The Key Role of Porphyromonas gingivalis in the Pathogenesis of Periodontitis Linked with Systemic Diseases
Abstract
1. Introduction of Periodontitis
1.1. Overview of Periodontitis and Its Prevalence
- Host Susceptibility: Genetic and immune system factors that predispose individuals to periodontal disease;
- Environmental Factors: External influences, such as environmental pollutants, diet, and microbiome composition, that may exacerbate or mitigate the progression of periodontal disease;
- Behavioral Factors: Lifestyle choices, including oral hygiene practices, smoking, and dietary habits, which significantly impact the onset and severity of periodontal conditions.
1.2. Importance of Oral Microbiome in Periodontal Disease
1.3. Introduction to P. gingivalis and Its Role as a Keystone Pathogen
2. Materials and Methods
Inclusion and Exclusion Criteria
3. Pathogenic Mechanisms of P. gingivalis
3.1. Microbiome Formation and Microbial Synergy
3.2. Immune Evasion Strategies
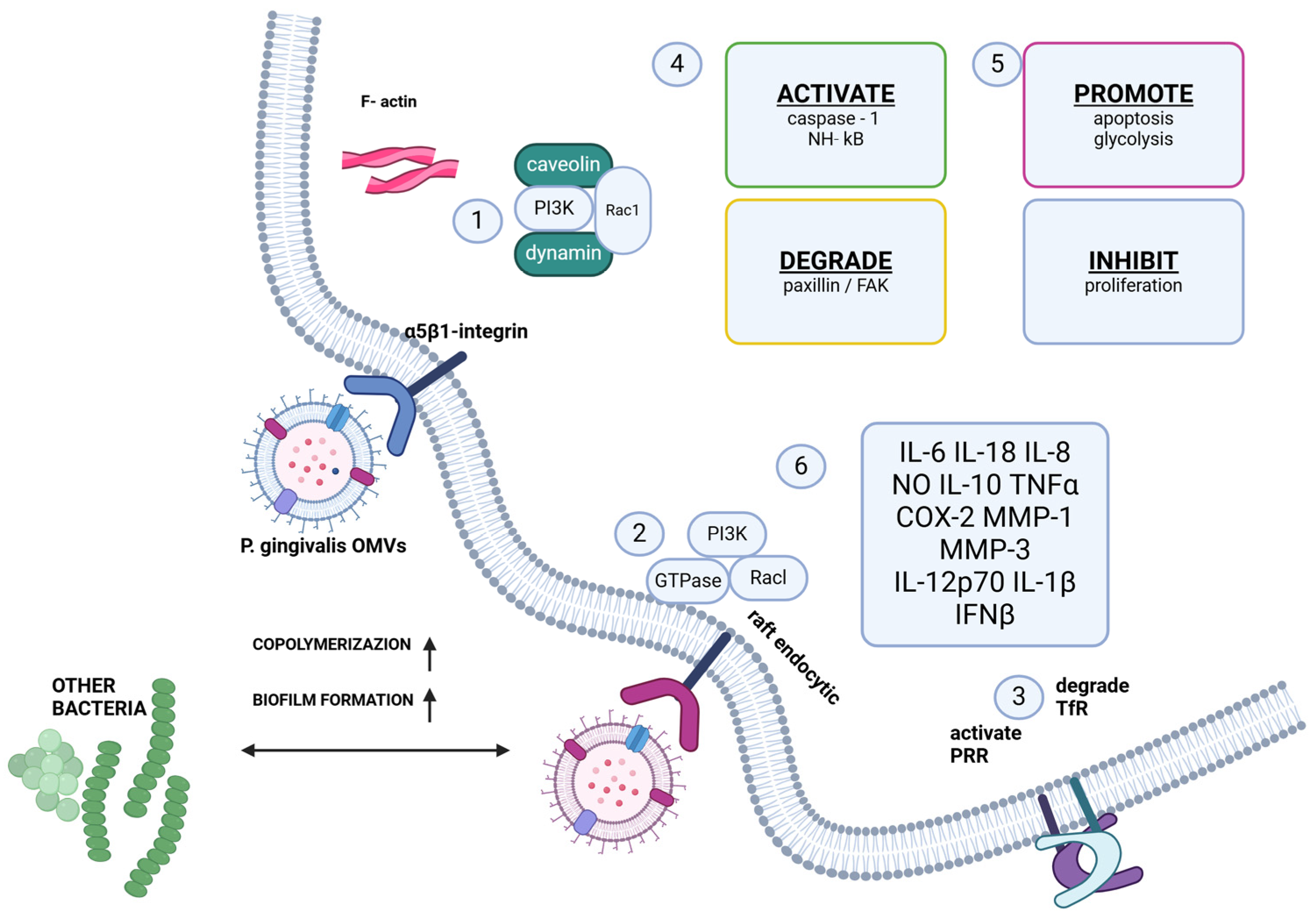
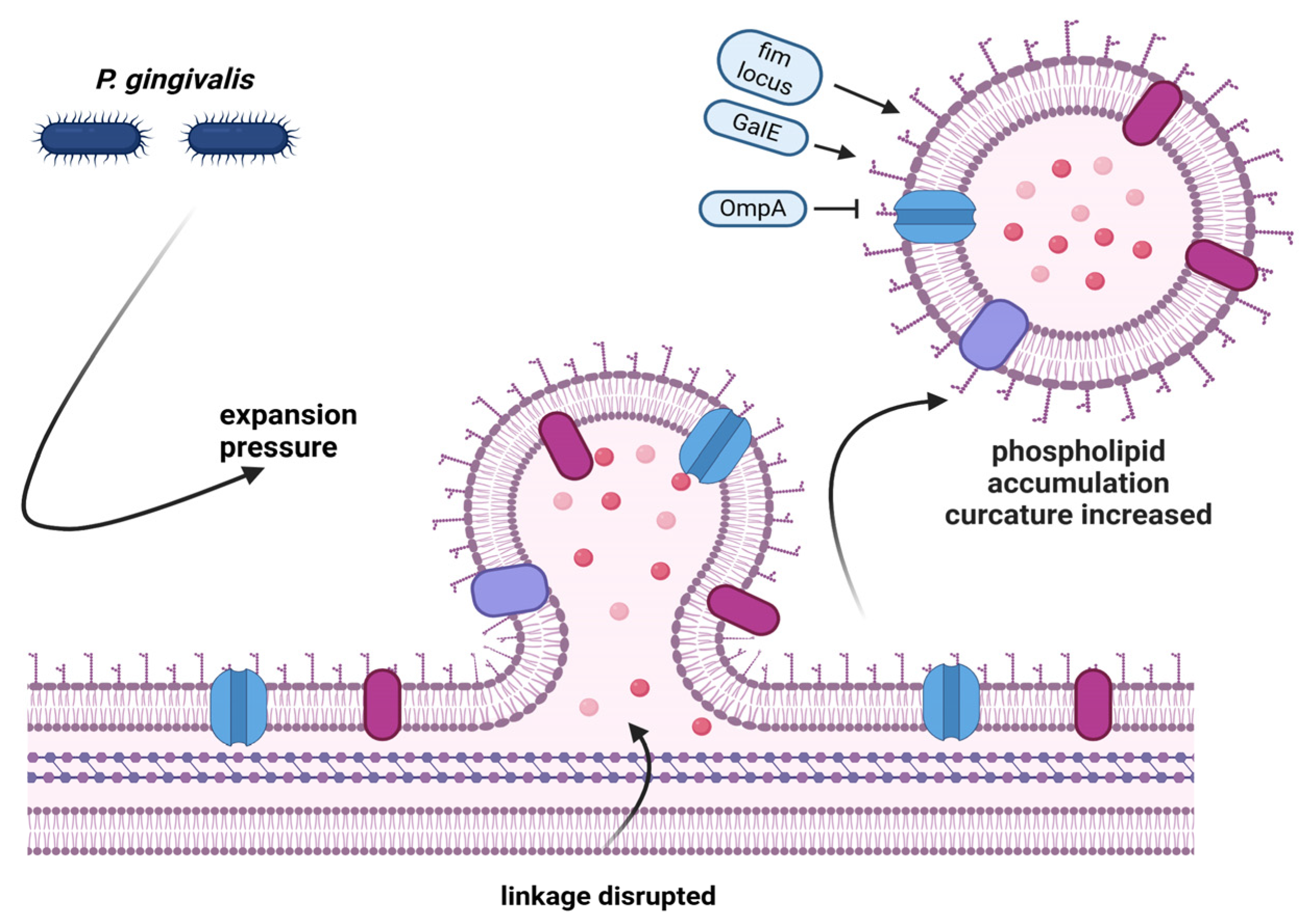
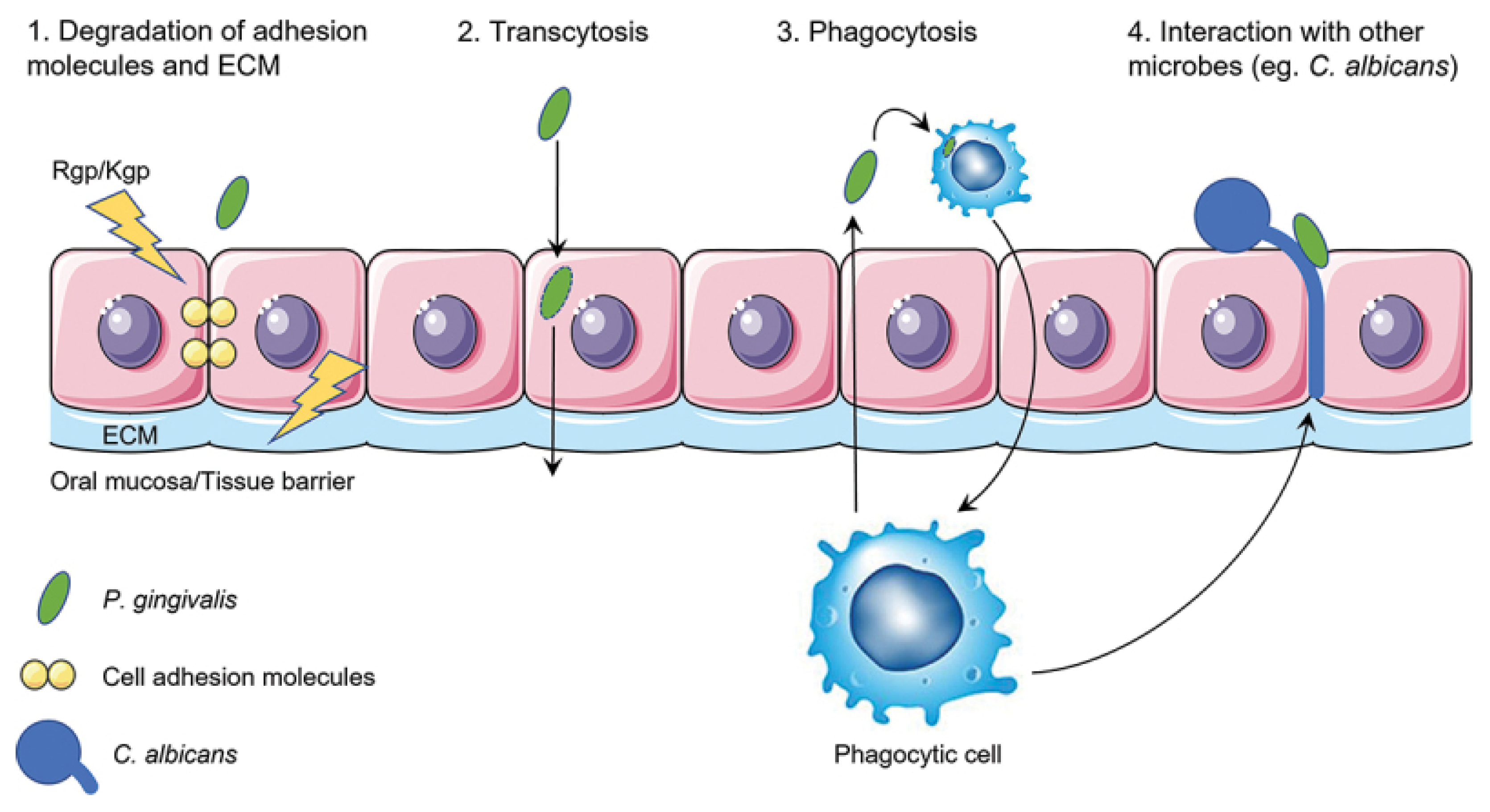
3.3. Virulence Factors and Tissue Invasion
3.4. Molecular Mimicry and Systemic Dissemination
4. P. gingivalis and Systemic Diseases
4.1. Cardiovascular Diseases
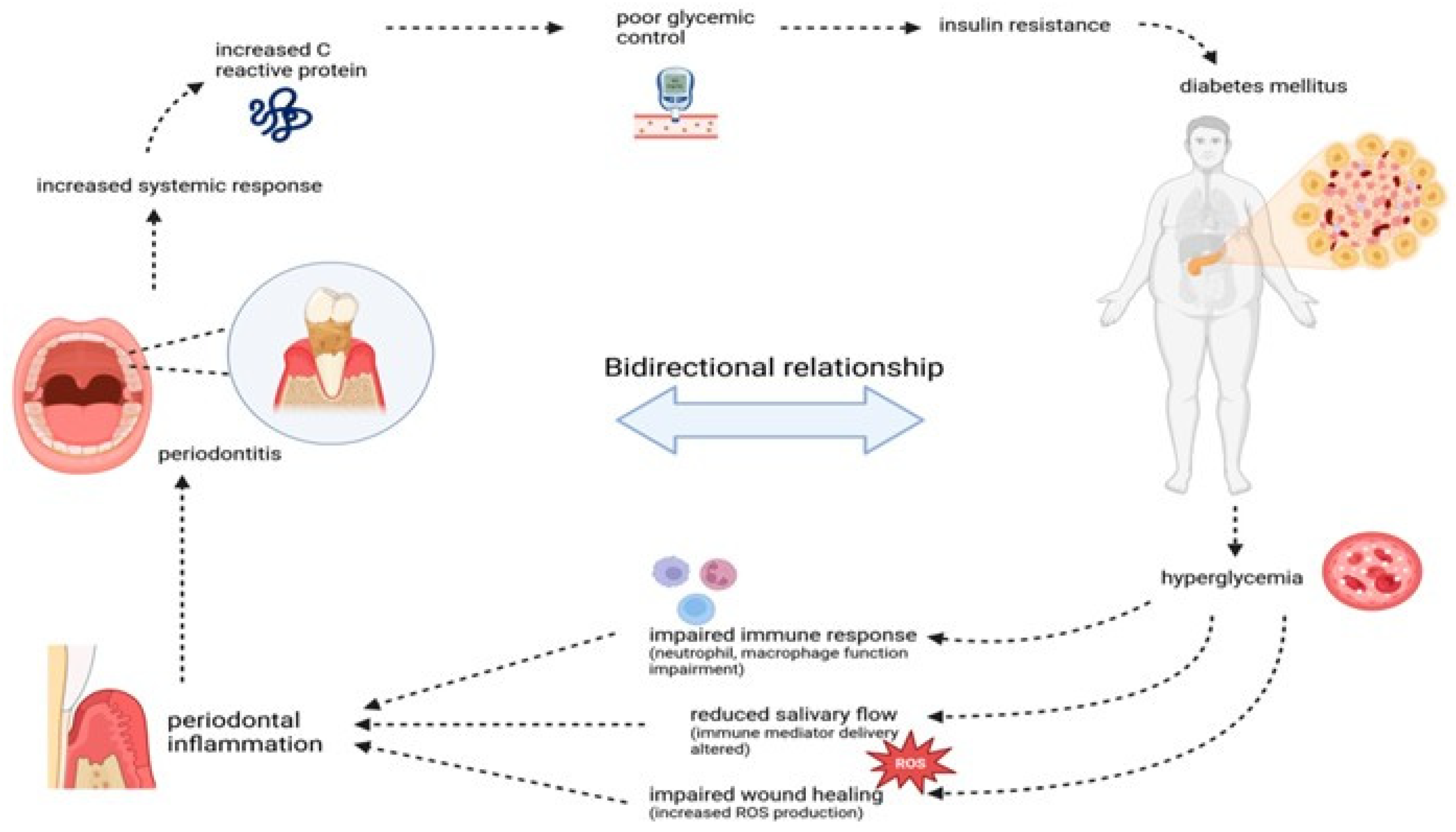
4.2. Diabetes Mellitus
- Increases the production of matrix metalloproteinases (MMPs);
- Promotes the secretion of prostaglandin E2 (PGE2);
- Activates osteoclasts and promotes bone resorption by inhibiting osteoblast differentiation;
- Induces apoptosis, preventing tissue regeneration and exacerbating periodontal destruction. Elevated levels of TNF-α, caused by inflammation, are associated with increased levels of HbA1c, apoptosis in pancreatic cells, and reduced insulin secretion, ultimately leading to insulin resistance [50].
4.3. Alzheimer’s Disease
4.4. Rheumatoid Arthritis
4.5. Other Diseases
4.5.1. Oncology
4.5.2. Biology and Immunology
5. Diagnostic and Therapeutic Implications
- Identification of patients suspected of having periodontitis;
- Confirmation of the diagnosis of periodontitis;
- Staging of the periodontitis case;
- Classification of the periodontitis case.
- Enzyme-linked immunosorbent assays (ELISAs);
- DNA probes;
- Real-time polymerase chain reactions (PCRs).
6. Future Directions and Research Gaps: Probiotics and Vaccines
- Vaccines derived from pure cultures of Streptococcus and other oral microorganisms;
- Autogenous vaccines, which are derived from the patient’s own microbial flora;
- Serial vaccines, such as the Van Cott vaccine, the Goldenberg vaccine, and the Inava Endocorps vaccine.
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45, S162–S170. [Google Scholar] [CrossRef] [PubMed]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions—Introduction and key changes from the 1999 classification. J. Clin. Periodontol. 2018, 45, S1–S8. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Berglundh, T.; Sculean, A.; Tonetti, M.S.; Consultants, T.E.W.P.A.M.; Lambert, N.L.F. Treatment of stage I–III periodontitis—The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47, 4–60. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Santonocito, S.; Lupi, S.M.; Polizzi, A.; Sclafani, R.; Patini, R.; Marchetti, E.; Brzozowski, T. Periodontal Health and Disease in the Context of Systemic Diseases. Mediat. Inflamm. 2023, 2023, 9720947. [Google Scholar] [CrossRef]
- Kassebaum, N.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.; Marcenes, W. Global Burden of Severe Periodontitis in 1990–2010: A Systematic Review and Meta-regression. J. Dent. Res. 2014, 93, 1045–1053. [Google Scholar] [CrossRef]
- Kwon, T.; Lamster, I.B.; Levin, L. Current Concepts in the Management of Periodontitis. Int. Dent. J. 2021, 71, 462–476. [Google Scholar] [CrossRef]
- Nazir, M.A. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int. J. Health Sci. 2025, 11, 72. [Google Scholar]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L., Jr. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef]
- Chen, W.A.; Dou, Y.; Fletcher, H.M.; Boskovic, D.S. Local and Systemic Effects of Porphyromonas gingivalis Infection. Microorganisms 2023, 11, 470. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D. Periodontal microbial ecology. Periodontol. 2000 2005, 38, 135–187. [Google Scholar] [CrossRef]
- Yilmaz, Ö. The chronicles of Porphyromonas gingivalis: The microbium, the human oral epithelium and their interplay. Microbiology 2008, 154, 2897–2903. [Google Scholar] [CrossRef] [PubMed]
- Bodet, C.; Chandad, F.; Grenier, D. Pathogenic potential of Porphyromonas gingivalis, Treponema denticola and Tannerella forsythia, the red bacterial complex associated with periodontitis. Pathol. Biol. 2007, 55, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Mysak, J.; Podzimek, S.; Sommerova, P.; Lyuya-Mi, Y.; Bartova, J.; Janatova, T.; Prochazkova, J.; Duskova, J. Porphyromonas gingivalis: Major Periodontopathic Pathogen Overview. J. Immunol. Res. 2014, 2014, 476068. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, F.; Murakami, Y.; Nishikawa, K.; Hasegawa, Y.; Kawaminami, S. Surface components of Porphyromonas gingivalis. J. Periodontal Res. 2009, 44, 1–12. [Google Scholar] [CrossRef]
- Antonyuk, S.V.; Siemińska, K.; Śmiga, M.; Strange, R.W.; Wagner, M.; Barnett, K.J.; Olczak, T. Bacteroides fragilis expresses three proteins similar to Porphyromonas gingivalis HmuY: Hemophore-like proteins differentially evolved to participate in heme acquisition in oral and gut microbiomes. FASEB J. 2023, 37, e22981. [Google Scholar] [CrossRef]
- Marshall, R.I. Gingival defensins: Linking the innate and adaptive immune responses to dental plaque. Periodontol. 2000 2004, 35, 14–20. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Tribble, G.D.; Baker, H.V.; Mans, J.J.; Handfield, M.; Lamont, R.J. Role of Porphyromonas gingivalis SerB in Gingival Epithelial Cell Cytoskeletal Remodeling and Cytokine Production. Infect. Immun. 2008, 76, 2420–2427. [Google Scholar] [CrossRef]
- Nisapakultorn, K.; Ross, K.F.; Herzberg, M.C.; Tuomanen, E.I. Calprotectin Expression In Vitro by Oral Epithelial Cells Confers Resistance to Infection by Porphyromonas gingivalis. Infect. Immun. 2001, 69, 4242–4247. [Google Scholar] [CrossRef]
- Weinberg, A.; Krisanaprakornkit, S.; Dale, B. Epithelial Antimicrobial Peptides: Review and Significance for Oral Applications. Crit. Rev. Oral Biol. Med. 1998, 9, 399–414. [Google Scholar] [CrossRef]
- Curtis, M.; Aduse-Opoku, J.; Rangarajan, M. Cysteine Proteases of Porphyromonas gingivalis. Crit. Rev. Oral Biol. Med. 2001, 12, 192–216. [Google Scholar] [CrossRef]
- Bao, K.; Belibasakis, G.N.; Thurnheer, T.; Aduse-Opoku, J.; Curtis, M.A.; Bostanci, N. Role of Porphyromonas gingivalis gingipains in multi-species biofilm formation. BMC Microbiol. 2014, 14, 258. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Krauss, J.L.; Domon, H.; McIntosh, M.L.; Hosur, K.B.; Qu, H.; Li, F.; Tzekou, A.; Lambris, J.D.; Hajishengallis, G. The C5a Receptor Impairs IL-12–Dependent Clearance of Porphyromonas gingivalis and Is Required for Induction of Periodontal Bone Loss. J. Immunol. 2011, 186, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Ikegami, A.; Kuramitsu, H.K. Synergistic biofilm formation by Treponema denticola and Porphyromonas gingivalis. FEMS Microbiol. Lett. 2005, 250, 271–277. [Google Scholar] [CrossRef]
- Lamont, R.J.; Jenkinson, H.F. Subgingival colonization by Porphyromonas gingivalis. Oral Microbiol. Immunol. 2000, 15, 341–349. [Google Scholar] [CrossRef]
- Medzhitov, R. Recognition of microorganisms and activation of the immune response. Nature 2007, 449, 819–826. [Google Scholar] [CrossRef]
- Darveau, R.P. Periodontitis: A polymicrobial disruption of host homeostasis. Nat. Rev. Microbiol. 2010, 8, 481–490. [Google Scholar] [CrossRef]
- Hajishengallis, G. Immune Evasion Strategies of Porphyromonas gingivalis. J. Oral Biosci. 2011, 53, 233–240. [Google Scholar] [CrossRef]
- Denis, F.K.P. P. gingivalis interactions with epithelial cells. Front. Biosci. 2008, 13, 966–984. [Google Scholar] [CrossRef]
- Bostanci, N.; Belibasakis, G.N. Porphyromonas gingivalis: An invasive and evasive opportunistic oral pathogen. FEMS Microbiol. Lett. 2012, 333, 1–9. [Google Scholar] [CrossRef]
- Bélanger, M.; Rodrigues, P.H.; Dunn, W.A., Jr.; Progulske-Fox, A. Autophagy: A Highway for Porphyromonas gingivalis in Endothelial Cells. Autophagy 2006, 2, 165–170. [Google Scholar] [CrossRef]
- Clais, S.; Boulet, G.; Kerstens, M.; Horemans, T.; Teughels, W.; Quirynen, M.; Lanckacker, E.; De Meester, I.; Lambeir, A.-M.; Delputte, P.; et al. Importance of biofilm formation and dipeptidyl peptidase IV for the pathogenicity of clinical Porphyromonas gingivalis isolates. Pathog. Dis. 2014, 70, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Bramanti, T.E.; Holt, S.C. Roles of porphyrins and host iron transport proteins in regulation of growth of Porphyromonas gingivalis W50. J. Bacteriol. 1991, 173, 7330–7339. [Google Scholar] [CrossRef] [PubMed]
- Genco, C.A.; Odusanya, B.M.; Brown, G. Binding and accumulation of hemin in Porphyromonas gingivalis are induced by hemin. Infect. Immun. 1994, 62, 2885–2892. [Google Scholar] [CrossRef]
- Pérez-Cruz, C.; Delgado, L.; López-Iglesias, C.; Mercade, E.; Rudel, T. Outer-Inner Membrane Vesicles Naturally Secreted by Gram-Negative Pathogenic Bacteria. PLoS ONE 2015, 10, e0116896. [Google Scholar] [CrossRef]
- Ellis, T.N.; Kuehn, M.J. Virulence and Immunomodulatory Roles of Bacterial Outer Membrane Vesicles. Microbiol. Mol. Biol. Rev. 2010, 74, 81–94. [Google Scholar] [CrossRef]
- Carrizales-Sepúlveda, E.F.; Ordaz-Farías, A.; Vera-Pineda, R.; Flores-Ramírez, R. Periodontal Disease, Systemic Inflammation and the Risk of Cardiovascular Disease. Heart Lung Circ. 2018, 27, 1327–1334. [Google Scholar] [CrossRef]
- Bahekar, A.A.; Singh, S.; Saha, S.; Molnar, J.; Arora, R. The prevalence and incidence of coronary heart disease is significantly increased in periodontitis: A meta-analysis. Am. Heart J. 2007, 154, 830–837. [Google Scholar] [CrossRef]
- Leng, Y.; Hu, Q.; Ling, Q.; Yao, X.; Liu, M.; Chen, J.; Yan, Z.; Dai, Q. Periodontal disease is associated with the risk of cardiovascular disease independent of sex: A meta-analysis. Front. Cardiovasc. Med. 2023, 10, 1114927. [Google Scholar] [CrossRef]
- Geerts, S.O.; Nys, M.; De Mol, P.; Charpentier, J.; Albert, A.; Legrand, V.; Rompen, E.H. Systemic Release of Endotoxins Induced by Gentle Mastication: Association with Periodontitis Severity. J. Periodontol. 2002, 73, 73–78. [Google Scholar] [CrossRef]
- Figuero, E.; Sánchez-Beltrán, M.; Cuesta-Frechoso, S.; Tejerina, J.M.; del Castro, J.A.; Gutiérrez, J.M.; Herrera, D.; Sanz, M. Detection of Periodontal Bacteria in Atheromatous Plaque by Nested Polymerase Chain Reaction. J. Periodontol. 2011, 82, 1469–1477. [Google Scholar] [CrossRef]
- Szulc, M.; Kustrzycki, W.; Janczak, D.; Michalowska, D.; Baczynska, D.; Radwan-Oczko, M. Presence of Periodontopathic Bacteria DNA in Atheromatous Plaques from Coronary and Carotid Arteries. BioMed Res. Int. 2015, 2015, 825397. [Google Scholar] [CrossRef] [PubMed]
- Salhi, L.; Rompen, E.; Sakalihasan, N.; Laleman, I.; Teughels, W.; Michel, J.-B.; Lambert, F. Can Periodontitis Influence the Progression of Abdominal Aortic Aneurysm? A Systematic Review. Angiology 2018, 70, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Persson, G.R.; Persson, R.E. Cardiovascular disease and periodontitis: An update on the associations and risk. J. Clin. Periodontol. 2008, 35, 362–379. [Google Scholar] [CrossRef]
- Pink, C.; Holtfreter, B.; Völzke, H.; Nauck, M.; Dörr, M.; Kocher, T. Periodontitis and systemic inflammation as independent and interacting risk factors for mortality: Evidence from a prospective cohort study. BMC Med. 2023, 21, 430. [Google Scholar] [CrossRef]
- Winning, L.; Patterson, C.C.; Neville, C.E.; Kee, F.; Linden, G.J. Periodontitis and incident type 2 diabetes: A prospective cohort study. J. Clin. Periodontol. 2017, 44, 266–274. [Google Scholar] [CrossRef]
- Ranbhise, J.S.; Ju, S.; Singh, M.K.; Han, S.; Akter, S.; Ha, J.; Choe, W.; Kim, S.S.; Kang, I. Chronic Inflammation and Glycemic Control: Exploring the Bidirectional Link Between Periodontitis and Diabetes. Dent. J. 2025, 13, 100. [Google Scholar] [CrossRef]
- Mirnic, J.; Djuric, M.; Brkic, S.; Gusic, I.; Stojilkovic, M.; Tadic, A.; Veljovic, T. Pathogenic Mechanisms That May Link Periodontal Disease and Type 2 Diabetes Mellitus—The Role of Oxidative Stress. Int. J. Mol. Sci. 2024, 25, 9806. [Google Scholar] [CrossRef]
- Blasco-Baque, V.; Garidou, L.; Pomié, C.; Escoula, Q.; Loubieres, P.; Le Gall-David, S.; Lemaitre, M.; Nicolas, S.; Klopp, P.; Waget, A.; et al. Periodontitis induced by Porphyromonas gingivalis drives periodontal microbiota dysbiosis and insulin resistance via an impaired adaptive immune response. Gut 2016, 66, 872–885. [Google Scholar] [CrossRef]
- Sahota, J.; Bakshi, D.; Kaur, G.; Singh, D.; Thakur, A.; Grover, S. Estimation of Plasma Levels of Tumor Necrosis Factor-α, Interleukin-4 and 6 in Patients with Chronic Periodontitis and Type II Diabetes Mellitus. J. Contemp. Dent. Pract. 2018, 19, 166–169. [Google Scholar] [CrossRef]
- Choubaya, C.; Chahine, N.; Aoun, G.; Anil, S.; Zalloua, P.; Salameh, Z. Expression of Inflammatory Mediators in Periodontitis Over Established Diabetes: An Experimental Study in Rats. Med. Arch. 2021, 75, 436–443. [Google Scholar] [CrossRef]
- Zheng, S.; Yu, S.; Fan, X.; Zhang, Y.; Sun, Y.; Lin, L.; Wang, H.; Pan, Y.; Li, C. Porphyromonas gingivalis survival skills: Immune evasion. J. Periodontal Res. 2021, 56, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sun, X.; Yang, Y.; Qie, Y. Insight of the interrelationship and association mechanism between periodontitis and diabetes mellitus. Regen. Ther. 2024, 26, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Zhang, X.; Chen, R.; Li, Y.; Yang, Y.; Deng, K.; Cai, Z.; Lai, H.; Shi, J. Impact of periodontitis on type 2 diabetes: A bioinformatic analysis. BMC Oral Health 2024, 24, 635. [Google Scholar] [CrossRef]
- Zhao, M.; Xie, Y.; Gao, W.; Li, C.; Ye, Q.; Li, Y. Diabetes mellitus promotes susceptibility to periodontitis—Novel insight into the molecular mechanisms. Front. Endocrinol. 2023, 14, 1192625. [Google Scholar] [CrossRef]
- Brookmeyer, R.; Corrada, M.M.; Curriero, F.C.; Kawas, C. Survival Following a Diagnosis of Alzheimer Disease. Arch. Neurol. 2002, 59, 1764–1767. [Google Scholar] [CrossRef]
- Brookmeyer, R.; Johnson, E.; Ziegler-Graham, K.; Arrighi, H.M. Forecasting the global burden of Alzheimer’s disease. Alzheimer’s Dement. 2007, 3, 186–191. [Google Scholar] [CrossRef]
- Chen, M.X.; Zhong, Y.J.; Dong, Q.Q.; Wong, H.M.; Wen, Y.F. Global, regional, and national burden of severe periodontitis, 1990–2019: An analysis of the Global Burden of Disease Study 2019. J. Clin. Periodontol. 2021, 48, 1165–1188. [Google Scholar] [CrossRef]
- Liccardo, D.; Marzano, F.; Carraturo, F.; Guida, M.; Femminella, G.D.; Bencivenga, L.; Agrimi, J.; Addonizio, A.; Melino, I.; Valletta, A.; et al. Potential Bidirectional Relationship Between Periodontitis and Alzheimer’s Disease. Front. Physiol. 2020, 11, 683. [Google Scholar] [CrossRef]
- Villar, A.; Paladini, S.; Cossatis, J. Periodontal Disease and Alzheimer’s: Insights from a Systematic Literature Network Analysis. J. Prev. Alzheimer’s Dis. 2024, 11, 1148–1165. [Google Scholar] [CrossRef]
- Zeng, F.; Liu, Y.; Huang, W.; Qing, H.; Kadowaki, T.; Kashiwazaki, H.; Ni, J.; Wu, Z. Receptor for advanced glycation end products up-regulation in cerebral endothelial cells mediates cerebrovascular-related amyloid β accumulation after Porphyromonas gingivalis infection. J. Neurochem. 2020, 158, 724–736. [Google Scholar] [CrossRef]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The amyloid-β pathway in Alzheimer’s disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.S.; Landry, K.S. Oral Dysbiosis and Neurodegenerative Diseases: Correlations and Potential Causations. Microorganisms 2022, 10, 1326. [Google Scholar] [CrossRef] [PubMed]
- Darveau, R.P.; Hajishengallis, G.; Curtis, M.A. Porphyromonas gingivalis as a Potential Community Activist for Disease. J. Dent. Res. 2012, 91, 816–820. [Google Scholar] [CrossRef]
- Dominy, S.S.; Lynch, C.; Ermini, F.; Benedyk, M.; Marczyk, A.; Konradi, A.; Nguyen, M.; Haditsch, U.; Raha, D.; Griffin, C.; et al. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 2019, 5, eaau3333. [Google Scholar] [CrossRef]
- Singhrao, S.K. Porphyromonas gingivalis-lipopolysaccharide and amyloid-β: A dangerous liaison for impairing memory? J. Alzheimers Dis. 2025. [Google Scholar] [CrossRef]
- Hao, X.; Li, Z.; Li, W.; Katz, J.; Michalek, S.M.; Barnum, S.R.; Pozzo-Miller, L.; Saito, T.; Saido, T.C.; Wang, Q.; et al. Periodontal Infection Aggravates C1q-Mediated Microglial Activation and Synapse Pruning in Alzheimer’s Mice. Front. Immunol. 2022, 13, 816640. [Google Scholar] [CrossRef]
- Brahmbhatt, Y.; Alqaderi, H.; Chinipardaz, Z. Association Between Severe Periodontitis and Cognitive Decline in Older Adults. Life 2024, 14, 1589. [Google Scholar] [CrossRef]
- de Molon, R.S.; Rossac, C., Jr.; Thurlings, R.M.; Cirelli, J.A.; Koenders, M.I. Linkage of Periodontitis and Rheumatoid Arthritis: Current Evidence and Potential Biological Interactions. Int. J. Mol. Sci. 2019, 20, 4541. [Google Scholar] [CrossRef]
- Laugisch, O.; Wong, A.; Sroka, A.; Kantyka, T.; Koziel, J.; Neuhaus, K.; Sculean, A.; Venables, P.J.; Potempa, J.; Möller, B.; et al. Citrullination in the periodontium—A possible link between periodontitis and rheumatoid arthritis. Clin. Oral Investig. 2015, 20, 675–683. [Google Scholar] [CrossRef]
- Routsias, J.G.; Goules, J.D.; Goules, A.; Charalampakis, G.; Pikazis, D. Autopathogenic correlation of periodontitis and rheumatoid arthritis. Rheumatology 2011, 50, 1189–1193. [Google Scholar] [CrossRef]
- Dalix, E.; Marotte, H. From a better knowledge of periodontal disease to Porphyromonas gingivalis target for rheumatoid arthritis disease activity. Jt. Bone Spine 2024, 92, 105822. [Google Scholar] [CrossRef] [PubMed]
- Mangat, P.; Wegner, N.; Venables, P.J.; Potempa, J. Bacterial and human peptidylarginine deiminases: Targets for inhibiting the autoimmune response in rheumatoid arthritis? Arthritis Res. Ther. 2010, 12, 209. [Google Scholar] [CrossRef] [PubMed]
- Sherina, N.; de Vries, C.; Kharlamova, N.; Sippl, N.; Jiang, X.; Brynedal, B.; Kindstedt, E.; Hansson, M.; Mathsson-Alm, L.; Israelsson, L.; et al. Antibodies to a Citrullinated Porphyromonas gingivalis Epitope Are Increased in Early Rheumatoid Arthritis, and Can Be Produced by Gingival Tissue B Cells: Implications for a Bacterial Origin in RA Etiology. Front. Immunol. 2022, 13, 804822. [Google Scholar] [CrossRef]
- Hitchon, C.A.; Chandad, F.; Ferucci, E.D.; Willemze, A.; Ioan-Facsinay, A.; van der Woude, D.; Markland, J.; Robinson, D.; Elias, B.; Newkirk, M.; et al. Antibodies to Porphyromonas gingivalis Are Associated with Anticitrullinated Protein Antibodies in Patients with Rheumatoid Arthritis and Their Relatives. J. Rheumatol. 2010, 37, 1105–1112. [Google Scholar] [CrossRef]
- Yu, X.; Mankia, K.; Do, T.; Meade, J. Oral Microbiome Dysbiosis and Citrullination in Rheumatoid Arthritis. Adv. Exp. Med. Biol. 2025, 1472, 185–199. [Google Scholar] [CrossRef]
- Dolcezza, S.; Flores-Fraile, J.; Lobo-Galindo, A.B.; Montiel-Company, J.M.; Zubizarreta-Macho, Á. Relationship Between Rheumatoid Arthritis and Periodontal Disease—Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 14, 10. [Google Scholar] [CrossRef]
- de Jongh, C.A.; de Vries, T.J.; Bikker, F.J.; Gibbs, S.; Krom, B.P. Mechanisms of Porphyromonas gingivalis to translocate over the oral mucosa and other tissue barriers. J. Oral Microbiol. 2023, 15, 2205291. [Google Scholar] [CrossRef]
- Murray, P.E.; Coffman, J.A.; Garcia-Godoy, F. Oral Pathogens’ Substantial Burden on Cancer, Cardiovascular Diseases, Alzheimer’s, Diabetes, and Other Systemic Diseases: A Public Health Crisis—A Comprehensive Review. Pathogens 2024, 13, 1084. [Google Scholar] [CrossRef]
- Gholizadeh, P.; Eslami, H.; Yousefi, M.; Asgharzadeh, M.; Kafil, H.S. Role of oral microbiome on oral cancers, a review. Biomed. Pharmacother. 2016, 84, 552–558. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, Y.; Shi, X.; Zhao, Y.; Tang, Y.; Liu, S.; Zhu, X. Porphyromonas gingivalis outer membrane vesicles augments proliferation and metastasis of oral squamous cell carcinoma cells. BMC Oral Health 2025, 25, 701. [Google Scholar] [CrossRef]
- Jia, G.; Zhi, A.; Lai, P.F.H.; Wang, G.; Xia, Y.; Xiong, Z.; Zhang, H.; Che, N.; Ai, L. The oral microbiota—A mechanistic role for systemic diseases. Br. Dent. J. 2018, 224, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Makkawi, H.; Hoch, S.; Burns, E.; Hosur, K.; Hajishengallis, G.; Kirschning, C.J.; Nussbaum, G. Porphyromonas gingivalis Stimulates TLR2-PI3K Signaling to Escape Immune Clearance and Induce Bone Resorption Independently of MyD88. Front. Cell. Infect. Microbiol. 2017, 7, 359. [Google Scholar] [CrossRef] [PubMed]
- Michaud, D.S. Role of bacterial infections in pancreatic cancer. Carcinogenesis 2013, 34, 2193–2197. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Zhu, H.; Mou, Q.; Wong, P.Y.; Lan, L.; Ng, C.W.K.; Lei, P.; Cheung, M.K.; Wang, D.; Wong, E.W.Y.; et al. Integrative analysis reveals associations between oral microbiota dysbiosis and host genetic and epigenetic aberrations in oral cavity squamous cell carcinoma. npj Biofilms Microbiomes 2024, 10, 39. [Google Scholar] [CrossRef]
- Xie, H. Biogenesis and Function of Porphyromonas Gingivalis Outer Membrane Vesicles. Futur. Microbiol. 2015, 10, 1517–1527. [Google Scholar] [CrossRef]
- Tiantian, M.; Xin, L. Promotion of Porphyromonas gingivalis to viral disease. West China J. Stomatol. 2016, 34, 425–428. [Google Scholar] [CrossRef]
- Grover, V.; Kapoor, A.; Malhotra, R.; Kaur, G. Porphyromonas gingivalis Antigenic Determinants—Potential Targets for the Vaccine Development against Periodontitis. Infect. Disord.-Drug Targets 2014, 14, 1–13. [Google Scholar] [CrossRef]
- Imai, K.; Ochiai, K. Role of histone modification on transcriptional regulation and HIV-1 gene expression: Possible mechanisms of periodontal diseases in AIDS progression. J. Oral Sci. 2011, 53, 1–13. [Google Scholar] [CrossRef]
- Fiorillo, L.; Cervino, G.; Laino, L.; D’amico, C.; Mauceri, R.; Tozum, T.F.; Gaeta, M.; Cicciù, M. Porphyromonas gingivalis, Periodontal and Systemic Implications: A Systematic Review. Dent. J. 2019, 7, 114. [Google Scholar] [CrossRef]
- Niemczyk, W.; Niemczyk, S.; Odrzywolska, O.; Doroz, P.; Hochuł, D.; Zawadzka, K. Application of i-PRF in dentistry. Wiadomosci Lek. 2024, 77, 2348–2352. [Google Scholar] [CrossRef]
- Niemczyk, W.; Janik, K.; Żurek, J.; Skaba, D.; Wiench, R. Platelet-Rich Plasma (PRP) and Injectable Platelet-Rich Fibrin (i-PRF) in the Non-Surgical Treatment of Periodontitis—A Systematic Review. Int. J. Mol. Sci. 2024, 25, 6319. [Google Scholar] [CrossRef] [PubMed]
- Polizzi, A.; Leanza, Y.; Belmonte, A.; Grippaudo, C.; Leonardi, R.; Isola, G. Impact of Hyaluronic Acid and Other Re-Epithelializing Agents in Periodontal Regeneration: A Molecular Perspective. Int. J. Mol. Sci. 2024, 25, 12347. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Lau, L.; Herrera, D.; Morillo, J.M.; Silva, A. Methods of detection of Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis and Tannerella forsythensis in periodontal microbiology, with special emphasis on advanced molecular techniques: A review. J. Clin. Periodontol. 2004, 31, 1034–1047. [Google Scholar] [CrossRef] [PubMed]
- Kuret, S.; Kalajzic, N.; Ruzdjak, M.; Grahovac, B.; Buselic, M.A.J.; Sardelić, S.; Delic, A.; Susak, L.; Sutlovic, D. Real-Time PCR Method as Diagnostic Tool for Detection of Periodontal Pathogens in Patients with Periodontitis. Int. J. Mol. Sci. 2024, 25, 5097. [Google Scholar] [CrossRef]
- Williams, N.T. Probiotics. Am. J. Health Syst. Pharm. 2010, 67, 449–458. [Google Scholar] [CrossRef]
- Walker, C.B.; Gordon, J.M.; Socransky, S.S. Antibiotic susceptibility testing of subgingival plaque samples. J. Clin. Periodontol. 1983, 10, 422–432. [Google Scholar] [CrossRef]
- Chandrasekaran, P.; Weiskirchen, S.; Weiskirchen, R. Effects of Probiotics on Gut Microbiota: An Overview. Int. J. Mol. Sci. 2024, 25, 6022. [Google Scholar] [CrossRef]
- Kudyar, N.; Dani, N.; Mahale, S. Periodontal vaccine: A dream or reality. J. Indian Soc. Periodontol. 2011, 15, 115–120. [Google Scholar] [CrossRef]
| Mechanism | Effector Molecules |
|---|---|
| Counteraction of oxidative damage; resistance to environmental oxidative stress and oxidative killing by phagocytes | Rubrerythrin (nonheme iron protein), alkyl hydroperoxide reductase, FeoB2 |
| Inherent resistance to complement-mediated lysis | LPS with anionic polysaccharide repeat units A-LPS |
| Hijacking complement regulatory proteins (C4b) | HrgpA |
| Inhibitor of complement activation through digestion of the central complement component C3 | Gingipains HrgpA RgpB |
| TLR4 evasion by expressing dephosphorylated and tetra-acylated Lipid A | Lipid A-1 deacylase, 4’-phosphatase, and deacylase |
| TLR4 antagonism by expressing monophosphorylated treta-acylated Lipid A | Lipid A 4’-phosphatase and deacylase (Lipid A 1-phosphatase suppressed by hemin) |
| Shedding and proteolysis of complement regulatory protein CD46 from oral epithelial cells | Kgp |
| Upregulation of negative regulators of TLR signaling (IRAK-M) in monocytes | LPS |
| Degradation of TLR coreceptors, cytokines, or antimicrobial peptides | Gingipains |
| Inhibition of phagocyte killing via instigation of C5aR-TLR2 crosstalk | HrgpA RgpB |
| Inhibition of phagocyte killing via instigation of CXCR4-TLR2 crosstalk | Fimbriae |
| Suppression of TLR-2-induced IL-12 via CR3 binding | Fimbriae |
| Promotion of intracellular survival via CR3 mediated entry | Fimbriae |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Messina, B.M.; Grippaudo, C.; Polizzi, A.; Blasi, A.; Isola, G. The Key Role of Porphyromonas gingivalis in the Pathogenesis of Periodontitis Linked with Systemic Diseases. Appl. Sci. 2025, 15, 6847. https://doi.org/10.3390/app15126847
Messina BM, Grippaudo C, Polizzi A, Blasi A, Isola G. The Key Role of Porphyromonas gingivalis in the Pathogenesis of Periodontitis Linked with Systemic Diseases. Applied Sciences. 2025; 15(12):6847. https://doi.org/10.3390/app15126847
Chicago/Turabian StyleMessina, Bianca Maria, Cristina Grippaudo, Alessandro Polizzi, Andrea Blasi, and Gaetano Isola. 2025. "The Key Role of Porphyromonas gingivalis in the Pathogenesis of Periodontitis Linked with Systemic Diseases" Applied Sciences 15, no. 12: 6847. https://doi.org/10.3390/app15126847
APA StyleMessina, B. M., Grippaudo, C., Polizzi, A., Blasi, A., & Isola, G. (2025). The Key Role of Porphyromonas gingivalis in the Pathogenesis of Periodontitis Linked with Systemic Diseases. Applied Sciences, 15(12), 6847. https://doi.org/10.3390/app15126847








