1. Introduction
Isobutyraldehyde is a strong-smelling, colorless liquid, commonly used as a solvent and chemical intermediate, and its derivatives have applications in pharmaceuticals, fertilizers, paints and coatings, gasoline additives, inks, fragrances, and more.
Isobutyraldehyde is commonly produced from propylene and isobutene [
1], and it is also primarily produced as a byproduct of the hydrogenation of propylene [
2]. Recently, with the rise in environmental awareness, the shift toward bio-based alternatives and sustainable practices could influence the isobutyraldehyde market dynamics [
3,
4]. Along with the production of byproducts, the treatment of surplus isobutyraldehyde has clearly become an important issue. In addition to its production as isobutanol, the process of oxidizing isobutyraldehyde to isobutyric acid is also one of the main options in the market.
Isobutyric acid is a versatile chemical raw material, used as a pharmaceutical intermediate, organic synthesis intermediate, and food flavoring, among other applications. Due to its distinctive odor, isobutyric acid has been increasingly used in food, fragrances, and perfumes in recent years. In addition, the market’s growing preference for environmentally friendly substances has significantly increased the demand for isobutyric acid [
5,
6]. Isobutyric acid can be produced from renewable feedstocks and can also be generated through microbial fermentation. Furthermore, it is biodegradable. Those characteristics contribute positively to sustainable development and a green economy.
Isobutyric acid is primarily produced through two methods: biological and oxidation methods. In the biological method, using sugars as raw materials for the fermentation of engineered bacteria is the dominant approach [
7]. However, commercialized biological methods still face certain limitations that are difficult to overcome, such as low production capacity, sensitivity to pH value, and formation of byproducts. As a result, the oxidation method using isobutanol or isobutyraldehyde as raw materials has become the mainstream process in the global market. Companies like Oxea GmbH, Mitsubishi Chemical, Eastman Chemical, BASF SE, and Dow Chemical all utilize this oxidation method [
8]. The new isobutyric acid production plants of Oxea GmbH and Eastman Chemical were completed and commissioned in 2017 and 2018, respectively. This demonstrates the growing demand for isobutyric acid in the market.
The oxidation of isobutyraldehyde to isobutyric acid is typically carried out within a temperature range of 20–100 °C. Hydrogen peroxide, oxygen, or air can be used as oxidizing agents. The operating pressure ranges from 20 psig to 75 psig. Additionally, acetone can be used as a co-solvent, and alkaline metal catalysts may be employed [
9]. Depending on the choice of oxidizing agent, the reaction can be either a gas–gas or gas–liquid reaction.
In actual process cases, Mitsubishi Chemical’s patented process [
10] employs hydrogen peroxide as the oxidizing agent and acetone as a co-solvent. The reaction is conducted at an operating temperature of 50–70 °C and a pressure of 5 bar. The reaction time typically ranges from 4 to 7 h, and it is carried out as a batch production process.
On the other hand, Eastman Chemical’s patented process [
11] uses oxygen as the oxidizing agent, with a temperature range of 20–100 °C and operating pressures between 1.4 bar and 3.8 bar. The reaction duration is 5–7 h, and like the Mitsubishi process, it is also conducted in batch production.
These two processes highlight practical approaches to isobutyraldehyde oxidation in industrial settings as well as two issues with the existing processes: first, the relatively long reaction time, and second, the low conversion rate and poor selectivity caused by the formation of byproducts. In this study, the aim is to overcome the challenges encountered by existing processes through the use of microreactor technology.
Microreactor technology (MRT) is centered around microstructured components, where chemical reactions and substance separation processes are carried out in flow channels on the millimeter or even micron scale. By miniaturizing the system and designing specialized flow channels, it enhances the mass and heat transfer efficiency, thereby improving the process’s efficiency and controllability [
12].
In addition, the scale-up design of microreactor technology involves amplifying the number of parallel units, such as flow channels, and corresponding reductions in equipment size [
13]. As a result, it avoids the scaling effect commonly encountered in traditional technologies. The research findings obtained on this platform can be quickly translated into practical production capabilities.
In this study, laboratory-scale experiments will be conducted using Ehrfeld’s MMRS modular microreactor. This system modularizes the various functional units of the process, allowing for flexible assembly and adjustment based on experimental needs, making it convenient for testing and optimizing experimental conditions. Once the optimal operating conditions are achieved, production tests will be conducted using a pilot-scale system, with the aim of quickly applying the results in the industry after achieving stable outcomes.
2. Experiment
2.1. Materials
All materials are reagent grade. Isobutyraldehyde, isobutyric acid, and acetone were purchased from Sigma-Aldrich (St. Louis, MO, USA). Hydrogen peroxide (30 w/t%) was obtained from J.T. Baker (Phillipsburg, NJ, USA).
2.2. Instruments
Laboratory-scale experiments were primarily conducted using Ehrfeld’s MMRS modular system, with supporting equipment including a PTFE high-pressure flow control pump (Sanotac SF1005B, Sanotac, Münster, Germany), liquid flow control pump (HITACHI L-7150, HITACHI, Tokyo, Japan), mass flow controller (MCQ-2SLPM-D-PCV10, Alicat Scientific, Tucson, AZ, USA), and Swagelok’s backpressure regulator (KBP1J0A4A5A20000, Swagelok, Solon, OH, USA), among others.
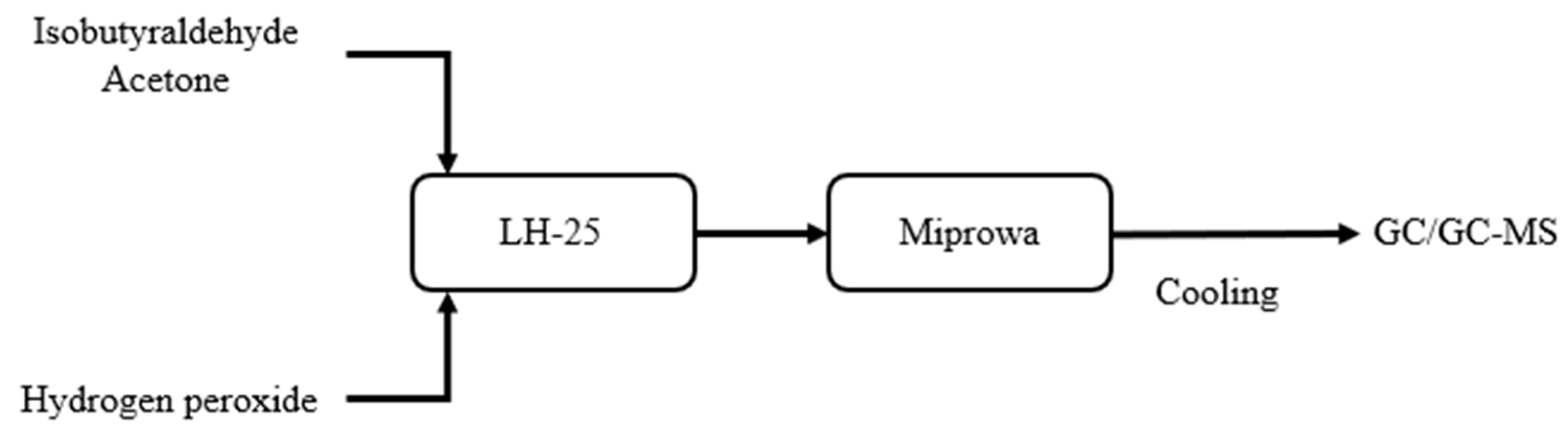
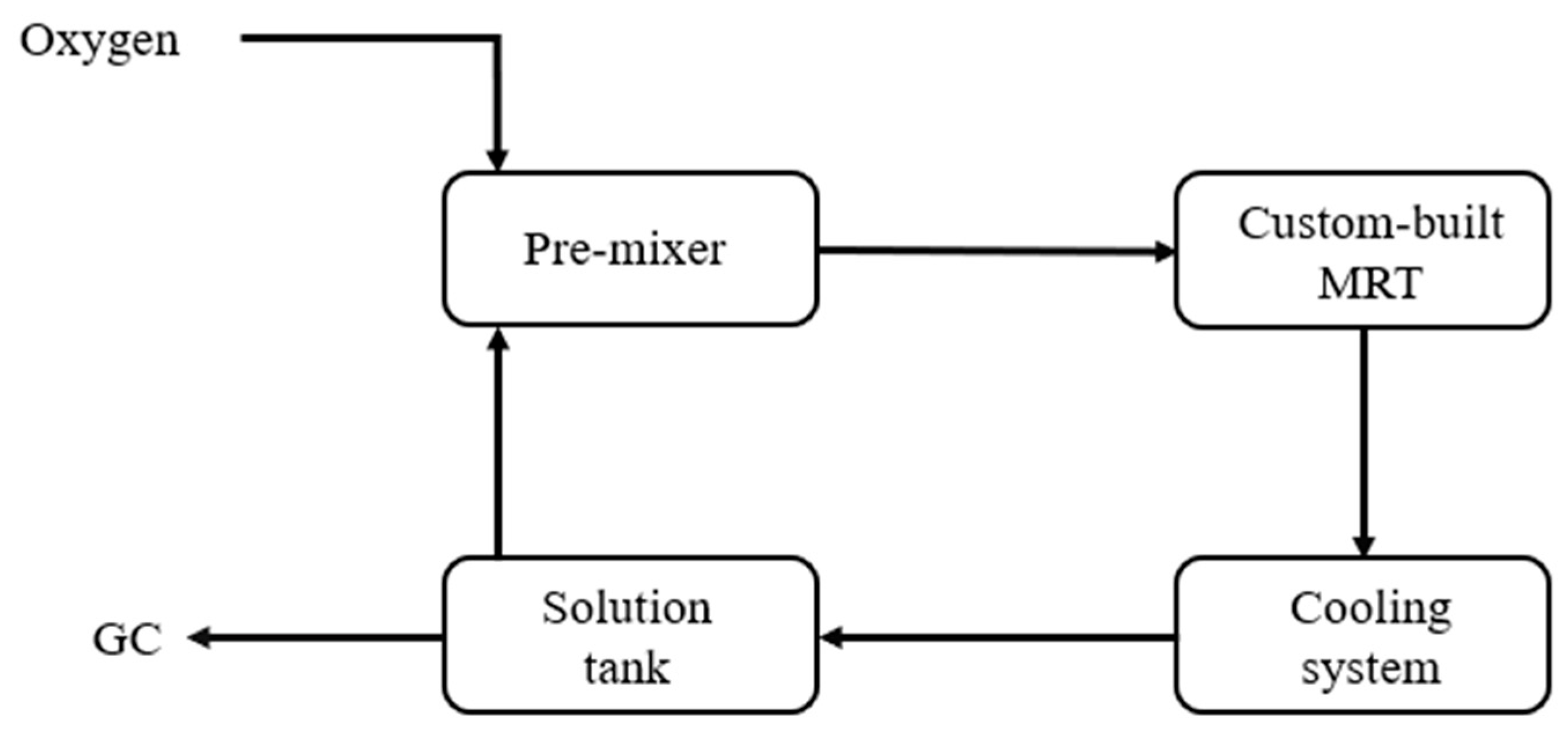
In the experiments using hydrogen peroxide as the oxidant, this study employed the MMRS system to establish a continuous production process, with the process flow shown in
Figure 1. The operating temperature ranged from 20 to 80 °C, and the experiments were conducted under 1 to 10 bar. The feed ratio of isobutyraldehyde to hydrogen peroxide is operated between 2:1 and 1:8, with total flow of 5 mL/min.
In the experiments using oxygen as the oxidant, a recirculating process was designed to extend the residence time, with the process flow illustrated in a later section. The operating temperature ranged from 20 to 80 °C, the system pressure was maintained between 5 and 15 bar, and the reaction time was between 1 and 2 h.
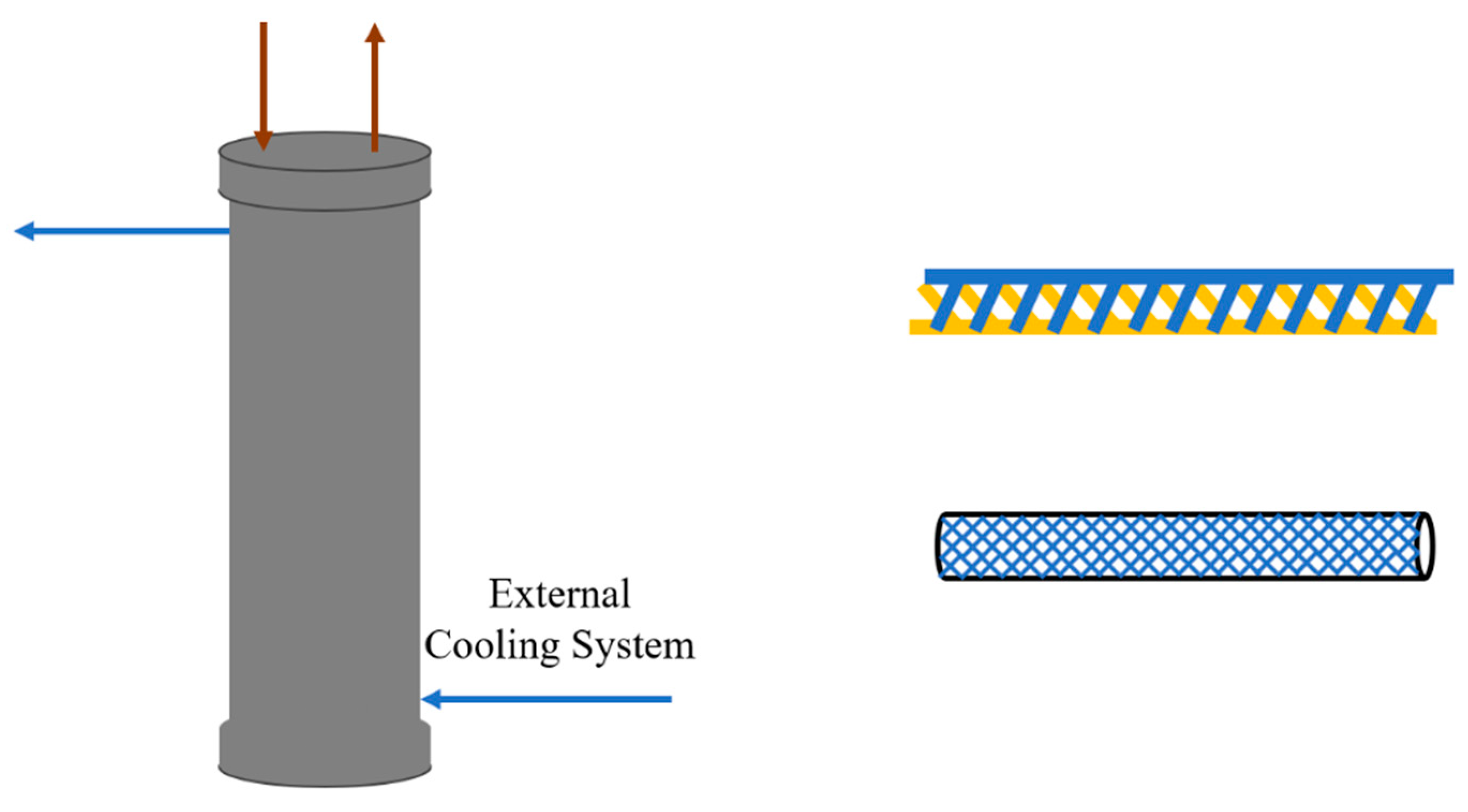
Pilot-scale experiments, on the other hand, were performed using a self-designed microreactor system, which includes custom-built microchannel piping, an external cooling system, and a pipeline support box. Supporting equipment used in the pilot-scale experiments is similar to that used in the laboratory-scale experiments.
In the design of the custom-built microreactor system, a recirculation unit was also incorporated to increase the residence time. The process flow and internal design are shown in a later section. The experiments were conducted at an operating temperature of 20 °C, a pressure of 10 bar, and a reaction time ranging from 1 to 3 h.
2.3. Analysis of Products
Gas chromatography (Agilent 8890, G3540A, Agilent, Santa Clara, CA, USA) and gas chromatography mass spectrometry (Agilent 7890B GC—5977A MSD) were used to analyze the substances in the experimental samples.
3. Results and Discussion
3.1. Laboratory-Scale Experiments with Hydrogen Peroxide as Oxidizing Agent
The batch experiments for this study were conducted in a beaker prior to the microreactor system experiments. In the batch experiments, 120 mL of isobutyraldehyde was placed in a beaker, and hydrogen peroxide was fed at a rate of 2 mL per minute using a pump for the oxidation reaction. The hydrogen peroxide was stored at 0 °C, while the beaker containing the reaction mixture was maintained at approximately 25 °C. After 3 h of operation, the conversion rate of isobutyraldehyde was only around 15%, with almost no formation of isobutyric acid. It is presumed that the reaction performance in the batch beaker setup was very limited. Therefore, to achieve better reaction performance and optimize conditions, subsequent experiments will be conducted using a microreactor system.
For microreactor system, the MMRS Miprowa modular reactor will be used as the main reactor for the reaction. The reactor is equipped with an external jacket through which liquid is passed for temperature control. A LH-25 mixer will be installed before the reactor to pre-mix the two feedstreams. The system is also equipped with a backpressure valve at the downstream section for pressure control. Before exiting the reactor, the pipeline will pass through an ice bath for cooling. Samples will be taken for analysis using GC and GC-MS. A simplified experimental flowchart is shown in
Figure 1.
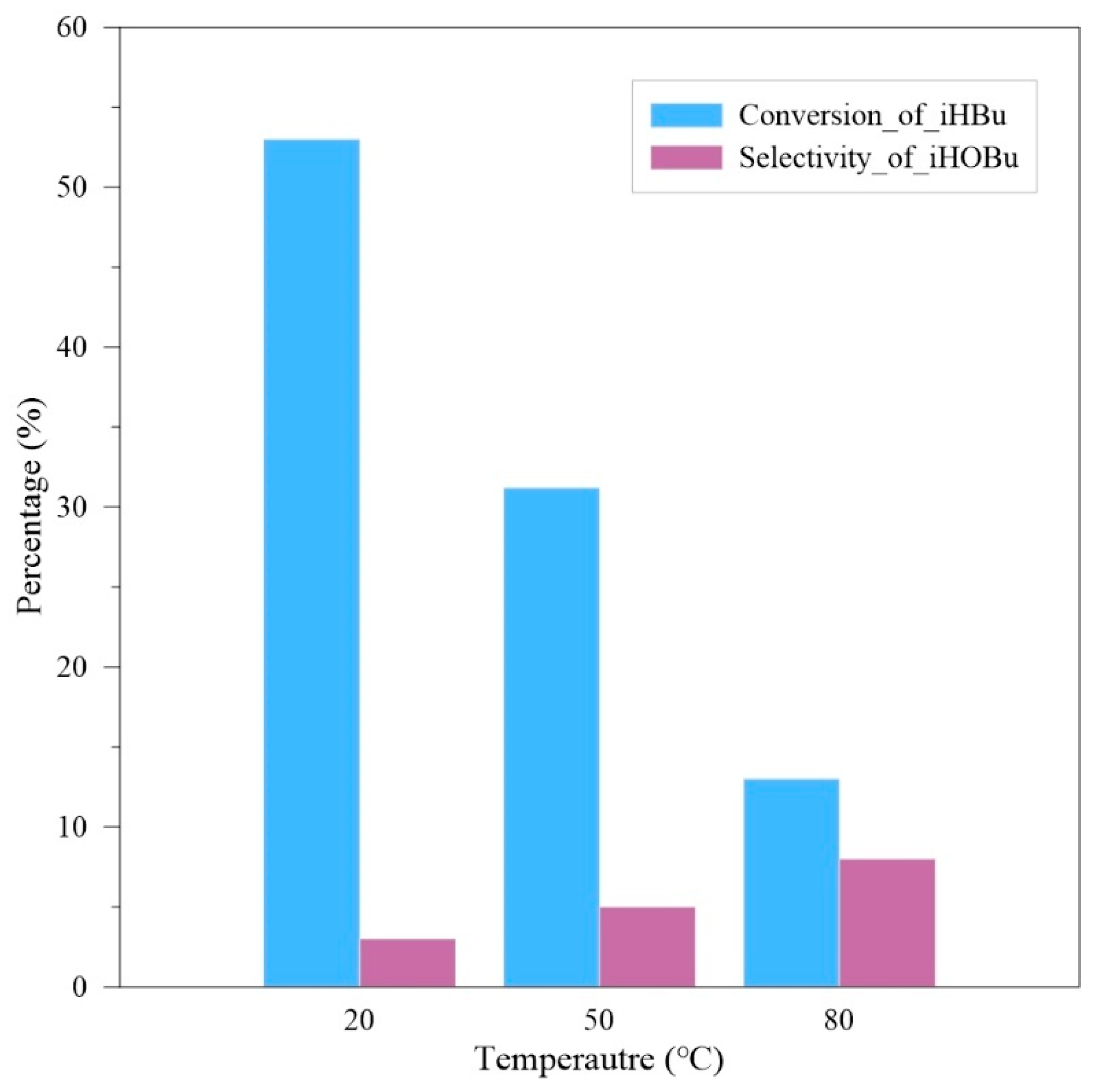
In the experimental design, the primary objective is to determine the appropriate experimental temperature. The feed ratio of isobutyraldehyde/acetone/hydrogen heroxide is 1:1:1, with isobutyraldehyde and acetone pre-mixed. The total feed flow rate is 5 mL/min, and the reaction time is 6 min. The experimental operating pressure is 10 bar, and the operating temperature is designed between 20 °C and 80 °C. The experimental results are shown in
Figure 2.
The experimental results indicate that the optimal condition is 20 °C, which is close to room temperature. There are two main reasons for this outcome. First, the reaction is exothermic, and according to Le Chatelier principle, an increase in temperature will hinder the progress of the oxidation reaction. Second, an increase in temperature will cause the decomposition of hydrogen peroxide, leading to a loss of its oxidizing ability. Therefore, 20 °C will be selected as the testing temperature for subsequent experiments.
In the experimental results of
Figure 2, although the optimal conversion rate of isobutyraldehyde (iHBu) reaches nearly 50%, the selectivity for isobutyric acid (iHOBu) does not exceed 10%. Therefore, the next step is to modify the feed ratio.
The system design still follows the process outlined in
Figure 1. As for the experimental conditions, the total liquid feed flow rate is 5 mL/min, the reaction time is 6 min, the experimental operating pressure is 10 bar, and the operating temperature is 20 °C. The feed ratio of isobutyraldehyde to hydrogen peroxide is operated between 2:1 and 1:8. The experimental results are shown in
Figure 3.
In the experimental results of
Figure 3, when the isobutyraldehyde to hydrogen peroxide ratio exceeds 1:4, the conversion rate reaches a critical value of 90%. However, the selectivity for isobutyric acid remains very low, staying around 5% regardless of the ratio. Although the addition of acetone as a co-solvent did indeed address the challenges of the heterogeneous reaction between isobutyraldehyde and hydrogen peroxide, it still could not direct the reaction towards the synthesis of isobutyric acid.
According to Eastman’s patented process [
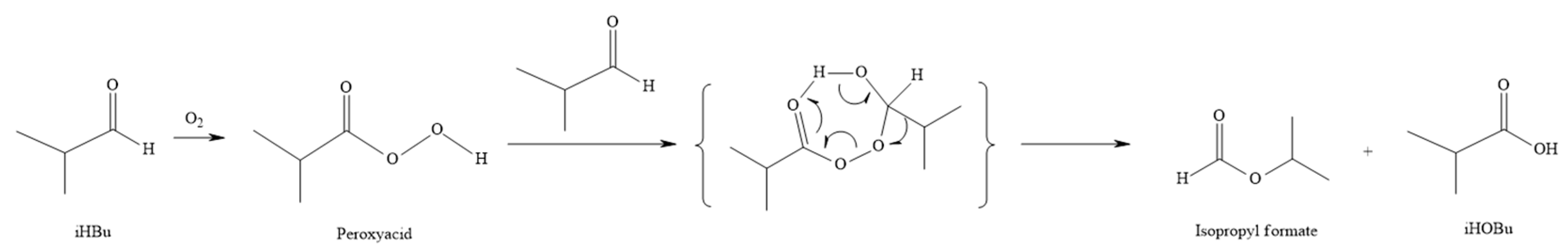
11], there are two main pathways for the oxidation of isobutyraldehyde to isobutyric acid:
In the oxidation reaction, isobutyraldehyde first combines with oxygen to form a peroxyacid. This peroxyacid then reacts with another molecule of isobutyraldehyde, leading to two different outcomes depending on the molecular arrangement.
In
Figure 4, Electrons move from a C–H bond on the peroxy acid–aldehyde intermediate, leading to the formation of two moles of isobutyric acid. In
Figure 5, electrons move from a C–C bond on the peroxy acid–aldehyde intermediate, leading to the formation of one mole of isobutyric and one mole of isopropyl formate.
The reaction pathway in
Figure 5 is referred to as the Bayer–Villiger oxidation. According to the study by Schweitzer-Chaput et al. [
14], using hydrogen peroxide as the oxidant is unfavorable for the occurrence of the Bayer–Villiger reaction.
Based on the results shown in
Figure 2 and
Figure 3, isobutyric acid is not effectively formed under the experimental conditions described. It is therefore evident that pathway A in
Figure 4 is not the main reaction pathway. Moreover, the Bayer–Villiger reaction in
Figure 5, which could occur as a side reaction, is reported in the literature to be unlikely under such conditions.
In summary, the results indicate that the strong oxidizing power of hydrogen peroxide is unfavorable for the formation of isobutyric acid. The complex GC signals indicate the formation of multiple byproducts, which also supports the discussion presented above. Compared with previous research findings [
9,
15], it can be concluded that the use of a catalyst may be one of the necessary conditions for the hydrogen peroxide-based process. However, the use of solid catalysts also significantly increases the risk of microchannel clogging.
At this point, this study concludes that hydrogen peroxide is not suitable as the oxidizing agent for this reaction. In addition to its inability to effectively produce isobutyric acid, the use of acetone as a co-solvent also increases the cost of product separation and purification. Therefore, the next step will be to use oxygen as the oxidizing agent for further testing.
3.2. Laboratory-Scale Experiments with Oxygen as Oxidizing Agent
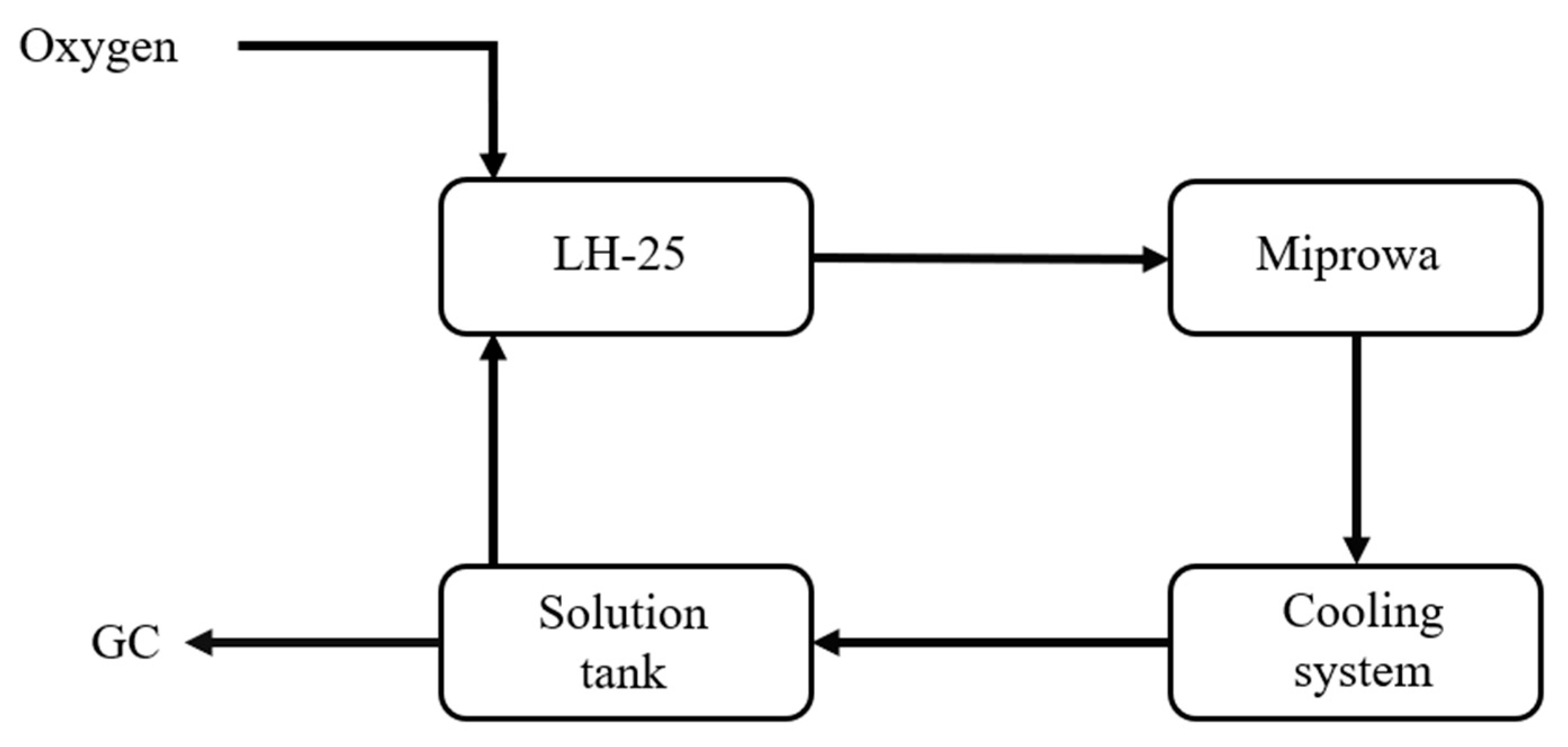
The system design is as shown in
Figure 6. The main difference from the previous design is the addition of a reflux setup, aimed at extending the reaction time of isobutyraldehyde.
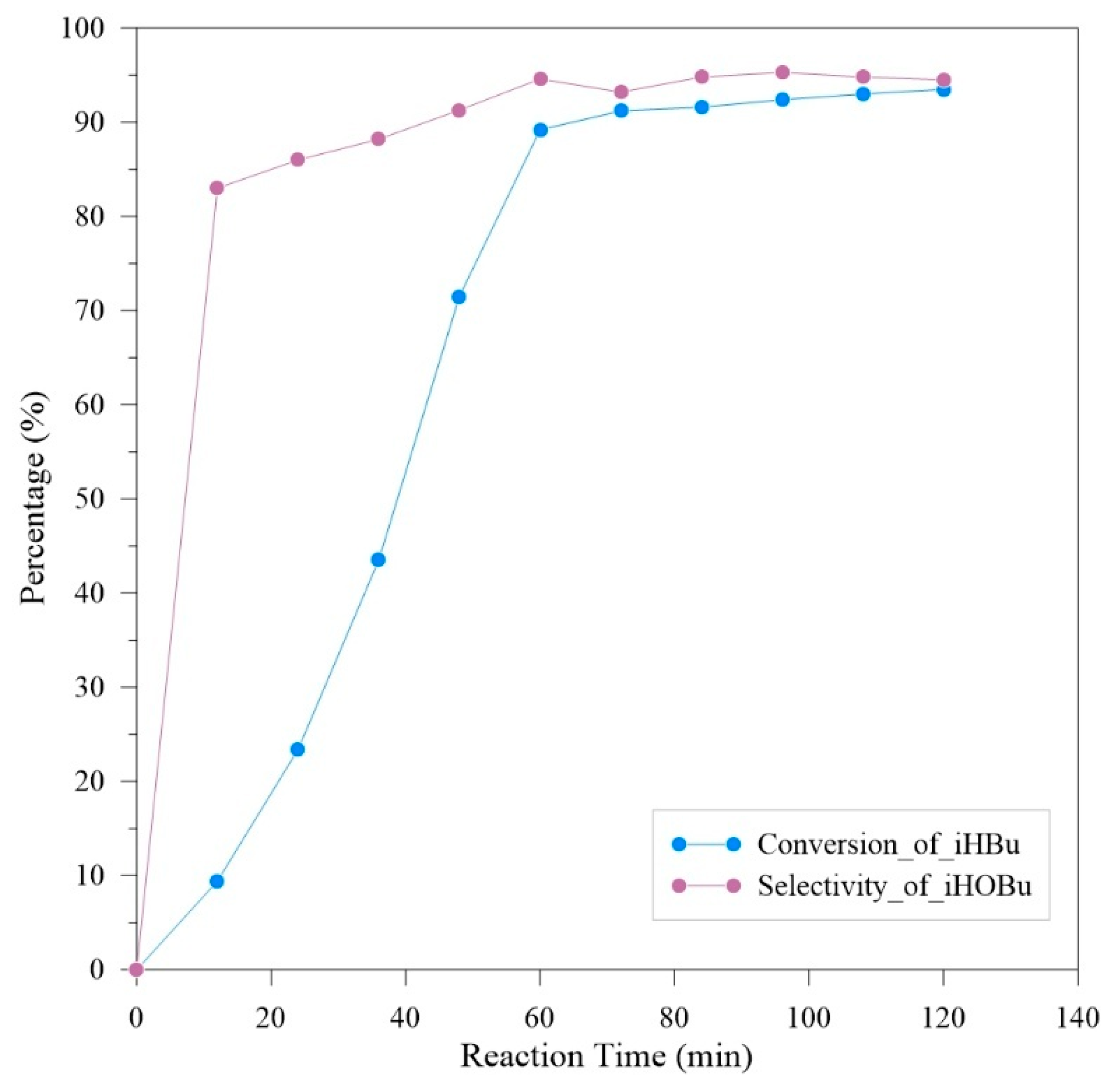
Isobutyraldehyde is initially placed in a solution tank. The oxygen flow rate is controlled at 1 SLPM, with a liquid flow rate of 5 mL/min, an operating pressure of 10 bar, and an operating temperature of 20 °C. Sampling is performed every 12 min, and the total experimental reaction time is 2 h. The experimental results are shown in
Figure 7.
The experimental results in
Figure 7 show that after 1 h of reaction time, the conversion rate reaches its plateau. After a total reaction time of 2 h, a 93.5% conversion rate of isobutyraldehyde and a 94.4% selectivity for isobutyric acid are achieved. In the first three sampling points of the experiment, peroxyacid and isopropyl formate were detected as byproducts. After that, isopropyl formate became the main byproduct. Considering the efficiency for subsequent production, a reaction time of 1 h is considered the optimal experimental condition.
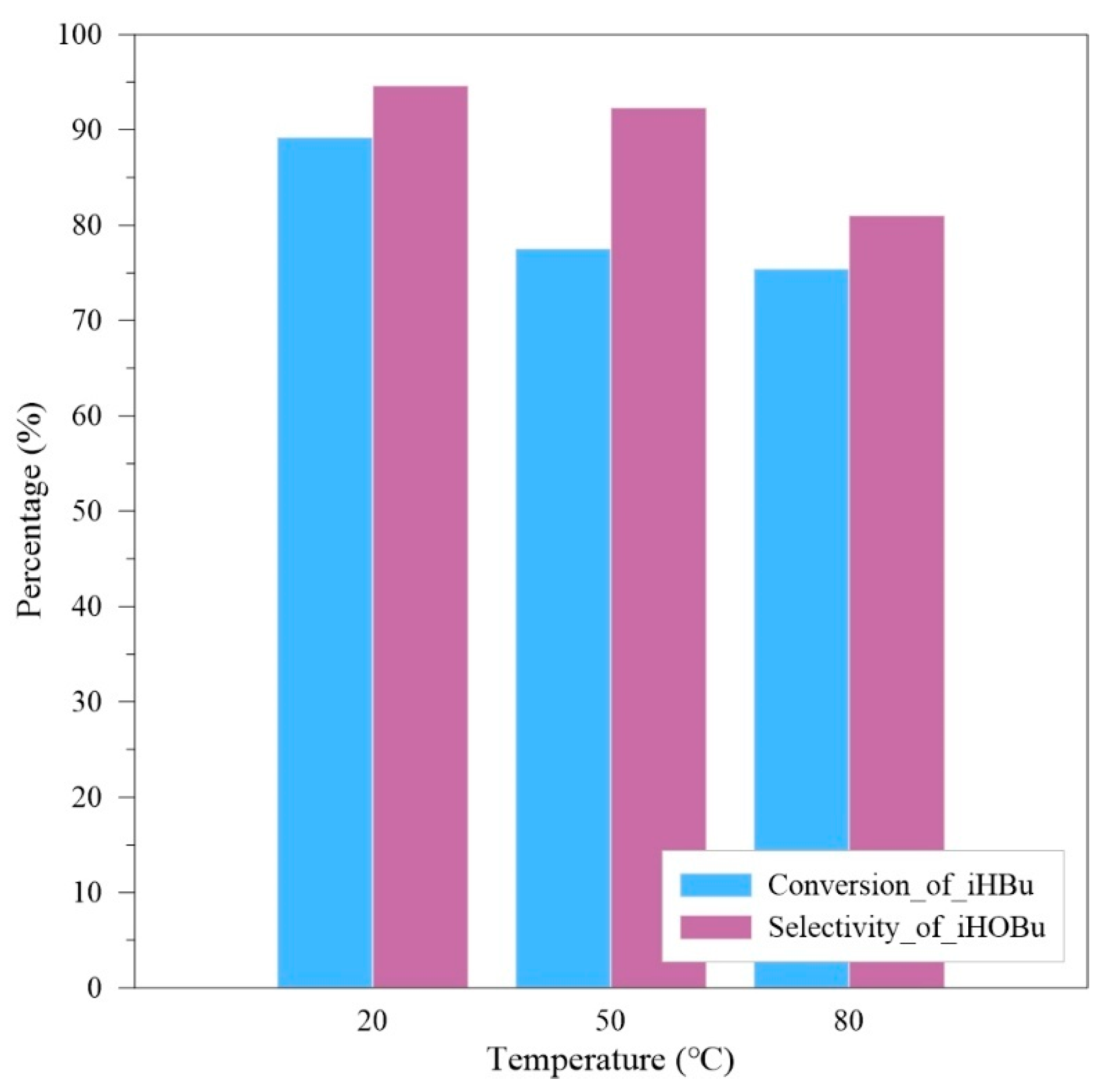
The next step is to confirm the effect of temperature in the experiment. The oxygen flow rate is controlled at 1 SLPM, with a liquid flow rate of 5 mL/min, an operating pressure of 10 bar, and experimental reaction time is 1 h. Operating temperature is designed between 20 °C and 80 °C. The experimental results are shown in
Figure 8.
According to the results from
Figure 8, under the system temperature condition of 20 °C, the optimal conversion rate of isobutyraldehyde is 89.2%, and the selectivity for isobutyric acid is 94.6%, with isopropyl formate as the main byproduct. This indicates that excessively high temperatures are unfavorable for this exothermic reaction and can also reduce the solubility of oxygen in the solution.
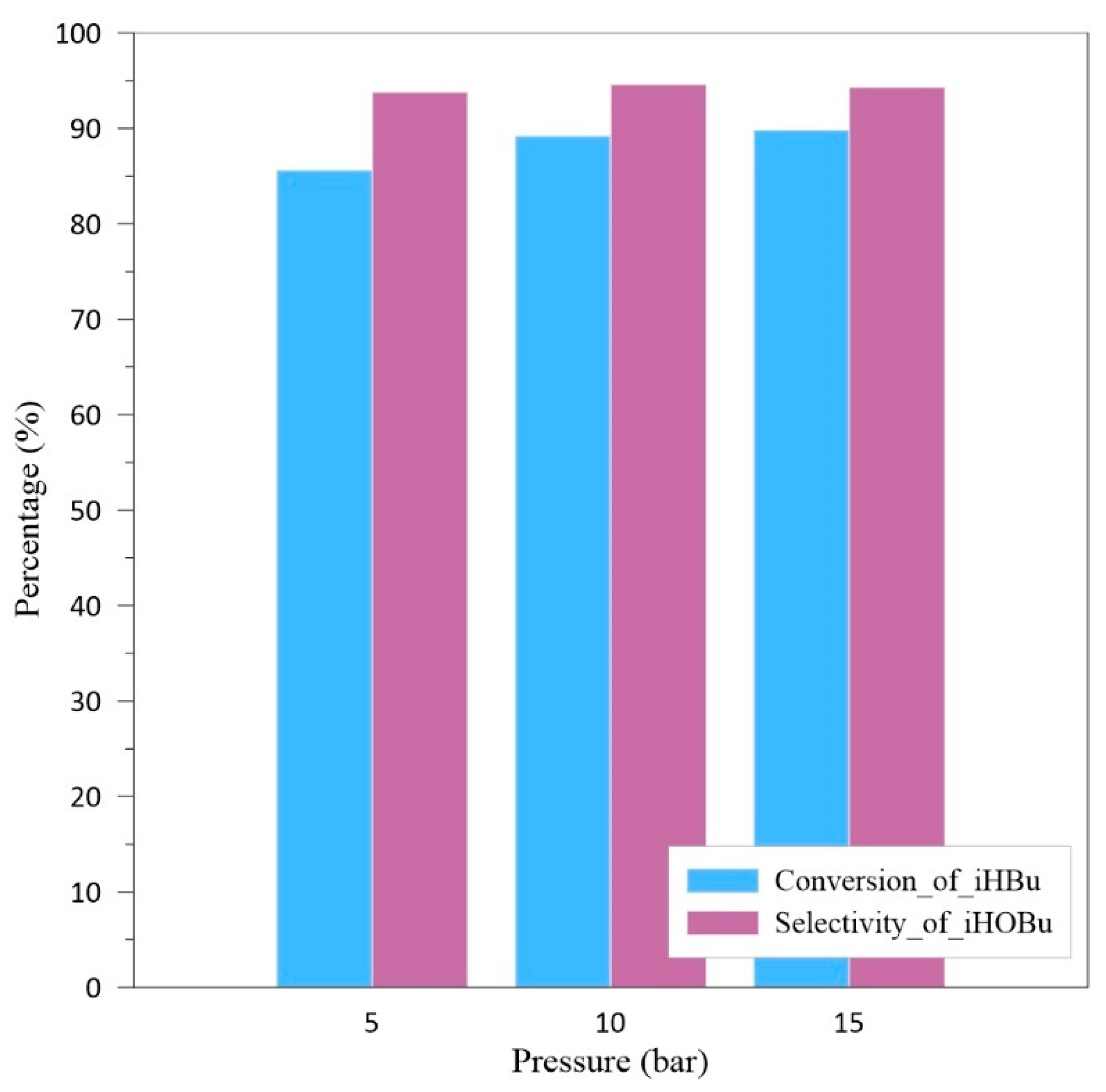
As the final step, this study will confirm the impact of pressure on the experimental results. The oxygen flow rate is controlled at 1 SLPM, with a liquid flow rate of 5 mL/min, an operating temperature of 20 °C, and experimental reaction time is 1 h. Operating pressure is designed between 5bar and 15bar. The experimental results are shown in
Figure 9.
According to the results from
Figure 9, under the system pressure of 15 bar, the highest isobutyraldehyde conversion rate of 90.2% can be achieved. However, this result is only 1% higher than the experimental result under the condition of 10 bar. Furthermore, higher system pressure will lead to increased operating costs and greater instability. Therefore, operating pressure of 10 bar is considered the optimal experimental condition.
At this point, the laboratory-scale experiments have obtained the optimal experimental parameters and results. The oxygen flow rate is 1 SLPM, the liquid flow rate is 5 mL/min, the operating pressure is 10 bar, the operating temperature is 20 °C, and the reaction time is 1 h. Under these conditions, a conversion rate of 89.2% for isobutyraldehyde and a selectivity of 94.6% for isobutyric acid can be achieved, with isopropyl formate as the main byproduct.
Compared with the experimental results reported in the literature [
11], the process design in this study significantly reduced the required reaction time. However, due to the limited reactor volume of the MMRS system, the current production capacity remains relatively low. Therefore, this study aimed to design a custom-built microreactor to increase the production of isobutyric acid, with the goal of reproducing the laboratory-scale test results.
3.3. Pilot-Scale Experiment
In pilot-scale experiment, the test is carried out using a custom-built microreactor system, and the production scale is scaled up. The process is shown in
Figure 10, and the simplified structure of the reactor is shown in
Figure 11.
In the custom-built microreactor system, the main reactor consists of a cylindrical shell filled with microchannel tubing of a specific length. Referring to the Miprowa reactor design by Ehrfeld (Köln, Germany), 3D-printed staggered inserts were placed inside the custom-built micro-channel. By stacking these inserts, efficient mixing of the fluid was achieved as it passed through.
In terms of experimental parameters, the process was scaled up proportionally based on the optimal conditions identified at the laboratory-scale. The oxygen flow rate is 4 SLPM, the liquid flow rate is 20 mL/min, the operating pressure is 10 bar, the operating temperature is 20 °C, and the reaction time is between 1 and 3 h. The experimental results are shown in
Figure 12.
Based on the experimental results from
Figure 12, similar results to the laboratory-scale experiment can be achieved within two hours of reaction time. After three hours of reaction, a conversion rate of 91.57% and a selectivity of 96.09% for isobutyric acid can be obtained.
The results of the experiment demonstrate the effectiveness of the custom-built microchannel system. It achieves experimental outcomes comparable to those obtained with the Ehrfeld microreactor system, proving the advantage of microreactor technology in theory, which exhibits no scaling-effects.
Under the current experimental conditions, a single custom-built microreactor system can achieve an annual production of 7–8 tons of isobutyric acid. The results of this study have been granted a patent in Taiwan. Additionally, a company has shown strong interest in this technology, and discussions for potential collaboration are currently underway. It is hoped that this research will serve as the foundation for establishing a production process for isobutyric acid at a scale of hundreds of tons per year.
4. Conclusions
In this study, hydrogen peroxide was first tested as the oxidant. Although a conversion rate of nearly 90% for isobutyraldehyde was achieved in the Miprowa process, it was almost impossible to control the reaction to proceed towards isobutyric acid, with the selectivity for the product being less than 10%. Moreover, the hydrogen peroxide process is originally a heterogeneous reaction, requiring the addition of acetone as a co-solvent, which significantly increases the cost of subsequent separation. Therefore, it was decided to abandon the use of hydrogen peroxide as the oxidant for this study and switch to using oxygen for the oxidation process.
The experimental results of the oxygen-based process showed that in the laboratory-scale process, with an isobutyraldehyde flow rate of 5 mL/min, an air flow rate of 1 SLPM, a process back pressure of 10 bar, and a reaction time of 1 h, the optimal isobutyraldehyde conversion rate of 90% and the isobutyric acid selectivity of 95% were achieved. In the pilot-scale system, with an isobutyraldehyde flow rate of 20 mL/min, an air flow rate of 4 SLPM, a process back pressure of 10 bar, and a reflux time of 3 h, the optimal isobutyraldehyde conversion rate of 91% and the isobutyric acid selectivity of 97% were achieved. Under the optimal experimental conditions, a single custom-built microreactor system can achieve an annual production of 7–8 tons of isobutyric acid.
However, microreactor technology still has certain limitations in practical use. Firstly, the cost is relatively high. For instance, the Ehrfeld MMRS system used in this study costs over USD 300,000, which poses a significant financial burden for research laboratories and small to medium-sized enterprises. Secondly, microchannels are highly prone to clogging. The formation of solid precipitates or crystal deposits from reaction products can lead to an increase in system pressure, ultimately causing the experiment to halt.
Therefore, this study attempted to use a custom-built microreactor system to reduce the cost to below USD 100,000. In addition, catalysts were deliberately avoided in the experimental setup to minimize the risk of channel clogging during the reaction. This study demonstrates a value-added process by converting isobutyraldehyde (valued at USD 0.8–1.0/kg) into isobutyric acid (valued at USD 1.3–1.6/kg). Through technology transfer, the process is expected to be scaled up to a hundred-ton level and developed into a commercial-scale mass production process.
In summary, this study has improved the isobutyraldehyde oxidation process using microreactor technology, achieving better production efficiency and demonstrating that there is no scaling-effect in the amplification of the process using custom-built MRT. Furthermore, the patent for this study has been successfully filed, and efforts are actively being made to scale up the production process to a hundred-ton level. It is hoped that this environmentally friendly and efficient process will be realized in the industry.