Investigating the Secondary Thermal Neutron Intensity of Neutron Capture-Enhanced Proton Therapy
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
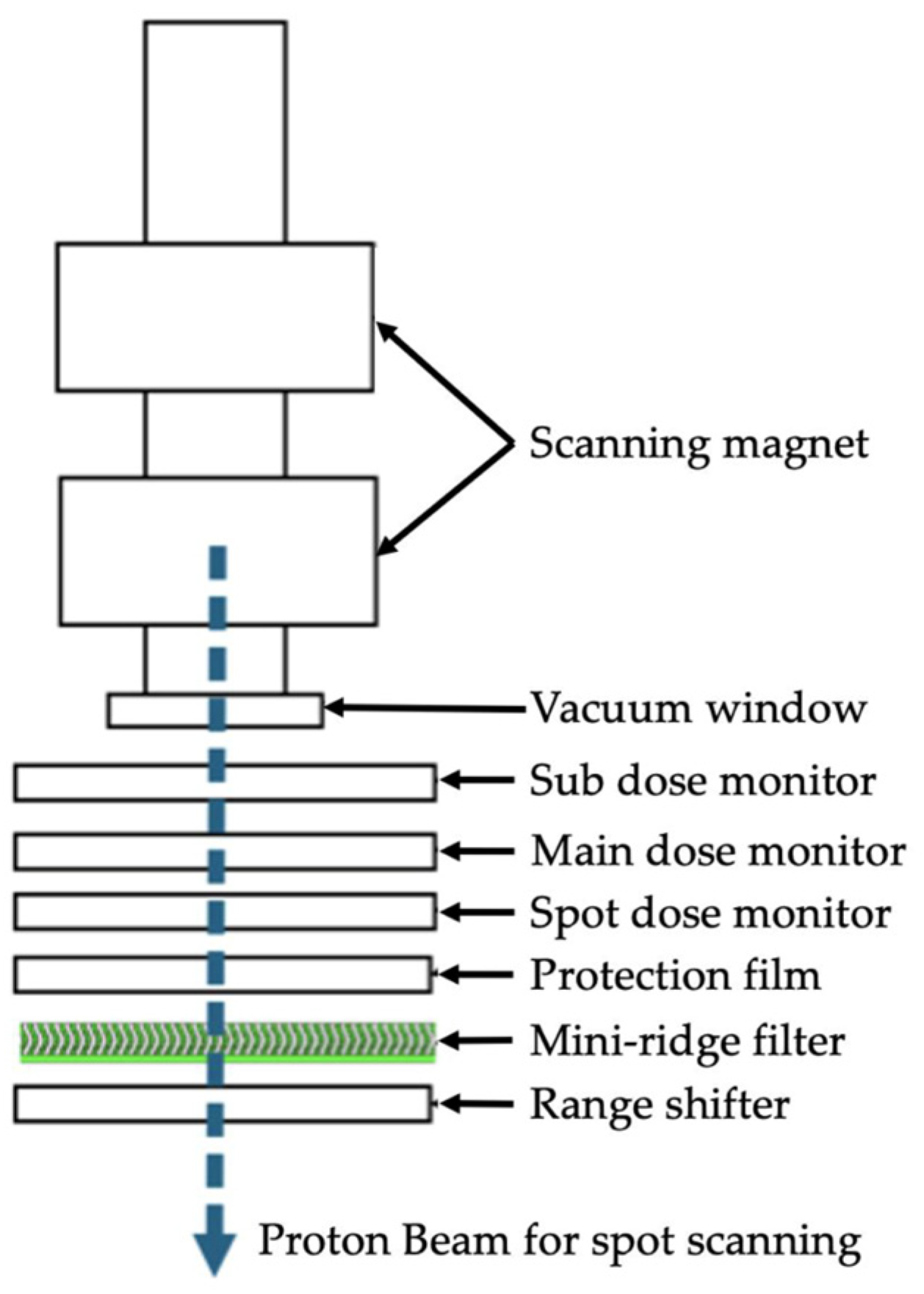
2.1. Monte Carlo Simulation
2.2. Neutron Fluence Measurement
2.3. Energy Influence Assessment
2.4. Depth Influence Assessment
3. Results
3.1. RR and Neutron Fluence Measurement
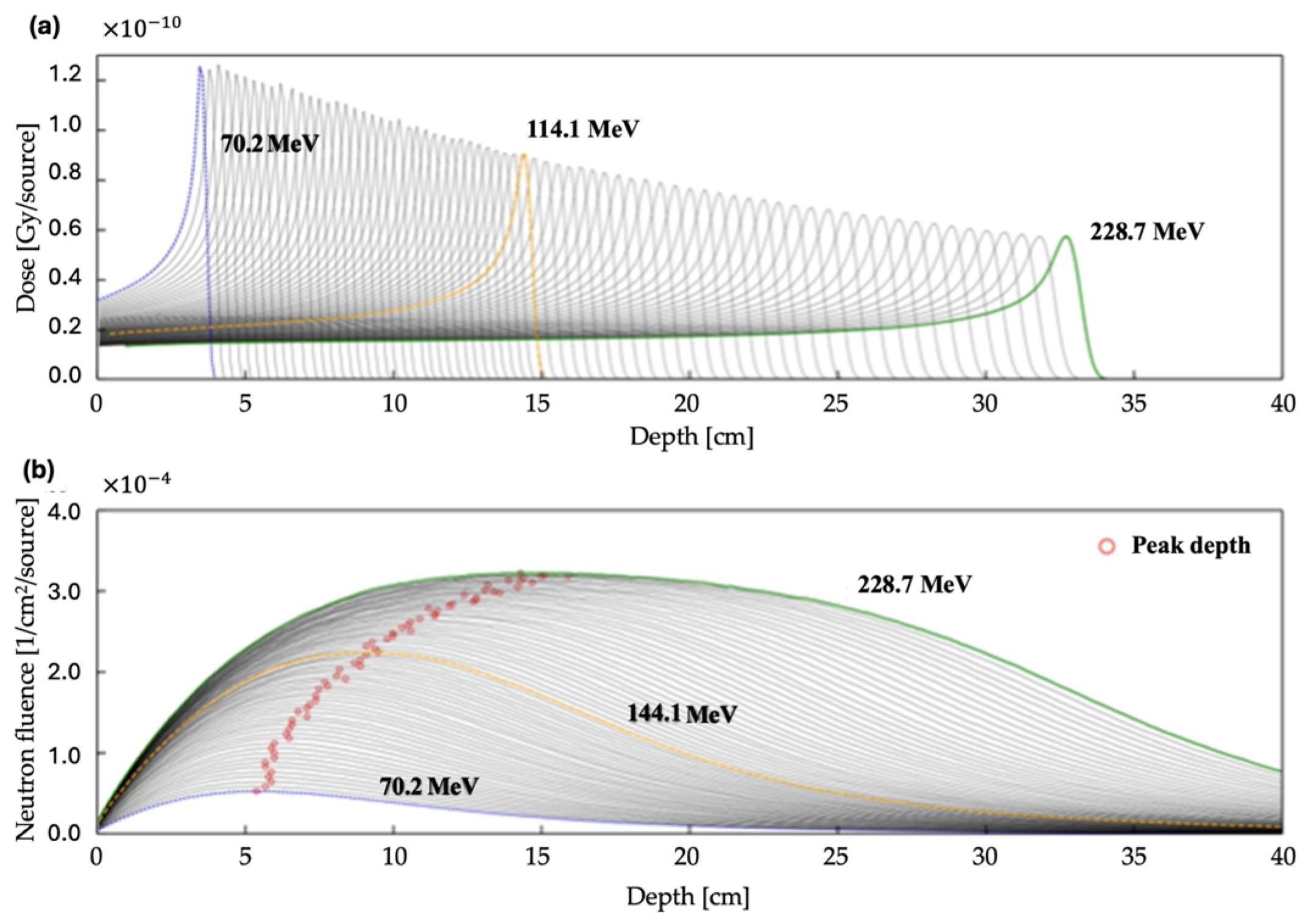
3.2. Proton Energy Simulations
3.3. Effect of Target Depth
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BNCR | Boron Neutron Capture Reaction |
| PBT | Proton-Beam Therapy |
| SOBP | Spread-out Bragg Peak |
| NCEPT | Neutron Capture-Enhanced Proton Therapy |
| LET | Linear Energy Transfer |
| MCS | Monte Carlo Simulation |
| PHITS | Particle and Heavy Ion Treatment Code System |
References
- Wilson, R.R. Radiological use of fast protons. Radiology 1946, 47, 487–491. [Google Scholar] [CrossRef] [PubMed]
- PTCOG Report No. 1. 2010. Available online: https://www.ptcog.site/index.php/ptcog-publications (accessed on 15 January 2025).
- Mohan, R.; Grosshans, D. Proton therapy—Present and future. Adv. Drug Deliv. Rev. 2017, 109, 26–44. [Google Scholar] [CrossRef] [PubMed]
- Paganetti, H.; Niemierko, A.; Ancukiewicz, M.; Gerweck, L.E.; Goitein, M.; Loeffler, J.S.; Suit, H.D. Relative biological effectiveness (RBE) values for proton beam therapy. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 407–421. [Google Scholar] [CrossRef]
- Bláha, P.; Feoli, C.; Agosteo, S.; Calvaruso, M.; Cammarata, F.P.; Catalano, R.; Ciocca, M.; Cirrone, G.A.P.; Conte, V.; Cuttone, G.; et al. The Proton-Boron Reaction Increases the Radiobiological Effectiveness of Clinical Low- and High-Energy Proton Beams: Novel Experimental Evidence and Perspectives. Front. Oncol. 2021, 11, 682647. [Google Scholar] [CrossRef] [PubMed]
- Safavi-Naeini, M.; Chacon, A.; Guatelli, S.; Franklin, D.R.; Bambery, K.; Gregoire, M.C.; Rosenfeld, A. Opportunistic dose amplification for proton and carbon ion therapy via capture of internally generated thermal neutrons. Sci. Rep. 2018, 8, 16257. [Google Scholar] [CrossRef]
- Yeo, J.J.W.; Tan, H.Q.; Ang, K.W.; Lee, J.C.L.; Bettiol, A.A. Quantifying the spatial and angular distribution of lethal neutrons for treating planning. In World Congress on Medical Physics and Biomedical Engineering 2018. IFMBE Proceedings; Lhotska, L., Sukupova, L., Lacković, I., Ibbott, G.S., Eds.; Springer: Singapore, 2019; Volume 68, p. 1. [Google Scholar]
- El-Asery, M.; Sadoune, Z.; El Bekkouri, H.; Didi, A.; Chakir, E.M. Evaluation of secondary neutron produced in proton therapy using Phits. Moscow Univ. Phys. 2023, 78, 155–160. [Google Scholar] [CrossRef]
- El-Asery, M.; Sadoune, Z.; El Bekkouri, H.; Al Ibrahmi, E.M.; Didi, A.; Chakir, E.M. Characterization of secondary neutron spectra from therapeutic proton and carbon ion beams using PHITS Simulation. Moscow Univ. Phys. 2023, 78, 810–816. [Google Scholar] [CrossRef]
- Barth, R.F.; Vicente, M.G.; Harling, O.K.; Kiger, W.S.; Riley, K.J.; Binns, P.J.; Wagner, F.M.; Suzuki, M.; Aihara, T.; Kato, I.; et al. Current status of boron neutron capture therapy of high grade gliomas and recurrent head and neck cancer. Radiat. Oncol. 2012, 7, 146. [Google Scholar] [CrossRef]
- Dell’Oro, M.; Short, M.; Wilson, P.; Peukert, D.; Hua, C.H.; Merchant, T.E.; Bezak, E. Lifetime attributable risk of radiation induced second primary cancer from scattering and scanning proton therapy—A model for out-of-field organs of paediatric patients with cranial cancer. Radiother. Oncol. 2022, 172, 65–75. [Google Scholar] [CrossRef]
- Barth, R.F.; Coderre, J.A.; Vicente, M.G.; Blue, T.E. Boron neutron capture therapy of cancer: Current status and future prospects. Clin. Cancer Res. 2005, 11, 3987–4002. [Google Scholar] [CrossRef]
- Shimo, T.; Shiba, S.; Watanabe, H.; Yamanaka, M.; Matsumoto, K.; Yamano, A.; Tokuuye, K. Evaluation of the mini-ridge filter’s impact on the generation of secondary radiation in synchrotron-based proton beam therapy. J. Med. Phys. 2025, 50, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Iwamoto, Y.; Hashimoto, S.; Ogawa, T.; Furuta, T.; Abe, S.I.; Kai, T.; Matsuya, Y.; Matsuda, N.; Hirata, Y.; et al. Recent improvements of the particle and heavy ion transport code system–PHITS version 3.33. J. Nucl. Sci. Technol. 2023, 61, 127–135. [Google Scholar] [CrossRef]
- Shibata, K.; Iwamoto, O.; Nakagawa, T.; Iwamoto, N.; Ichihara, A.; Kunieda, S.; Chiba, S.; Furutaka, K.; Otuka, N.; Ohsawa, T.; et al. JENDL-4.0: A new library for nuclear science and engineering. J. Nucl. Sci. Technol. 2011, 48, 1–30. [Google Scholar] [CrossRef]
- Lynch, G.R.; Dahl, O.I. Approximations to multiple Coulomb scattering. Nucl. Inst. Methods Phys. Res. B 1991, 58, 6–10. [Google Scholar] [CrossRef]
- Lee, C.; Lee, S.; Lee, S.J.; Song, H.; Kim, D.H.; Cho, S.; Jo, K.; Han, Y.; Chung, Y.H.; Kim, J.S. Monte Carlo simulation of secondary neutron dose for scanning proton therapy using FLUKA. PLoS ONE 2017, 12, e0186544. [Google Scholar] [CrossRef] [PubMed]
- Alinejad, A.H.; Kakavand, T.; Aboudzadeh Rovais, M.R.; Kakaei, S. Study of the 199Au nanoparticles production parameters via irradiation of platinum target by using thermal neutrons. Appl. Radiat. Isot. 2022, 184, 110187. [Google Scholar] [CrossRef]
- Suzuki, S.; Nitta, K.; Yagihashi, T.; Eide, P.; Koivunoro, H.; Sato, N.; Gotoh, S.; Shiba, S.; Omura, M.; Nagata, H.; et al. Initial evaluation of accelerator-based neutron source system at the Shonan Kamakura General Hospital. Appl. Radiat. Isot. 2023, 199, 110898. [Google Scholar] [CrossRef]
- Hirose, K.; Kato, T.; Harada, T.; Motoyanagi, T.; Tanaka, H.; Takeuchi, A.; Kato, R.; Komori, S.; Yamazaki, Y.; Arai, K.; et al. Determining a methodology of dosimetric quality assurance for commercially available accelerator-based boron neutron capture therapy system. J. Radiat. Res. 2022, 63, 620–635. [Google Scholar] [CrossRef]
- Tanaka, H.; Sakurai, Y.; Suzuki, M.; Masunaga, S.; Mitsumoto, T.; Fujita, K.; Kashino, G.; Kinashi, Y.; Liu, Y.; Takada, M.; et al. Experimental verification of beam characteristics for cyclotron-based epithermal neutron source (C-BENS). Appl. Radiat. Isot. 2011, 69, 1642–1645. [Google Scholar] [CrossRef]
- Moreno, A.C.; Frank, S.J.; Garden, A.S.; Rosenthal, D.I.; Fuller, C.D.; Gunn, G.B.; Reddy, J.P.; Morrison, W.H.; Williamson, T.D.; Holliday, E.B.; et al. Intensity modulated proton therapy (IMPT)—The future of IMRT for head and neck cancer. Oral Oncol. 2019, 88, 66–74. [Google Scholar] [CrossRef]
- Pedroni, E.; Scheib, S.; Böhringer, T.; Coray, A.; Grossmann, M.; Lin, S.; Lomax, A. Experimental characterization and physical modelling of the dose distribution of scanned proton pencil beams. Phys. Med. Biol. 2005, 50, 541–561. [Google Scholar] [CrossRef] [PubMed]
- Yonai, S.; Matsumoto, S. Secondary neutron dose in carbon-ion radiotherapy: Investigations in QST–NIRS. J. Radiat. Prot. Res. 2021, 46, 39–47. [Google Scholar] [CrossRef]
- Barth, R.F.; Mi, P.; Yang, W. Boron delivery agents for neutron capture therapy of cancer. Cancer Commun. 2018, 38, 35. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Kirihata, M. Boron compounds: New candidates for boron carriers in BNCT. In Neutron Capture Therapy; Sauerwein, W., Wittig, A., Moss, R., Nakagawa, Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; p. 9783642313349. [Google Scholar]
- Atkinson, J.; Bezak, E.; Le, H.; Kempson, I. The current status of FLASH particle therapy: A systematic review. Phys. Eng. Sci. Med. 2023, 46, 529–560. [Google Scholar] [CrossRef]
- Chingyun, C.; Liming, X.; Hao, J.; Balaji, S.; Haibo, L.; Michael, P.; Arpit, M.C.; Shaakir, H.; Huifang, Z.; Yin, Z.; et al. The Potential and Challenges of Proton FLASH in Head and Neck Cancer Reirradiation. Cancers 2024, 16, 3249. [Google Scholar] [CrossRef]


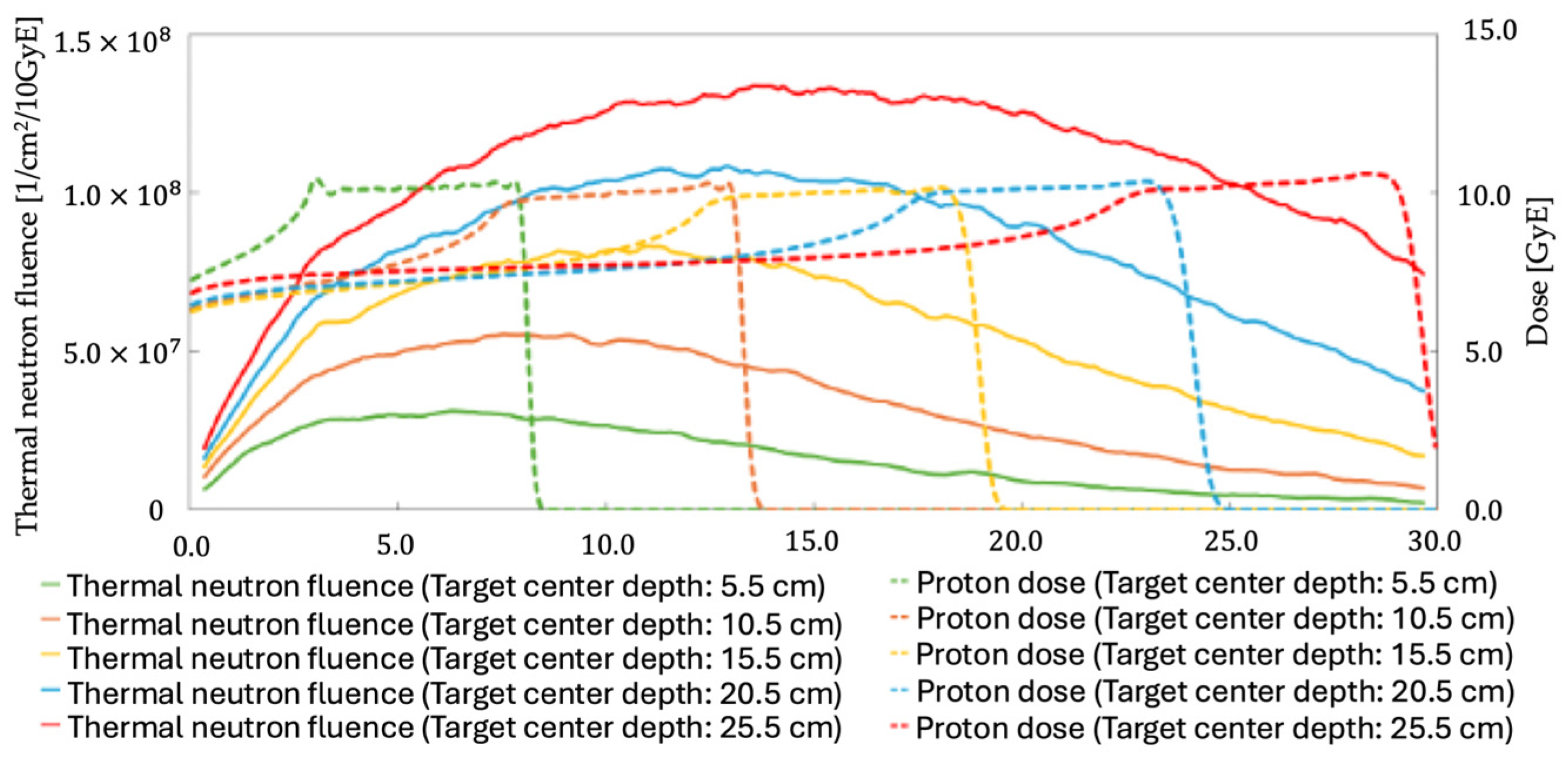
| Target Center Depth [cm] | Number of Proton-Beam Particles [n] | Average Thermal Neutron Fluence Within the Target [1/cm2/Gy] |
|---|---|---|
| 5.5 | 3.39 × 1011 | 2.98 × 107 |
| 10.5 | 3.45 × 1011 | 5.23 × 107 |
| 15.5 | 3.86 × 1011 | 7.14 × 107 |
| 20.5 | 4.35 × 1011 | 8.70 × 107 |
| 25.5 | 4.91 × 1011 | 1.01 × 108 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimo, T.; Shiba, S.; Watanabe, H.; Yamanaka, M.; Matsumoto, K.; Yamano, A.; Nagano, H.; Tokuuye, K. Investigating the Secondary Thermal Neutron Intensity of Neutron Capture-Enhanced Proton Therapy. Appl. Sci. 2025, 15, 6833. https://doi.org/10.3390/app15126833
Shimo T, Shiba S, Watanabe H, Yamanaka M, Matsumoto K, Yamano A, Nagano H, Tokuuye K. Investigating the Secondary Thermal Neutron Intensity of Neutron Capture-Enhanced Proton Therapy. Applied Sciences. 2025; 15(12):6833. https://doi.org/10.3390/app15126833
Chicago/Turabian StyleShimo, Takahiro, Shintaro Shiba, Hiroyuki Watanabe, Masashi Yamanaka, Kazuki Matsumoto, Akihiro Yamano, Hisato Nagano, and Kohichi Tokuuye. 2025. "Investigating the Secondary Thermal Neutron Intensity of Neutron Capture-Enhanced Proton Therapy" Applied Sciences 15, no. 12: 6833. https://doi.org/10.3390/app15126833
APA StyleShimo, T., Shiba, S., Watanabe, H., Yamanaka, M., Matsumoto, K., Yamano, A., Nagano, H., & Tokuuye, K. (2025). Investigating the Secondary Thermal Neutron Intensity of Neutron Capture-Enhanced Proton Therapy. Applied Sciences, 15(12), 6833. https://doi.org/10.3390/app15126833






