Abstract
Numerous remediation strategies exist for cyanobacterial harmful algal blooms (cyanoHABs); however, most are limited by challenges of scalability and adverse off-target effects on the surrounding ecosystem. Germicidal ultraviolet light (UV-C) has emerged as a promising method for suppressing cyanoHABs in a sustainable, chemical-free manner that is both scalable and results in limited off-target ecological effects in the surrounding area. In this study, the US Army Engineer Research and Development Center’s (ERDC)’s CyanoSTUNTM (Cyanobacterial Suppression Through Ultraviolet-Light-C Neutralization) vessel was deployed to a cyanoHAB as part of a field trial to determine whether UV-C could effectively suppress cellular growth, degrade associated cyanotoxins, and inhibit harmful phytoplankton species more readily than beneficial species without the addition of chemicals. The cyanoHAB exhibited an average cyanobacteria abundance of 3.75 × 105 cells/mL (n = 5, SD = 6.76 × 104 cells/mL) and average total microcystin concentration of 3.5 µg/L (n = 5; SD = 0.24 µg/L). Pre- and post-treatment samples were collected and re-grown for 9 days in the laboratory to observe differences in microcystin, chlorophyll a, and phycocyanin concentrations, optical density, cell density, and community composition. The results of the field trial showed that the CyanoSTUN UV-C treatment effectively suppressed the growth of the cyanobacteria community for approximately two days at the three tested UV-C doses. The CyanoSTUN UV-C treatment also demonstrated a sustained, dose-dependent effect on microcystin concentration; the average reduction in microcystin concentration for 15, 30, and 45 mJ/cm2 treatment doses was 31.6% (n = 10, SD = 20.1%; 1.3 µg/L reduced), 45.7% (n = 10, SD = 10.8%; 1.9 µg/L reduced), and 49.9% (n = 10, SD = 8.2%; 1.7 µg/L reduced), respectively, over the 9-day regrowth period. Non-cyanobacteria were too scarce in this CyanoHAB to conclude whether the CyanoSTUN UV-C inhibits harmful phytoplankton species more readily than beneficial species. Further field studies with the CyanoSTUNTM are required to validate performance under more severe cyanoHAB conditions, however the results reported herein from the first field trial with the CyanoSTUNTM suggest that this treatment method may offer water managers confronted with a CyanoHAB the ability to rapidly and safely pause a bloom for multiple days and reduce the risks posed by its associated cyanotoxins without adding chemicals.
1. Introduction
Cyanobacterial harmful algal blooms (cyanoHABs) are increasing in frequency, duration, scale, and severity in freshwater systems across the globe, and their adverse ecological and human health impacts are predicted to be exacerbated by the impacts of climate change [1,2]. CyanoHABs are caused by cyanobacteria that proliferate under favorable nutrient, water temperature, weather, and light conditions to form areas of high cell density that often release potent cyanotoxins, rendering water bodies unusable and potentially hazardous [1,3,4]. Numerous remediation strategies exist for cyanoHABs, including mechanical harvesting (i.e., physical removal of floating cyanobacteria), reduction in nutrients such as phosphorus through the use of sorbents or dredging, ultrasonic treatment to impair cell buoyancy, application of biocontrol/biomanipulation agents to stimulate growth of species competitive with or predatory to cyanobacteria (e.g., macrophytes and zooplankton), and application of chemical algicides and cyanocides (e.g., copper and aluminum salts), oxidants (e.g., hydrogen peroxide), and coagulants/flocculants (e.g., polyaluminum chloride and chitosan) [3,5]. Each of these remediation strategies has unique benefits and limitations; however, the two shortcomings consistent across all of the strategies are scalability and adverse off-target effects on the surrounding ecosystem due to imprecise, wide-area application [6]. One treatment technology that has shown promise in suppressing cyanoHABs in both laboratory and field studies and may face fewer obstacles to achieve scalability and ensure limited off-target ecological effects in the surrounding area is irradiation of cyanobacteria with germicidal ultraviolet light (UV-C).
Disinfection of water using UV-C is ubiquitous at drinking water and wastewater facilities across the United States and has demonstrated high efficacy in protecting human health through the inactivation of harmful pathogens [7,8]. The U.S. Environmental Protection Agency (EPA) (1999) states that UV disinfection is effective at inactivating most viruses, spores, and cysts, and that it has a shorter contact time when compared with other disinfectants. Additionally, there is no residual effect that can be harmful to humans or aquatic life [9]. UV-C has also been used in surface waters as a chemical-free biocide/herbicide to suppress the growth of unwanted flora and fauna for decades without introducing potentially harmful chemicals into the ecosystem [10]. For example, in situ exposure of certain submerged aquatic vegetation (SAV) to UV-C has been shown to significantly reduce plant biomass and height. In a field study, Carlson et al. (2022) reported a 100% reduction in biomass (complete eradication) of invasive curly leaf pondweed (Potamogeton crispus) after in situ irradiation with germicidal UV-C, and found mature plants were the most vulnerable/susceptible life stage to germicidal UV-C, with observed loss of turgor pressure and chlorophyll a [11]. Similarly, a pilot study conducted by the Lake Tahoe Resource Conservation District, in which a boat equipped with a UV-C lamp array (not contained within a reactor) irradiated various SAV species in both an enclosed waterbody (i.e., marinas) and open waterbody (i.e., beach littoral zone), showed that the application of UV-C results in mortality of SAV, and most macrophytes (macrophytes in the study included Eurasian watermilfoil (Myriophyllum spicatum), curly leaf pondweed (Potamogeton crispus), Richardson’s pondweed (Potamogeton richardsonii), Leafy pondweed (Potamogeton foliosus), Coontail (Ceratophyllum demersum), Elodea spp., and Sago pondweed (Stuckenia pectinate)) treated with UV-C exhibited signs of deterioration within 7 to 10 days following treatment [12].
Following a similar mechanism of action that affects waterborne pathogens and SAV, UV-C damages the genetic material within cyanobacterial cells (e.g., through the formation of pyrimidine dimers), resulting in either sublethal (e.g., growth suppression) or lethal effects. Tao et al. (2010) demonstrated in laboratory studies that UV-C at a wavelength of 254 nm (“UV254”) can disrupt intracellular processes within Microcystis aeruginosa, leading to both reversible and irreversible damage depending on the intensity of the radiation and the amount of time the cells are exposed. The UV-C dose can be tuned to a low/medium range (10–50 mJ/cm2) to either stun M. aeruginosa cells for a period of time without killing the cells or impacting their structural integrity—an important consideration if the cells contain potent cyanotoxins—or a high range (>50 mJ/cm2) to destroy the cells and prevent possible regrowth. The same study also revealed that cyanobacteria are more susceptible to damage from UV254 than other phytoplankton (e.g., green microalgae), suggesting that UV-C may be employed to suppress harmful cyanobacteria without significantly impacting microalgae that are beneficial to the ecosystem [13]. McGivney et al. (2015) also support this finding, reporting that the freshwater microalga Pseudokirchneriella subcapitata and the marine microalga Tetraselmis suecica both had a high tolerance to UV254 (LC50 = 236 mJ/cm2 and 353 mJ/cm2, respectively [14]. Related, bench-scale studies showed that UV254 photodegrades the cyanotoxin microcystin-LR (MC-LR), transforming it into non-toxic photolytic products [15].
Outside of the laboratory, the authors are only aware of two field-scale studies that attempted to suppress phytoplankton growth using UV-C, both of which were conducted in Japan. In Iseri and Sasaki (1994), a boat equipped with UV lamps was developed to suppress freshwater red tide of the dinoflagellate Peridinium bipes. The researchers showed 90–100% lethality of P. bipes depending on in situ exposure time (20 or 50 s) and cell concentration (<5.0 × 103 or >1.0 × 104 cells/mL) [16]. In Iseri and Maeda (2004), researchers developed a plankton control ship equipped with a UV irradiation system to examine the relationship between UV254 dose and inhibition of harmful red tide algae growth in the Ariake Sea, Kyushu, Japan. The study reported that a 40 s UV treatment with the experimental equipment immediately destroyed the cells or stopped activity in about 80% of the Chattonella spp. that dominated the bloom (neither UV intensity nor dose was reported [17].
Given the safety and efficacy that UV-C has proven for water and wastewater disinfection, as well as the promise that it has shown as an herbicide for suppressing invasive SAV, phytoplankton, and cyanobacteria, the goal of this study was to conduct a field trial to determine whether UV-C could be deployed to U.S. surface waters affected by cyanobacteria to sustainably and effectively suppress/stun cellular growth, degrade associated cyanotoxins, and inhibit harmful phytoplankton species more readily than beneficial species without the addition of chemicals. To achieve these ends, the US Army Engineer Research and Development Center (ERDC) engineered the CyanoSTUNTM (Cyanobacterial Suppression Through Ultraviolet-Light-C Neutralization) vessel, which irradiated cyanoHAB-contaminated surface waters with UV-C delivered by a wastewater-grade UV reactor mounted to the underside of a pontoon boat (Figure 1; [18,19]). The UV-C was therefore contained within the UV reactor (rather than applied to a wider area like many HAB remediation chemicals), so organisms that did not pass through the reactor control volume were unaffected. To the best of our knowledge, this study represents the first field-scale study in the United States to attempt to quantify the potential for UV-C to suppress phytoplankton and degrade harmful cyanotoxins.
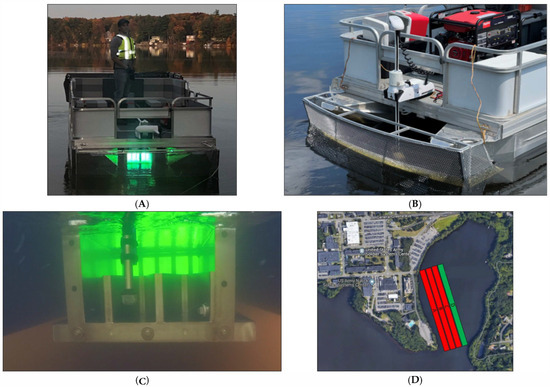
Figure 1.
(A) The ERDC CyanoSTUNTM vessel during operation. (B) A screen protects the UV reactor from debris, and a bow-mounted trolling motor maximizes mixing within the UV-C reaction zone. (C) The UV-C reactor beneath the CyanoSTUN pontoon platform (stern side depicted with weir and effluent sampling device)—water flows through the reactor as the boat moves forward. (D) Treatment areas as linear routes (image not to scale). Treatment Area A—low dose = 15 mJ/cm2. Treatment Area B—medium dose = 30 mJ/cm2. Treatment Area C—high dose = 45 mJ/cm2. Control Area D—no UV-C irradiation = 0 mJ/cm2.
2. Methods
2.1. Field Collection
The CyanoSTUN vessel was deployed to Lake Cochituate (Natick, MA, USA) on 12 September 2023 (Figure 1A–C). At the time of the field trial, all ponds associated with Lake Cochituate were under a Massachusetts Department of Public Health (DPH) “Harmful Cyanobacterial Bloom Advisory” and were closed to swimming and wading. The Massachusetts DPH recommends issuing a public health advisory for HABs at recreational freshwater locations when at least one of the following criteria is met: (1) a visible cyanobacteria scum or mat is evident, (2) total cell count of cyanobacteria exceeds 7.00 × 104 cells/mL, (3) concentration of the toxin microcystin exceeds 8 µg/L, or 4) concentration of the toxin cylindrospermopsin exceeds 15 µg/L [20].
Three treatment areas and a control area were examined for depth and submerged hazards and defined in Pegan Cove near the US Army Natick Soldier Systems Center (approximate coordinates 42°17′10.3″ N 71°21′35.7″ W, Figure 1D). The depth of the treatment areas ranged from 5 to 10 feet, and the pond bottom in these areas consisted primarily of mud. The three treatment areas corresponded to three UV-C experimental doses: low (15 mJ/cm2), medium (30 mJ/cm2), and high (45 mJ/cm2); the control area corresponded to no UV-C irradiation (0 mJ/cm2).
The CyanoSTUN UV-C reactor lamps (Trojan UV3000PTP, TrojanUV, London, ON, Canada) were turned on and given a 10 min warm-up period. Prior to field testing, the reactor lamps had been burned-in (i.e., run continuously) for 100 h to burn off impurities and stabilize lamp output, per EPA recommendations for low-pressure mercury vapor lamp operation [21]. Two composite sampler intake strainers (Global Water WS755, Global Water Resources, Phoenix, AZ, USA; size 20 mesh (841 microns)) were fastened at 10.16 cm (4 in.) water depth, one at the influent (herein referred to as front) end and one at the effluent (herein referred to as rear) end of the reactor. Total composite sample collection was set to 2.5 L with a sampling interval of 5 min and a sampling volume of 500 mL. A flow meter (FloWatch FW450, JDC Electronic SA, Yverdon-les-Bains, Switzerland) was fastened to the bow of the boat with the impeller set to 20.32 cm (8 in.) depth to monitor CyanoSTUN velocity.
The UV transmittance (UVT) of the water was measured using a Real UV254 P200 (Real Tech Inc., Whitby, ON, Canada). After the 10 min warm-up period, the UV intensity within the reactor was observed on the Trojan UV3000PTP System Monitor (TrojanUV, London, ON, Canada). Because the observed UV intensity represented a single point value at the exact location of the UV sensor within the CyanoSTUN reactor rather than the desired average UV intensity within the reactor, a conversion factor was applied to derive the average UV intensity from the point value measurement using Equation (1). The conversion factor (f) was determined by modeling the CyanoSTUN reactor design in UV Calc Premium (Bolton Photosciences Inc., Edmonton, AB, Canada) and identifying the relationship between the sensor intensity reading and average reactor intensity at various UV transmittances.
where
IA = IP × f
IA = Average UV-C Intensity within CyanoSTUN Reactor (mW/cm2);
IP = Point UV Intensity Detected by CyanoSTUN Sensor (mW/cm2);
f = Conversion Factor (unitless) = 0.3483e0.0078 * T;
T = UV Transmittance (%).
The CyanoSTUN target velocity was calculated to achieve the target treatment doses using Equation (2). Water temperature was recorded along with chlorophyll a and phycocyanin concentrations (ug/L) at depths of 15.24 cm, 30.48 cm, 60.96 cm, and 91.44 cm (6 in., 12 in., 24 in., 36 in.) using the YSI ProDSS Multiparameter Digital Water Quality Meter (YSI Inc., Yellow Springs, OH, USA). The YSI ProDSS contained a Total Algae PC sensor, TAL-PC, as well as a Conductivity and Temperature Sensor.
where
v = [V/(A × (D/IA))]/44.704
v = CyanoSTUN velocity (miles/hour);
V = CyanoSTUN Reactor Volume = 66,020 cm3;
A = CyanoSTUN Reactor Cross-Sectional Area (Front) = 483.87 cm2;
D = Target Treatment Dose (mJ/cm2);
IA = Average UV-C Intensity within CyanoSTUN Reactor (mW/cm2).
The target velocity for the control run (0 mJ/cm2) was set to the same target velocity as the low-dose treatment run (15 mJ/cm2), which was the fastest of the three treatment runs. For the control run, lamps were given 10 min to cool to ambient water temperature.
Treatment routes were navigated at the target velocity for 25 min or until 2.5 L had been collected in both the front and rear containers of the composite sampler. At the end of each treatment, 2 L from each composite sample container was transferred to two 1 L NalgeneTM Amber Bottles (Thermo Fisher Scientific Inc., Waltham, MA, USA) filled to the top to prevent any sloshing during transport to the laboratory. An additional 44.6 mL from each composite sample container was transferred to 50 mL falcon tubes pre-filled with 5.4 mL formaldehyde (final concentration of 4% formaldehyde) for Day 0 analysis, and 10 mL from each composite sample container was transferred to 15 mL falcon tubes for Day 0 toxin analysis.
Samples were shipped overnight to the ERDC Environmental Laboratory (Vicksburg, MS, USA) for analysis.
2.2. Laboratory Analysis
Upon arrival, the samples were kept at room temperature before the regrowth experiment was set up. The 2 L samples from each treatment run were transferred to three 1 L Erlenmeyer flasks with 600 mL of culture in each flask for triplicate (n = 24 flasks (4 treatment areas × 2 (front/back) × 3 replicates)), and no additional media was added to the cultures. Samples were incubated in a Percival Growth Chamber (Percival Scientific, Perry, IA, USA) at 25 °C with fluorescent lamps as the light source, an automated light/dark cycle of 12 h/12 h, with a light intensity of 2220 Lx during the lighting phase, and a UV-C intensity of 0 mJ/cm2. Flasks were kept on a ChemCell Orbital Shaker (Chemglass Life Sciences, Vineland, NJ, USA) at 85 rpm. Samples were grown for 9 days (a duration within which cellular and water quality changes were expected to be detected based on Tao et al. (2010) [13]) and measured daily for microcystin, chlorophyll a, phycocyanin, and optical density. Samples were also collected daily for cell density and community composition analyses. For each microcystin and cell density/community composition measurement, triplicate samples were composited into a single sample.
2.2.1. Microcystin
Composite samples were taken over the 9-day period to measure the change in microcystin samples. Microcystin was measured with the ABRAXIS® Microcystins-ADDA ELISA immunoassay (Gold Standard Diagnostics Horsham Inc., Warminster, PA, USA). Total microcystin was determined by first homogenizing samples in a Lysis Matrix B tube on a Fast Prep homogenizer (MP Biomedicals, Santa Ana, CA, USA) at 4.0 m/s for 1 min. This homogenization step was repeated two additional times, with samples being placed on ice for 1 min. After homogenization, samples were centrifuged at 12,000 rpm, and the resulting supernatant was retained and stored in a glass vial. Lysed samples were stored at −20 °C until experimentation and then thawed to room temperature to run the ELISA immunoassay.
2.2.2. Chlorophyll, Phycocyanin, and Optical Density
Chlorophyll a and phycocyanin were measured with the YSI ProDSS, and in vivo chlorophyll a and phycocyanin were measured with the AquaPen-C (Photon Systems Instruments, Drasov, Czech Republic). Chlorophyll a and phycocyanin were measured on the YSI ProDSS by placing the probe into the 1 L flask and waiting for the reading to stabilize before recording the measurements for chlorophyll a and phycocyanin. For in vivo chlorophyll a and phycocyanin, samples were collected into a cuvette and placed in the dark until ready for measurement. Measurements were made by recording the Ft 450 for in vivo chlorophyll a and Ft 620 for in vivo phycocyanin (Ft stands for continuous fluorescence yield in non-actinic light). Optical density was recorded utilizing a Shimadzu UV-1800 UV-VIS Spectrophotometer (Schimadzu Scientific Instruments, Columbia, MD, USA). The instrument was blanked using a cuvette filled with filtered water from Lake Cochituate, and then samples were read at 680 nm, and the absorbance was recorded.
2.2.3. Cell Density and Community Composition
Samples were preserved for analysis of cell density and community composition at the University of Florida Institute of Food and Agricultural Sciences (UF/IFAS). Briefly, researchers combined 15 mL from each triplicate into a 50 mL falcon tube and added 5.4 mL formaldehyde/formalin (4%) to the composite sample for preservation. The Utermöhl method [22] was performed, and samples were enumerated using an inverted microscope to determine cell density (cells/mL) for the lowest taxonomic unit possible for all phytoplankton identified in the samples, as well as community composition and relative abundance (% composition) for each of the lowest taxonomic units identified. Samples were also examined for any lysing or distortion of cells. Due to contractual limitations and cost constraints, only 5 days of samples (instead of all 9 days) could be analyzed post-treatment for cell density and community composition. The “Day 5 Rear (45 mJ/cm2)” sample was compromised during transport to UF/IFAS and could not be analyzed.
2.3. Statistical Analysis
A statistical analysis was conducted to assess whether “front” labeled data were statistically different than “rear” labeled data associated with varied UV treatment. Due to the low number of replicate measurements (n = 3), two methods of data aggregation were used to prepare datasets for statistical analysis. In the first “pooled” approach, untreated sample data were pooled across all treatment days to inform a “control” distribution of values. Comparison of treatment values to this common control distribution will establish the meaning of statistical difference. Thus, we made two major assumptions regarding the data collected in the field. Our first assumption is that control measurements are free of UV treatment at the time of data collection. Our second assumption regards null treatment data as identical and independently distributed random variables. Under this pooling procedure, a deviation in rear measurements from those of the pooled front control samples would provide fidelity of the statistical significance generally associated with UV treatment. Aside from these front “control” data, treatment measurements were analyzed on a per-day basis; that is, assays allowed to mature over time were analyzed individually and independently of data reported for other durations. For each such duration, front-labeled data were pooled across UV treatment intensity for each assay type (e.g., Chlorophyll), resulting in 12 samples. These 12 samples were compared against 3 replicates reported for each UV treatment intensity per assay type using methods of statistical significance described below.
This pooled approach should be contrasted against the alternative “unpooled” approach, wherein front and rear labeled measurements are jointly conditioned on each treatment day and no others. Although this unpooled approach avoids bias from groupings with correlation spanning treatment days, it otherwise reflects a low number of replicate measurements, and statistical confidence is therefore limited.
There is a risk that, for data collected in the field on each day, the front (UV = 0 mJ/cm2) data will be correlated across UV treatment conditions, potentially due to imperfect sampling, drift in water currents, mixing from the boat wake, etc. If so, then the pooling methodology described above would be invalidated. Analysis of Variance (ANOVA) was used to test for these potential, albeit linear correlations. Briefly, we use ANOVA to test whether, for each observable (Chlorophyll, Phycocyanin, Temperature, etc.), the front data will be linearly correlated with the magnitude of UV treatment, and the algorithm tests whether the means of each treatment grouping are statistically equal to all others by assuming the data are independent and identically distributed (iid) random variables with equal population variances. Given the low number of replicate samples per UV treatment group (n = 3), a rejection of the null hypothesis is not a strong indicator that front treatments are correlated with UV treatment intensity, and so would not necessarily require that results from a pooled methodology be invalidated. It is nevertheless useful to assess these statistics because they contextualize the degree of confidence we can assign to these pooled results. The results from this analysis are illustrated in Table 1. The entries of this table quantify the probability, p, that the means of all front-collected data are statistically identical. Thus, for values p < 0.05, all front-collected data cannot be considered as statistically identical for each condition (i.e., at least 1 UV treatment front-collected grouping differs from the others), and thus the non-pooled methodology would be the best choice for data comparison.

Table 1.
Empirical assessment of correlations in ‘front’ labeled data associated with each observation category. Entries represent the probability, p, that ‘front’ labeled data are statistically similar (one-way ANOVA). A value p < 0.05 indicates that ‘front’ labeled data in which UV = 0 mJ/cm2 are not statistically identical, and thus could represent a linear trend.
Given control data derived from either the pooled and unpooled approaches, we chose two common methods for which to quantify statistical significance: the nonparametric Kolmogorov–Smirnov (KS) and the parametric Student’s t-test (T). The two-sample KS test makes few assumptions about the distributions from which the data are drawn. To determine whether two sets of data samples are drawn from the same distribution, the cumulative distributions are calculated, and a distance measure (the KS statistic) is used to assess whether the distributions are different. Confidence in the truth of this method increases with the number of samples, because it is easier for the method to distinguish between distributions (the KS statistic is compared against a value which decreases with the square root of the sample size). The Student’s t-test assumes the data are unimodal and can be modeled using a Student’s t-distribution. To apply the t-test, we transform each replicate from each UV-treatment by the mean value of either the pooled or unpooled “control” distribution. The output of this method is the probability that the shifted distribution has a mean equal to zero, considering that the variance follows that of the Student’s t-distribution. For both the KS and T tests, we apply a desired level of significance of 0.05 in accordance with water quality practice [23].
3. Results and Discussion
A blue-green sheen was noticeable at the US Army Natick Soldier Systems Center boat ramp extending into Pegan Cove, indicating the likely presence of cyanobacteria. Throughout the four runs, water temperature ranged from 24.7 to 25.0 °C (n = 8; M = 24.8 °C, SD = 0.15 °C), UVT ranged from 48.9 to 50.1% (n = 8; M = 49.4%, SD = 0.45%), chlorophyll a concentration ranged from 3.8 to 21.0 µg/L (n = 48; M = 8.16 µg/L, SD = 4.71 µg/L), and phycocyanin concentration ranged from 0.97 to 3.76 µg/L (n = 48; M = 2.52 µg/L, SD = 1.20 µg/L). In general, chlorophyll a and phycocyanin concentrations showed a slight positive correlation to depth, with the lowest readings occurring at 15.24 cm (6 in.), slight increases at 30.48 cm (12 in.) and 60.06 cm (24 in.), and the highest readings occurring at 91.44 cm (36 in.).
Using Equation (2), the CyanoSTUN target velocities were calculated, and the vessel was driven at 0.55 miles per hour (mph), 0.29 mph, and 0.18 mph to achieve the target treatment doses of (15 mJ/cm2), (30 mJ/cm2), and (45 mJ/cm2), respectively. Water flow rates through the CyanoSTUN reactor were therefore 11.9 L/s (5.55 s exposure time), 6.3 L/s (10.5 s exposure time), and 3.9 L/s (16.9 s exposure time) for the 15 mJ/cm2, 30 mJ/cm2, and 45 mJ/cm2 runs, respectively. The target velocity for the control run (0 mJ/cm2) was 0.55 mph (the same target velocity as the 15 mJ/cm2 treatment run).
3.1. Microcystin
The CyanoSTUN UV-C treatment demonstrated a sustained, dose-dependent effect on microcystin concentration over the 9-day regrowth period. Figure 2A shows that samples collected from the rear of the vessel had a significantly reduced microcystin concentration compared to samples collected from the front of the vessel. Figure 2B shows that the average reduction in microcystin concentration for 15, 30, and 45 mJ/cm2 treatment doses was 31.6% (n = 10, SD = 20.1%; 1.3 µg/L reduced), 45.7% (n = 10, SD = 10.8%; 1.9 µg/L reduced), and 49.9% (n = 10, SD = 8.2%; 1.7 µg/L reduced), respectively, over the 9-day regrowth period. These findings suggest that the UV254 emitted from the reactor may have induced direct photolysis of microcystin. It is also possible that the low-pressure mercury lamps within the Trojan UV3000PTP reactor emitted radiation at other wavelengths than 254 nm, which had an impact on microcystin degradation. For example, low-pressure mercury lamps typically emit 185 nm radiation at approximately 8% of the intensity of 254 nm radiation [24]. If this 185 nm radiation is not completely eliminated by the quartz sleeve, then the 185 nm radiation would be absorbed by the ambient water, leading to photochemical ionization and the production of hydroxyl radicals capable of oxidizing microcystin. It is unclear why the vessel had an effect on microcystin concentration at the control dose (0 mJ/cm2; 17.8% reduction (n = 10, SD = 13.3%; 0.8 µg/L reduced), however it is possible that some microcystin adsorption occurred on the polytetrafluorethylene diffuse reflector sheeting that lined the interior stainless-steel walls of the reactor to increase UV254 reflectance.
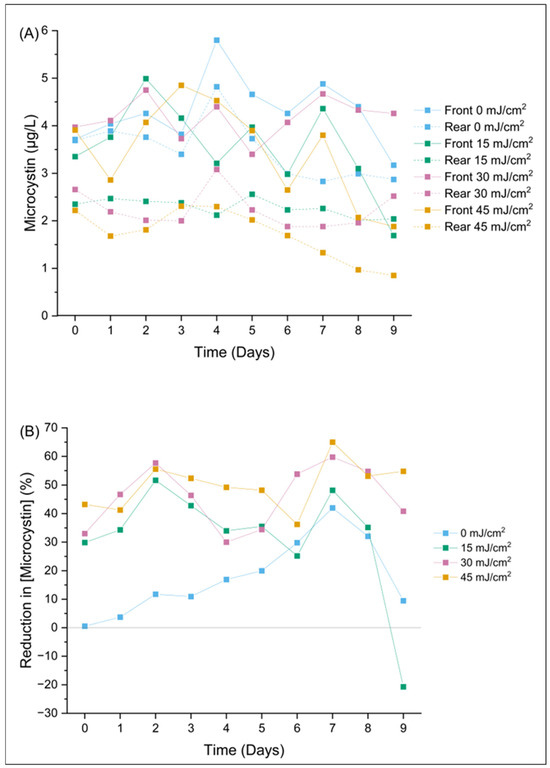
Figure 2.
Changes in composite microcystin samples are shown by (A) the concentration of microcystin (µg/L) over a 9-day period post-field trial and (B) the percent reduction in microcystin concentration over a 9-day period post-field trial.
3.2. Chlorophyll
Results from the Kolmogorov–Smirnov and Student’s t-test suggest that chlorophyll a concentrations were reduced by the CyanoSTUN UV-C treatment at a statistically significant level (Figure 3; Table 1, Table 2, and Table S1), but this determination is not definitive, because the statistical significance of treatment conditions varied across the regrowth period. For example, for Day 1 of the regrowth period (Table 2), chlorophyll a data at the 15 mJ/cm2 dose exhibited statistical significance (p < 0.05) from control data for KS and T tests applied using both pooled and unpooled analysis methods. However, none of the other doses exhibited this level of statistical significance on Day 1 of the regrowth period. Importantly, for the control dose (0 mJ/cm2), the front data were not statistically different from the rear measurements (Table 1), indicating that measurements were reliable despite the few replicates provided. Across Days 2–9 of the regrowth period, chlorophyll a concentrations affected by UV-C treatment demonstrated significant differences between treatment doses and control demonstrated on some days but not others. The authors conclude that it is likely that, at least in the earlier days of the 9-day regrowth period, chlorophyll a is affected by the UV-C treatment.
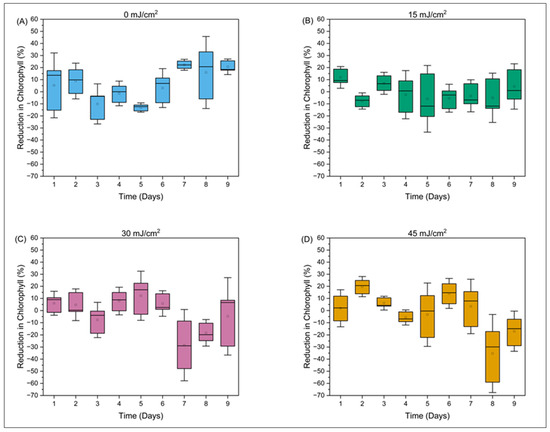
Figure 3.
The percent reduction in chlorophyll a over a 9-day period post-field trial is shown at different concentrations (A) 0 mJ/cm2, (B) 15 mJ/cm2, (C) 30 mJ/cm2, and (D) 30 mJ/cm2.

Table 2.
Representative statistics of the Kolmogorov–Smirnov (KS) and the parametric Student’s t-test (T), for ‘front’ pooled control data and unpooled alternative. Shown is the probability, p, that the representative statistic is as ‘extreme’ or more so than the null hypothesis; for example, p < 0.05 can typically be associated with statistical significance in relation to aqueous environments. Data shown for observables reported for ‘Day 1’ assays.
3.3. Phycocyanin and Other Measured Parameters
Statistical analyses showed that no other measured parameters—phycocyanin, optical density at 680 nm, relative fluorescence yield (Ft) at 450 nm (in vivo chlorophyll a), Ft at 620 nm (in vivo phycocyanin), and quantum yield—were affected by UV treatment at a statistically significant level (see, e.g., Table 1 and Table 2). Table S2 shows a general decrease in phycocyanin concentrations over the 9-day regrowth period; however, this decrease is independent of treatment (front vs. rear) or dose. Figure 4 shows that the percent reduction in phycocyanin shows no discernible trend and no correlation to UV-C dose.
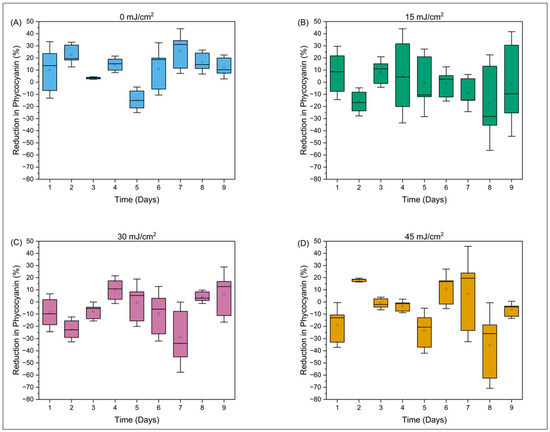
Figure 4.
The percent reduction in phycocyanin over a 9-day period post-field trial is shown at different concentrations (A) 0 mJ/cm2, (B) 15 mJ/cm2, (C) 30 mJ/cm2, and (D) 45 mJ/cm2.
3.4. Cell Density and Community Composition
Results from the algal identification services provided by UF/IFAS showed that the most prominent families in the Lake Cochituate cyanoHAB included Bacillariophyta, Charophyta, Chlorophyta, Cryptophyta, Cyanobacteria, Dinophyta, and Euglenophyta. The family that dominated this cyanoHAB was Cyanobacteria. The 5 most prominent species were Planktolyngbya cf. limnetica, Raphidiopsis spp., Planktothrix agardhii, Dolichospermum planctonicum, and Aphanizomenon cf. gracile, combining for 94.3% of the community composition (60.9%, 17.9%, 10.4%, 2.8%, and 2.4%, respectively), on average throughout the regrowth period. Individual cells in each strand of filament were counted to make comparisons in cell density between filamentous and free-swimming cyanobacterial cells.
In general, for total cyanobacteria present (16 species) in the samples, the CyanoSTUN UV-C treatment suppressed growth for approximately two days, with pre-treatment samples showing a recovery of cell density around Day 1 or 2 of the regrowth period and post-treatment samples showing recovery around Day 4 (Figure 5 and Figure 6). This trend appears to be independent of UV dose; however, the sample for Day 5 for the 45 mJ/cm2 dose was cracked during shipment and could not be analyzed, so it is unclear whether the cyanobacteria population exposed to the highest treatment dose rebounded after Day 4 or remained suppressed. Additionally, the trend varied slightly at the genus level, with the 2-day suppression appearing most distinctly with Planktolyngbya cf. limnetica, which dominates the community (60.9%) in the samples and likely skews the results for total cyanobacteria. In Figure 5, the initial decrease in cell density in the early timepoints for both the front and rear samples may be attributable to the shock of collection, shipment, and introduction to a growth chamber, all within 24 h.
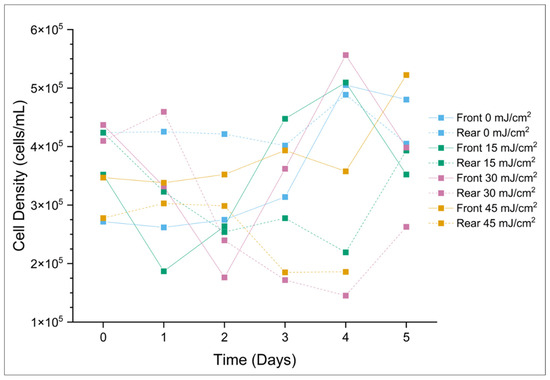
Figure 5.
Total cyanobacterial cell density (cells/mL) over a 5-day period post-field trial. The “Day 5 Rear (45 mJ/cm2)” sample was compromised during transport to UF/IFAS and could not be analyzed.
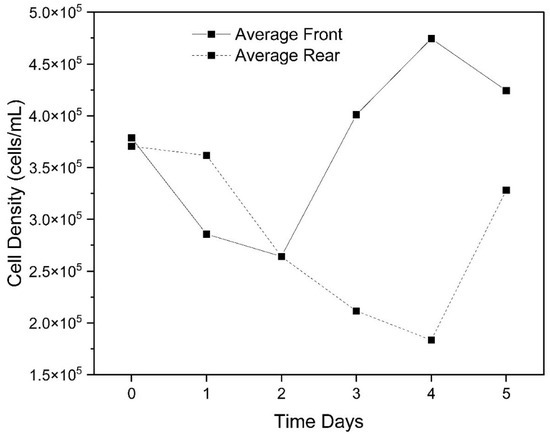
Figure 6.
Average of total cyanobacterial cell density (cells/mL) in the front (pre-treatment) vs. the rear (post-treatment samples) over a 5-day period post-field trial (excluding control dose).
Reduction in total cyanobacteria cell density due to the CyanoSTUN UV-C treatment peaked around Day 4 (Figure 7), with the 30 mJ/cm2 dose yielding the greatest difference (4.11 × 105 cells/mL; 73.9%) of the treatments, followed by the 15 mJ/cm2 dose (2.90 × 105 cells/mL; 57.0%), 45 mJ/cm2 dose (1.72 × 105 cells/mL; 48.0%) and control (1.70 × 105 cells/mL; 3.3%).
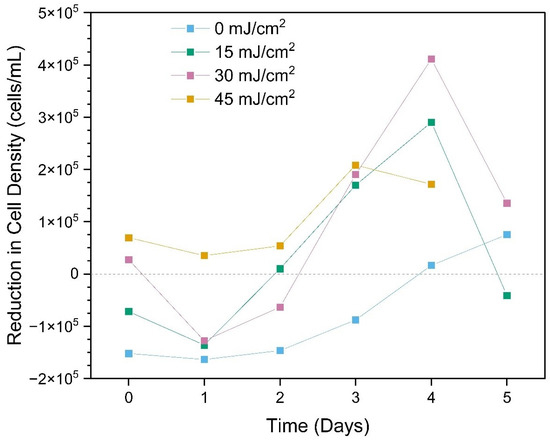
Figure 7.
Reduction in total cyanobacterial cell density due to treatment over a 5-day period post-field trial. The “Day 5 Rear (45 mJ/cm2)” sample was compromised during transport to UF/IFAS and could not be analyzed.
It should be noted that microcystin-producing species were relatively scarce (<15% composition), which explains the relatively low levels of microcystin detected (Figure 2). In addition to growth suppression, the CyanoSTUN UV-C treatment appeared to have some adverse effect on cellular integrity for specific cyanobacteria species. The UF/IFAS laboratory reported distortion of cells where post-treatment samples appeared to be more degraded; filaments on the Aphanizomenon, Raphidiopsis, and Dolichospermum species showed the most pronounced distortion and fragmentation, whereas Planktothrix showed the least.
Non-cyanobacteria were very scarce, comprising only 0.26% of the community, on average. In general, this group of species shows slow but consistent growth in pre-treatment samples over the regrowth period, whereas post-treatment samples show a decline in cell density from Day 0 to Day 1 and then steady maintenance of the population (neither growth nor decline) through Day 5 (Figure 8). At such low cell densities, we are unable to conclude whether the CyanoSTUN UV-C inhibits cyanobacteria more readily than non-cyanobacteria.
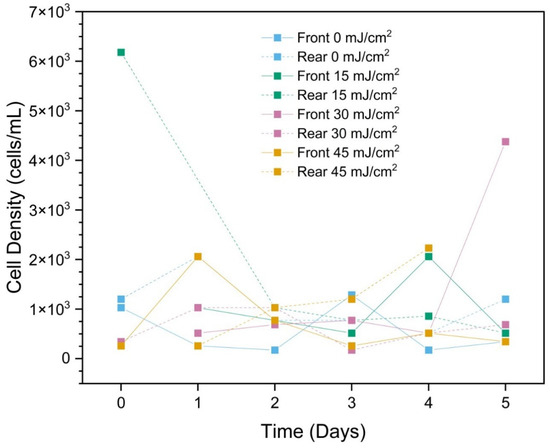
Figure 8.
Total non-cyanobacterial cell density (cells/mL) over a 5-day period post-field trial. The “Day 5 Rear (45 mJ/cm2)” sample was compromised during transport to UF/IFAS and could not be analyzed.
3.5. Future Field Studies
As the cell density and microcystin concentration within this particular cyanoHAB were comparatively low, additional field studies with the CyanoSTUNTM should be conducted in surface waters experiencing more severe cyanoHABs to validate performance under more challenging conditions. It may also be advantageous to conduct future field studies in the morning when many species of planktonic cyanobacteria accumulate at the surface after consuming a significant portion of their reserve carbohydrates during the dark, and form floating scums [3]. Future field studies should examine the effects of the CyanoSTUN UV-C treatment on additional cyanotoxins, including cylindrospermopsin, saxitoxin, and anatoxin-a, as there is little data on methods to control these toxins.
Additionally, future studies should measure the CyanoSTUN’s impact on zooplankton and other small aquatic organisms. While the CyanoSTUN’s design restricts all UV-C radiation to the control volume of its reactor, thereby preventing off-target ecological effects from occurring in the surrounding water, it is still possible that off-target effects will occur inside the reactor should non-target organisms pass through the 0.5-inch diameter holes of the CyanoSTUN’s bow-mounted screen. Due to funding constraints, zooplankton and other small aquatic organisms could not be monitored in this study, but they are expected to experience some effects from UV-C exposure should they enter the reactor. For example, zooplankton are known to be susceptible to DNA damage from UV-B radiation (Hansson and Hylander, 2009), and because UV-C is more reactive than UV-B and also absorbed by DNA, UV-C may be more harmful to zooplankton than UV-B. Thus, exposure to the UV-C from the CyanoSTUN treatment may adversely impact behavioral responses, fecundity, and, at a sufficient dose, cause increased mortality of zooplankton, among other possible adverse effects. However, Hansson and Hylander (2009) note that some zooplankton are less affected by UV radiation either due to possessing photoprotective compounds that prevent UV damage or having the capability to repair UV damage through photoenzymatic repair, so the precise impacts of the CyanoSTUN on zooplankton and other small aquatic organisms cannot be discerned without further study [25].
Scalability of UV-C is a key advantage of the technology. In terms of treatment scale for the current CyanoSTUN design, our modeling in UV Calc Premium software 2023 showed that the CyanoSTUN can treat a HAB with a surface area of 4.05 × 103 m2 (1 acre) to 12.7 cm depth (5 in.) in 11.9 h at a UV dose of 20 mJ/cm2 assuming a bloom UVT of 50% and 9.13 h assuming a bloom UVT of 60% [18]. Currently, the CyanoSTUN vessel is designed with one wastewater-grade UV reactor because the chassis vessel is small (4.14 m in length); however, the treatment capability of the CyanoSTUN scales linearly with each additional reactor. For example, a vessel equipped with two of the Trojan UV3000PTP reactors used here could treat the same area as our study in either half the time or with double the UV-C dose, or it could provide equivalent treatment to this study but in surface waters with lower UVTs than the approximately 50% encountered in Lake Cochituate. Adding UV-C reactors to the underside of pontoon- or catamaran-style hulls (i.e., two hulls connected by a platform) is straightforward but does require longer vessels with higher weight capacities (e.g., barges) as the number of reactors increases.
In this study, we chose to monitor pre- and post-treatment samples in a controlled laboratory environment rather than in the treated areas in Lake Cochituate due to challenges in controlling environmental conditions in open water, such as surface and water column mixing between the treatment zones. However, it may be beneficial to test the CyanSTUNTM in controlled waterbodies such as experimental ponds to observe changes in the complete water body post-treatment. Future field studies with the CyanoSTUNTM should also consider alternative motor configurations and improved flow metering capabilities, as it was challenging for the field researchers to maintain the speed of the vessel at the low velocities required in this study using the current electric motor configuration. Additionally, the CyanoSTUNTM had a fixed reactor depth, which was challenging to establish at 12.7 cm (5 in.) and keep constant throughout the field studies as the field researchers moved about the vessel, causing the boat platform and affixed reactor to tip side-to-side and front-to-back. An alternative engineering design should be considered to enable variable depth control of the reactor and potentially independent leveling relative to the boat platform, especially since chlorophyll a and phycocyanin concentrations showed a slight positive correlation to depth in this study, with the lowest readings occurring near the surface and the highest readings occurring at 91.44 cm (36 in.; farthest from the UV reactor). Improved depth control would also be advantageous when environmental conditions are such that the target cyanobacterial biomass is more distributed in the water column rather than concentrated at the surface.
4. Conclusions
In this study, the ERDC CyanoSTUNTM vessel was deployed to a cyanoHAB in Massachusetts as part of a field trial to determine whether UV-C could effectively suppress/stun cellular growth, degrade associated cyanotoxins, and inhibit harmful phytoplankton species more readily than beneficial species without the addition of chemicals. The results from this first field trial with the CyanoSTUNTM suggest that this treatment method may offer water managers confronted with a CyanoHAB the ability to rapidly and safely pause a bloom for multiple days and reduce the risks posed by its associated cyanotoxins without adding chemicals. The CyanoSTUN UV-C treatment effectively suppressed the growth of the cyanobacteria community for approximately two days at the three tested UV-C doses. This effect did not show a relationship with UV dose; thus, a dose–response relationship between UV-C dose and duration of cyanoHAB suppression could not be established. However, it is unclear whether the cyanobacteria population exposed to the highest treatment dose may have remained suppressed for longer than two days, due to the fact that a key sample was compromised during transport to UF/IFAS.
The CyanoSTUN UV-C treatment also demonstrated a sustained, dose-dependent effect on microcystin concentration, possibly as a result of direct photolysis by UV254 or indirect destruction by UV185-induced reactive oxygen species. Additionally, the CyanoSTUN UV-C treatment reduced chlorophyll a concentration at a statistically significant level for some of the regrowth period, but had no significant effect on phycocyanin, optical density at 680 nm, Ft 450 (in vivo chlorophyll a), Ft 620 (in vivo phycocyanin), or quantum yield. Analysis of cell density and community composition revealed that non-cyanobacteria were very scarce in this CyanoHAB (only 0.26% of the community, on average); at such low cell densities, we are unable to conclude whether the CyanoSTUN UV-C inhibits harmful phytoplankton species more readily than beneficial species without the addition of chemicals. As the cell density and microcystin concentration within this particular cyanoHAB were comparatively low, additional field studies with the CyanoSTUNTM should be conducted in surface waters experiencing more severe cyanoHABs to validate performance under more challenging conditions.
Because the CyanoSTUN treatment does not impact water quality parameters or environmental conditions that are supportive of HABs, it cannot permanently eliminate blooms from a water body. However, the results of this study suggest that the CyanoSTUN may be a useful tool for either mitigating an ongoing bloom (reactive treatment) or preventing a future bloom (proactive treatment) through the short-term suppression of cyanobacterial growth. By targeting the suppression of cyanobacterial cell growth, the CyanoSTUN appears capable of pausing a bloom so it cannot worsen. Extending these findings to the case of proactive treatment, the CyanoSTUN may be able to suppress cyanobacterial growth in a target area that is not experiencing a bloom but is suspected to experience one imminently, such that the suspected bloom’s development is delayed or does not occur at all. The results of this study indicate that the “pause” period will last for approximately two days before the cells within the bloom recover. During this two-day period, a water manager may take several courses of action including but not limited to using the CyanoSTUN again to extend the “pause” period, using a different treatment method to remove or kill the cells while they are stunned, or letting a natural event (e.g., rain event, cold front, etc.) take its course and disrupt the bloom naturally while the cells cannot self-regulate.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app15126765/s1, Table S1: Changes in chlorophyll a are shown by the concentration of chlorophyll a (µg/L) over 9-day period post-field trial; Table S2: Changes in phycocyanin are shown by the concentration of phycocyanin (µg/L) over 9-day period post-field trial.
Author Contributions
Conceptualization: T.R.; developing methods, data analysis: T.R., B.F., and M.L.M.; preparation of figures and tables: T.R., and B.F.; conducting the research, data interpretation: T.R., B.F., and M.L.M.; Writing: T.R., B.F., and M.L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Aquatic Nuisance Species Research Program (ANSRP) Harmful Algal Bloom Congressional Interest of the U.S. Army Engineer Research & Development Center.
Institutional Review Board Statement
Not applicable
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
At the time of publication, the ANSRP Manager was Michael Greer, the Strategic Initiatives Program Manager was Mandy Michalsen, and the Technical Director was Jennifer Seiter-Moser. The authors would like to acknowledge Dail Laughinghouse at the University of Florida Institute of Food and Agricultural Sciences at the Fort Lauderdale Research and Education Center for providing algae identification services, Jazmine Davalos and Griffin Donohue for providing field trial support, and Andrew McQueen and Alyssa Eck for providing internal peer review and editorial feedback. Correspondence concerning this article should be addressed to Taylor Rycroft at taylor.e.rycroft@usace.army.mil.
Conflicts of Interest
The authors declare no conflicts of interest. The scientific results and conclusions, as well as any views or opinions expressed here, are those of the authors and do not necessarily reflect those of the U.S. Army Engineer Research and Development Center, and should not be construed as an Official Department of the Army position or decision unless so designated by other official documentation.
References
- Pound, H.L.; Martin, R.M.; Sheik, C.S.; Steffen, M.M.; Newell, S.E.; Dick, G.J.; McKay, R.M.L.; Bullerjahn, G.S.; Wilhelm, S.W. Environmental studies of cyanobacterial harmful algal blooms should include interactions with the dynamic microbiome. Environ. Sci. Technol. 2021, 55, 12776–12779. [Google Scholar] [CrossRef] [PubMed]
- Mishra, D.R.; Kumar, A.; Ramaswamy, L.; Boddula, V.K.; Das, M.C.; Page, B.P.; Weber, S.J. CyanoTRACKER: A cloud-based integrated multi-platform architecture for global observation of cyanobacterial harmful algal blooms. Harmful Algae 2020, 96, 101828. [Google Scholar] [CrossRef] [PubMed]
- Sukenik, A.; Kaplan, A. Cyanobacterial harmful algal blooms in aquatic ecosystems: A comprehensive outlook on current and emerging mitigation and control approaches. Microorganisms 2021, 9, 1472. [Google Scholar] [CrossRef] [PubMed]
- Buratti, F.M.; Manganelli, M.; Vichi, S.; Stefanelli, M.; Scardala, S.; Testai, E.; Funari, E. Cyanotoxins: Producing organisms, occurrence, toxicity, mechanism of action and human health toxicological risk evaluation. Arch. Toxicol. 2017, 91, 1049–1130. [Google Scholar] [CrossRef] [PubMed]
- Anabtawi, H.M.; Lee, W.H.; Al-Anazi, A.; Mohamed, M.M.; Hassan, A.A. Advancements in Biological Strategies for Controlling Harmful Algal Blooms (HABs). Water 2024, 16, 224. [Google Scholar] [CrossRef]
- Anderson, D.M. Approaches to monitoring, control and management of harmful algal blooms (HABs). Ocean. Coast. Manag. 2009, 52, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Yoon, H.-W.; Lee, M.-A.; Kim, Y.-H.; Lee, C.J. Impact of UV-C Irradiation on Bacterial Disinfection in a Drinking Water Purification System. J. Microbiol. Biotechnol. 2023, 33, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Collivignarelli, M.C.; Abbà, A.; Miino, M.C.; Caccamo, F.M.; Torretta, V.; Rada, E.C.; Sorlini, S. Disinfection of Wastewater by UV-Based Treatment for Reuse in a Circular Economy Perspective. Where Are We at? Int. J. Environ. Res. Public Health 2021, 18, 77. [Google Scholar] [CrossRef] [PubMed]
- USEPA (US Environmental Protection Agency). Wastewater Technology Fact Sheet: Ultraviolet Disinfection. EPA 832-F-99-064. Available online: https://www3.epa.gov/npdes/pubs/uv.pdf (accessed on 21 December 2023).
- Li, S.; Tao, Y.; Zhan, X.M.; Dao, G.H.; Hu, H.Y. UV-C irradiation for harmful algal blooms control: A literature review on effectiveness, mechanisms, influencing factors and facilities. Sci. Total Environ. 2020, 723, 137986. [Google Scholar] [CrossRef] [PubMed]
- Carlson, E.; Everest, E.; Suenaga, E.; Chandra, S.; Paoluccio, J. Using Ultraviolet Light-C to Control Aquatic Invasive Plant Populations in Lake Tahoe: Lab Experiment to Field Testing. In Proceedings of the Joint Aquatic Sciences Meeting, Grand Rapids, MI, USA, 14–20 May 2022. [Google Scholar]
- Tahoe Resource Conservation District (RCD), UV-C Light Plant Control Advisory Team Aquatic Invasive Plant Control Pilot Project Final Monitoring Report. Available online: https://www.tahoercd.org/files/7ce178157/UV_Plant_Control_Pilot_2018_Monitoring_FINAL.pdf (accessed on 21 December 2023).
- Tao, Y.; Zhang, X.; Au, D.W.; Mao, X.; Yuan, K. The effects of sub-lethal UV-C irradiation on growth and cell integrity of cyanobacteria and green algae. Chemosphere 2010, 78, 541–547. [Google Scholar] [CrossRef] [PubMed]
- McGivney, E.; Carlsson, M.; Gustafsson, J.P.; Gorokhova, E. Effects of UV-C and Vacuum-UV TiO2 Advanced Oxidation Processes on the Acute Mortality of Microalgae. Photochem. Photobiol. 2015, 91, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Barancheshme, F.; Sikon, K.; Keen, O. Loss of toxicity of microcystins in UV/H2O2 and UV/Cl2 treatment. J. Water Process Eng. 2024, 57, 104707. [Google Scholar] [CrossRef]
- Iseri, Y.; Kawabata, Z.I.; Sasaki, M. Development of a boat equipped with UV lamps for suppression of freshwater red tide in a reservoir. Jpn. J. Water Treat. Biol. 1994, 29, 61–70. [Google Scholar] [CrossRef]
- Iseri, Y.; Nakamichi, M.; Maeda, T. Development of a red tide control ship equipped with a UV irradiation system-preliminary and field experiments on harmful marine algae. In Proceedings of the Oceans’ 04 MTS/IEEE Techno-Ocean’04 (IEEE Cat. No. 04CH37600), Kobe, Japan, 9–12 November 2004; Volume 3, pp. 1787–1792. [Google Scholar]
- Rycroft, T. Guidance for Managers of USACE Waterbodies: Deploying the ERDC CyanoSTUNTM Vessel for Suppression of Cyanobacterial Harmful Algal Blooms; US Army Engineer Research and Development Center: Vicksburg, MS, USA, 2025. [CrossRef]
- Rycroft, T.; Tomberg, Z.; Donohue, G. Floatation Apparatus and Method of Treating Liquid Medium. U.S. Patent No. 18/130,884, 4 April 2023. [Google Scholar]
- Department of Public Health Massachusetts. Guidelines for Cyanobacteria at Recreational Freshwater Locations. Available online: https://www.mass.gov/info-details/guidelines-for-cyanobacteria-at-recreational-freshwater-locations (accessed on 9 June 2025).
- USEPA (US Environmental Protection Agency). Ultraviolet Disinfection Guidance Manual for the Final Long Term 2 Enhanced Surface Water Treatment Rule. EPA 815-R-06-007. Available online: https://nepis.epa.gov/Exe/ZyNET.exe/600006T3.TXT?ZyActionD=ZyDocument&Client=EPA&Index=2006+Thru+2010&Docs=&Query=&Time=&EndTime=&SearchMethod=1&TocRestrict=n&Toc=&TocEntry=&QField=&QFieldYear=&QFieldMonth=&QFieldDay=&IntQFieldOp=0&ExtQFieldOp=0&XmlQuery=&File=D%3A%5Czyfiles%5CIndex%20Data%5C06thru10%5CTxt%5C00000000%5C600006T3.txt&User=ANONYMOUS&Password=anonymous&SortMethod=h%7C-&MaximumDocuments=1&FuzzyDegree=0&ImageQuality=r75g8/r75g8/x150y150g16/i425&Display=hpfr&DefSeekPage=x&SearchBack=ZyActionL&Back=ZyActionS&BackDesc=Results%20page&MaximumPages=1&ZyEntry=1&SeekPage=x&ZyPURL (accessed on 21 December 2023).
- Utermöhl, H. Zur Vervollkomnung der quantitativen Phytoplankton-Methodik. Mitt. Int. Ver. Ther. Angew. Limnol. 1958, 9, 1–38. [Google Scholar]
- Schreiber, S.G.; Schreiber, S.; Tanna, R.N.; Roberts, D.R.; Arciszewski, T.J. Statistical tools for water quality assessment and monitoring in river ecosystems–a scoping review and recommendations for data analysis. Water Qual. Res. J. 2022, 57, 40–57. [Google Scholar] [CrossRef]
- Zoschke, K.; Börnick, H.; Worch, E. Vacuum-UV radiation at 185 nm in water treatment—a review. Water Res. 2014, 52, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Hansson, L.A.; Hylander, S. Effects of ultraviolet radiation on pigmentation, photoenzymatic repair, behavior, and community ecology of zooplankton. Photochem. Photobiol. Sci. 2009, 8, 1266–1275. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).