The Effect of Stress Distribution on Tibial Implants with a Honeycomb Structure in Open-Wedge High Tibial Osteotomy
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Finite Element Analysis
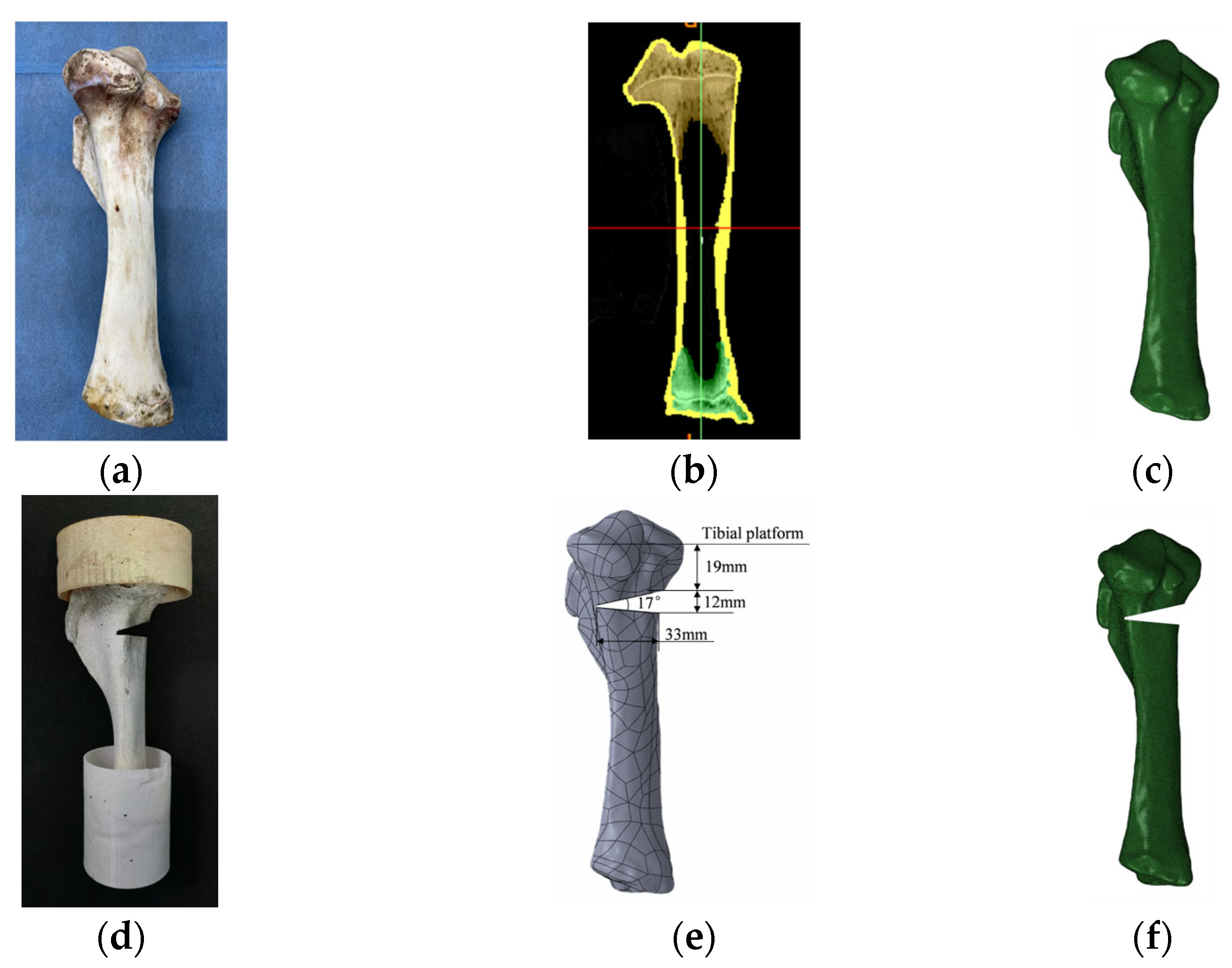
2.1.1. Model Construction
2.1.2. Materials Properties and Interactions
2.1.3. Boundary and Loading Conditions
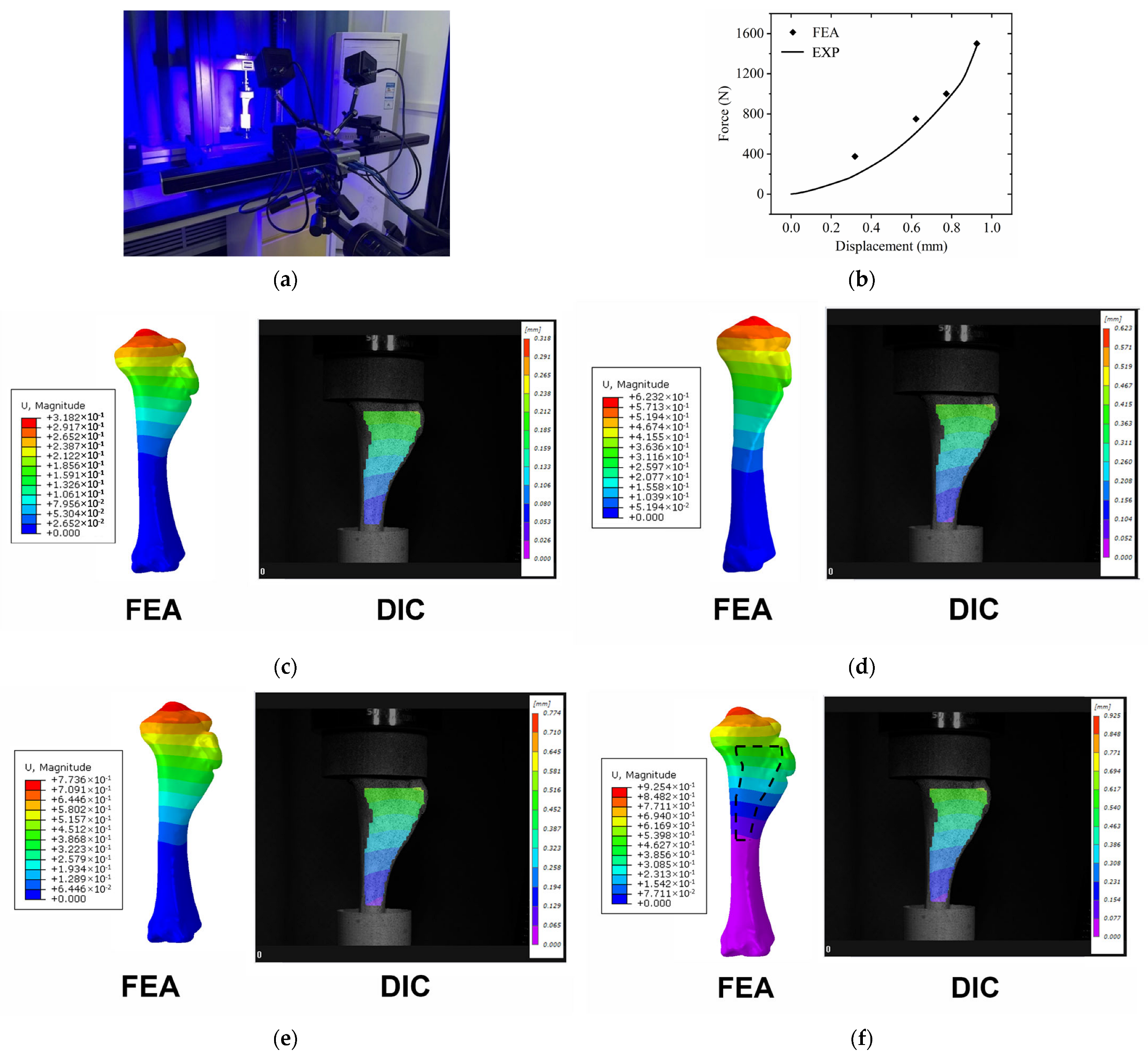
2.2. Experimental Research
2.2.1. Specimens
2.2.2. Experimental Stand
3. Results
3.1. Validation of FE Model
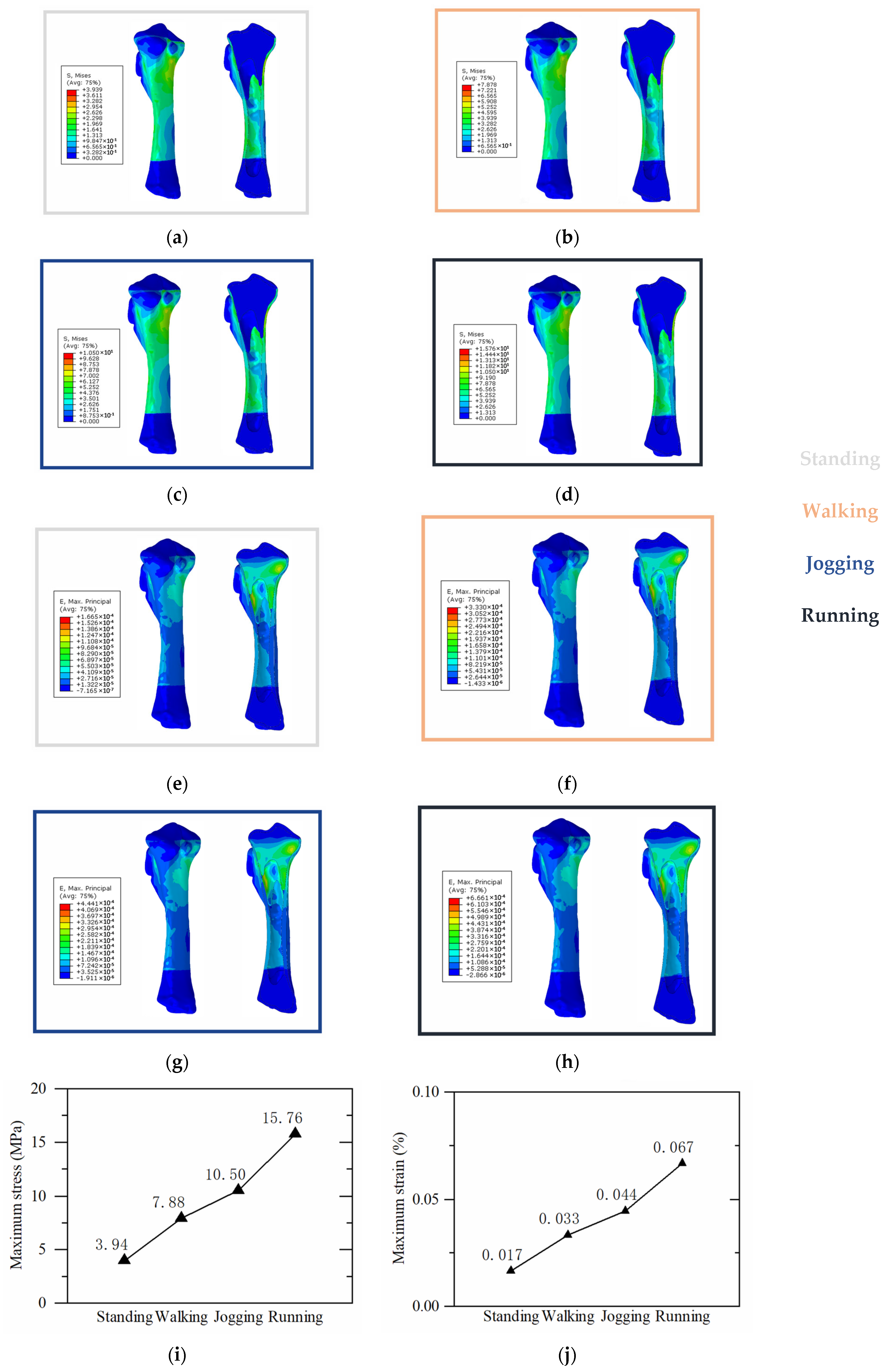
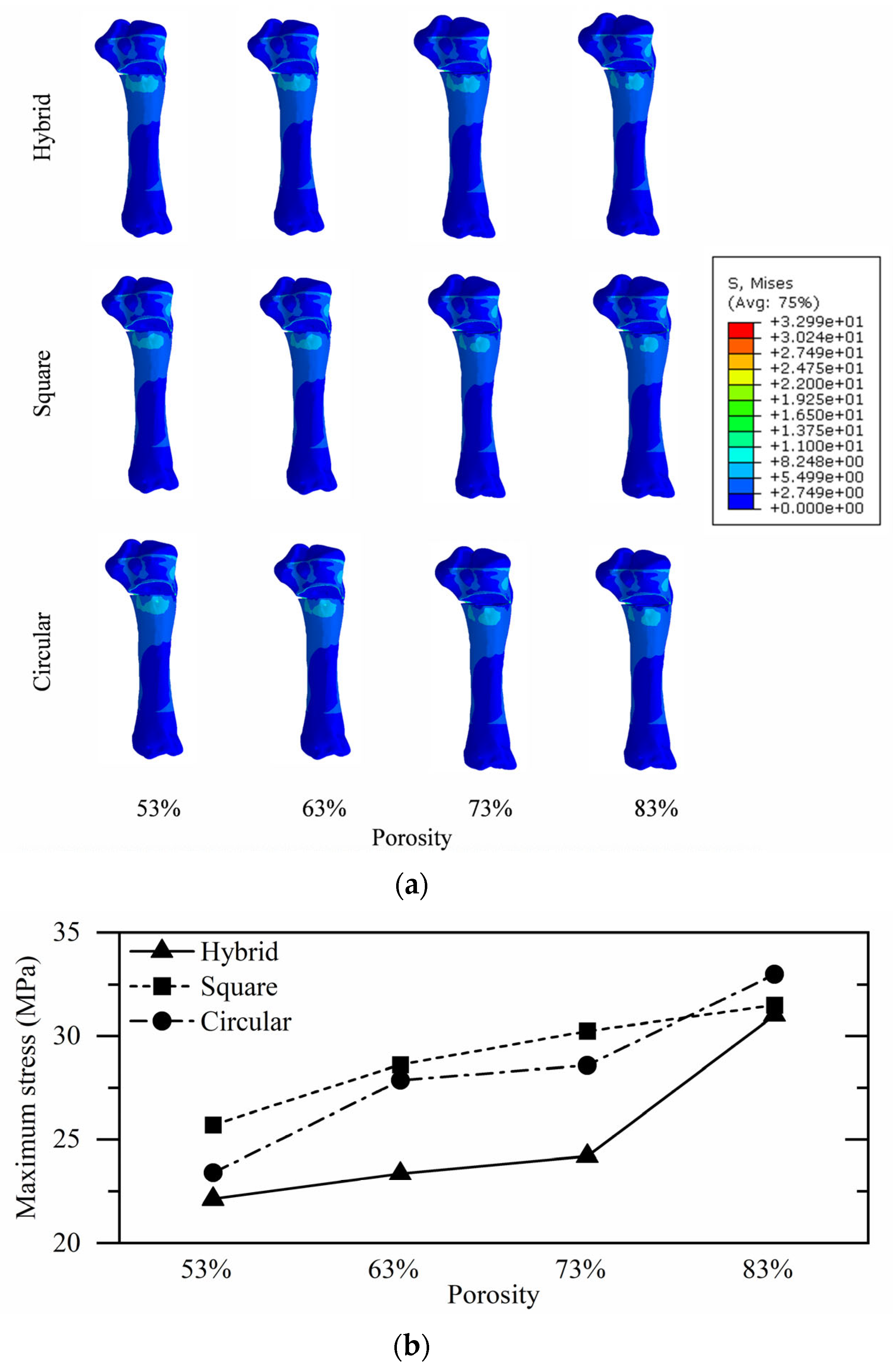
3.2. Proximal Tibial Stress Analysis
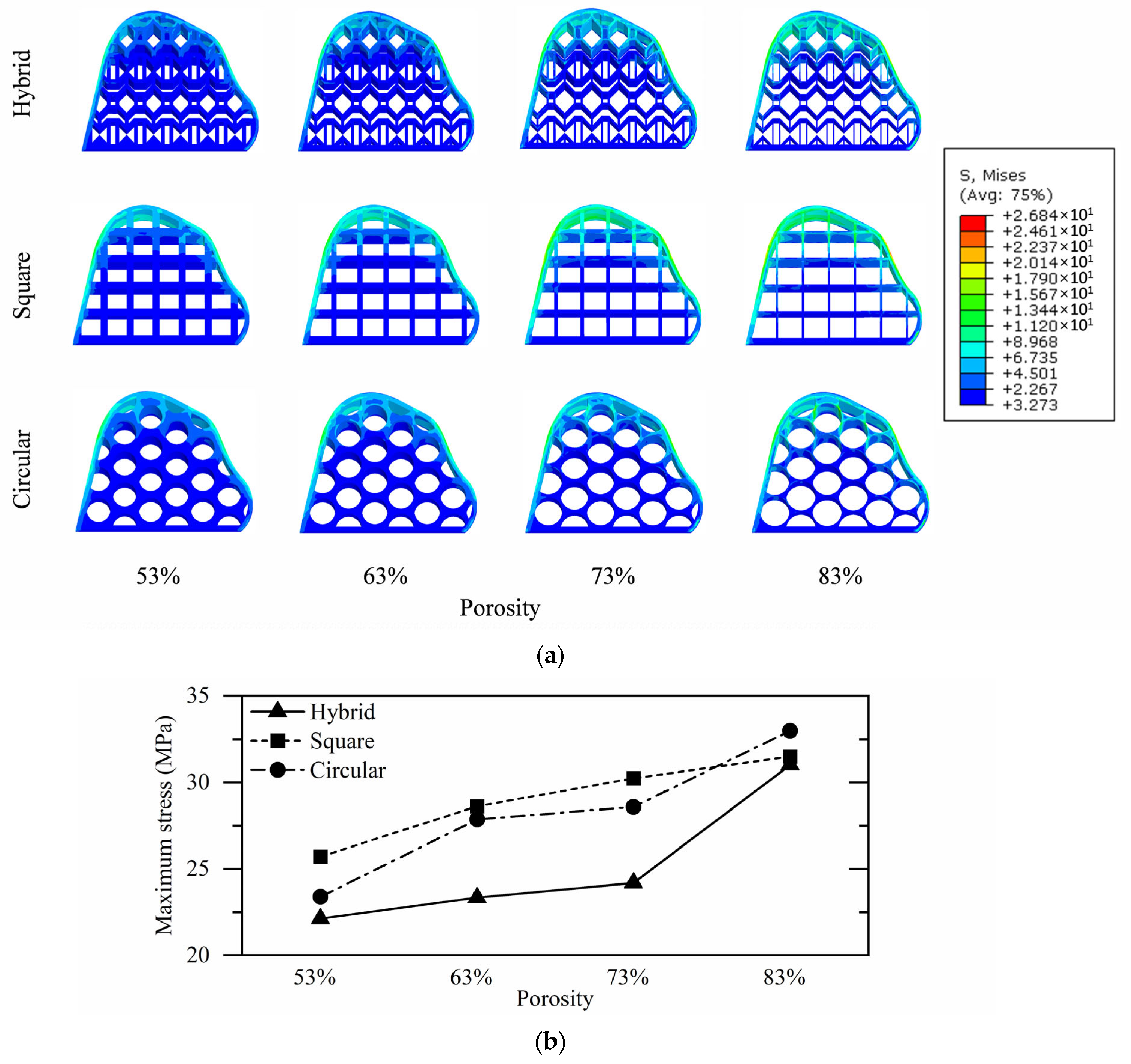
3.3. Tibial Implant Stress Analysis
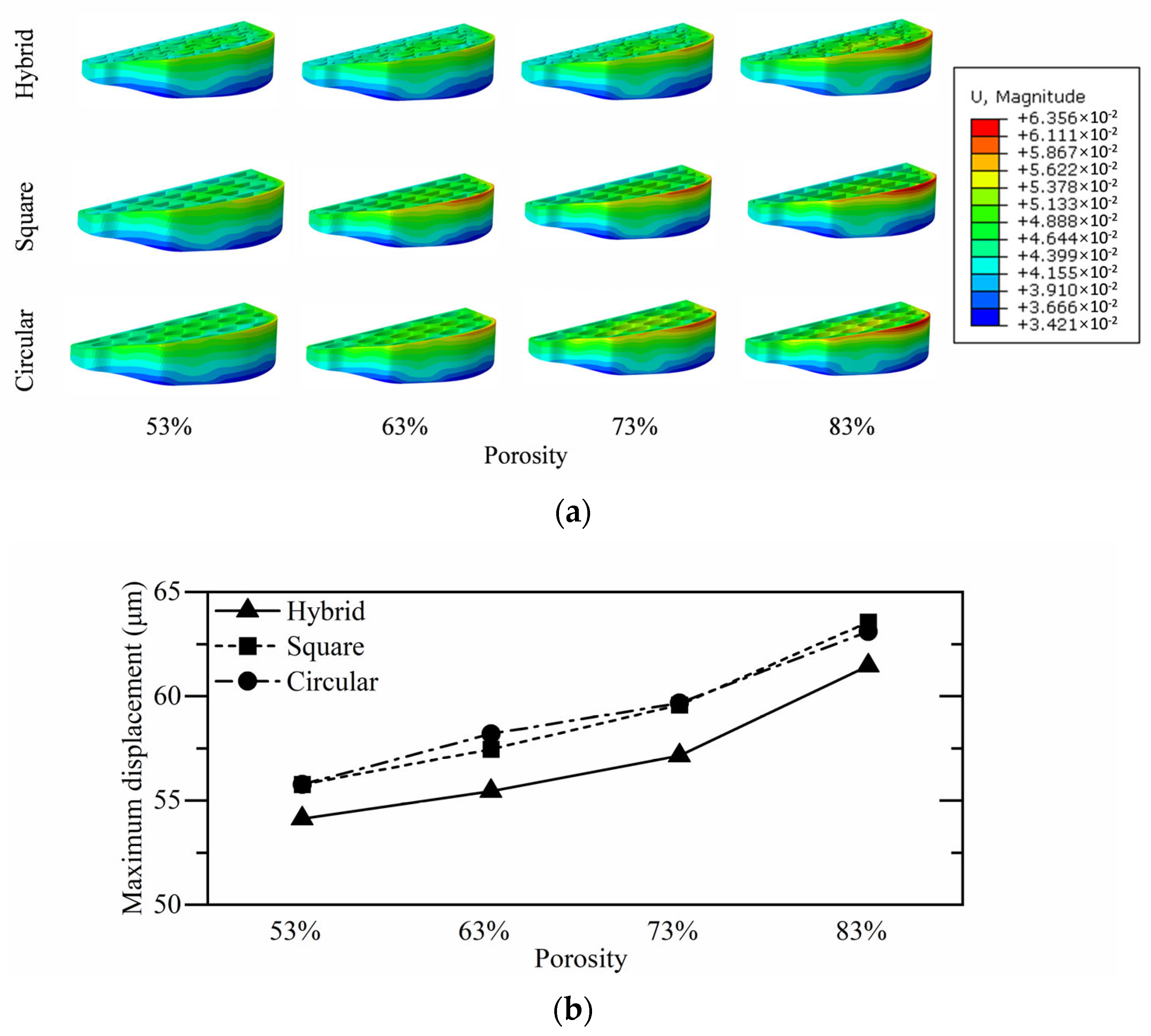
3.4. Tibial Implant Micromotion Analysis
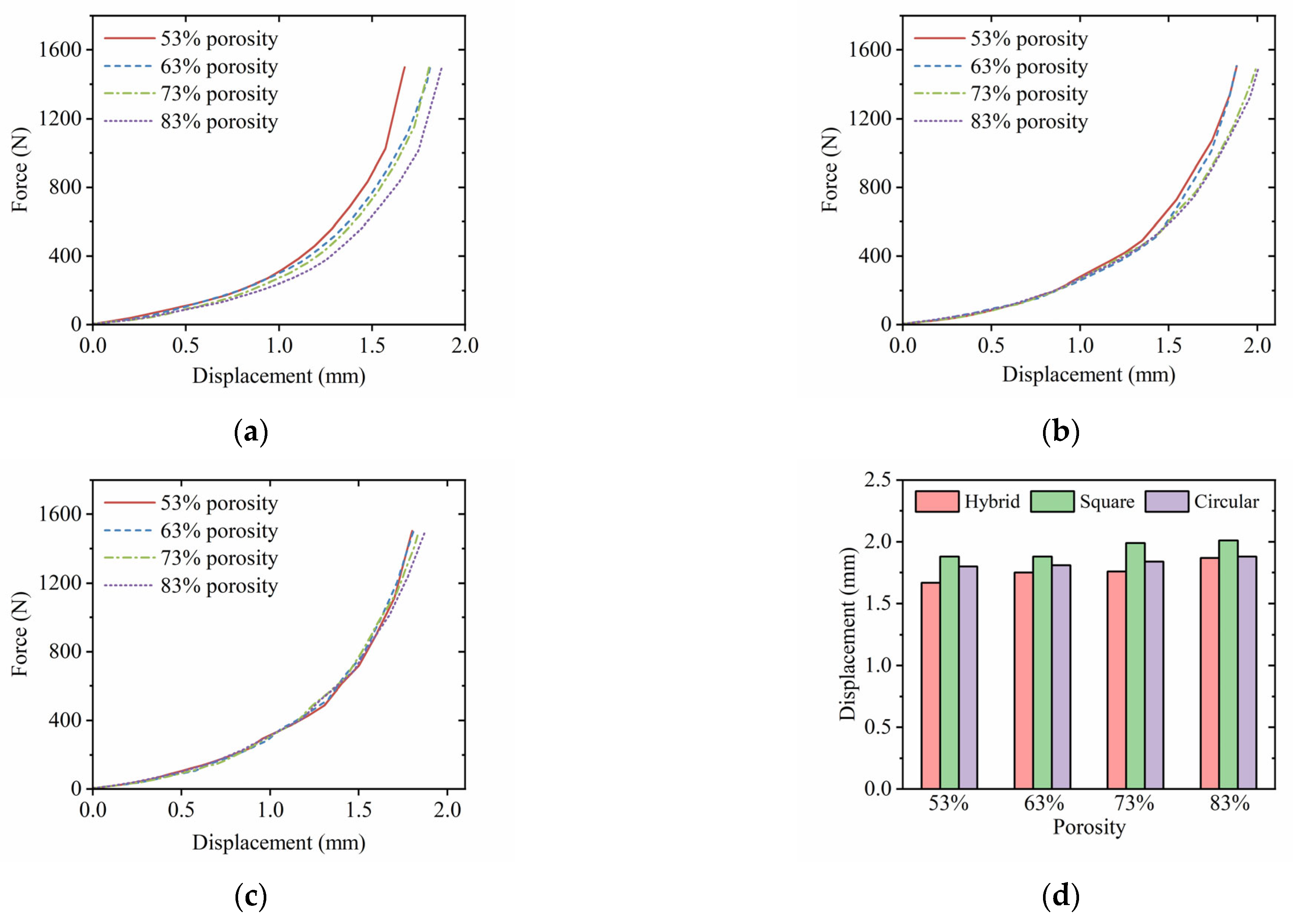
3.5. Postoperative Tibial Biomechanical Experiments
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cui, A.; Li, H.; Wang, D.; Zhong, J.; Chen, Y.; Lu, H. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EClinicalMedicine 2020, 29–30, 100587. [Google Scholar] [CrossRef] [PubMed]
- Schram, B.; Orr, R.; Pope, R.; Canetti, E.; Knapik, J. Risk factors for development of lower limb osteoarthritis in physically demanding occupations: A narrative umbrella review. J. Occup. Health 2020, 62, e12103. [Google Scholar] [CrossRef] [PubMed]
- Briggs, A.M.; Cross, M.J.; Hoy, D.G.; Sànchez-Riera, L.; Blyth, F.M.; Woolf, A.D.; March, L. Musculoskeletal Health Conditions Represent a Global Threat to Healthy Aging: A Report for the 2015 World Health Organization World Report on Ageing and Health. Gerontologist 2016, 56, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, J.D.; Culbreth, G.T.; Haile, L.M.; Rafferty, Q.; Lo, J.; Fukutaki, K.G.; Cruz, J.A.; Smith, A.E.; Vollset, S.E.; Brooks, P.M.; et al. Global, regional, and national burden of osteoarthritis, 1990–2020 and projections to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, 508–522. [Google Scholar] [CrossRef]
- Ryu, D.J.; Wang, J.H. Editorial Commentary: Posterolateral Malposition of the Cortical Hinge During Medial Open-Wedge High Tibial Osteotomy Increases Posterior Tibial Slope: Incomplete Posterior Osteotomy May Shift the Hinge From Lateral to Posterolateral. Arthroscopy 2021, 37, 2202–2203. [Google Scholar] [CrossRef]
- Deng, X.; Chen, W.; Zhao, K.; Zhu, J.; Hu, H.; Cheng, X.; Wang, Z.; Wang, Y.; Tan, Z.; Ye, Z.; et al. Changes in patellar height and posterior tibial slope angle following uniplanar medial opening wedge high tibial osteotomy using a novel wedge-shaped spacer implanation concurrent with proximal partial fibulectomy. Int. Orthop. 2020, 45, 109–115. [Google Scholar] [CrossRef]
- Greimel, F.; Maderbacher, G.; Baier, C.; Krieg, B.; Zeman, F.; Grifka, J.; Keshmiri, A. Medial Open Wedge High tibial Osteotomy (MOWHTO) does not relevantly alter patellar kinematics: A cadaveric study. Arch. Orthop. Trauma Surg. 2020, 142, 13–21. [Google Scholar] [CrossRef]
- Subasi, O.; Karaismailoglu, B.; Ashkani-Esfahani, S.; Lazoglu, I. Investigation of lattice infill parameters for additively manufactured bone fracture plates to reduce stress shielding. Comput. Biol. Med. 2023, 161, 107062. [Google Scholar] [CrossRef]
- Xu, S.; Ding, X.; Xiong, M.; Duan, P.; Zhang, H.; Li, Z. The optimal design of 3D-printed lattice bone plate by considering fracture healing mechanism. Int. J. Numer. Methods Biomed. Eng. 2023, 39, e3682. [Google Scholar] [CrossRef]
- Varaschin, A.; Gill, H.S.; Zaffagnini, S.; Leardini, A.; Ortolani, M.; Norvillo, F.; MacLeod, A.; Dal Fabbro, G.; Cassiolas, G.; Grassi, A.; et al. Personalised High Tibial Osteotomy Surgery Is Accurate: An Assessment Using 3D Distance Mapping. Appl. Sci. 2024, 14, 9033. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Song, B.; Zhang, J.; Fan, J.; Zhang, Z.; Han, Q.; Shi, Y. Anisotropic mechanical and mass-transport performance of Ti6Al4V plate-lattice scaffolds prepared by laser powder bed fusion. Acta Biomater. 2022, 148, 374–388. [Google Scholar] [CrossRef] [PubMed]
- Crook, C.; Bauer, J.; Guell Izard, A.; Santos de Oliveira, C.; Martins de Souza e Silva, J.; Berger, J.B.; Valdevit, L. Plate-nanolattices at the theoretical limit of stiffness and strength. Nat. Commun. 2020, 11, 1579. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Wen, W.; Fang, D. Additively-manufactured anisotropic and isotropic 3D plate-lattice materials for enhanced mechanical performance: Simulations & experiments. Acta Mater. 2020, 199, 397–412. [Google Scholar] [CrossRef]
- Al-Tamimi, A.A.; Tlija, M.; Alhamidi, A.; Abidi, M.H.; Al-Ahmari, A.; Al-Zahrani, S.M. A novel reinforced PLA locking compression plate to eliminate stress shielding using design for additive manufacturing. J. Mater. Res. Technol. 2024, 32, 2127–2139. [Google Scholar] [CrossRef]
- Al-Tamimi, A.A.; Almeida, H.; Bartolo, P. Structural optimisation for medical implants through additive manufacturing. Prog. Addit. Manuf. 2020, 5, 95–110. [Google Scholar] [CrossRef]
- Alkentar, R.; Mankovits, T. Development of Surrogate Model for Patient-Specific Lattice-Structured Hip Implant Design via Finite Element Analysis. Appl. Sci. 2025, 15, 3522. [Google Scholar] [CrossRef]
- Karuna, C.; Poltue, T.; Khrueaduangkham, S.; Promoppatum, P. Mechanical and fluid characteristics of triply periodic minimal surface bone scaffolds under various functionally graded strategies. J. Colloid Interface Sci. 2022, 9, 1258–1278. [Google Scholar] [CrossRef]
- Distefano, F.; Pasta, S.; Epasto, G. Titanium Lattice Structures Produced via Additive Manufacturing for a Bone Scaffold: A Review. J. Funct. Biomater. 2023, 14, 125. [Google Scholar] [CrossRef]
- Honda, T.; Katano, Y.; Kuzuya, T.; Hayashi, K.; Ishigami, M.; Itoh, A.; Hirooka, Y.; Nakano, I.; Ishikawa, T.; Toyoda, H.; et al. Comparison of the efficacy of ribavirin plus peginterferon alfa-2b for chronic hepatitis C infection in patients with and without coagulation disorders. J. Med. Virol. 2012, 85, 228–234. [Google Scholar] [CrossRef]
- Abate, K.M.; Nazir, A.; Jeng, J.-Y. Design, optimization, and selective laser melting of vin tiles cellular structure-based hip implant. Int. J. Adv. Manuf. Technol. 2021, 112, 2037–2050. [Google Scholar] [CrossRef]
- Torres-Sanchez, C.; Al Mushref, F.R.A.; Norrito, M.; Yendall, K.; Liu, Y.; Conway, P.P. The effect of pore size and porosity on mechanical properties and biological response of porous titanium scaffolds. Mater. Sci. Eng. C 2017, 77, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Al Zoubi, N.F.; Tarlochan, F.; Mehboob, H. Mechanical and Fatigue Behavior of Cellular Structure Ti-6Al-4V Alloy Femoral Stems: A Finite Element Analysis. Appl. Sci. 2022, 12, 4197. [Google Scholar] [CrossRef]
- Nazir, A.; Abate, K.M.; Kumar, A.; Jeng, J.-Y. A state-of-the-art review on types, design, optimization, and additive manufacturing of cellular structures. Int. J. Adv. Manuf. Technol. 2019, 104, 3489–3510. [Google Scholar] [CrossRef]
- Wangpraseurt, D.; You, S.; Azam, F.; Jacucci, G.; Gaidarenko, O.; Hildebrand, M.; Kühl, M.; Smith, A.G.; Davey, M.P.; Smith, A.; et al. Bionic 3D printed corals. Nat. Commun. 2020, 11, 1748. [Google Scholar] [CrossRef]
- Ghazlan, A.; Ngo, T.; Nguyen, T.; Linforth, S.; Van Le, T. Uncovering a high-performance bio-mimetic cellular structure from trabecular bone. Sci. Rep. 2020, 10, 14247. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Feng, q.; Hu, F.; Zhang, B.; Qi, F.; Zhao, N.; Ouyang, X. A composite nanofiller with a nail column void structure to imitate beetle shell fiber to enhance the impact resistance of polyurethane elastomer. Compos. Sci. Technol. 2022, 221, 109304. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Z.; Wang, Y.; Xu, D.; Zhao, Y. Butterfly inspired functional materials. Mater. Sci. Eng. Rep. 2021, 144, 100605. [Google Scholar] [CrossRef]
- Sun, H.; Li, H.; Dauletbek, A.; Lorenzo, R.; Corbi, I.; Corbi, O.; Ashraf, M. Review on materials and structures inspired by bamboo. Constr. Build. Mater. 2022, 325, 126656. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Wang, C.; Zeng, Y.; Chen, T. Crashworthiness of bionic fractal hierarchical structures. Mater. Des. 2018, 158, 147–159. [Google Scholar] [CrossRef]
- Zhang, T.; Wei, Q.; Zhou, H.; Jing, Z.; Liu, X.; Zheng, Y.; Cai, H.; Wei, F.; Jiang, L.; Yu, M.; et al. Three-dimensional-printed individualized porous implants: A new “implant-bone” interface fusion concept for large bone defect treatment. Bioact. Mater. 2021, 6, 3659–3670. [Google Scholar] [CrossRef]
- Mullen, L.; Stamp, R.C.; Brooks, W.K.; Jones, E.; Sutcliffe, C.J. Selective Laser Melting: A regular unit cell approach for the manufacture of porous, titanium, bone in-growth constructs, suitable for orthopedic applications. J. Biomater. Mater. Rse. B 2008, 89B, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Arabnejad, S.; Johnston, R.B.; Pura, J.A.; Singh, B.; Tanzer, M.; Pasini, D. High-strength porous biomaterials for bone replacement: A strategy to assess the interplay between cell morphology, mechanical properties, bone ingrowth and manufacturing constraints. Acta Biomater. 2016, 30, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, M.; Qin, D.; Chen, J.; Wu, H. Crashworthiness Analysis and Multi-Objective Optimization for Concave I-Shaped Honeycomb Structure. Appl. Sci. 2022, 12, 10420. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Z.; Zhang, Y.; Cui, S.; Yang, Z.; Lu, Z. Ultra-low density architectured metamaterial with superior mechanical properties and energy absorption capability. Compos. Part B 2020, 202, 108379. [Google Scholar] [CrossRef]
- Yong, J.; Dong, Y.; Wan, Z.; Li, W.; Chen, Y. Collaborative Design of Static and Vibration Properties of a Novel Re-Entrant Honeycomb Metamaterial. Appl. Sci. 2024, 14, 1497. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, J.; Hui, D. Mechanical behaviors of inclined cell honeycomb structure subjected to compression. Compos. Part B 2017, 110, 307–314. [Google Scholar] [CrossRef]
- Ha, N.S.; Lu, G. A review of recent research on bio-inspired structures and materials for energy absorption applications. Compos. Part B 2020, 181, 107496. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Hao, J.; Wang, Y.; Wang, K.; Tai, S. Study on mechanical properties of cellular structures with negative Poisson’s ratio based on the development of Abaqus plug-in tool. Compos. Struct. 2023, 322, 117348. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, Y.; Ren, X.; Han, D.; Jiang, W.; Zhang, X.G.; Luo, H.C.; Xie, Y.M. Design and mechanical characteristics of auxetic metamaterial with tunable stiffness. Int. J. Mech. Sci. 2022, 223, 107286. [Google Scholar] [CrossRef]
- Chen, S.; Tan, X.; Hu, J.; Zhu, S.; Wang, B.; Wang, L.; Jin, Y.; Wu, L. A novel gradient negative stiffness honeycomb for recoverable energy absorption. Compos. Part B 2021, 215, 108745. [Google Scholar] [CrossRef]
- Pan, Y.; Zhou, Y.; Wang, M.; Gao, Q.; Sun, B. A novel reinforced cylindrical negative stiffness metamaterial for shock isolation: Analysis and application. Int. J. Solids Struct. 2023, 279, 112391. [Google Scholar] [CrossRef]
- Chen, X.; He, T.; Hu, Y.; Feng, M. A 3D dislocated re-entrant structure with compression-twist coupling effect. Smart Mater. Struct. 2023, 32, 055009. [Google Scholar] [CrossRef]
- Yang, K.; Rao, L.; Hu, L.; Li, Z. 3D curved-walled same-phase chiral honeycombs with controllable compression-torsion coupling effect via variable cross-section design. Thin Walled Struct. 2023, 193, 111267. [Google Scholar] [CrossRef]
- Chen, P.; Zhan, Y.; Zhan, S.; Li, R.; Luo, C.; Xie, X. Biomechanical evaluation of different types of lateral hinge fractures in medial opening wedge high tibial osteotomy. Clin. Biomech. 2021, 83, 105295. [Google Scholar] [CrossRef]
- Simmen, H.-P.; Yang, J.C.-S.; Lin, K.-Y.; Lin, H.-H.; Lee, O.K. Biomechanical evaluation of high tibial osteotomy plate with internal support block using finite element analysis. PLoS ONE 2021, 16, e0247412. [Google Scholar] [CrossRef]
- Belvedere, C.; Gill, H.S.; Ortolani, M.; Sileoni, N.; Zaffagnini, S.; Norvillo, F.; MacLeod, A.; Dal Fabbro, G.; Grassi, A.; Leardini, A. Instrumental Gait Analysis and Tibial Plateau Modelling to Support Pre- and Post-Operative Evaluations in Personalized High Tibial Osteotomy. Appl. Sci. 2023, 13, 12425. [Google Scholar] [CrossRef]
- Cofaru, I.I.; Oleksik, M.; Cofaru, N.F.; Branescu, A.H.; Haşegan, A.; Roman, M.D.; Fleaca, S.R.; Dobrotă, R.D. A Computer-Assisted Approach Regarding the Optimization of the Geometrical Planning of Medial Opening Wedge High Tibial Osteotomy. Appl. Sci. 2022, 12, 6636. [Google Scholar] [CrossRef]
- Soltanihafshejani, N.; Bitter, T.; Verdonschot, N.; Janssen, D. The effect of periprosthetic bone loss on the failure risk of tibial total knee arthroplasty. J. Orthop. Res. 2023, 42, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Liu, Y.; Zhang, A.; Chen, H.; Wan, Q.; Zhong, L.; Wang, X.; Han, Q.; Wang, J. Medial-lateral translational malalignment of the prosthesis on tibial stress distribution in total knee arthroplasty: A finite element analysis. Front. Bioeng. Biotechnol. 2023, 11, 1119204. [Google Scholar] [CrossRef]
- Jyoti; Ghosh, R. Printable functionally graded tibial implant for TAR: FE study comparing implant materials, FGM properties, and implant designs. Comput. Biol. Med. 2024, 177, 108645. [Google Scholar] [CrossRef]
- Lerner, Z.F.; DeMers, M.S.; Delp, S.L.; Browning, R.C. How tibiofemoral alignment and contact locations affect predictions of medial and lateral tibiofemoral contact forces. J. Biomech. 2015, 48, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Frydrýšek, K.; Čepica, D.; Halo, T.; Skoupý, O.; Pleva, L.; Madeja, R.; Pometlová, J.; Losertová, M.; Koutecký, J.; Michal, P.; et al. Biomechanical Analysis of Staples for Epiphysiodesis. Appl. Sci. 2022, 12, 614. [Google Scholar] [CrossRef]
- Bori, E.; Innocenti, B. Biomechanical Analysis of Femoral Stem Features in Hinged Revision TKA with Valgus or Varus Deformity: A Comparative Finite Elements Study. Appl. Sci. 2023, 13, 2738. [Google Scholar] [CrossRef]
- Pan, C.S.; Wang, X.; Ding, L.Z.; Zhu, X.P.; Xu, W.F.; Huang, L.X. The best position of bone grafts in the medial open-wedge high tibial osteotomy: A finite element analysis. Comput. Methods Programs Biomed. 2023, 228, 107253. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.; Son, J.; Kim, H.; Kwon, S.K.; Kwon, O.; Kim, H.J.; Kang, K. Multi-objective design optimization of high tibial osteotomy for improvement of biomechanical effect by using finite element analysis. J. Orthop. Res. 2018, 36, 2956–2965. [Google Scholar] [CrossRef]
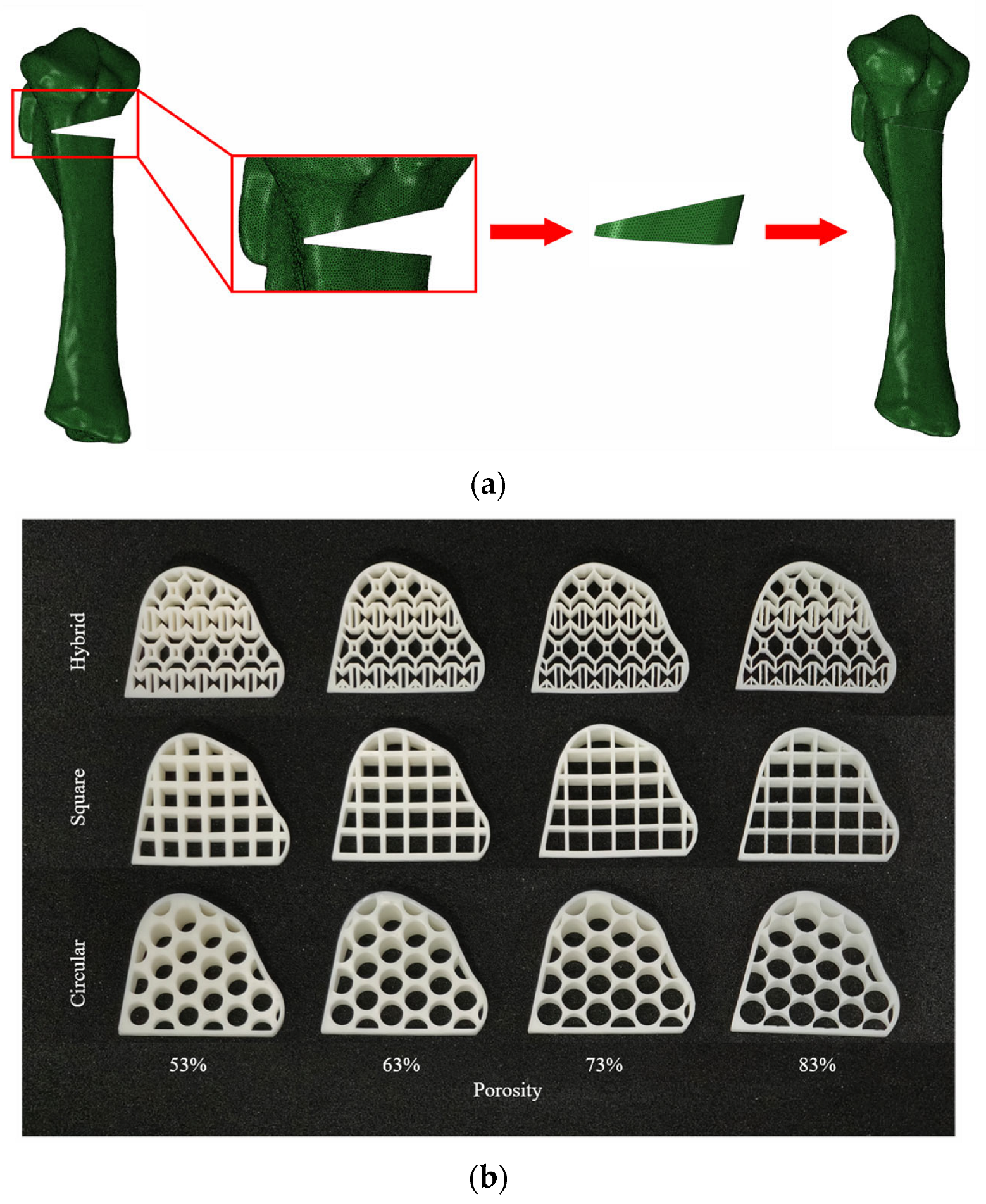
| Structure Parameters | Porosity | |||
|---|---|---|---|---|
| 53% | 63% | 73% | 83% | |
| Hybrid hole strut size | 0.9 mm | 0.7 mm | 0.5 mm | 0.3 mm |
| Square hole diameter | 4.4 mm | 4.8 mm | 5.1 mm | 5.5 mm |
| Circular hole diameter | 5.4 mm | 5.8 mm | 6.3 mm | 6.7 mm |
| Materials | Young’s Modulus (MPa) | Poisson’s Ratio |
|---|---|---|
| Cortical bone | 17,000 | 0.3 |
| Cancellous bone | 450 | 0.3 |
| Resin | 2700 | 0.42 |
| Structure | Porosity | |||
|---|---|---|---|---|
| 53% | 63% | 73% | 83% | |
| Hybrid | 22.12 | 23.34 | 24.20 | 31.01 |
| Square | 25.71 | 28.61 | 30.24 | 31.50 |
| Circular | 23.40 | 27.86 | 28.58 | 32.99 |
| Structure | Porosity | |||
|---|---|---|---|---|
| 53% | 63% | 73% | 83% | |
| Hybrid | 12.33 | 13.62 | 15.95 | 20.69 |
| Square | 16.67 | 17.76 | 23.32 | 26.84 |
| Circular | 14.41 | 14.55 | 18.09 | 21.91 |
| Structure | Porosity | |||
|---|---|---|---|---|
| 53% | 63% | 73% | 83% | |
| Hybrid | 54.13 | 55.45 | 57.15 | 61.47 |
| Square | 55.77 | 57.46 | 59.59 | 63.56 |
| Circular | 55.77 | 58.20 | 59.68 | 63.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Mu, C.; Xia, Y. The Effect of Stress Distribution on Tibial Implants with a Honeycomb Structure in Open-Wedge High Tibial Osteotomy. Appl. Sci. 2025, 15, 6467. https://doi.org/10.3390/app15126467
Xu Z, Mu C, Xia Y. The Effect of Stress Distribution on Tibial Implants with a Honeycomb Structure in Open-Wedge High Tibial Osteotomy. Applied Sciences. 2025; 15(12):6467. https://doi.org/10.3390/app15126467
Chicago/Turabian StyleXu, Zengbo, Chunhui Mu, and Yi Xia. 2025. "The Effect of Stress Distribution on Tibial Implants with a Honeycomb Structure in Open-Wedge High Tibial Osteotomy" Applied Sciences 15, no. 12: 6467. https://doi.org/10.3390/app15126467
APA StyleXu, Z., Mu, C., & Xia, Y. (2025). The Effect of Stress Distribution on Tibial Implants with a Honeycomb Structure in Open-Wedge High Tibial Osteotomy. Applied Sciences, 15(12), 6467. https://doi.org/10.3390/app15126467





