A Review of Innovative Medical Rehabilitation Systems with Scalable AI-Assisted Platforms for Sensor-Based Recovery Monitoring
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Medical Platforms for Rehabilitation
3.2. Role of IoT Sensors in Rehabilitation
| IoT Device | Primary Parameters | Specific Application | References |
|---|---|---|---|
| Signal-morphology Impedance Cardiography (thoracic surface electrodes) | Beat-to-beat stroke volume, cardiac output, HR | Predict responsiveness to exercise-based cardiac rehab | [26] |
| MotionSense™ triaxial IMU (accel-gyro-mag) worn on thigh and shank | Knee flexion/extension angles, gait spatiotemporal metrics | Objective monitoring after total-knee arthroplasty | [27] |
| Wearable Magnetic-IMU (MIMU) strapped to tibia | 3-D knee kinematics (flexion, varus/valgus, rotation) | Home-based knee rehab, validated vs. optoelectronic system | [28] |
| Wearable single-strap IMU on upper arm | Shoulder ROM, repetition quality | Home exercises for adhesive capsulitis (frozen-shoulder) | [29] |
| On-board IMU in 3D-printed hand exoskeleton + wireless MCU | Finger joint orientation and motion | Bilateral-mode hand exoskeleton control for stroke rehab | [31] |
| Conformal, stretchable, wireless epidermal sEMG array | Forearm muscle activity patterns | Hand-gesture recognition and stroke-hand functional training | [32] |
| EMG sensors | Gesture recognition, forearm muscle signals, mobile robot control | Restoring fine motor skills, interactive training for clinical and home use | [33] |
| Xbox One Kinect | Depth sensor-based pose estimation, joint position tracking | Stroke recovery, tracking upper limb movements | [34] |
| Wearable EMG smart sensors | Muscle activation signals (EMG data), movement response | Targeted stroke rehab via a closed-loop EMG system with Functional Electrical Stimulation (FES) and VR (Virtual Reality), used in hospital and home settings | [35] |
| Under-mattress ballistocardiography/pressure strip | Sleep stages, HR, movement counts | Overnight sleep monitoring during inpatient rehab | [36] |
| Wearable accelerometers, Bluetooth Low-Energy beacons | Activity levels, location tracking, time spent standing/lying down | Monitoring elderly patients in subacute rehabilitation, predicting hospital readmission outcomes | [37] |
| Microphone array + ambient sensor nodes | Speech acoustics, pronunciation metrics | Elderly speech-rehab learning assistance platform | [38] |
| Wireless Sensor Network (WSN) nodes integrating inertial and energy-monitoring chips | Limb kinematics + node energy status | Bilateral-mode hand exoskeleton control for stroke rehab | [42] |
3.3. AI and ML for Rehabilitation Systems
3.3.1. AI Algorithms and Machine Learning for Personalized Rehabilitation and Recovery Prediction
| Methods | Accuracy | Recall | Precision | F1-Score | Training/Validation | Dataset Composition | Provenance/Accessibility | Reference |
|---|---|---|---|---|---|---|---|---|
| Proposed FFO-BI-LSTM | 99.06 | 99.65 | 99 | 99.20 | Not reported | Only illustrative values are shown | Private/Institutional (Real-time VR rehabilitation sessions collected by the authors’ lab) | [44] |
| Deep Neural Network (DNN) | 92.50% | - | - | - | 58 patients with shoulder diseases (80/20) | Age: 60.5 ± 9.7 (37–82); gender: 46.6% M, 53.4% F; conditions: adhesive capsulitis, rotator cuff disease; severity: The Shoulder Pain and Disability Index (SPADI) 43.9 ± 22.7 (0–100). | Private/Institutional (IMU-sensor data recorded at Seoul Metropolitan Government Boramae Medical Center, South Korea) | [45] |
| Artificial Neural Network (ANN) | 86.4% | 71.2% | - | - | 128 subjects (one IRP per person); training (80.4%): 103 records/subjects; Validation/test (19.6 %): 25 records/subjects. | Age: 48.9 ± 10.5 yrs (range 37–82). Sex: 55.5% female (71 F/57 M); severity strata used in the model: mBI ≤ 30 (“severe”) and mBI ≤ 45 (“moderate”); SPMSQ 0–2, 3–4, ≥5. | Private/Institutional | [46] |
| Convolutional Neural Network (CNN) | 99.70% | 99.75% | 99.70% | 99.70% | 10 subjects (80/20) | Age: 29.3 ± 5.85 yrs (5M/5F); condition: all participants were healthy | UI-PRMD Dataset—Public, University of Idaho repository | [47] |
| Methods | Accuracy | Recall | Precision | F1-Score | Training/Validation | Dataset Composition | Provenance/Accessibility | Reference |
|---|---|---|---|---|---|---|---|---|
| Two-Layer Neural Network | 90.60% | - | - | - | 1280 tests (16 subjects, LOO-CV) | Ages 8–75, 56% male/44% female, post-abdominal surgery rehab | IEEE DataPort (public) | [48] |
| Logistic Regression (LR) | 100% | - | - | - | 22 patients (70/30) | Ages 50–75, knee osteoarthritis (TKA), KOOS-ADL functional score | Private/Institutional (GDPR, request from authors) | [49] |
| Linear Discriminant Analysis (LDA)—Support Vector Machine (SVM) | 86.98% | 67.44% | 67.84% | 67.48% | 645 samples (70/15/15 split), 43 participants | Healthy subjects, mixed gender, 5 gesture types | Public—GRABMyo dataset on PhysioNet | [50] |
| Genetic Algorithm-Based Clustering (GAClust) | 100% | - | - | - | 64 training/56 test | RTSA patients | Private/Institutional (Hygeia Group, Athens) | [51] |
| K-Nearest Neighbors (KNN) | 99% | 99% | 99% | 99% | 96 training/24 test | RTSA rehabilitation metrics | Private/Institutional | [52] |
| Adaptive Boosting (AdaBoost) | 84% | 91% | - | 65% | 16 training/4 test | Children, psychological subgroup classification | Private/Institutional (physiotherapy clinic, on request) | [53] |
3.3.2. Virtual Assistants and Chatbots Powered by LLMs for Patient Support
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| ML | Machine Learning |
| IoT | Internet of Things |
| LLM | Large Language Model |
| IMU | Inertial Measurement Unit |
| SLAM | Simultaneous Localization and Mapping |
| BCG | Ballistocardiography |
| HR | Heart Rate |
| VR | Virtual Reality |
| FES | Functional Electrical Stimulation |
| EMG | Electromyography |
| FFO | Firefly Optimization |
| DNN | Deep Neural Network |
| ANN | Artificial Neural Network |
| CNN | Convolutional Neural Network |
| LR | Logistic Regression |
| LDA | Linear Discriminant Analysis |
| SVM | Support Vector Machine |
| GAClust | Genetic Algorithm-Based Clustering |
| KNN | K-Nearest Neighbors |
| AdaBoost | Adaptive Boosting |
| RTSA | Reverse Total Shoulder Arthroplasty |
| PCA | Principal Component Analysis |
| SVD | Singular Value Decomposition |
| RF | Random forest |
| PROM | Patient-Reported Outcome Measure |
| CROM | Clinical-Reported Outcome Measurement |
| BI | Barthel Index |
| ICF | International Classification of Functioning |
| LSTM | Long Short-Term Memory |
| Bi-LSTM | Bidirectional Long Short-Term Memory |
| FTSTS | Five Time Sit To Stand |
| TUG | Timed Up and Go |
| UWB | Ultra-Wideband |
| NLP | Natural Language Processing |
| EFIC | Exception from Informed Consent |
References
- World Health Organization. Rehabilitation. Available online: https://www.who.int/ru/news-room/questions-and-answers/item/rehabilitation (accessed on 28 April 2025).
- Blas, H.S.S.; Mendes, A.S.; Encinas, F.G.; Silva, L.A.; González, G.V. A Multi-Agent System for Data Fusion Techniques Applied to the Internet of Things Enabling Physical Rehabilitation Monitoring. Appl. Sci. 2021, 11, 331. [Google Scholar] [CrossRef]
- Soumis, D.N.; Tselikas, N.D. A Web-Based Platform for Hand Rehabilitation Assessment. Big Data Cogn. Comput. 2025, 9, 52. [Google Scholar] [CrossRef]
- Rikhof, C.J.H.; Feenstra, Y.; Fleuren, J.F.M.; Buurke, J.H.; Prinsen, E.C.; Rietman, J.S.; Prange-Lasonder, G.B. Robot-Assisted Support Combined with Electrical Stimulation for the Lower Extremity in Stroke Patients: A Systematic Review. J. Neural Eng. 2024, 21, 021001. [Google Scholar] [CrossRef]
- Kairatova, G.K.; Khismetova, Z.A.; Smailova, D.S.; Serikova-Esengeldina, D.S.; Berikuly, D.; Akhmetova, K.M.; Shalgumbayeva, G.M. Assessment of Skills of Caregivers Providing Care for Stroke Patients in East Kazakhstan Region. Healthcare 2025, 13, 27. [Google Scholar] [CrossRef]
- Paladugu, P.; Kumar, R.; Ong, J.; Waisberg, E.; Sporn, K. Virtual Reality-Enhanced Rehabilitation for Improving Musculoskeletal Function and Recovery after Trauma. J. Orthop. Surg. Res. 2025, 20, 404. [Google Scholar] [CrossRef]
- Lee, P.; Chen, T.-B.; Lin, H.-Y.; Yeh, L.-R.; Liu, C.-H.; Chen, Y.-L. Integrating OpenPose and SVM for Quantitative Postural Analysis in Young Adults: A Temporal-Spatial Approach. Bioengineering 2024, 11, 548. [Google Scholar] [CrossRef]
- Sergazin, G.; Ozhiken, A.; Zhetenbayev, N.; Ozhikenov, K.; Tursunbayeva, G.; Nurgizat, Y.; Uzbekbayev, A.; Ayazbay, A.-A. Development of an Ankle Exoskeleton: Design, Modeling, and Testing. Sensors 2025, 25, 2020. [Google Scholar] [CrossRef] [PubMed]
- Amarelo, A.; Mota, M.; Amarelo, B.; Ferreira, M.C.; Fernandes, C.S. Technological Resources for Physical Rehabilitation in Cancer Patients Undergoing Chemotherapy: A Scoping Review. Cancers 2024, 16, 3949. [Google Scholar] [CrossRef]
- Ramírez-Sanz, J.M.; Garrido-Labrador, J.L.; Olivares-Gil, A.; García-Bustillo, Á.; Arnaiz-González, Á.; Díez-Pastor, J.-F.; Jahouh, M.; González-Santos, J.; González-Bernal, J.J.; Allende-Río, M. A Low-Cost System Using a Big-Data Deep-Learning Framework for Assessing Physical Telerehabilitation: A Proof-of-Concept. Healthcare 2023, 11, 507. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, J.; Gong, Z.; Lei, Y.; OuYang, X.; Chan, C.C.; Ruan, S. Wearable Physiological Monitoring System Based on Electrocardiography and Electromyography for Upper Limb Rehabilitation Training. Sensors 2020, 20, 4861. [Google Scholar] [CrossRef]
- Moghbelan, Y.; Esposito, A.; Zyrianoff, I.; Spaletta, G.; Borgo, S.; Masolo, C.; Ballarin, F.; Seidita, V.; Toni, R.; Barbaro, F.; et al. A Smart Motor Rehabilitation System Based on the Internet of Things and Humanoid Robotics. Appl. Sci. 2024, 14, 11489. [Google Scholar] [CrossRef]
- Faliagka, E.; Skarmintzos, V.; Panagiotou, C.; Syrimpeis, V.; Antonopoulos, C.P.; Voros, N. Leveraging Edge Computing ML Model Implementation and IoT Paradigm towards Reliable Postoperative Rehabilitation Monitoring. Electronics 2023, 12, 3375. [Google Scholar] [CrossRef]
- Ferraris, C.; Ronga, I.; Pratola, R.; Coppo, G.; Bosso, T.; Falco, S.; Amprimo, G.; Pettiti, G.; Lo Priore, S.; Priano, L.; et al. Usability of the REHOME Solution for the Telerehabilitation in Neurological Diseases: Preliminary Results on Motor and Cognitive Platforms. Sensors 2022, 22, 9467. [Google Scholar] [CrossRef]
- Vestito, L.; Ferraro, F.; Iaconi, G.; Genesio, G.; Bandini, F.; Mori, L.; Trompetto, C.; Dellepiane, S. STORMS: A Pilot Feasibility Study for Occupational TeleRehabilitation in Multiple Sclerosis. Sensors 2024, 24, 6470. [Google Scholar] [CrossRef] [PubMed]
- Neumann-Langen, M.V.; Ochs, B.G.; Lützner, J.; Postler, A.; Kirschberg, J.; Sehat, K.; Selig, M.; Grupp, T.M. Musculoskeletal Rehabilitation: New Perspectives in Postoperative Care Following Total Knee Arthroplasty Using an External Motion Sensor and a Smartphone Application for Remote Monitoring. J. Clin. Med. 2023, 12, 7163. [Google Scholar] [CrossRef]
- Porciuncula, F.; Roto, A.V.; Kumar, D.; Davis, I.; Roy, S.; Walsh, C.J.; Awad, L.N. Wearable Movement Sensors for Rehabilitation: A Focused Review of Technological and Clinical Advances. PMR 2018, 10 (Suppl. 2), S220–S232. [Google Scholar] [CrossRef]
- Galli, A.; Montree, R.J.H.; Que, S.; Peri, E.; Vullings, R. An Overview of the Sensors for Heart Rate Monitoring Used in Extramural Applications. Sensors 2022, 22, 4035. [Google Scholar] [CrossRef]
- Ianculescu, M.; Constantin, V.-Ș.; Gușatu, A.-M.; Petrache, M.-C.; Mihăescu, A.-G.; Bica, O.; Alexandru, A. Enhancing Connected Health Ecosystems Through IoT-Enabled Monitoring Technologies: A Case Study of the Monit4Healthy System. Sensors 2025, 25, 2292. [Google Scholar] [CrossRef]
- Moraes, J.L.; Rocha, M.X.; Vasconcelos, G.G.; Filho, J.E.V.; De Albuquerque, V.H.C.; Alexandria, A.R. Advances in Photopletysmography Signal Analysis for Biomedical Applications. Sensors 2018, 18, 1894. [Google Scholar] [CrossRef]
- Al-Ayyad, M.; Owida, H.A.; De Fazio, R.; Al-Naami, B.; Visconti, P. Electromyography Monitoring Systems in Rehabilitation: A Review of Clinical Applications, Wearable Devices and Signal Acquisition Methodologies. Electronics 2023, 12, 1520. [Google Scholar] [CrossRef]
- Tamura, T.; Huang, M. Unobtrusive Bed Monitor State of the Art. Sensors 2025, 25, 1879. [Google Scholar] [CrossRef]
- Habibzadeh, H.; Dinesh, K.; Rajabi Shishvan, O.; Boggio-Dandry, A.; Sharma, G.; Soyata, T. A Survey of Healthcare Internet of Things (HIoT): A Clinical Perspective. IEEE Internet Things J. 2020, 7, 53–71. [Google Scholar] [CrossRef]
- Čuljak, I.; Lučev Vasić, Ž.; Mihaldinec, H.; Džapo, H. Wireless Body Sensor Communication Systems Based on UWB and IBC Technologies: State-of-the-Art and Open Challenges. Sensors 2020, 20, 3587. [Google Scholar] [CrossRef]
- Hämäläinen, M.; Mucchi, L.; Caputo, S.; Biotti, L.; Ciani, L.; Marabissi, D.; Patrizi, G. Ultra-Wideband Radar-Based Indoor Activity Monitoring for Elderly Care. Sensors 2021, 21, 3158. [Google Scholar] [CrossRef]
- Bour, F.; Milstein, E.; Poty, A.; Garaud, Y.; Vitiello, D.; Leprêtre, P.M. Signal-Morphology Impedance Cardiography Is a Non-Invasive Tool for Predicting Responses to Exercise-Based Cardiac Rehabilitation. Int. J. Cardiol. 2024, 419, 132670. [Google Scholar] [CrossRef]
- Forsyth, L.; Ligeti, A.; Blyth, M.; Clarke, J.; Riches, P. Validity of Wearable Sensors for Total Knee Arthroplasty (TKA) Rehabilitation: A Study in Younger and Older Healthy Participants. Knee 2024, 51, 292–302. [Google Scholar] [CrossRef]
- Fezazi, M.E.; Achmamad, A.; Jbari, A.; Jilbab, A. A Convenient Approach for Knee Kinematics Assessment Using Wearable Inertial Sensors during Home-Based Rehabilitation: Validation with an Optoelectronic System. Sci. Afr. 2023, 20, e01676. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, C.; Tsai, M.; Chuang, T.; Lee, O.K. Wearable Motion Sensor Device to Facilitate Rehabilitation in Patients with Shoulder Adhesive Capsulitis: Pilot Study to Assess Feasibility. J. Med. Internet Res. 2020, 22, e17032. [Google Scholar] [CrossRef]
- Oshomoji, O.I.; Ajiroba, J.O.; Semudara, S.O.; Olayemi, M.A. Tele-Rehabilitation in African Rural Areas: A Systematic Review. Bull. Fac. Phys. Ther. 2024, 29, 1. [Google Scholar] [CrossRef]
- Triwiyanto, T.; Luthfiyah, S.; Pawana, I.P.A.; Ahmed, A.A.; Andrian, A. Bilateral Mode Exoskeleton for Hand Rehabilitation with Wireless Control Using 3D Printing Technology Based on IMU Sensor. HardwareX 2023, 14, e00432. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, S.; Yang, Y.; Liu, X.; Li, J.; Bao, B.; Liu, C.; Yang, H.; Guo, K.; Cheng, H. Conformal, Stretchable, Breathable, Wireless Epidermal Surface Electromyography Sensor System for Hand Gesture Recognition and Rehabilitation of Stroke Hand Function. Mater. Des. 2024, 243, 113029. [Google Scholar] [CrossRef]
- S, G.R.; Reka, S.S.; Keisuke, S.; Venugopal, P. EMG Controlled Mobile Robot Equipped with Gripper Mechanism for Fine Motor Skills Training in Rehabilitation. Results Eng. 2025, 26, 104563. [Google Scholar]
- Sarsfield, J.; Brown, D.; Sherkat, N.; Langensiepen, C.; Lewis, J.; Taheri, M.; McCollin, C.; Barnett, C.; Selwood, L.; Standen, P.; et al. Clinical Assessment of Depth Sensor Based Pose Estimation Algorithms for Technology Supervised Rehabilitation Applications. Int. J. Med. Inform. 2018, 121, 30–38. [Google Scholar] [CrossRef]
- Spinelli, G.; Ennes, K.P.; Chauvet, L.; Kilbride, C.; Jesutoye, M.; Harabari, V. A Wearable Device Employing Biomedical Sensors for Advanced Therapeutics: Enhancing Stroke Rehabilitation. Electronics 2025, 14, 1171. [Google Scholar] [CrossRef]
- Hendriks, M.M.S.; Van Lotringen, J.H.; Hulst, M.V.D.; Keijsers, N.L.W. Bed Sensor Technology for Objective Sleep Monitoring within the Clinical Rehabilitation Setting: Observational Feasibility Study. JMIR Mhealth Uhealth 2021, 9, e24339. [Google Scholar] [CrossRef]
- Ramezani, R.; Zhang, W.; Xie, Z.; Shen, J.; Elashoff, D.; Roberts, P.; Stanton, A.; Eslami, M.; Wenger, N.; Sarrafzadeh, M.; et al. A Combination of Indoor Localization and Wearable Sensor–Based Physical Activity Recognition to Assess Older Patients Undergoing Subacute Rehabilitation: Baseline Study Results. JMIR Mhealth Uhealth 2019, 7, e14090. [Google Scholar] [CrossRef]
- Lai, L.; Gaohua, Z. Intelligent Speech Elderly Rehabilitation Learning Assistance System Based on Deep Learning and Sensor Networks. Meas. Sens. 2024, 33, 101191. [Google Scholar] [CrossRef]
- Reddick, C.G.; Enriquez, R.; Harris, R.J.; Sharma, B. Determinants of Broadband Access and Affordability: An Analysis of a Community Survey on the Digital Divide. Cities 2020, 106, 102904. [Google Scholar] [CrossRef]
- Gong, E.; Wang, H.; Zhu, W.; Galea, G.; Xu, J.; Yan, L.L.; Shao, R. Bridging the Digital Divide to Promote Prevention and Control of Non-Communicable Diseases for All in China and Beyond. BMJ 2024, 387, e076768. [Google Scholar] [CrossRef]
- Maita, K.C.; Maniaci, M.J.; Haider, C.R.; Avila, F.R.; Torres-Guzman, R.A.; Borna, S.; Lunde, J.J.; Coffey, J.D.; Demaerschalk, B.M.; Forte, A.J. The Impact of Digital Health Solutions on Bridging the Health Care Gap in Rural Areas: A Scoping Review. Perm. J. 2024, 28, 130–143. [Google Scholar] [CrossRef]
- Ke, Y.; Xing, X. Construction of Rehabilitation Training Simulation Model Based on Energy Monitoring and Transmission by Wireless Sensor Networks. Alex. Eng. J. 2023, 85, 19–28. [Google Scholar] [CrossRef]
- Tahsin, T.; Mumenin, K.M.; Akter, H.; Tiang, J.J.; Nahid, A.-A. Machine Learning-Based Stroke Patient Rehabilitation Stage Classification Using Kinect Data. Appl. Sci. 2024, 14, 6700. [Google Scholar] [CrossRef]
- Alsheikhy, A.A.; Shawly, T.; Said, Y.E.; Ahmed, H.E.; Alazzam, M.B. Developing Machine Learning Models for Personalized Game-Based Stroke Rehabilitation Therapy in Virtual Reality. Alex. Eng. J. 2025, 121, 358–369. [Google Scholar] [CrossRef]
- Lee, K.; Kim, J.; Hong, H.; Jeong, Y.; Ryu, H.; Kim, H.; Lee, S. Deep Learning Model for Classifying Shoulder Pain Rehabilitation Exercises Using IMU Sensor. J. Neuroeng. Rehabil. 2024, 21, 42. [Google Scholar] [CrossRef]
- Santilli, G.; Mangone, M.; Agostini, F.; Paoloni, M.; Bernetti, A.; Diko, A.; Tognolo, L.; Coraci, D.; Vigevano, F.; Vetrano, M.; et al. Evaluation of Rehabilitation Outcomes in Patients with Chronic Neurological Health Conditions Using a Machine Learning Approach. J. Funct. Morphol. Kinesiol. 2024, 9, 176. [Google Scholar] [CrossRef]
- Zaher, M.; Ghoneim, A.S.; Abdelhamid, L.; Atia, A. Unlocking the Potential of RNN and CNN Models for Accurate Rehabilitation Exercise Classification on Multi-Datasets. Multimed. Tools Appl. 2024, 84, 1261–1301. [Google Scholar] [CrossRef]
- Procházka, A.; Martynek, D.; Vitujová, M.; Janáková, D.; Charvátová, H.; Vyšata, O. Mobile Accelerometer Applications in Core Muscle Rehabilitation and Pre-Operative Assessment. Sensors 2024, 24, 7330. [Google Scholar] [CrossRef]
- Emmerzaal, J.; De Brabandere, A.; Van Der Straaten, R.; Bellemans, J.; De Baets, L.; Davis, J.; Jonkers, I.; Timmermans, A.; Vanwanseele, B. Can the Output of a Learned Classification Model Monitor a Person’s Functional Recovery Status Post-Total Knee Arthroplasty? Sensors 2022, 22, 3698. [Google Scholar] [CrossRef]
- Kok, C.L.; Ho, C.K.; Tan, F.K.; Koh, Y.Y. Machine Learning-Based Feature Extraction and Classification of EMG Signals for Intuitive Prosthetic Control. Appl. Sci. 2024, 14, 5784. [Google Scholar] [CrossRef]
- Vrouva, S.; Koumantakis, G.A.; Sopidou, V.; Tatsios, P.I.; Raptis, C.; Adamopoulos, A. Comparison of Machine Learning Algorithms and Hybrid Computational Intelligence Algorithms for Rehabilitation Classification and Prognosis in Reverse Total Shoulder Arthroplasty. Bioengineering 2025, 12, 150. [Google Scholar] [CrossRef]
- Hussain, A.N.; Abboud, S.A.; Jumaa, B.A.B.; Abdullah, M.N. Impact of Feature Reduction Techniques on Classification Accuracy of Machine Learning Techniques in Leg Rehabilitation. Meas. Sens. 2022, 25, 100544. [Google Scholar] [CrossRef]
- Romaniszyn-Kania, P.; Pollak, A.; Kania, D.; Mitas, A.W. Longitudinal Observation of Psychophysiological Data as a Novel Approach to Personalised Postural Defect Rehabilitation. Sci. Rep. 2025, 15, 92368. [Google Scholar] [CrossRef]
- Santilli, V.; Mangone, M.; Diko, A.; Alviti, F.; Bernetti, A.; Agostini, F.; Palagi, L.; Servidio, M.; Paoloni, M.; Goffredo, M.; et al. The Use of Machine Learning for Inferencing the Effectiveness of a Rehabilitation Program for Orthopedic and Neurological Patients. Int. J. Environ. Res. Public Health 2023, 20, 5575. [Google Scholar] [CrossRef]
- Tschuggnall, M.; Grote, V.; Pirchl, M.; Holzner, B.; Rumpold, G.; Fischer, M.J. Machine Learning Approaches to Predict Rehabilitation Success Based on Clinical and Patient-Reported Outcome Measures. Inform. Med. Unlocked 2021, 24, 100598. [Google Scholar] [CrossRef]
- Mennella, C.; Maniscalco, U.; De Pietro, G.; Esposito, M. A Deep Learning System to Monitor and Assess Rehabilitation Exercises in Home-Based Remote and Unsupervised Conditions. Comput. Biol. Med. 2023, 166, 107485. [Google Scholar] [CrossRef]
- Vourganas, I.; Stankovic, V.; Stankovic, L. Individualised Responsible Artificial Intelligence for Home-Based Rehabilitation. Sensors 2021, 21, 2. [Google Scholar] [CrossRef]
- Kurniawan, M.H.; Handiyani, H.; Nuraini, T.; Hariyati, R.T.S.; Sutrisno, S. A Systematic Review of Artificial Intelligence-Powered (AI-Powered) Chatbot Intervention for Managing Chronic Illness. Ann. Med. 2024, 56, 2302980. [Google Scholar] [CrossRef]
- Koulouri, T.; Macredie, R.D.; Olakitan, D. Chatbots to Support Young Adults’ Mental Health: An Exploratory Study of Acceptability. ACM Trans. Interact. Intell. Syst. 2022, 12, 1–39. [Google Scholar] [CrossRef]
- Hussain, S.; Alarcon, A.; Pisharody, S.; Shrestha, A.; Ma, H.A.; Ahmed, K.; Kothiya, A.; Rahman, M.A.; Shah, R.; Islam, M.F.; et al. Therapeutic Exercise Recognition Using a Single UWB Radar with AI-Driven Feature Fusion and ML Techniques in a Real Environment. Sensors 2024, 24, 5533. [Google Scholar] [CrossRef]
- Zhang, L.; Tashiro, S.; Mukaino, M.; Yamada, S. Use of Artificial Intelligence Large Language Models as a Clinical Tool in Rehabilitation Medicine: A Comparative Test Case. J. Rehabil. Med. 2023, 55, jrm13373. [Google Scholar] [CrossRef]
- Sivarajkumar, S.; Zhang, S.; Kaji, M.; Aliberti, S.; Zukotynski, K.; Laranjo, L. Mining Clinical Notes for Physical Rehabilitation Exercise Information: Natural Language Processing Algorithm Development and Validation Study. JMIR Med. Inform. 2024, 12, e52289. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Lu, R.; Su, L.; Song, G.; Wu, M.; Liu, Y.; Gao, S.; Li, J.; Zhao, Z.; Lin, J.; et al. The Application of Large Language Models in Medicine: A Scoping Review. iScience 2024, 27, 109713. [Google Scholar] [CrossRef]
- Cascella, M.; Montomoli, J.; Bellini, V.; Bignami, E. Evaluating the Feasibility of ChatGPT in Healthcare: An Analysis of Multiple Clinical and Research Scenarios. J. Med. Syst. 2023, 47, 33. [Google Scholar] [CrossRef]
- Kornblith, A.E.; Wu, M.; Kim, J.; Sun, H.; Wu, A.V.; Ferket, B.S.; Ghassemi, M. Analyzing Patient Perspectives with Large Language Models: A Cross-Sectional Study of Sentiment and Thematic Classification on Exception from Informed Consent. Sci. Rep. 2025, 15, 6179. [Google Scholar] [CrossRef] [PubMed]
- Bui, N.; Ngo, M.; Lam, V.T.; Ho, L.M.; Nguyen, M.T.; Nguyen, A.Q.; Tran, V.K.; Nguyen, N.V.; Pham, H.T.; Nguyen, T.P.; et al. Fine-Tuning Large Language Models for Improved Health Communication in Low-Resource Languages. Comput. Methods Programs Biomed. 2025, 263, 108655. [Google Scholar] [CrossRef]
- Şişman, A.Ç.; Acar, A.H. Artificial Intelligence-Based Chatbot Assistance in Clinical Decision-Making for Medically Complex Patients in Oral Surgery: A Comparative Study. BMC Oral Health 2025, 25, 351. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.T.; Yang, Z.; Dai, X.; Chen, X.; He, J.; Lu, Y.; Peng, Y.; Liu, C.; Chang, S.; Yu, H.; et al. Red Teaming ChatGPT in Medicine to Yield Real-World Insights on Model Behavior. Digit. Med. 2025, 8, 149. [Google Scholar] [CrossRef]
- Yang, Z.; Yao, Z.; Tasmin, M.; Vashisht, P.; Jang, W.S.; Ouyang, F.; Wang, B.; McManus, D.; Berlowitz, D.; Yu, H. Unveiling GPT-4V’s Hidden Challenges Behind High Accuracy on USMLE Questions: Observational Study. J. Med. Internet Res. 2025, 27, e65146. [Google Scholar] [CrossRef]
- Chelli, M.; Descamps, J.; Lavoué, V.; Trojani, C.; Azar, M.; Deckert, M.; Raynier, J.L.; Clowez, G.; Boileau, P.; Ruetsch-Chelli, C. Hallucination Rates and Reference Accuracy of ChatGPT and Bard for Systematic Reviews: Comparative Analysis. J. Med. Internet Res. 2024, 26, e53164. [Google Scholar] [CrossRef]
- Bäck, M.; Leosdottir, M.; Ekström, M.; Hambraeus, K.; Ravn-Fischer, A.; Öberg, B.; Östlund, O.; James, S. The Remote Exercise SWEDEHEART Study–Rationale and Design of a Multicenter Registry-Based Cluster Randomized Crossover Clinical Trial (RRCT). Am. Heart J. 2023, 262, 110–118. [Google Scholar] [CrossRef]
- Ramachandran, H.J.; Jiang, Y.; Tam, W.W.S.; Yeo, T.J.; Wang, W. Effectiveness of Home-Based Cardiac Telerehabilitation as an Alternative to Phase 2 Cardiac Rehabilitation of Coronary Heart Disease: A Systematic Review and Meta-Analysis. Eur. J. Prev. Cardiol. 2021, 29, 1017–1043. [Google Scholar] [CrossRef] [PubMed]
- Tam, P.K.; Tang, N.; Kamsani, N.S.B.; Chua, P.K.; Fong, E.T.; Yong, A.G.; Ng, D.C.; Lim, A.Y.; Chia, L.K.; Loh, Y.Y.; et al. Overground Robotic Exoskeleton vs. Conventional Therapy in Inpatient Stroke Rehabilitation: Results from a Pragmatic, Multicentre Implementation Programme. J. Neuroeng. Rehabil. 2025, 22, 3. [Google Scholar] [CrossRef] [PubMed]
- Louie, D.R.; Mortenson, W.B.; Durocher, M.; Lloyd, D.K.; Ouellette, M.R.; Teasell, R.W.; Handy, J.M.; Loh, E.; Gagnon, B.; Knorr, A.E.; et al. Efficacy of an Exoskeleton-Based Physical Therapy Program for Non-Ambulatory Patients During Subacute Stroke Rehabilitation: A Randomized Controlled Trial. J. Neuroeng. Rehabil. 2021, 18, 149. [Google Scholar] [CrossRef]
- Calabrò, R.S.; Naro, A.; Russo, M.; Leo, A.; De Luca, R.; Balletta, T.; Marra, A.; Bramanti, P.; Bramanti, A. Shaping Neuroplasticity by Using Powered Exoskeletons in Patients with Stroke: A Randomized Clinical Trial. J. Neuroeng. Rehabil. 2018, 15, 35. [Google Scholar] [CrossRef]
- Ullah, E.; Parwani, A.; Baig, M.M.; Saeed, W.; Niaz, R.; Shahbaz, M.; Al-Sharif, M.A.; Rehman, N.; Abid, A.; Baig, M.M. Challenges and Barriers of Using Large Language Models (LLM) Such as ChatGPT for Diagnostic Medicine with a Focus on Digital Pathology—A Recent Scoping Review. Diagn. Pathol. 2024, 19, 43. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Sha, L.; Zhao, L.; Li, Y.; Martinez-Maldonado, R.; Chen, G.; Li, X.; Jin, Y.; Gašević, D. Practical and Ethical Challenges of Large Language Models in Education: A Systematic Scoping Review. Br. J. Educ. Technol. 2023, 55, 90–112. [Google Scholar] [CrossRef]
- Shahzad, T.; Mazhar, T.; Tariq, M.U.; Farrukh, M.; Asif, S.; Anjum, K.; Alarood, A. A Comprehensive Review of Large Language Models: Issues and Solutions in Learning Environments. Discov. Sustain. 2025, 6, 27. [Google Scholar] [CrossRef]
- Singhal, K.; Azizi, S.; Tu, T.; Liu, J.; Parsons, N.; Hoffman, C.; Cole, J.; Tan, D.; Gleason, M.; Khan, T.; et al. Large Language Models Encode Clinical Knowledge. Nature 2023, 620, 172–180. [Google Scholar] [CrossRef]
- Wu, C.; Lin, W.; Zhang, X.; Zhang, Y.; Xie, W.; Wang, Y. PMC-LLaMA: Toward Building Open-Source Language Models for Medicine. J. Am. Med. Inform. Assoc. 2024, 31, 1833–1843. [Google Scholar] [CrossRef]
- Yao, Y.; Duan, J.; Xu, K.; Cai, Y.; Sun, Z.; Zhang, Y. A Survey on Large Language Model (LLM) Security and Privacy: The Good, The Bad, and The Ugly. High-Confid. Comput. 2024, 4, 100211. [Google Scholar] [CrossRef]
- Zhang, R.; Li, H.; Qian, X.; Jiang, W.; Chen, H. On Large Language Models Safety, Security, and Privacy: A Survey. J. Electron. Sci. Technol. 2025, 23, 100301. [Google Scholar] [CrossRef]
- Ong, J.C.L.; Chang, S.Y.; William, W.; Butte, A.J.; Shah, N.H.; Chew, L.S.T.; Liu, N.; Doshi-Velez, F.; Lu, W.; Savulescu, J.; et al. Ethical and Regulatory Challenges of Large Language Models in Medicine. Lancet Digit. Health 2024, 6, e428–e432. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Phillips, R.V.; Malenica, I.; Coorey, M.; Singh, H.; Dunn, A.G.; Laranjo, L. Clinical Artificial Intelligence Quality Improvement: Towards Continual Monitoring and Updating of AI Algorithms in Healthcare. Npj Digit. Med. 2022, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Bayram, F.; Ahmed, B.S. Towards Trustworthy Machine Learning in Production: An Overview of the Robustness in MLOps Approach. ACM Comput. Surv. 2025, 57, 121. [Google Scholar] [CrossRef]
- Abdul Razak, M.S.; Nirmala, C.R.; Sreenivasa, B.R.; Lahza, H.; Lahza, H.F.M. A Survey on Detecting Healthcare Concept Drift in AI/ML Models from a Finance Perspective. Front. Artif. Intell. 2023, 5, 955314. [Google Scholar] [CrossRef]
- Bayram, F.; Ahmed, B.S.; Kassler, A. From Concept Drift to Model Degradation: An Overview on Performance-Aware Drift Detectors. Knowl.-Based Syst. 2022, 245, 108632. [Google Scholar] [CrossRef]
- Adibi, S.; Rajabifard, A.; Shojaei, D.; Wickramasinghe, N. Enhancing Healthcare through Sensor-Enabled Digital Twins in Smart Environments: A Comprehensive Analysis. Sensors 2024, 24, 2793. [Google Scholar] [CrossRef]
- Baldini, G.; Botterman, M.; Neisse, R.; del Rio, A.; Flegar, G.; Ma, Y.; Miron, V. Ethical Design in the Internet of Things. Sci. Eng. Ethics 2018, 24, 905–925. [Google Scholar] [CrossRef]
- Kumar, S.; Tiwari, P.; Zymbler, M. Internet of Things Is a Revolutionary Approach for Future Technology Enhancement: A Review. J. Big Data 2019, 6, 111. [Google Scholar] [CrossRef]
- Gupta, B.; Quamara, M. An Overview of Internet of Things (IoT): Architectural Aspects, Challenges, and Protocols. Concurrency Computat. Pract. Exp. 2018, 32, e4946. [Google Scholar] [CrossRef]
- Gilman, E.; Tamminen, S.; Yasmin, R.; Ristimella, E.; Peltonen, E.; Harju, M.; Lovén, L.; Riekki, J.; Pirttikangas, S. Internet of Things for Smart Spaces: A University Campus Case Study. Sensors 2020, 20, 3716. [Google Scholar] [CrossRef] [PubMed]
- Ndiaye, M.; Oyewobi, S.S.; Abu-Mahfouz, A.M.; Hancke, G.P.; Kurien, A.M.; Djouani, K. IoT in the Wake of COVID-19: A Survey on Contributions, Challenges and Evolution. IEEE Access 2020, 8, 186821–186839. [Google Scholar] [CrossRef]
- Albreem, M.A.; Sheikh, A.M.; Alsharif, M.H.; Jusoh, M.; Mohd Yasin, M.N. Green Internet of Things (GIoT): Applications, Practices, Awareness, and Challenges. IEEE Access 2021, 9, 38833–38858. [Google Scholar] [CrossRef]
- Cenci, M.P.; Scarazzato, T.; Munchen, D.D.; Dartora, P.C.; Veit, H.M.; Bernardes, A.M.; Dias, P.R. Eco-Friendly Electronics—A Comprehensive Review. Adv. Mater. Technol. 2021, 7, 22001263. [Google Scholar] [CrossRef]
- Dhar, P. The Carbon Impact of Artificial Intelligence. Nat. Mach. Intell. 2020, 2, 423–425. [Google Scholar] [CrossRef]
- Buyya, R.; Ilager, S.; Arroba, P. Energy-Efficiency and Sustainability in New Generation Cloud Computing: A Vision and Directions for Integrated Management of Data Centre Resources and Workloads. Softw. Pract. Exp. 2023, 54, 24–38. [Google Scholar] [CrossRef]
- Iftikhar, S.; Davy, S. Reducing Carbon Footprint in AI: A Framework for Sustainable Training of Large Language Models. In Proceedings of the Future Technologies Conference (FTC) 2024; Lecture Notes in Networks and Systems; Springer: Cham, Switzerland, 2024; pp. 325–336. [Google Scholar] [CrossRef]
- Bach, A.J.; Wolfson, T.; Crowell, J.K. Poverty, Literacy, and Social Transformation: An Interdisciplinary Exploration of the Digital Divide. J. Media Lit. Educ. 2018, 10, 22–41. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT01801527 (accessed on 7 June 2025).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT06968923 (accessed on 7 June 2025).
- Leochico, C.F.D.; Espiritu, A.I.; Ignacio, S.D.; Mojica, J.P. Challenges to the Emergence of Telerehabilitation in a Developing Country: A Systematic Review. Front. Neurol. 2020, 11, 1007. [Google Scholar] [CrossRef] [PubMed]
- Nizeyimana, E.; Joseph, C.; Plastow, N.; Dawood, G.; Louw, Q.A. A Scoping Review of Feasibility, Cost, Access to Rehabilitation Services and Implementation of Telerehabilitation: Implications for Low- and Middle-Income Countries. Digit. Health 2022, 8, 205520762211316. [Google Scholar] [CrossRef]
- Sia, L.L.; Sharma, S.; Kumar, S.; Singh, D.K.A. Exploring Physiotherapists’ Perceptions of Telerehabilitation for Musculoskeletal Disorders: Insights from Focus Groups. Digit. Health 2024, 10, 20552076241248916. [Google Scholar] [CrossRef]
- Hoffman, L.C. Reconnecting the Patient: Why Telehealth Policy Solutions Must Consider the Deepening Digital Divide. Indiana Health Law Rev. 2022, 19, 351–385. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.; Koola, J.; Contreras, A.; Castillo, A.; Ruiz, M.; Tedone, K.; Yakuta, M.; Schiaffino, M. Consumer Health Informatics Adoption among Underserved Populations: Thinking beyond the Digital Divide. Yearb. Med. Inform. 2018, 27, 146–155. [Google Scholar] [CrossRef] [PubMed]
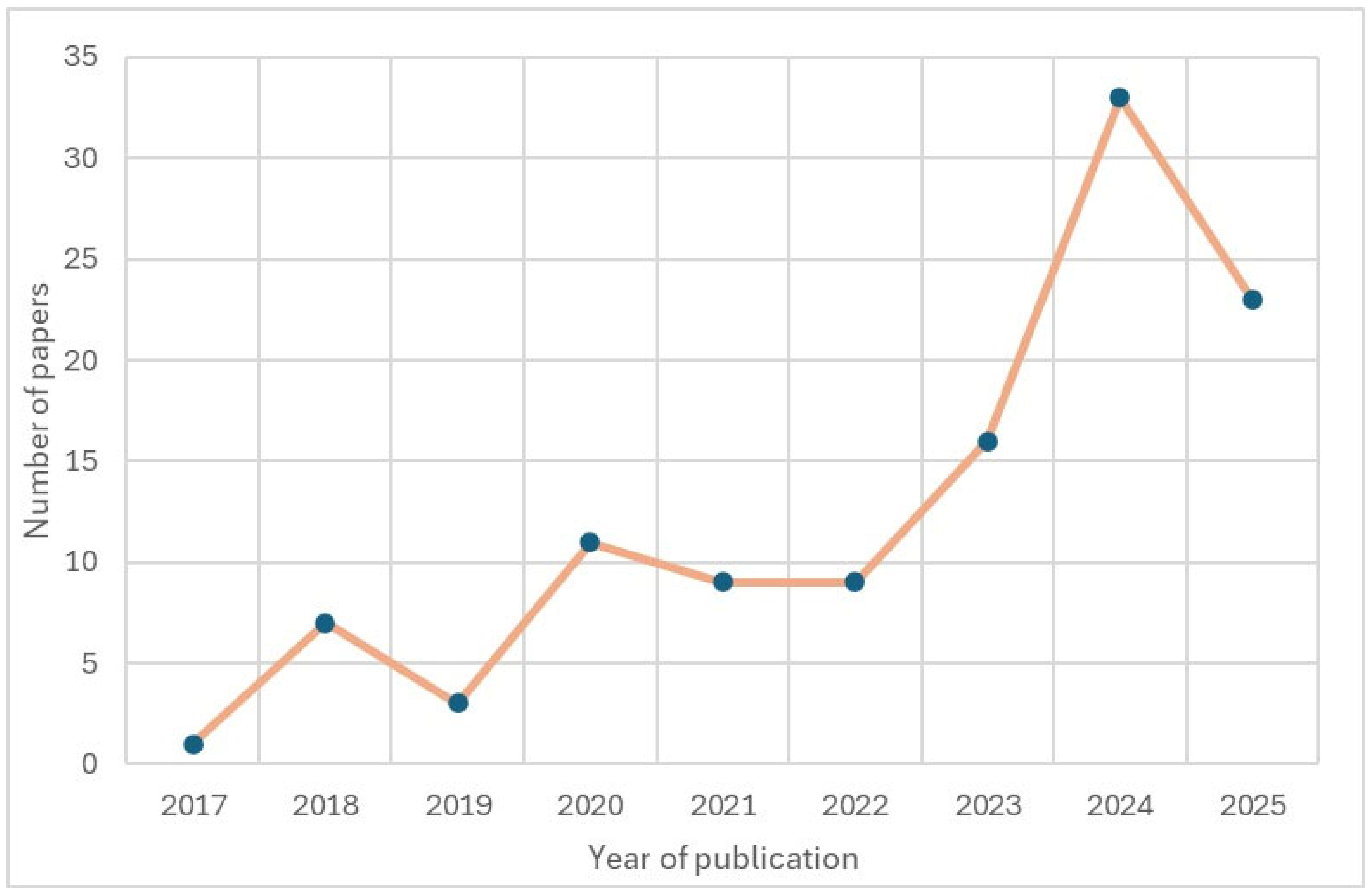

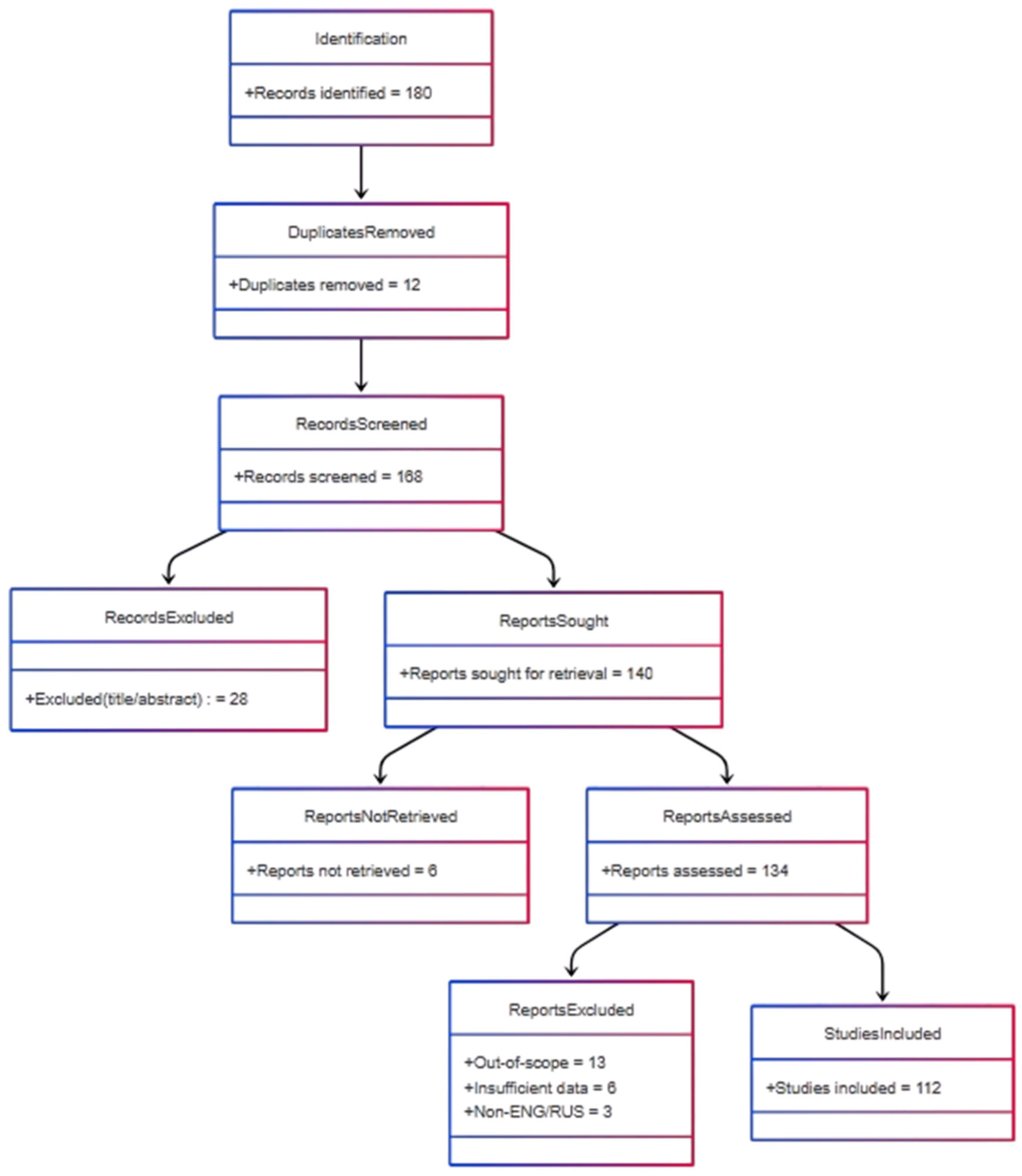
| Platform/Source | Architecture | Key Technologies | Purpose | Personalization (Qualitative) | Evidence Strength | Clinical Maturity | Cost-Effectiveness | Reference |
|---|---|---|---|---|---|---|---|---|
| REHOME | IoT + local interfaces | sensors, cognitive games, VR | comprehensive cognitive and motor telerehabilitation | patient data use: 100%, adaptation: personalized | Pilot studies, n = 28–27–15; SUS > 68 | Advanced; multi-domain remote rehab in hospitals | Likely cost savings via reduced hospital use | [14] |
| ReMoVES | IoMT + remote | telemedicine, IoT | rehabilitation for multiple sclerosis | patient data use: 100%, adaptation: weekly | Feasibility study, n = 2 | Rehab for MS; positive user engagement | Potential savings from remote sessions | [13] |
| I-TROPHYTS | IoT + humanoid robotics | wearable IoT, sensors, AI, robotics | autonomous monitoring and physiotherapy | patient data use: 100% adaptation: real-time | Small trial, n = 6; ~100% accuracy | Partial; home rehab via AI + robot | Scalable; one therapist for many patients | [12] |
| 3D-printing + AI | computer-aided design (CAD) + cloud system | AI optimization, bioscanning | custom prosthetics and orthotics | patient data use: 100%, adaptation: personalized | Conceptual; no clinical trial | Limited; wrist rehab prototype | Possible savings via 3D-printing | [15] |
| AI + CT Diagnostics | cloud-based | ResNet50, segmentation, AI | diagnostics/monitoring | patient data use: 100%, adaptation: real-time | No data available | No data available | No data available | [12] |
| Pheno4U Platform | cloud-based + Mobile App | motion sensors, data tracking, integration with hospital Information system (HIS) | remote monitoring and rehabilitation | patient data use: 100%, adaptation: weekly | Clinical study, n = 98; p < 0.001 | TKA aftercare with sensors/app; well-accepted | Supports autonomy; reduces inpatient needs | [16] |
| Haodf (ind./comm. model) | online service platform | multilevel interaction | virtual consultations | patient data use: 100%, adaptation: continuous | No data available | No data available | No data available | [13] |
| Methods | Accuracy | Recall | Precision | F1-Score | Training/Validation | Dataset Composition | Provenance/Accessibility | Reference |
|---|---|---|---|---|---|---|---|---|
| BloomZ-3B (Fine-tuned) | - | 0.8355 | 0.7876 | 0.81 | 337,000 QA/578 | Various health topics; CVD 26.05%, musculoskeletal 7.53%; no demographics or severity | Compiled from ViHealthQA, PubmedQA, etc.; Private/Institutional, on request | [66] |
| LLaMA2-7B (Fine-tuned) | - | 0.8335 | 0.836 | 0.8343 | 337,000 QA/578 | Same as BloomZ-3B | Same as BloomZ-3B | [66] |
| LLaMA2-13B (Fine-tuned) | - | 0.8119 | 0.8109 | 0.8109 | 337,000 QA/578 | Same as BloomZ-3B | Same as BloomZ-3B | [66] |
| GPT-4 | 87% | - | - | - | NR/3692, 123, 188 (EFIC), 102 interviews | EFIC interviews; no demographics or severity reported | OpenAI GPT-4 via Azure; data not shareable; Private/Institutional | [65] |
| GPT-3.5 | 93.4% | - | - | - | NR/64 × 2 (OMFS), 123 (EFIC), used in QA dataset | OMFS QA; EFIC interviews; no patient data or severity | OpenAI GPT-3.5 via Azure; Private/Institutional | [67] |
| Claude-Instant | 95.2% | - | - | - | NR/64 × 2 (OMFS) | OMFS QA; no demographics or severity | Anthropic Claude-Instant; online; Private/Institutional | [67] |
| ChatGPT (zero-shot) | - | 0.33 | 0.8 | 0.37 | NR/50 notes (13,605 patients) | Stroke patients; age 75 ± 16, 51% female, some race/ethnicity data; no severity scale | UPMC stroke notes; IRB-approved; Private/Institutional | [62] |
| ChatGPT (few-shot) | - | 0.27 | 0.82 | 0.35 | NR/50 notes (13,605 patients) | Same as zero-shot | Same as zero-shot | [62] |
| Model | Hallucination Rate | Medical Exam Score (USMLE): Step 1 | Medical Exam Score (USMLE): Step 2 | Medical Exam Score (USMLE): Step 3 | References |
|---|---|---|---|---|---|
| GPT-4 | 28.6% | 63.2% | 64.3% | 66.7% | [69,70] |
| GPT-3.5 | 39.6% | - | - | - | [70] |
| GPT-3.5 Turbo | - | 42.1% | 50% | 50% | [69] |
| GPT-4V | - | 84.2% | 85.7% | 88.9% | [69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boltaboyeva, A.; Baigarayeva, Z.; Imanbek, B.; Ozhikenov, K.; Getahun, A.J.; Aidarova, T.; Karymsakova, N. A Review of Innovative Medical Rehabilitation Systems with Scalable AI-Assisted Platforms for Sensor-Based Recovery Monitoring. Appl. Sci. 2025, 15, 6840. https://doi.org/10.3390/app15126840
Boltaboyeva A, Baigarayeva Z, Imanbek B, Ozhikenov K, Getahun AJ, Aidarova T, Karymsakova N. A Review of Innovative Medical Rehabilitation Systems with Scalable AI-Assisted Platforms for Sensor-Based Recovery Monitoring. Applied Sciences. 2025; 15(12):6840. https://doi.org/10.3390/app15126840
Chicago/Turabian StyleBoltaboyeva, Assiya, Zhanel Baigarayeva, Baglan Imanbek, Kassymbek Ozhikenov, Aliya Jemal Getahun, Tanzhuldyz Aidarova, and Nurgul Karymsakova. 2025. "A Review of Innovative Medical Rehabilitation Systems with Scalable AI-Assisted Platforms for Sensor-Based Recovery Monitoring" Applied Sciences 15, no. 12: 6840. https://doi.org/10.3390/app15126840
APA StyleBoltaboyeva, A., Baigarayeva, Z., Imanbek, B., Ozhikenov, K., Getahun, A. J., Aidarova, T., & Karymsakova, N. (2025). A Review of Innovative Medical Rehabilitation Systems with Scalable AI-Assisted Platforms for Sensor-Based Recovery Monitoring. Applied Sciences, 15(12), 6840. https://doi.org/10.3390/app15126840






