Postbiotic Potential of Lactic Acid Bacteria Strains in Functional Minimally Processed Oranges
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Identification of LAB Strains
2.1.1. Molecular Identification of LAB Isolates
2.1.2. Pulsed Field Gel Electrophoresis (PFGE) Analysis
2.2. Safety Evaluation
2.2.1. DNase, Gelatinase, and Hemolytic Assays
2.2.2. Antimicrobial Susceptibility Test
2.3. Bacterial Growth and Cell Suspension Standardisation
2.4. Antibacterial Activity
2.5. Evaluation of Functional Traits of Strains
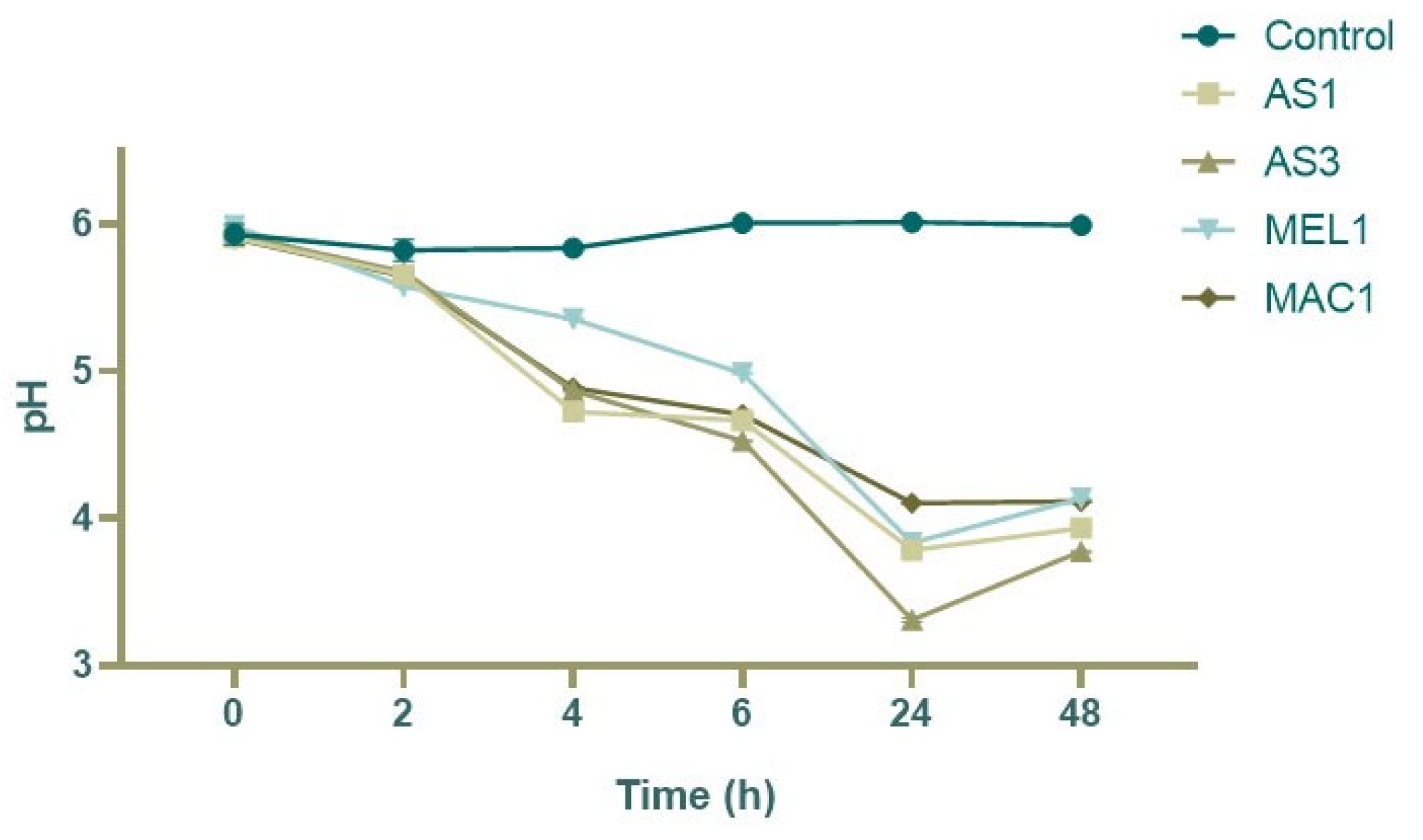
2.5.1. Tolerance to Acidic Conditions and Low Temperatures
2.5.2. Acidification Capacity Test
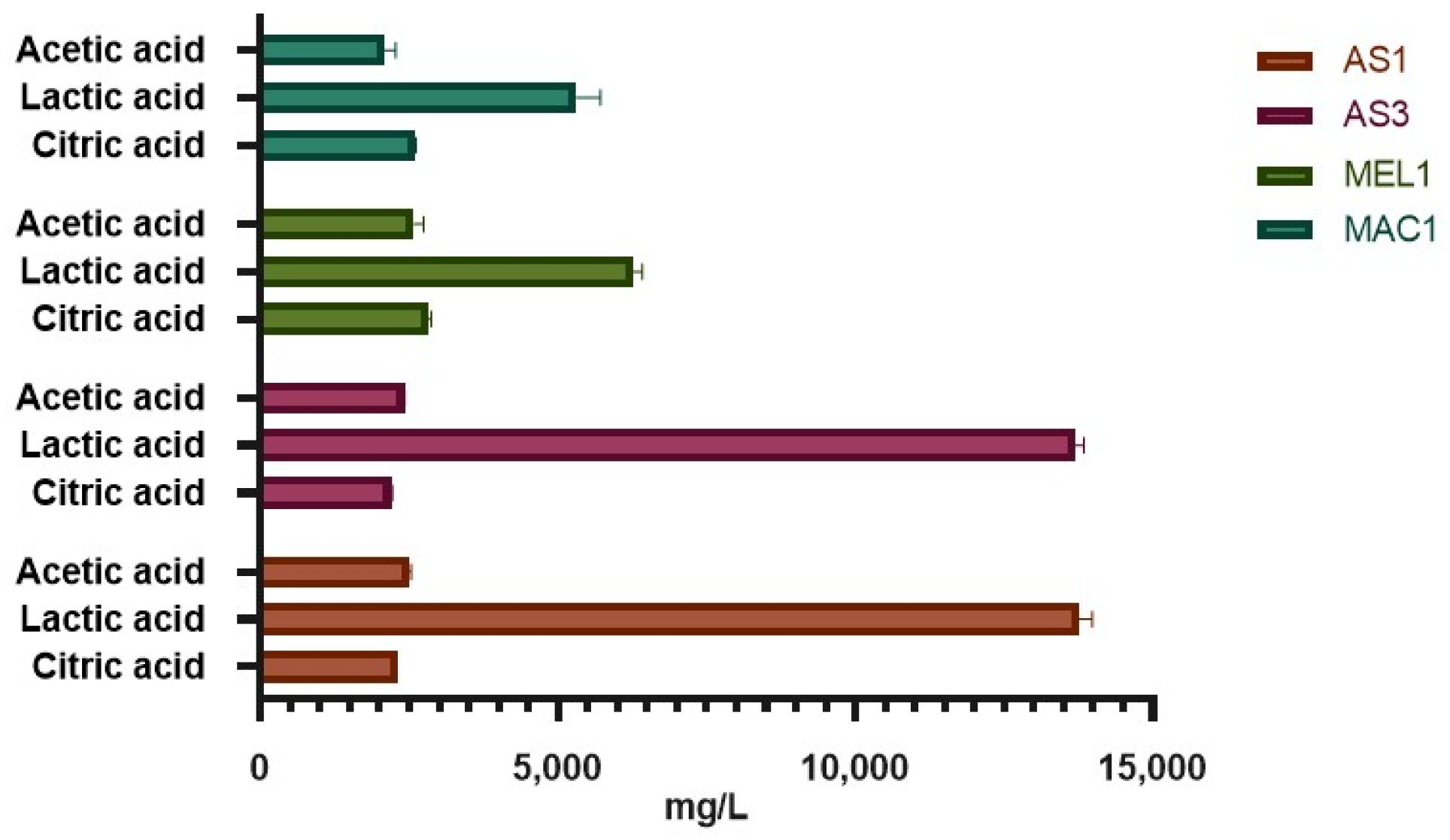
2.5.3. Production of Organic Acids
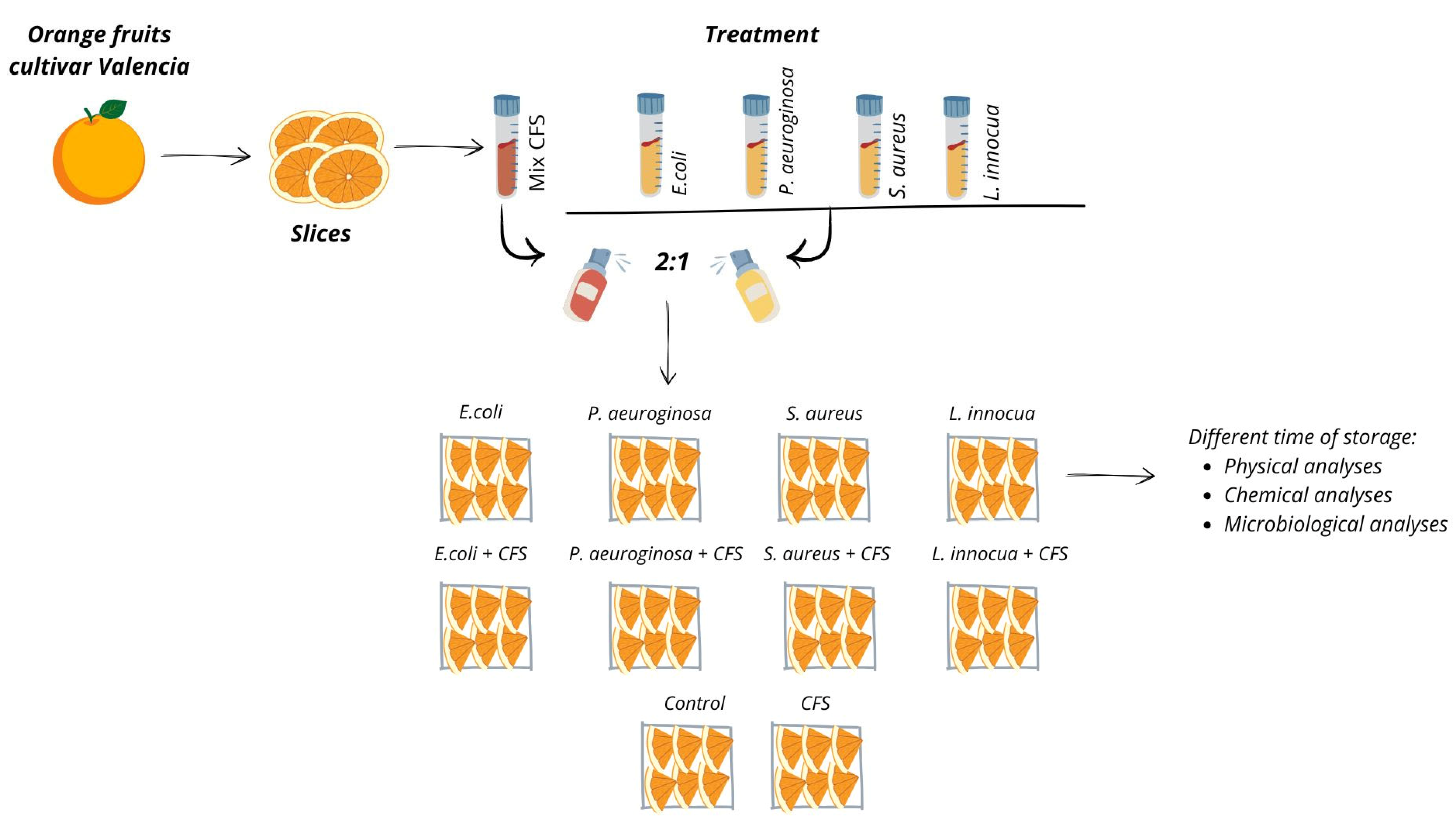
2.6. Processing and Treatments of Orange Slices
2.6.1. Vegetal Matrix
2.6.2. Fruit Treatments
2.6.3. Physical Analyses
2.6.4. Microbiological Analyses
2.6.5. Chemical Analyses
2.7. Statistical Analysis
3. Results and Discussion
3.1. Phenotypic and Genotypic Characterisation of LAB Isolates
3.2. Safety Evaluation Results
3.3. Antimicrobial Activity of LAB Isolates
3.4. Evaluation of Functional Traits of Isolates
3.5. Physico-Chemical Analyses of Minimally Processed Orange Slices Treated Differently During Refrigerated Storage
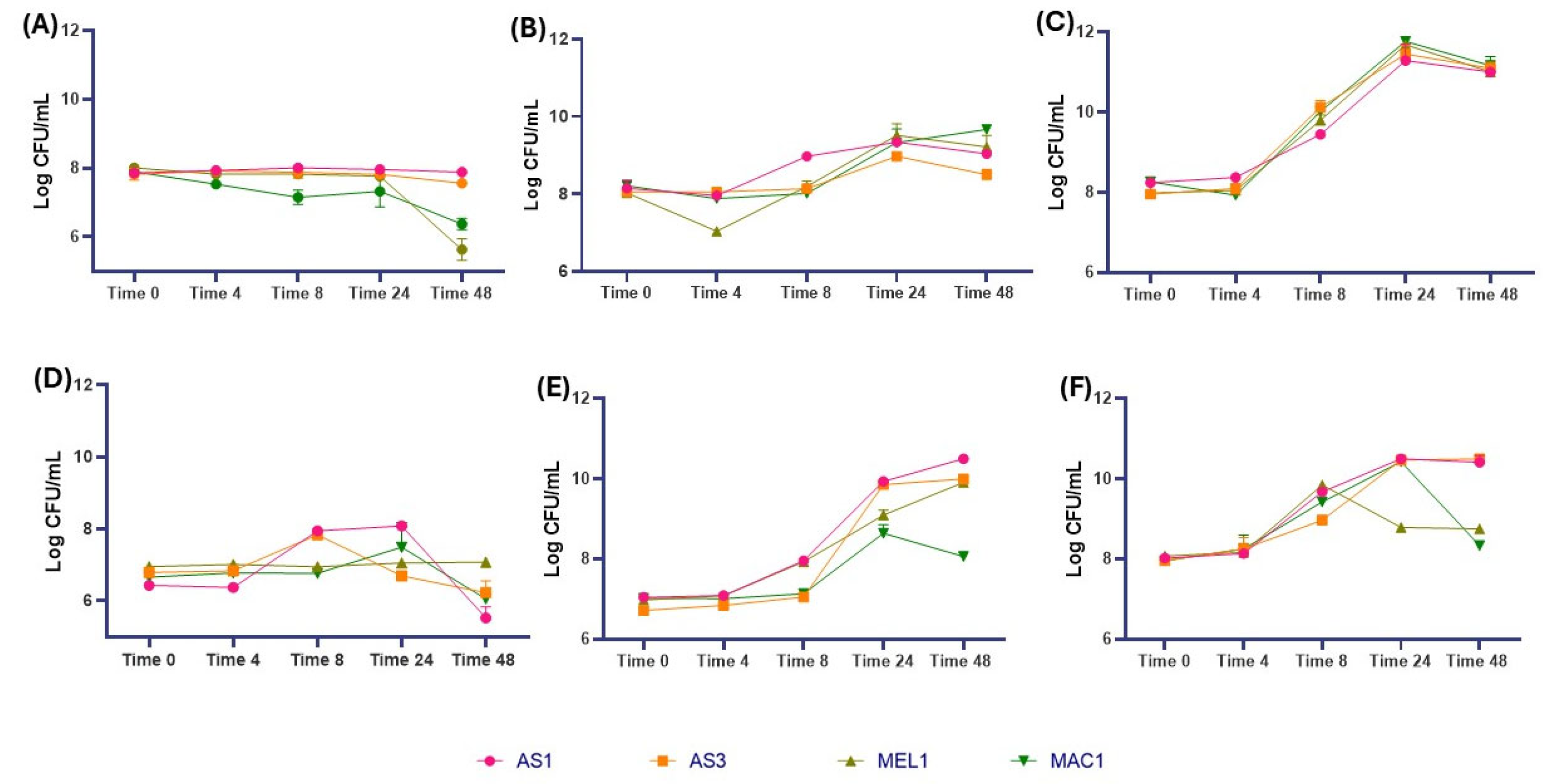
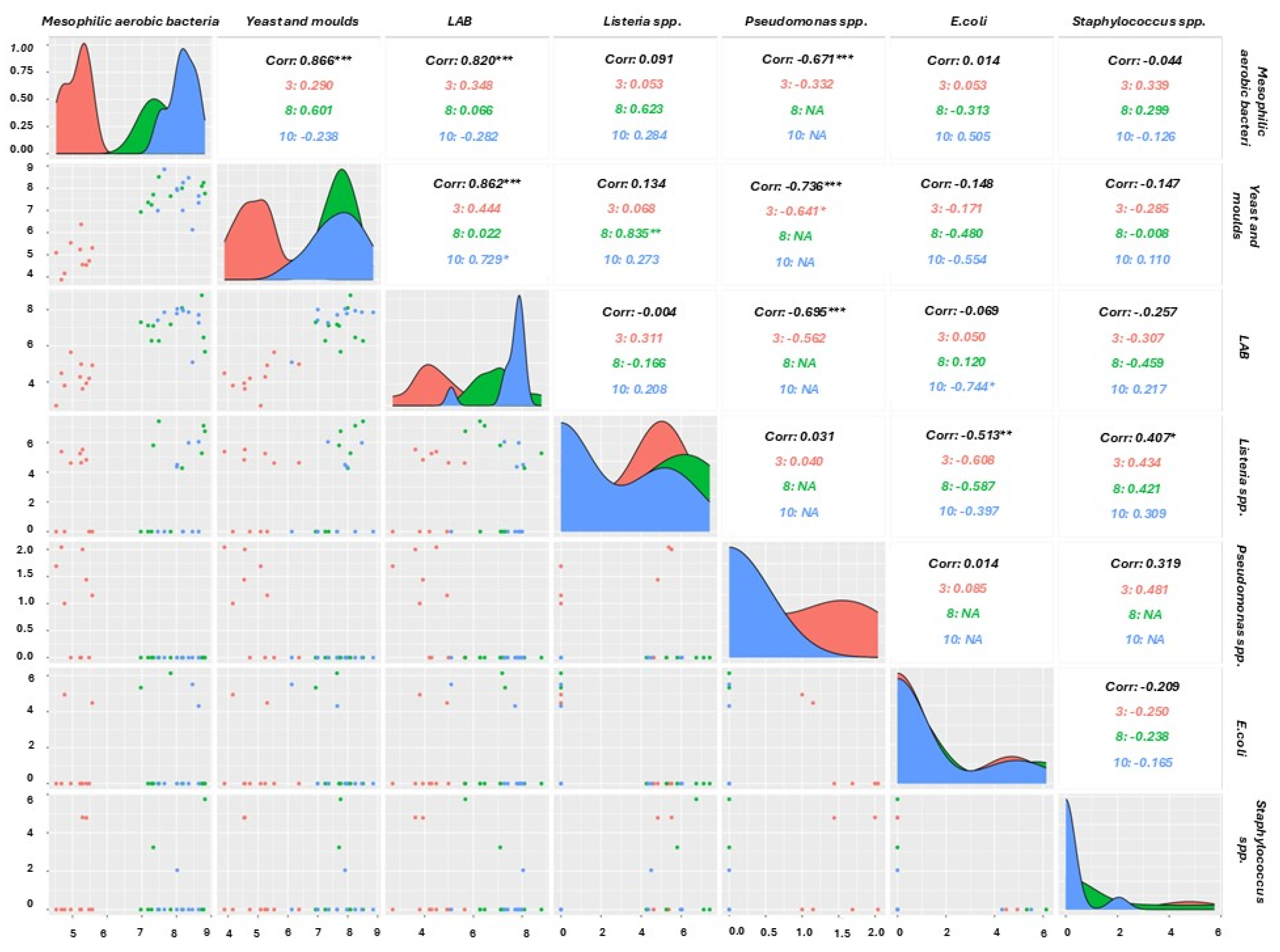
3.6. Dynamics of the Main Microbial Groups in Minimally Processed Orange Slices Treated Differently During Storage at Refrigerated Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pilone, V.; Stasi, A.; Baselice, A. Quality preferences and pricing of fresh-cut salads in Italy: New evidence from market data. Brit. Food J. 2017, 119, 1473–1486. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). Current Good Manufacturing Practice, Hazard Analysis, and Risk-Based Preventive Controls for Human Food. 2015. Available online: http://federalregister.gov/a/2015-21920 (accessed on 15 April 2025).
- Russo, P.; Botticella, G.; Capozzi, V.; Massa, S.; Spano, G.; Beneduce, L. A Fast, Reliable, and Sensitive Method for Detection and Quantification of Listeria monocytogenes and Escherichia coli O157:H7 in Ready-to-Eat Fresh-Cut Products by MPN-QPCR. BioMed Res. Int. 2014, 2014, 608296. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Belgacem, I.; Schena, L.; Teixidó, N.; Romeo, F.V.; Ballistreri, G.; Abadias, M. Effectiveness of a pomegranate peel extract (PGE) in reducing Listeria monocytogenes in vitro and on fresh-cut pear, apple and melon. Eur. Food Res. Technol. 2020, 246, 1765–1772. [Google Scholar] [CrossRef]
- Sharafi, H.; Divsalar, E.; Rezaei, Z.; Liu, S.-Q.; Moradi, M. The Potential of Postbiotics as a Novel Approach in Food Packaging and Biopreservation: A Systematic Review of the Latest Developments. Crit. Rev. Food Sci. Nutr. 2023, 64, 12524–12554. [Google Scholar] [CrossRef]
- Aguirre-Garcia, Y.L.; Nery-Flores, S.D.; Campos-Muzquiz, L.G.; Flores-Gallegos, A.C.; Palomo-Ligas, L.; Ascacio-Valdés, J.A.; Sepúlveda-Torres, L.; Rodríguez-Herrera, R. Lactic Acid Fermentation in the Food Industry and Bio-Preservation of Food. Fermentation 2024, 10, 168. [Google Scholar] [CrossRef]
- Obis, D.; Paris, M.; Ouwehand, A.C. The safety of lactic acid bacteria for use in foods. In Lactic Acid Bacteria; CRC Press: Boca Raton, FL, USA, 2019; pp. 355–369. [Google Scholar] [CrossRef]
- Mani-López, E.; Arrioja-Bretón, D.; López-Malo, A. The impacts of antimicrobial and antifungal activity of cell-free supernatants from lactic acid bacteria in vitro and foods. Compr. Rev. Food Sci. Food Saf. 2022, 21, 604–641. [Google Scholar] [CrossRef]
- Foti, P.; Ballistreri, G.; Timpanaro, N.; Rapisarda, P.; Romeo, F.V. Prebiotic Effects of Citrus Pectic Oligosaccharides. Nat. Prod. Res. 2022, 36, 3173–3176. [Google Scholar] [CrossRef]
- Cenci-Goga, B.T.; Sechi, P.; Iulietto, M.F.; Amirjalali, S.; Barbera, S.; Karama, M.; Aly, S.S.; Grispoldi, L. Characterization and growth under different storage temperatures of ropy slime-producing Leuconostoc mesenteroides isolated from cooked meat products. J. Food Prot. 2020, 83, 1043–1049. [Google Scholar] [CrossRef]
- Torriani, S.; Felis, G.E.; Dellaglio, F. Differentiation of Lactobacillus plantarum, L. pentosus, and L. paraplantarum by recA gene sequence analysis and multiplex PCR assay with recA gene-derived primers. Appl. Environ. Microbiol. 2001, 67, 3450–3454. [Google Scholar] [CrossRef]
- Coudeyras, S.; Marchandin, H.; Fajon, C.; Forestier, C. Taxonomic and Strain-Specific Identification of the Probiotic Strain Lactobacillus rhamnosus 35 within the Lactobacillus casei Group. Appl. Environ. Microbiol. 2008, 74, 2679–2689. [Google Scholar] [CrossRef]
- Behare, P.V.; Mazhar, S.; Pennone, V.; McAuliffe, O. Evaluation of lactic acid bacteria strains isolated from fructose-rich environments for their mannitol-production and milk-gelation abilities. J. Dairy Sci. 2020, 103, 11138–11151. [Google Scholar] [CrossRef] [PubMed]
- Russo, N.; Caggia, C.; Pino, A.; Coque, T.M.; Arioli, S.; Randazzo, C.L. Enterococcus spp. in Ragusano PDO and Pecorino Siciliano cheese types: A snapshot of their antibiotic resistance distribution. Food Chem. Toxicol. 2018, 120, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Tenover, F.C.; Arbeit, R.D.; Goering, R.V.; Mickelsen, P.A.; Murray, B.E.; Persing, D.H.; Swaminathan, B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: Criteria for bacterial strain typing. J. Clin. Microbiol. 1995, 33, 2233–2239. [Google Scholar] [CrossRef] [PubMed]
- Ben Farhat, L.; Romeo, F.V.; Foti, P.; Russo, N.; Randazzo, C.L.; Caggia, C.; Abidi, F. Multi-Functional Potential of Lactic Acid Bacteria Strains and Antimicrobial Effects in Minimally Processed Pomegranate (Punica granatum L. cv Jolly Red) Arils. Microorganisms 2022, 10, 1876. [Google Scholar] [CrossRef]
- Mokdad, F.H.; Benmechernene, Z.; Benyoucef, A.; Russo, N.; Randazzo, C.L.; Caggia, C.; Kihal, M. Characterization of bioactive Leuconostoc mesenteroides producing bacteriocin strains isolated from camel’s and goat’s Algerian raw milks. Int. J. Sci. Res. 2020, 76, 32–61. [Google Scholar] [CrossRef]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA J. 2012, 10, 2740. [Google Scholar] [CrossRef]
- Montville, T.J.; Matthews, K.R. Physiology, growth, and inhibition of microbes in foods. Food Microbiol. Fundam. Front. 2012, 2013, 3–18. [Google Scholar] [CrossRef]
- Yolmeh, M.; Khomeiri, M.; Ahmadi, Z. Application of mixture design to introduce an optimum cell-free supernatant of multiple-strain mixture (MSM) for Lactobacillus against food-borne pathogens. LWT Food Sci. Technol. 2017, 83, 298–304. [Google Scholar] [CrossRef]
- Bangar, S.P.; Suri, S.; Trif, M.; Ozogul, F. Organic acids production from lactic acid bacteria: A preservation approach. Food Biosci. 2022, 46, 101615. [Google Scholar] [CrossRef]
- Özcelik, S.; Kuley, E.; Özogul, F. Formation of lactic, acetic, succinic, propionic, formic and butyric acid by lactic acid bacteria. LWT 2016, 73, 536–542. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). Guidance for Industry: Guide to Minimize Microbial Food Safety Hazards of Fresh-Cut Fruits and Vegetables. 2008. Available online: https://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/ProducePlantProducts/ucm064458.htm (accessed on 28 March 2025).
- Vaccalluzzo, A.; Pino, A.; De Angelis, M.; Bautista-Gallego, J.; Romeo, F.V.; Foti, P.; Caggia, C.; Randazzo, C.L. Effects of Different Stress Parameters on Growth and on Oleuropein-Degrading Abilities of Lactiplantibacillus plantarum Strains Selected as Tailored Starter Cultures for Naturally Table Olives. Microorganisms 2020, 8, 1607. [Google Scholar] [CrossRef] [PubMed]
- Macarisin, D.; Sheth, I.; Hur, M.; Wooten, A.; Kwon, H.J.; Gao, Z.; De Jesus, A.; Jurick, W.; Chen, Y. Survival of outbreak, food, and environmental strains of Listeria monocytogenes on whole apples as affected by cultivar and wax coating. Sci. Rep. 2019, 9, 12170. [Google Scholar] [CrossRef] [PubMed]
- Kho, K.; Kadar, A.D.; Bani, M.D.; Pramanda, I.T.; Martin, L.; Chrisdianto, M.; Pratama, F.; Devanthi, P.V.P. The Potential of Pediococcus acidilactici Cell-Free Supernatant as a Preservative in Food Packaging Materials. Foods 2024, 13, 644. [Google Scholar] [CrossRef] [PubMed]
- Carballo, S.; Zingarello, F.A.; Maestre, S.E.; Todoli, J.L.; Prats, M.S. Optimisation of analytical methods for the characterisation of oranges, clementines and citrus hybrids cultivated in spain on the basis of their composition in ascorbic acid, citric acid and major sugars. Int. J. Food Sci. Technol. 2014, 49, 146–152. [Google Scholar] [CrossRef]
- Xie, X.L.; Yin, X.R.; Chen, K.S. Roles of APETALA2/ethylene-response factors in regulation of fruit quality. Crit. Rev. Plant Sci. 2016, 35, 120–130. [Google Scholar] [CrossRef]
- Carstens, C.K.; Salazar, J.K.; Darkoh, C. Multistate Outbreaks of Foodborne Illness in the United States Associated with Fresh Produce from 2010 to 2017. Front. Microbiol. 2019, 10, 2667. [Google Scholar] [CrossRef]
- Bian, L.; Molan, A.L.; Maddox, I.; Shu, Q. Antimicrobial activity of Lactobacillus reuteri DPC16 supernatants against selected food borne pathogens. World J. Microbiol. Biotechnol. 2011, 27, 991–998. [Google Scholar] [CrossRef]
- Ozogul, F.; Tabanelli, G.; Toy, N.; Gardini, F. Impact of cell-free supernatant of lactic acid bacteria on putrescine and other polyamine formation by foodborne pathogens in ornithine decarboxylase broth. J. Agric. Food Chem. 2015, 63, 5828–5835. [Google Scholar] [CrossRef]
- Sanpa, S.; Sanpa, S.; Suttajit, M. Lactic acid bacteria isolates from Pla-som, their antimicrobial activities and fermentation properties in Pla-som. J. Food Health Bioenviron. Sci. 2019, 12, 36–43. Available online: https://li01.tci-thaijo.org/index.php/sdust/article/view/186555 (accessed on 17 April 2025).
- Riesute, R.; Salomskiene, J.; Moreno, D.S.; Gustiene, S. Effect of yeasts on food quality and safety and possibilities of their inhibition. Trends Food Sci. Technol. 2021, 108, 1–10. [Google Scholar] [CrossRef]
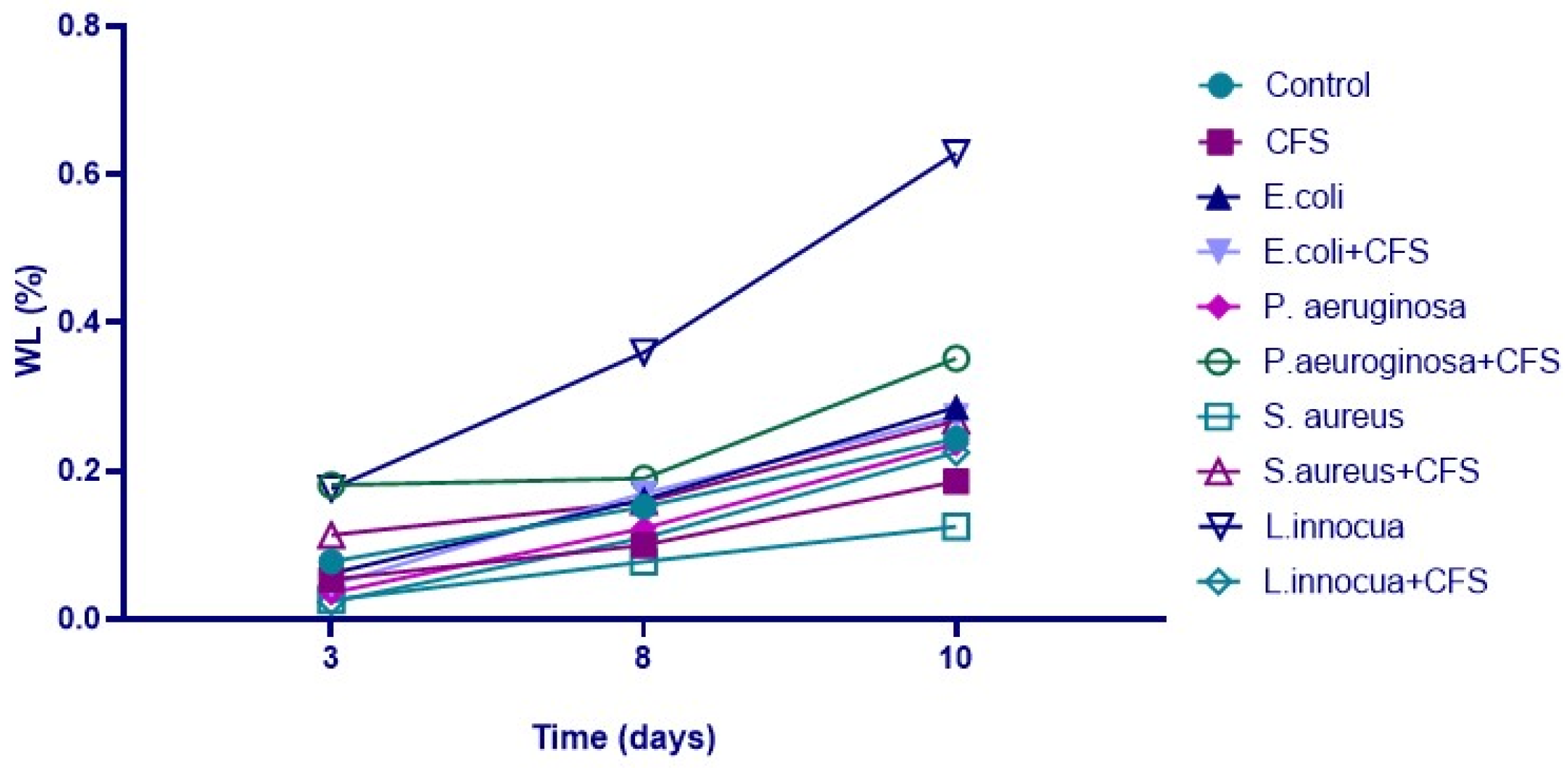
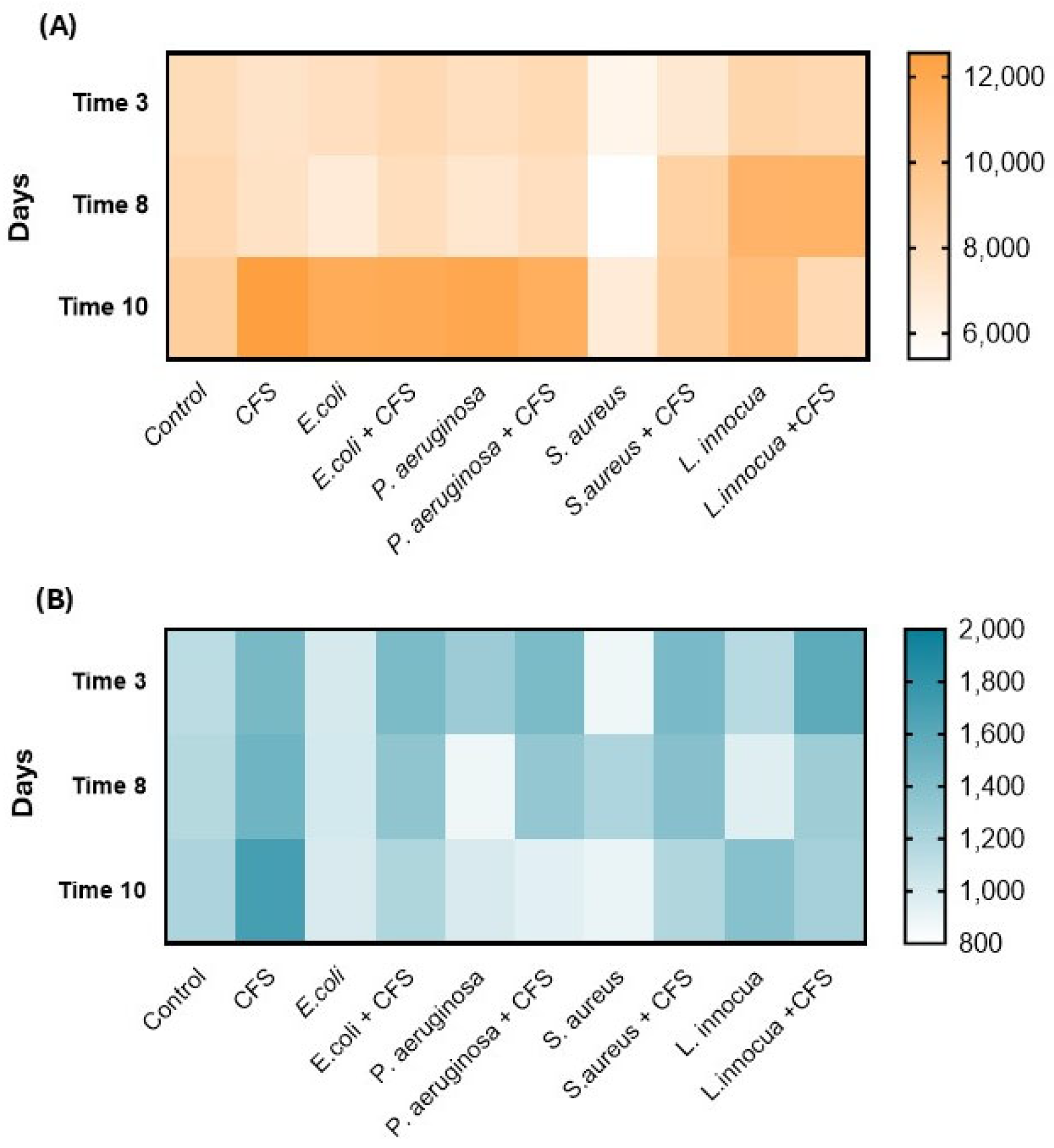
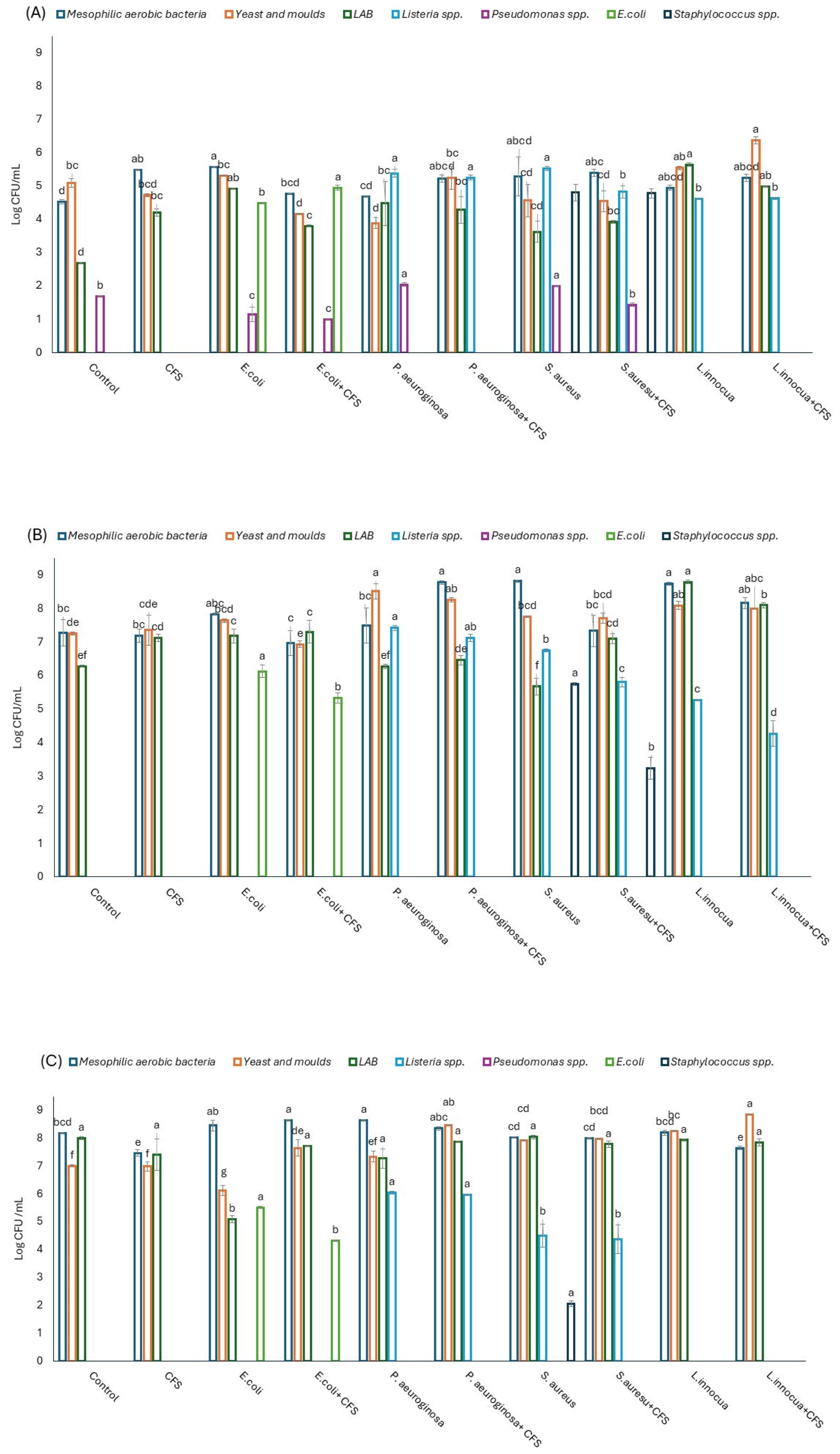
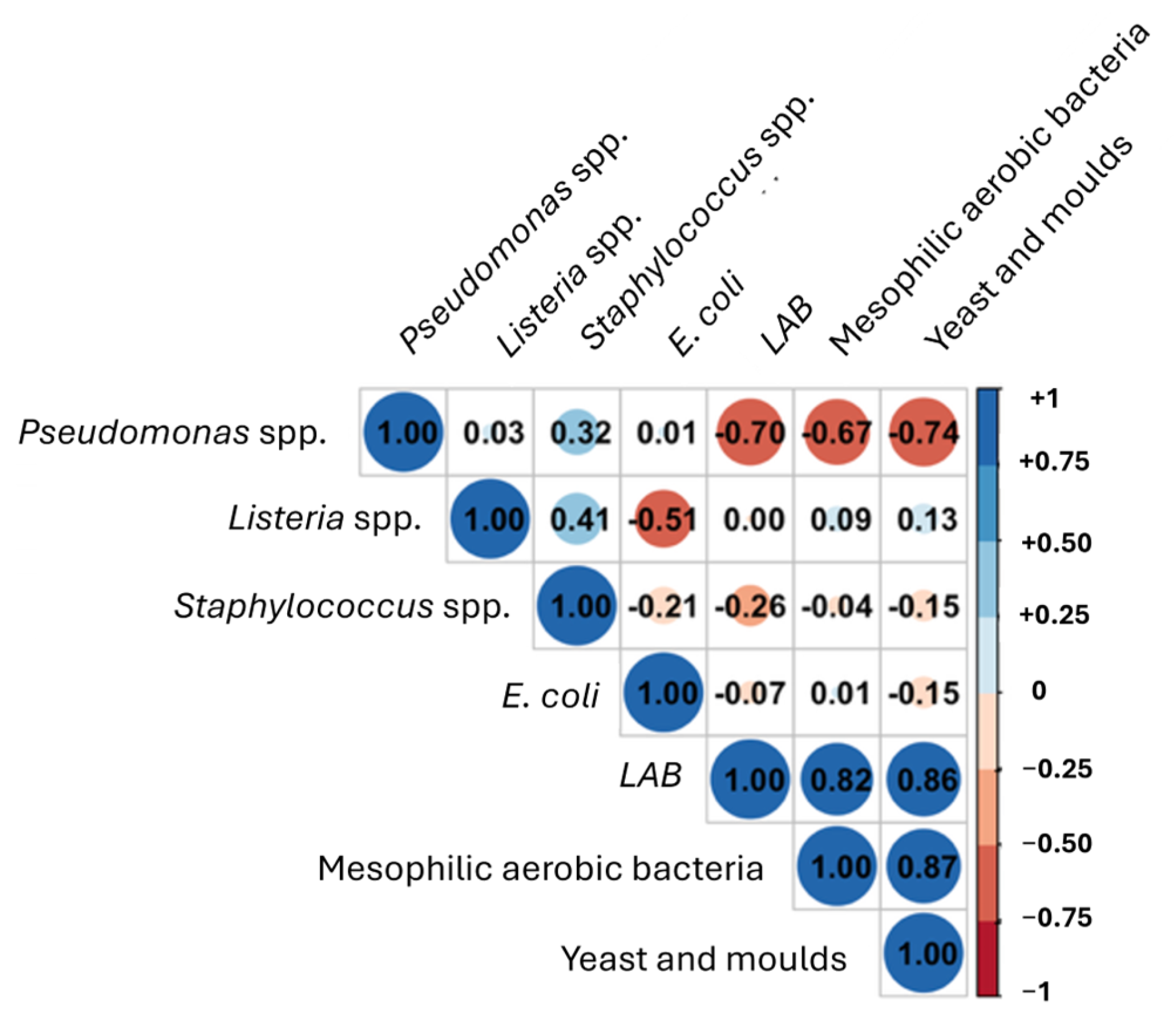
| Sample | Time | L*(D65) | a*(D65) | b*(D65) | Firmness (N) |
|---|---|---|---|---|---|
| Fresh orange slices | 0 | 49.88 ± 1.74 | −2.30 ± 2.29 | 30.23 ± 3.23 | 3.47 ± 0.64 |
| Control | 3 | 46.07 ± 1.44ab | −3.68 ± 0.14 | 28.20 ± 1.21abcd | 2.02 ± 0.67 |
| CFS | 3 | 47.26 ± 0.39ab | −2.28 ± 0.03 | 29.64 ± 0.79abc | 2.39 ± 0.65 |
| E. coli | 3 | 50.15 ± 1.64a | −3.33 ± 0.16 | 30.83 ± 1.58ab | 1.67 ± 0.61 |
| E. coli+CFS | 3 | 50.12 ± 3.23a | −2.74 ± 1.61 | 32.73 ± 2.78a | 2.24 ± 1.03 |
| P. aeruginosa | 3 | 46.43 ± 1.53ab | −3.20 ± 1.02 | 25.68 ± 3.44bcd | 2.22 ± 0.45 |
| P. aeruginosa+CFS | 3 | 46.03 ± 1.03ab | −2.60 ± 0.17 | 24.76 ± 1.53bcd | 2.56 ± 0.97 |
| S. aureus | 3 | 44.69 ± 0.14b | −3.36 ± 0.20 | 23.07 ± 0.10cd | 1.96 ± 0.37 |
| S. aureus+CFS | 3 | 43.77 ± 0.40b | −3.06 ± 0.12 | 22.73 ± 2.21d | 2.15 ± 0.71 |
| L. innocua | 3 | 46.70 ± 0.10ab | −2.52 ± 1.39 | 27.04 ± 3.73abcd | 2.75 ± 1.28 |
| L. innocua+CFS | 3 | 47.37 ± 1.62ab | −2.47 ± 0.77 | 28.10 ± 1.75abcd | 5.03 ± 6.90 |
| ** | n.s. | *** | n.s. | ||
| Control | 8 | 46.58 ± 0.27b | −2.64 ± 0.02ab | 26.36 ± 1.80ab | 2.37 ± 0.69 |
| CFS | 8 | 45.58 ± 0.17b | −3.59 ± 0.24abc | 23.36 ± 0.71ab | 2.18 ± 0.68 |
| E. coli | 8 | 45.89 ± 0.26b | −2.21 ± 0.30a | 26.65 ± 0.08ab | 2.21 ± 0.62 |
| E. coli+CFS | 8 | 45.90 ± 1.40b | −3.92 ± 1.22abc | 23.62 ± 0.64ab | 2.14 ± 0.63 |
| P. aeruginosa | 8 | 45.79 ± 1.50b | −2.83 ± 0.13ab | 25.56 ± 2.08ab | 2.53 ± 0.81 |
| P. aeruginosa+CFS | 8 | 45.22 ± 0.54b | −3.99 ± 0.00abc | 22.16 ± 0.55b | 2.61 ± 0.7 |
| S. aureus | 8 | 45.14 ± 0.30b | −3.29 ± 0.53abc | 24.92 ± 0.73ab | 1.96 ± 0.77 |
| S. aureus+CFS | 8 | 45.97 ± 0.10b | −2.74 ± 0.03ab | 27.18 ± 0.03ab | 2.15 ± 0.88 |
| L. innocua | 8 | 52.32 ± 0.68a | −4.84 ± 0.54c | 27.49 ± 0.27ab | 2.75 ± 0.91 |
| L. innocua+CFS | 8 | 51.35 ± 0.62a | −4.41 ± 0.64bc | 29.05 ± 5.54a | 5.03 ± 1.12 |
| ** | *** | *** | n.s. | ||
| Control | 10 | 47.60 ± 0.16bc | −3.70 ± 1.06ab | 27.44 ± 0.57ab | 2.52 ± 0.38abc |
| CFS | 10 | 48.36 ± 0.87bc | −4.47 ± 0.38ab | 26.95 ± 2.71ab | 1.91 ± 0.91bc |
| E. coli | 10 | 50.11 ± 4.03abc | −4.02 ± 0.35ab | 32.30 ± 6.96a | 2.04 ± 0.77bc |
| E. coli+CFS | 10 | 49.65 ± 3.72abc | −4.50 ± 0.40ab | 26.62 ± 1.52ab | 1.78 ± 0.71bc |
| P. aeruginosa | 10 | 47.70 ± 0.34bc | −3.26 ± 0.33ab | 26.66 ± 3.32ab | 1.59 ± 0.93c |
| P. aeruginosa+CFS | 10 | 50.02 ± 1.12abc | −4.53 ± 0.02ab | 28.65 ± 0.37ab | 2.70 ± 0.37abc |
| S. aureus | 10 | 47.09 ± 0.07bc | −3.10 ± 0.79a | 27.93 ± 0.79ab | 2.84 ± 0.79ab |
| S. aureus+CFS | 10 | 45.50 ± 1.35c | −3.12 ± 0.22a | 24.82 ± 1.45b | 2.62 ± 0.60abc |
| L. innocua | 10 | 51.36 ± 0.58ab | −4.39 ± 0.49ab | 26.37 ± 0.68ab | 1.99 ± 0.77bc |
| L. innocua+CFS | 10 | 53.75 ± 0.06a | −5.12 ± 0.32b | 29.91 ± 0.41ab | 3.43 ± 1.16a |
| ** | *** | ** | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foti, P.; Romeo, F.V.; Sciuto, G.; Strano, M.C.; Allegra, M.; Russo, N.; Caggia, C. Postbiotic Potential of Lactic Acid Bacteria Strains in Functional Minimally Processed Oranges. Appl. Sci. 2025, 15, 6736. https://doi.org/10.3390/app15126736
Foti P, Romeo FV, Sciuto G, Strano MC, Allegra M, Russo N, Caggia C. Postbiotic Potential of Lactic Acid Bacteria Strains in Functional Minimally Processed Oranges. Applied Sciences. 2025; 15(12):6736. https://doi.org/10.3390/app15126736
Chicago/Turabian StyleFoti, Paola, Flora Valeria Romeo, Gloria Sciuto, Maria Concetta Strano, Maria Allegra, Nunziatina Russo, and Cinzia Caggia. 2025. "Postbiotic Potential of Lactic Acid Bacteria Strains in Functional Minimally Processed Oranges" Applied Sciences 15, no. 12: 6736. https://doi.org/10.3390/app15126736
APA StyleFoti, P., Romeo, F. V., Sciuto, G., Strano, M. C., Allegra, M., Russo, N., & Caggia, C. (2025). Postbiotic Potential of Lactic Acid Bacteria Strains in Functional Minimally Processed Oranges. Applied Sciences, 15(12), 6736. https://doi.org/10.3390/app15126736












