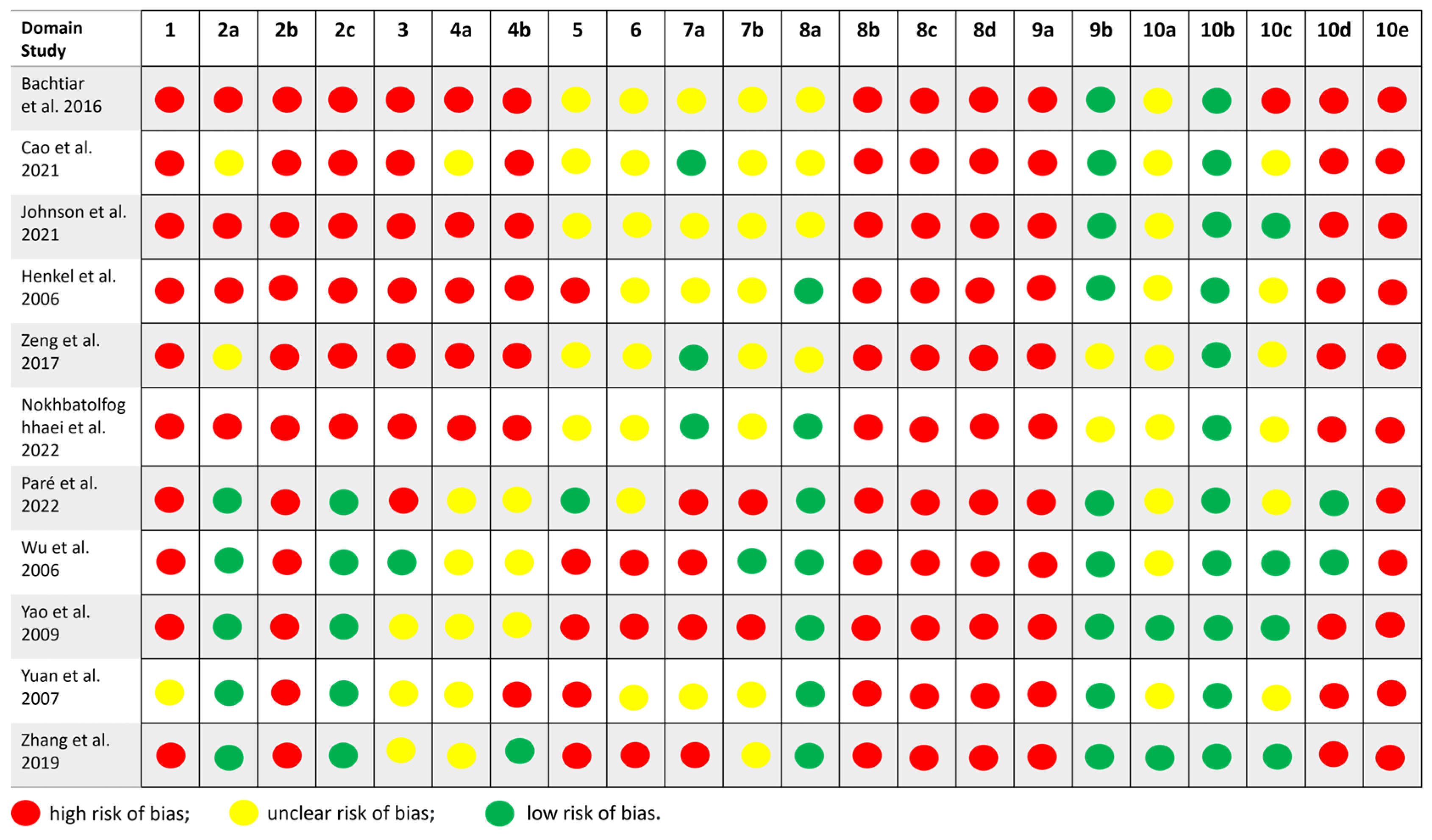
| Characteristics Studies | Animal | Strain/Breed | Age | Weight | Defect Size (mm) | Defect Region | Period of Analysis (Weeks) |
|---|---|---|---|---|---|---|---|
| Bachtiar et al. 2016 [34] | Primate | Macaca nemestrina | Young adult | NS | 10 × 20 mm | Mandible | 0, 2, and 4 weeks |
| Cao et al. 2021 [26] | Primate | Rhesus monkeys | 6–9 years | 6–12 kg | approximately 30 × 30 mm | Mandible | 12 weeks |
| Henkel et al. 2006 [28] | Minipig | Göttingen | 1 year | 20–25 kg | >50 mm in diameter | Mandible | 34 weeks |
| Johnson et al. 2021 [27] | Swine | Yorkshire | 2–3 months | NS | 30 mm in diameter | Calvaria | 8 and 12 weeks |
| Nokhbatolfoghahaei et al. 2022 [36] | Canine | Mongrel | NS | 15–25 kg | 25 × 10 × 8 mm | Mandible | 12 weeks |
| Paré et al. 2022 [32] | Sheep | Vendean strain | 4–7 years | 75–84 kg | 35 mm × 55 mm | Mandible | 13, 21, and 52 weeks |
| Wu et al. 2006 [29] | Canine | Beagle | 10 months | 11–13 kg | 30 × 20 mm | Mandible | 12 weeks |
| Yao et al. 2009 [33] | Canine | NS | Skeletal mature | 13–15 kg | 20 mm × 10 mm | Mandible | 8 weeks |
| Yuan et al. 2007 [35] | Canine | Mongrel | 1 year and 6 months | 20 kg | 30 mm in diameter | Mandible | 4, 12, 26, and 32 weeks |
| Zeng et al. 2017 [31] | Sheep | Dutch Texel | 2 and 3 years | NS | 20 mm in diameter | Calvaria | 13 and 39 weeks |
| Zhang et al. 2019 [30] | Minipig | NS | 10 months | 35–40 kg | 15 × 60 × 45 mm | Mandible | 14 days and 17 months |
| Studies | Groups | Outcome Parameters | Main Findings | ||
|---|---|---|---|---|---|
| Imaging | Histology | Biomechanical Testing | |||
| Bachtiar et al. 2016 [34] | Group 1: HA/TCP with 70:30 ratio; Group 2: HA/TCP with 50:50 ratio; Group 3: HA/TCP/chitosan. This study did not report the number of animals used in each group (n = 6 in total). | NS | (1) H&E | NS | The inflammatory cells were still present in areas where active bone and blood vessel formation was occurring. The remnants of scaffold biomaterials were rarely seen. |
| Cao et al. 2021 [26] | Group 1: TCP (n = 3); Group 2: PLGA/TCP (n = 3); Group 3: rhBMP-2-coated TCP (n = 3); Group 4: rhBMP-2-coated PLGA/TCP (n = 3); Group 5: prefabricated rhBMP-2-coated TCP (n = 3). | (1) PET/CT (2) Micro-CT | (1) H&E | (1) Uniaxial compressive strength testing | The original 3D architecture of the PLGA/TCP-BMP scaffold was found to be completely lost, whereas it was properly maintained in TCP-BMP scaffolds. Regular bone formation was observed with TCP-BMP scaffolds. The TCP-BMP scaffold was successfully fabricated, exhibiting pronounced effects on bone regeneration and vascularization. |
| Henkel et al. 2006 [28] | Group 1: CaP matrix, type I (60% HA 40%TCP) (n = 6); Group 2: CaP matrix, type II (100% HA) (n = 6); Group 3: HA (n = 6); Group 4: TCP (n = 6); Group 5: control group (n = 6). | NS | (1) Giemsa–toluidine (2) Hemalau–eosin (3) Goldner technique | NS | Complete bone formation was observed in the defect area, and simultaneously, the foreign material was resorbed almost completely. After implantation of the classical types of ceramics, only incomplete bone formation and a lower resorption rate of the foreign bodies were noted. |
| Johnson et al. 2021 [27] | Group 1: HA/TCP (n = 3); Group 2: DPNCC + HA/TCP (n = 3); Group 3: BMA + HA/TCP (n = 3); Group 4: native bone (n = 3). | (1) Micro-CT | (1) H&E | (1) Compressive strength testing (2) Mechanical strength testing | Both DPNCCs and BMA loaded into the 3D-printed osteoconductive scaffold support the regeneration of calvarial bone with density, compression strength, and trabecular structures similar to those of native bone. |
| Nokhbatolfoghahaei et al. 2022 [36] | Group 1: βTCP (n = 4); Group 2: βTCP/rhBMP2 (n = 4); Group 3: βTCP/MSCs (n = 4); Group 4: PCL/βTCP (n = 4); Group 5: PCL/βTCP/rhBMP2 (n = 4); Group 6: PCL/βTCP/MSCs (n = 4). | (1) CT scan | (1) H&E | NS | β-TCP scaffold groups resulted in significantly greater rates of new bone formation. Scaffold composition, pedicle preservation, and treatment with MSCs or rhBMP2 influence new bone formation and scaffold degradation rates in the prefabrication technique. |
| Paré et al. 2022 [32] | Group 1: βCP (n = 6); Group 2: clinical control (n = 6); Group 3: cadaveric study (n = 3). | (1) CT scan (2) Micro-CT | (1) HES (2) Movat pentachrome | NS | SMD regeneration was successful with the custom vascularized bone construct. Implants were well osseointegrated and vascularized after only 3 months of implantation and were completely entrapped in lamellar bone after 12 months; a healthy, yellow bone marrow filled the remaining space. |
| Wu et al. 2006 [29] | Group 1: MSC/βTCP; Group 2: autologous bone; Group 3: βTCP. This study did not report the number of animals used in each group. | (1) CT scan | (1) H&E (2) Masson’s trichrome | NS | New bone grafts were successfully developed 12 weeks after implantation, restoring the continuity of the mandible. Histologically, newly formed bone could be observed on the surface and in the pores of β-TCP in the cell/scaffold group, whereas incomplete bone repair was found in the pure β-TCP group. |
| Yao et al. 2009 [33] | Group 1: βCP (n = 10); Group 2: βCP of 8 weeks after implantation in muscle (n = 10). | (1) 99mTc-MDP SPECT/CT | (1) H&E (2) Masson’s trichrome | (1) Compressive strength testing | The in vivo TE ceramic bone grafts were involved in the host’s bone metabolism and fused well with the host bone. It is feasible to construct a live bone graft with osteoinductive CaP ceramics in vivo, then repair a mandibular bone defect, and support a dental implant. |
| Yuan et al. 2007 [35] | Group 1: BMSCs/βTCP constructs (n = 6); Group 2: autograft group (n = 4); Group 3: βTCP (n = 6). | (1) CT scan | (1) H&E | (1) Bending system | The engineered bone with BMSCs/b-TCP achieved a satisfactory biomechanical property in terms of bending load strength, bending displacement, bending stress, and Young’s modulus, which was very close to those of the contralateral edentulous mandible and autograft bone. |
| Zeng et al. 2017 [31] | Group 1: Control (unfilled); Group 2: PTMC; Group 3: PTMC-TCPc; Group 4: PTMC-TCPx; Group 5: PTMC-βCP; Group 6: βTCP. This study did not report the number of animals used in each group. | (1) Micro-CT | (1) 1% methylene blue (2) 0.3% basic fuchsine | NS | Porous β-TCP scaffolds as a control led to a larger amount of newly formed bone in the defects than all other materials. Histology revealed abundant new bone formation in the defects filled with porous b-TCP scaffolds. New bone formation was limited in defects filled with PTMC scaffolds or different PTMC-CaP matrices. |
| Zhang et al. 2019 [30] | Group 1: HA (n = 3); Group 2: BCP CaP (n = 3). | (1) Radiographs | (1) Toluidine blue dye | (1) Pullout test | Enhanced bone formation in the BCP group compared to the HA group. The pullout force was greater in the BCP group. |
| Characteristics Studies | 3D Printed | Scaffold Size (mm) | Groups | Porosity % | Pore Size | Macroscopy |
|---|---|---|---|---|---|---|
| Bachtiar et al. 2016 [34] | No | NS | Group 1: HA/TCP with 70:30 ratio; Group 2: HA/TCP with 50:50 ratio; Group 3: HA/TCP/chitosan. This study did not report the number of animals used in each group (n = 6 in total). | NS | NS | NS |
| Cao et al. 2021 [26] | Yes | 5 mm × 5 mm × 5mm | Group 1: TCP (n = 3); Group 2: PLGA/TCP (n = 3); Group 3: rhBMP-2-coated TCP (n = 3); Group 4: rhBMP-2-coated PLGA/TCP (n = 3); Group 5: prefabricated rhBMP-2-coated TCP (n = 3). | PLGA/TCP scaffolds = 63.7% ± 4.0%; TCP scaffolds = 74.2% ± 2.2% | TCP scaffolds = 345 ± 10 μm; PLGA/TCP scaffolds = 365 ± 30 μm. Only the rhBMP2-TCP group was used for mandibular reconstruction | Rectangular |
| Henkel et al. 2006 [28] | No | NS | Group 1: CaP matrix, type I (60% HA; 40% -TCP) (n = 6); Group 2: CaP matrix, type II (100% HA) (n = 6); Group 3: HA (n = 6); Group 4: TCP (n = 6); Group 5: control group (n = 6). | CaP matrices = 60–80%; HA and TCP = NS | CaP matrices: macropores diameter = 0.1–1 mm | Granulate |
| Johnson et al. 2021 [27] | Yes | 30 mm in diameter | Group 1: HA/TCP (n = 3); Group 2: DPNCC + HA/TCP (n = 3); Group 3: BMA + HA/TCP (n = 3); Group 4: native bone (n = 3). | NS | 4 mm | The top surface of the designed scaffold was a circle with a diameter of 3.8 cm, and it tapered down to a bottom surface with a diameter of 3.5 cm. The bottom was concave and closely matched the curvature of the inner skull surface |
| Nokhbatolfoghahaei et al. 2022 [36] | Yes | 25 mm × 10 mm × 8 mm | Group 1: βTCP (n = 4); Group 2: βTCP/rhBMP2 (n = 4); Group 3: βTCP/MSCs (n = 4); Group 4: PCL/βTCP (n = 4); Group 5: PCL/βTCP/rhBMP2 (n = 4); Group 6: PCL/βTCP/MSCs (n = 4). | PCL/βTCP scaffolds = 50% porosity and 100% interconnectivity; βTCP scaffolds = 70.6% porosity and 100% interconnectivity | PCL/βTCP scaffolds = 500 μm; βTCP scaffolds = 191.9 ± 74.6 μm | Rectangular |
| Paré et al. 2022 [32] | Yes | 54.80 mm × 34.4 mm × 11.58 mm | Group 1: βCP (n = 6); Group 2: clinical control (n = 6); Group 3: cadaveric study (n = 3). | BCP scaffolds = 40% | The implant surface displayed a roughness at the microscale (1–10 μm), evaluated to be under 2 μm and approximately 10% s/s of submicronic pores | Disc |
| Wu et al. 2006 [29] | Yes | 30 mm × 8 mm × 20 mm | Group 1: MSC/βTCP; Group 2: autologous bone; Group 3: βTCP. This study did not report the number of animals used in each group. | βTCP scaffolds = 58% | βTCP scaffolds = 100–250 μm | Rectangular |
| Yao et al. 2009 [33] | No | 20 mm × 10 mm × 8 mm | Group 1: βCP (n = 10); Group 2: βCP of 8 weeks after implantation in muscle (n = 10). | BCP scaffolds = 60% | BCP scaffolds = 300–400 μm | Cylinder |
| Yuan et al. 2007 [35] | Yes | 30 mm × 15 mm × 10 mm | Group 1: BMSCs/βTCP constructs (n = 6); Group 2: autograft group (n = 4); Group 3: βTCP (n = 6). | BCP scaffolds = 70% | BCP scaffolds = 450 ± 750 μm | Cuboid |
| Zeng et al. 2017 [31] | Yes | 20 mm in diameter × 5 mm in height | Group 1: Control (unfilled); Group 2: PTMC; Group 3: PTMC-TCPc; Group 4: PTMC-TCPx; Group 5: PTMC-βCP; Group 6: βTCP. This study did not report the number of animals used in each group. | PTMC scaffold = 70%; PTMC-TCPc = 70%; PTMC-TCPx = 70%; PTMC-BCP composite = 70%; βTCP scaffold = 60% | PTMC scaffold = 200–435 μm; PTMC-TCPc = 200–435 μm; PTMC-TCPx = 200–435 μm; PTMC-BCP = 200–435 μm; b-TCP scaffold = 400–700 μm | Disc |
| Zhang et al. 2019 [30] | No | NS | Group 1: HA (n = 3); Group 2: BCP CaP (n = 3). | NS | HA and BCP CaP ceramics = 300–400 μm | Trapezoid |
| Studies | Groups | Biomechanical Testing | |||
|---|---|---|---|---|---|
| Stiffness | Energy Absorption | Toughness | Mechanical Strengths | ||
| Cao et al. 2021 [26] | Group 1: TCP (n = 3); Group 2: PLGA/TCP (n = 3); Group 3: rhBMP-2-coated TCP (n = 3); Group 4: rhBMP-2-coated PLGA/TCP (n = 3); Group 5: prefabricated rhBMP-2-coated TCP (n = 3). | No | No | No | Yes |
| Johnson et al. 2021 [27] | Group 1: HA/TCP (n = 3); Group 2: DPNCC + HA/TCP (n = 3); Group 3: BMA + HA/TCP (n = 3); Group 4: native bone (n = 3). | No | No | No | Yes |
| Yao et al. 2009 [33] | Group 1: βCP (n = 10); Group 2: βCP of 8 weeks after implantation in muscle (n = 10). | No | No | Yes | Yes |
| Yuan et al. 2007 [35] | Group 1: BMSCs/βTCP constructs (n = 6); Group 2: autograft group (n = 4); Group 3: βTCP (n = 6). | No | No | No | Yes |
| Zhang et al. 2019 [30] | Group 1: HA (n = 3); Group 2: BCP CaP (n = 3). | No | Yes | No | Yes |
| Studies | Methods | System Used | Main Findings |
|---|---|---|---|
| Cao et al. 2021 [26] | Uniaxial compression test on PLGA/TCP and TCP scaffolds, comparing the groups with and without BMP. All tests were conducted at a crosshead speed of 0.5 mm/min, and the compressive strength (MPa) was calculated by dividing the maximum applied load by the initial cross-sectional area of the sample. | NS | The PLGA/TCP scaffolds without BMP exhibited significantly lower strength (0.1 MPa) than those with BMP (0.7 MPa), indicating that adding BMP improves compressive strength. Furthermore, the S-TCP-BMP scaffolds exhibited significantly higher strength (57.6 MPa), indicating a positive effect on strength enhancement with the use of TCP-BMP. |
| Johnson et al. 2021 [27] | Compression and mechanical strength tests were performed on calvarial regenerated bone with DPNCCs and HA/TCP, comparing it with native bone. The test was conducted at three locations within the defect area (with or without bone regeneration) and at three locations on the surrounding native bone for each sample. The compressive arm moved at a rate of 0.2 cm/s. For each location, the arm’s force and the arm’s displacement were measured. The end-of-test criteria were as follows: (a) the force that needed to be applied exceeded the capacity of the system, (b) a 40% drop in force was observed, or (c) the moving arm met the stationary stage. | INSTRON 5944 Universal Testing Systems up to 2 kN (450 lbf) force capacity. | The compressive strength of the regenerated bone was equivalent to that of native bone, with the formation of bone of comparable quality. This suggests that the use of the HA/TCP scaffold was effective in bone regeneration, achieving biomechanical properties similar to native bone. |
| Yao et al. 2009 [33] | Compression and bending tests of Ca-P ceramics implanted in the muscle for 8 weeks. The same mechanical tests were also performed on the same BCP ceramics, which were not implanted in muscle as a control. The samples were explanted from the femoral muscles, and the surrounding soft tissues were carefully stripped. The sample was loaded to failure at a loading rate. Electrical resistance strain gages were mounted on two lateral surfaces to measure longitudinal elastic strain in compression. The loading rate was 1 mm/min. The three-point bending test was conducted at a crosshead speed of 0.5 mm/min. | Electric universal testing machine (AG-10 TA Type; Shimadzu, Japan). | The compressive and bending strength of the ceramics significantly increased after implantation in the muscle, indicating that the in vivo process promotes the improvement of mechanical properties. The Ca-P ceramic showed remarkable improvements due to the integration of organic and inorganic elements, resulting in higher toughness. |
| Yuan et al. 2007 [35] | Three-point bending test on repaired mandibles, comparing the groups with BMSCs/b-TCP and autograft. Load was applied in the occlusal-to-inferior direction to the midpoint of the unsupported length, approximately in the middle of the repaired defect. Deformation was measured using a strain gauge at a loading rate of 0.5 mm/min. The bending stress was calculated with the equation, bending stress ¼ 3LF = ð2WT2Þ, where L, F, W, and T are the test span (mm), the bending strength load (KN), the width (mm), and the thickness of the specimen (mm), respectively. Young’s modulus was achieved from the linear region of the stress–strain curve as the slope. | Hydraulic materials testing machine (Shimadzu AG-5KN, Kyoto, Japan) | The experimental group BMSCs/b-TCP exhibited bending biomechanical properties similar to normal mandibles and the autograft group, with bending load and bending stress results close to those of native bone. This suggests that the BMSCs/b-TCP scaffold may provide adequate biomechanical support for bone regeneration in segmental defects. |
| Zhang et al. 2019 [30] | Pullout and removal torque tests were performed to evaluate the mechanical fixation of the implants in BCP and HA scaffolds. The testing was performed using a compression speed of 1 mm/min. | Multifunction electronic meter (model M10 M200; Minfeng Trading Co. Ltd., Shanghai, China) and BSC30; Ningbo Yinbo Scientific Equipment, Ningbo, China. | The BCP group exhibited significantly stronger fixation (310.4 N) compared to the HA group (74.6 N), indicating that BCP offers superior mechanical strength and bone integration, which is crucial for implant stability. |
| Study | Animal Species | Defect Location | Main Findings | Clinical Implications and Correlations |
|---|---|---|---|---|
| Bachtiar et al. 2016 [34] | Rat | Calvaria | Active bone formation, vascularization, and residual inflammation; few biomaterial remnants. | Bone formation and resorption compatible with small critical defects; potential for cranial regeneration. |
| Cao et al. 2021 [26] | Rat | Mandible | TCP-BMP preserved 3D architecture and increased compressive strength (0.7 → 57.6 MPa). | BMP enhances bone regeneration and mechanical strength; mandible suitable for functional testing. |
| Henkel et al. 2006 [28] | Rabbit | Mandible | CaP matrix promoted resorption and superior bone formation compared to classic HA/TCP. | Customized matrices favor bone integration; mandible allows detailed functional evaluation. |
| Johnson et al. 2021 [27] | Rat | Calvaria | Stem cells with 3D scaffolds achieved bone strength and density similar to native bone. | Stem cells feasible for small-defect regeneration; craniofacial model relevant for bone quality assessment. |
| Nokhbatolfoghahaei et al. 2022 [36] | Rabbit | Mandible | βTCP + MSCs or BMP enhanced bone formation; preserved vascularization was crucial. | Highlights importance of biofunctionalization and vascular support in mandibular repair. |
| Paré et al. 2022 [32] | Minipig | Mandible | Good osseointegration and lamellar bone encapsulating implants after 12 months. | Translational model; closely simulates human mandibular regeneration. |
| Wu et al. 2006 [29] | Canine | Mandible | MSCs increased bone formation in βTCP pores; incomplete repair without MSCs. | Confirms the need for stem cells in large defects; mandible allows robust functional evaluation. |
| Yao et al. 2009 [33] | Canine | Mandible | Pre-muscle implantation created viable grafts, enhancing compressive strength and bending resistance. | Pre-formed structures are promising for functional mandibular repair. |
| Yuan et al. 2007 [35] | Canine | Mandible | BMSCs/βTCP achieved biomechanical properties similar to autologous bone. | Viable alternative to autologous grafts in mandibular reconstructions. |
| Zeng et al. 2017 [31] | Canine | Mandible | βTCP led to greater bone formation compared to PTMC or composites. | TCP alone is more effective than polymers for mandibular regeneration. |
| Zhang et al. 2019 [30] | Canine | Mandible | BCP showed higher fixation strength compared to HA. | BCP is more promising than pure HA for implant fixation in mandible. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baggio, A.M.P.; Sillmann, Y.M.; Eber, P.; Michallek, F.R.S.; Monteiro, J.L.G.C.; Bassi, A.P.F.; Guastaldi, F.P.S. How Does Ceramic-Based Scaffold Microarchitecture Impact Maxillofacial Bone Regeneration? A Systematic Review of Large Animal Models. Appl. Sci. 2025, 15, 6899. https://doi.org/10.3390/app15126899
Baggio AMP, Sillmann YM, Eber P, Michallek FRS, Monteiro JLGC, Bassi APF, Guastaldi FPS. How Does Ceramic-Based Scaffold Microarchitecture Impact Maxillofacial Bone Regeneration? A Systematic Review of Large Animal Models. Applied Sciences. 2025; 15(12):6899. https://doi.org/10.3390/app15126899
Chicago/Turabian StyleBaggio, Ana M. P., Yannick M. Sillmann, Pascal Eber, Felicia R. S. Michallek, Joao L. G. C. Monteiro, Ana P. F. Bassi, and Fernando P. S. Guastaldi. 2025. "How Does Ceramic-Based Scaffold Microarchitecture Impact Maxillofacial Bone Regeneration? A Systematic Review of Large Animal Models" Applied Sciences 15, no. 12: 6899. https://doi.org/10.3390/app15126899
APA StyleBaggio, A. M. P., Sillmann, Y. M., Eber, P., Michallek, F. R. S., Monteiro, J. L. G. C., Bassi, A. P. F., & Guastaldi, F. P. S. (2025). How Does Ceramic-Based Scaffold Microarchitecture Impact Maxillofacial Bone Regeneration? A Systematic Review of Large Animal Models. Applied Sciences, 15(12), 6899. https://doi.org/10.3390/app15126899









