Healing Ability of Endodontic Filling Materials in Retrograde Treatment: A Systematic Review of Clinical Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Information Sources and Search Strategy
2.3. Selection Process and Data Collection
2.4. Quality Assessment
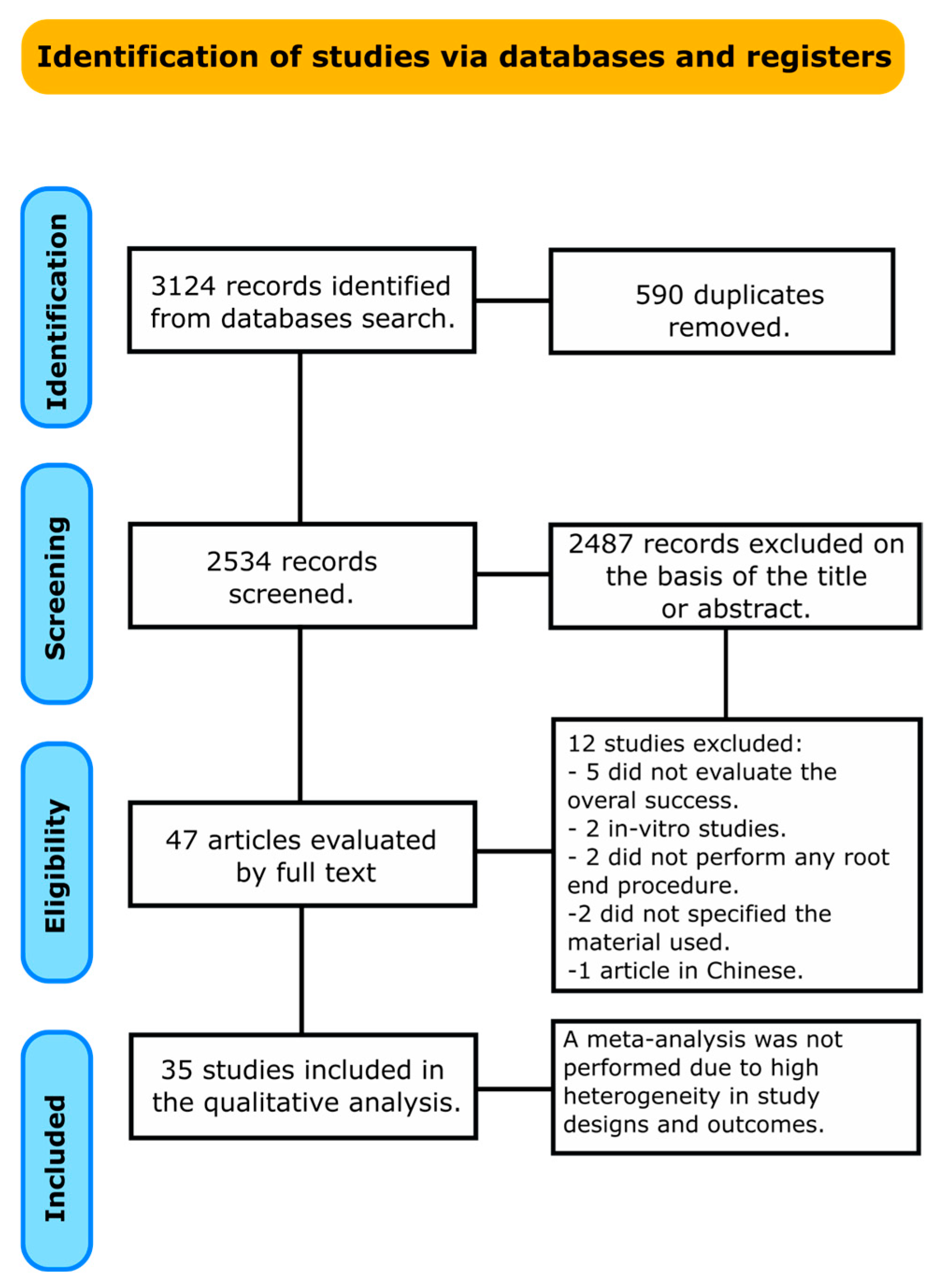
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Valenti, C.; Pagano, S.; Bozza, S.; Ciurnella, E.; Lomurno, G.; Capobianco, B.; Coniglio, M.; Cianetti, S.; Marinucci, L. Use of the Er: YAG Laser in Conservative Dentistry: Evaluation of the Microbial Population in Carious Lesions. Materials 2021, 14, 2387. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhou, J.; Guo, L. Impact of Different Regenerative Techniques and Materials on the Healing Outcome of Endodontic Surgery: A Systematic Review and Meta-analysis. Int. Endod. J. 2021, 54, 536–555. [Google Scholar] [CrossRef] [PubMed]
- Bodrumlu, E. Biocompatibility of Retrograde Root Filling Materials: A Review. Aust. Endod. J. 2008, 34, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Ng, Y.; Gulabivala, K. Factors That Influence the Outcomes of Surgical Endodontic Treatment. Int. Endod. J. 2023, 56, 116–139. [Google Scholar] [CrossRef]
- Gomes, L.L.; Dutra, J.C. Endodontic Surgery: A Review of Postoperative and Healing Outcome. J. Surg. Clin. Res. 2021, 12, 24–39. [Google Scholar] [CrossRef]
- Chao, Y.-C.; Chen, P.-H.; Su, W.-S.; Yeh, H.-W.; Su, C.-C.; Wu, Y.-C.; Chiang, H.-S.; Jhou, H.-J.; Shieh, Y.-S. Effectiveness of Different Root-End Filling Materials in Modern Surgical Endodontic Treatment: A Systematic Review and Network Meta-Analysis. J. Dent. Sci. 2022, 17, 1731–1743. [Google Scholar] [CrossRef]
- Mestry, S.U.; Kalmegh, S.; Mhaske, S. Mineral Trioxide Aggregates (MTA) in Dentistry: A Review on Chemistry, Synthesis Methods, and Critical Properties. Silicon 2023, 15, 2231–2249. [Google Scholar] [CrossRef]
- Markova, K.; Manchorova, N.; Pecheva, A. Classification of Dental Materials for Retrograde Endodontic Filling—An Overview. IOSR J. Dent. Med. Sci. 2021, 20, 1–5. [Google Scholar]
- Caron, G.; Azérad, J.; Faure, M.-O.; Machtou, P.; Boucher, Y. Use of a New Retrograde Filling Material (Biodentine) for Endodontic Surgery: Two Case Reports. Int. J. Oral Sci. 2014, 6, 250–253. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Syst. Rev. 2021, 10, 1–11. [Google Scholar] [CrossRef]
- Sterne, J.A.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Carra, M.C.; Romandini, P.; Romandini, M. Risk of Bias Evaluation of Cross-Sectional Studies: Adaptation of the Newcastle-Ottawa Scale. J. Periodontal Res. 2025. [CrossRef]
- Koçak, M.M.; Koçak, S.; Aktuna, S.; Görücü, J.; Yaman, S.D. Sealing Ability of Retrofilling Materials Following Various Root-End Cavity Preparation Techniques. Lasers Med. Sci. 2011, 26, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Grech, L.; Mallia, B.; Camilleri, J. Characterization of Set Intermediate Restorative Material, B Iodentine, B Ioaggregate and a Prototype Calcium Silicate Cement for Use as Root-end Filling Materials. Int. Endod. J. 2013, 46, 632–641. [Google Scholar] [CrossRef]
- Von Arx, T.; Marwik, E.; Bornstein, M.M. Effects of Dimensions of Root-End Fillings and Peripheral Root Dentine on the Healing Outcome of Apical Surgery. Eur. Endod. J. 2019, 4, 49–56. [Google Scholar] [CrossRef]
- Alagl, A.S.; Bedi, S.; Hassan, K.; Al-Aql, Z. Impact of Cone Beam Computed Tomography on Measurement of Bone Density in Periapical Lesions Following Endodontic Treatment: A Clinical Study. Biomed. Res. 2017, 28, 7669–7674. [Google Scholar]
- Saatchi, M.; Shadmehr, E.; Talebi, S.M.; Nazeri, M. A Prospective Clinical Study on Blood Mercury Levels Following Endodontic Root-End Surgery with Amalgam. Iran. Endod. J. 2013, 8, 85. [Google Scholar]
- Maheshwari, P. A Clinical Study to Evaluate the Efficacy of Apicoectomy Procedure Performed With or Without Root-End Filling: An Original Research Study. Int. J. Life Sci. Biotechnol. Pharma. Res. 2025, 14, 55–60. [Google Scholar]
- Zheng, C.; Wu, W.; Zhang, Y.; Tang, Z.; Xie, Z.; Chen, Z. A Novel Simplified Approach for Endodontic Retrograde Surgery in Short Single-Rooted Teeth. BMC Oral Health 2024, 24, 150. [Google Scholar] [CrossRef]
- Shao, W.; Xiao, F.; Xu, Z.; Ren, R.; Wang, Y.; Wu, Y. Treatment of Severe Periodontic-endodontic Combined Lesions with Minocycline Hydrochloride Ointment Combined with Mineral Trioxide Aggregate. Exp. Ther. Med. 2018, 16, 1389–1396. [Google Scholar] [CrossRef]
- Riis, A.; Taschieri, S.; Del Fabbro, M.; Kvist, T. Tooth Survival after Surgical or Nonsurgical Endodontic Retreatment: Long-Term Follow-up of a Randomized Clinical Trial. J. Endod. 2018, 44, 1480–1486. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.-C.; Lee, Y.-L.; Tsai, Y.-L.; Lin, H.-J.; Chang, M.-C.; Chang, S.-F.; Chang, S.-H.; Jeng, J.-H. Outcome Assessment of Apical Surgery: A Study of 234 Teeth. J. Formos. Med. Assoc. 2019, 118, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- von Arx, T.; Janner, S.F.; Hänni, S.; Bornstein, M.M. Scarring of Soft Tissues Following Apical Surgery: Visual Assessment of Outcomes One Year After Intervention Using the Bern and Manchester Scores. Int. J. Periodontics Restor. Dent. 2016, 36, 817–823. [Google Scholar] [CrossRef][Green Version]
- Sun, Y.-N.; Zhao, L.; Liu, W.; Yin, X.-P. Comparison of the Effects of Using iRoot BP plus and MTA Apical Barrier Surgery in Young Permanent Teeth with Chronic Apical Periodontitis. Shanghai J. Stomatol. 2024, 33, 260. [Google Scholar]
- Chong, B.; Pitt Ford, T.; Hudson, M. A Prospective Clinical Study of Mineral Trioxide Aggregate and IRM When Used as Root-end Filling Materials in Endodontic Surgery. Int. Endod. J. 2003, 36, 520–526. [Google Scholar] [CrossRef]
- Lindeboom, J.A.; Frenken, J.W.; Kroon, F.H.; van den Akker, H.P. A Comparative Prospective Randomized Clinical Study of MTA and IRM as Root-End Filling Materials in Single-Rooted Teeth in Endodontic Surgery. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2005, 100, 495–500. [Google Scholar] [CrossRef]
- Christiansen, R.; Kirkevang, L.; Hørsted-Bindslev, P.; Wenzel, A. Randomized Clinical Trial of Root-end Resection Followed by Root-end Filling with Mineral Trioxide Aggregate or Smoothing of the Orthograde Gutta-percha Root Filling–1-year Follow-up. Int. Endod. J. 2009, 42, 105–114. [Google Scholar] [CrossRef]
- Kim, E.; Song, J.-S.; Jung, I.-Y.; Lee, S.-J.; Kim, S. Prospective Clinical Study Evaluating Endodontic Microsurgery Outcomes for Cases with Lesions of Endodontic Origin Compared with Cases with Lesions of Combined Periodontal–Endodontic Origin. J. Endod. 2008, 34, 546–551. [Google Scholar] [CrossRef]
- Song, M.; Kim, E. A Prospective Randomized Controlled Study of Mineral Trioxide Aggregate and Super Ethoxy–Benzoic Acid as Root-End Filling Materials in Endodontic Microsurgery. J. Endod. 2012, 38, 875–879. [Google Scholar] [CrossRef]
- Kruse, C.; Spin-Neto, R.; Christiansen, R.; Wenzel, A.; Kirkevang, L.-L. Periapical Bone Healing after Apicectomy with and without Retrograde Root Filling with Mineral Trioxide Aggregate: A 6-Year Follow-up of a Randomized Controlled Trial. J. Endod. 2016, 42, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.R.D.; Silva, J.D.D.; Schnaider, T.B.; Veiga, D.F.; Novo, N.F.; Mesquita Filho, M.; Ferreira, L.M. The Use of a Biocompatible Cement in Endodontic Surgery. A Randomized Clinical Trial 1. Acta Cir. Bras. 2016, 31, 422–427. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Öğütlü, F.; Karaca, İ. Clinical and Radiographic Outcomes of Apical Surgery: A Clinical Study. J. Maxillofac. Oral Surg. 2018, 17, 75–83. [Google Scholar] [CrossRef]
- Parmar, P.; Dhamija, R.; Tewari, S.; Sangwan, P.; Gupta, A.; Duhan, J.; Mittal, S. 2D and 3D Radiographic Outcome Assessment of the Effect of Guided Tissue Regeneration Using Resorbable Collagen Membrane in the Healing of Through-and-through Periapical Lesions—A Randomized Controlled Trial. Int. Endod. J. 2019, 52, 935–948. [Google Scholar] [CrossRef]
- Bharathi, J.; Mittal, S.; Tewari, S.; Tewari, S.; Duhan, J.; Sangwan, P.; Kumar, V. Effect of the Piezoelectric Device on Intraoperative Hemorrhage Control and Quality of Life after Endodontic Microsurgery: A Randomized Clinical Study. J. Endod. 2021, 47, 1052–1060. [Google Scholar] [CrossRef]
- Salah, H.M.; Hashem, A.A.R.; Mustafa, T.; Soliman, A.H.; Khallaf, M.; Haddadeen, H. The Impact of Root End Filling Material Type and the Application of Bone Graft on Healing of Periapical Tissues after Endodontic Microsurgery (a Clinical Randomized Controlled Trial). Sci. Rep. 2024, 14, 25378. [Google Scholar] [CrossRef]
- Gulsever, S.; Ersahan, S.; Hepsenoglu, Y.E.; Tekin, A. Apicoectomy versus Apical Curettage in Combination with or without L-PRF Application: A Randomized Clinical Trial. Sci. Rep. 2025, 15, 8121. [Google Scholar] [CrossRef]
- Dong, X.; Su, Q.; Li, W.; Yang, J.; Song, D.; Yang, J.; Xu, X. The Outcome of Combined Use of iRoot BP Plus and iRoot SP for Root-End Filling in Endodontic Microsurgery: A Randomized Controlled Trial. Clin. Oral Investig. 2024, 28, 188. [Google Scholar] [CrossRef]
- Dhamija, R.; Tewari, S.; Gupta, A. Two-and Three-dimensional Healing Assessment after Endodontic Microsurgery in Through-and-through Periapical Lesions: 5-year Follow-up from a Randomized Controlled Trial. Int. Endod. J. 2024, 57, 1180–1199. [Google Scholar] [CrossRef]
- von Arx, T.; Jensen, S.S.; Hänni, S. Clinical and Radiographic Assessment of Various Predictors for Healing Outcome 1 Year after Periapical Surgery. J. Endod. 2007, 33, 123–128. [Google Scholar] [CrossRef]
- von Arx, T.; Hänni, S.; Jensen, S.S. Clinical Results with Two Different Methods of Root-End Preparation and Filling in Apical Surgery: Mineral Trioxide Aggregate and Adhesive Resin Composite. J. Endod. 2010, 36, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Kim, S.G.; Shin, S.-J.; Kim, H.-C.; Kim, E. The Influence of Bone Tissue Deficiency on the Outcome of Endodontic Microsurgery: A Prospective Study. J. Endod. 2013, 39, 1341–1345. [Google Scholar] [CrossRef] [PubMed]
- von Arx, T.; Hänni, S.; Jensen, S.S. 5-Year Results Comparing Mineral Trioxide Aggregate and Adhesive Resin Composite for Root-End Sealing in Apical Surgery. J. Endod. 2014, 40, 1077–1081. [Google Scholar] [CrossRef]
- Tortorici, S.; Difalco, P.; Caradonna, L.; Tetè, S. Traditional Endodontic Surgery versus Modern Technique: A 5-Year Controlled Clinical Trial. J. Craniofac. Surg. 2014, 25, 804–807. [Google Scholar] [CrossRef]
- von Arx, T.; Janner, S.F.; Hänni, S.; Bornstein, M.M. Bioceramic Root Repair Material (BCRRM) for Root-End Obturation in Apical Surgery. An Analysis of 174 Teeth after 1 Year. SWISS Dent. J. SSO Sci. Clin. Top. 2020, 130, 390–396. [Google Scholar] [CrossRef]
- Kaur, I.P.; Sroa, R.B.; Debbarma, M.; Pallawi, S.; Kumar, A. Outcome Assessment of Three Different Methods of Root-End Preparation and Filling Materials in Endodontic Surgery: A Comparative Clinical Prospective Study. Contemp. Clin. Dent. 2024, 15, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-H.; Zhang, M.-M.; Wang, J.; Jiang, L.; Liang, Y.-H. Outcomes of Endodontic Microsurgery Using a Microscope and Mineral Trioxide Aggregate: A Prospective Cohort Study. J. Endod. 2017, 43, 694–698. [Google Scholar] [CrossRef]
- Ramis-Alario, A.; Tarazona-Álvarez, B.; Peñarrocha-Diago, M.; Soto-Peñaloza, D.; Peñarrocha-Diago, M.; Peñarrocha-Oltra, D. The Study of Bone Healing after Endodontic Microsurgery Using Cone Beam Computed Tomography: A Retrospective Cohort Study. J. Clin. Exp. Dent. 2022, 14, e652. [Google Scholar] [CrossRef]
- Yoo, Y.J.; Cho, E.B.; Perinpanayagam, H.; Gu, Y.; Zhu, Q.; Noblett, W.C.; Kum, K.Y. Endodontic Microsurgery Outcomes over 10 Years and Associated Prognostic Factors: A Retrospective Cohort Study. J. Endod. 2024, 50, 934–943. [Google Scholar] [CrossRef]
- Liu, H.; Lu, L.; Xu, K.; Shen, Y. Treatment Outcomes and Prognostic Factors of the Apical Barrier Technique with Premixed Calcium Silicate-Based Putty in Necrotic Permanent Teeth with Open Apices: A Retrospective Cohort Study with up to Six Years Follow-Up. Clin. Oral Investig. 2024, 28, 425. [Google Scholar] [CrossRef]
- Saunders, W.P. A Prospective Clinical Study of Periradicular Surgery Using Mineral Trioxide Aggregate as a Root-End Filling. J. Endod. 2008, 34, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Pantchev, A.; Nohlert, E.; Tegelberg, Å. Endodontic Surgery with and without Inserts of Bioactive Glass PerioGlas®—A Clinical and Radiographic Follow-Up. Oral Maxillofac. Surg. 2009, 13, 21–26. [Google Scholar] [CrossRef]
- Shinbori, N.; Grama, A.M.; Patel, Y.; Woodmansey, K.; He, J. Clinical Outcome of Endodontic Microsurgery That Uses EndoSequence BC Root Repair Material as the Root-End Filling Material. J. Endod. 2015, 41, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhang, H.; Gao, J.; Du, X.; Chen, Y.; Han, L. Short-Term Observation of Clinical and Radiographic Results of Periapical Microsurgery: A Prospective Study. Biomed. Res. 0970-938X 2016, 27, 923–928. [Google Scholar]
- Sutter, E.; Valdec, S.; Bichsel, D.; Wiedemeier, D.; Rücker, M.; Stadlinger, B. Success Rate 1 Year after Apical Surgery: A Retrospective Analysis. Oral Maxillofac. Surg. 2020, 24, 45–49. [Google Scholar] [CrossRef]
- Truschnegg, A.; Rugani, P.; Kirnbauer, B.; Kqiku, L.; Jakse, N.; Kirmeier, R. Long-Term Follow-up for Apical Microsurgery of Teeth with Core and Post Restorations. J. Endod. 2020, 46, 178–183. [Google Scholar] [CrossRef]
- Van, C.L.; Thu, H.P.T.; Sangvanich, P.; Chuenchompoonut, V.; Thunyakitpisal, P. Acemannan Induces Rapid Early Osseous Defect Healing after Apical Surgery: A 12-Month Follow-up of a Randomized Controlled Trial. J. Dent. Sci. 2020, 15, 302–309. [Google Scholar] [CrossRef]
- Stueland, H.; Ørstavik, D.; Handal, T. Treatment Outcome of Surgical and Non-surgical Endodontic Retreatment of Teeth with Apical Periodontitis. Int. Endod. J. 2023, 56, 686–696. [Google Scholar] [CrossRef]
- Yamada, M.; Kasahara, N.; Matsunaga, S.; Fujii, R.; Miyayoshi, N.; Sekiya, S.; Ding, I.; McCulloch, C.A. Critical Factors Affecting Outcomes of Endodontic Microsurgery: A Retrospective Japanese Study. Dent. J. 2024, 12, 266. [Google Scholar] [CrossRef]
- Roa Flórez, C.M.; Espinosa Giraldo, L.M.; Palma Motta, I.R.; Castillo Delgado, M.A.; López Pérez, L.A.; Bermúdez Alarcón, J.; Rivera Barrero, J.R. Tasa de Éxito de Cirugías Apicales Realizadas En Un Posgrado de Endodoncia. Un Estudio Observacional Retrospectivo. Rev. Cuba. Estomatol. 2024, 61, e4884. [Google Scholar]
- Ørstavik, D. Endodontic Filling Materials. Endod. Top. 2014, 31, 53–67. [Google Scholar] [CrossRef]
- Ivanov, I.; Radeva, E.; Uzunov, T. Endodontic Surgical Treatment-A Literature Review. Int. J. Sci. Res. Publ. 2015, 5, 1–5. [Google Scholar]
- ElReash, A.A.; Hamama, H.; Comisi, J.C.; Zaeneldin, A.; Xiaoli, X. The Effect of Retrograde Material Type and Surgical Techniques on the Success Rate of Surgical Endodontic Retreatment: Systematic Review of Prospective Randomized Clinical Trials. BMC Oral Health 2021, 21, 375. [Google Scholar] [CrossRef] [PubMed]
- Algar, J.; Docampo-Vázquez, C.; Rico-Romano, C.; Boquete-Castro, A.; Obispo-Díaz, C.; Aragoneses, J.M. Randomised Clinical Trial: Effect of AH Plus and Neosealer Flo on Postoperative Pain and Healing of Periapical Lesions. Bioengineering 2025, 12, 376. [Google Scholar] [CrossRef]
- Seedat, H.C.; Van der Vyver, P.J.; De Wet, F.A. Micro-Endodontic Surgery Part 2: Root-End Filling Materials. S. Afr. Dent. J. 2018, 73, 336–342. [Google Scholar] [CrossRef]
- Ford, T.P.; Andreasen, J.; Dorn, S.; Kariyawasam, S. Effect of Super-EBA as a Root End Filling on Healing after Replantation. J. Endod. 1995, 21, 13–15. [Google Scholar] [CrossRef]
- Ford, T.P.; Andreasen, J.; Dorn, S.; Kariyawasam, S. Effect of Various Zinc Oxide Materials as Root-end Fillings on Healing after Replantation. Int. Endod. J. 1995, 28, 273–278. [Google Scholar] [CrossRef]
- Amezcua, O.; Gonzalez, A.; Borges, A.; Bandeca, M.C.; Estrela, C.; Estrela, C. Sealing Ability of Root-End Filling Materials. J. Contemp. Dent. Pr. 2015, 16, 210–214. [Google Scholar]
- Poggio, C.; Lombardini, M.; Alessandro, C.; Simonetta, R. Solubility of Root-End–Filling Materials: A Comparative Study. J. Endod. 2007, 33, 1094–1097. [Google Scholar] [CrossRef]
- Castillo-Villagomez, P.; Madla-Cruz, E.; Lopez-Martinez, F.; Rodriguez-Delgado, I.; Flores-Treviño, J.J.; Malagon-Santiago, G.I.; Garza-Ramos, M.A. de L. Antimicrobial Effectiveness of Root Canal Sealers against Enterococcus Faecalis. Biomater. Investig. Dent. 2022, 9, 47. [Google Scholar] [CrossRef]
- Weldon Jr, J.K.; Pashley, D.H.; Loushine, R.J.; Weller, R.N.; Kimbrough, W.F. Sealing Ability of Mineral Trioxide Aggregate and Super-EBA When Used as Furcation Repair Materials: A Longitudinal Study. J. Endod. 2002, 28, 467–470. [Google Scholar] [PubMed]
- Chong, B.S.; Ford, T.R.; Kariyawasam, S.P. Tissue Response to Potential Root-End Filling Materials in Infected Root Canals. Int. Endod. J. 1997, 30, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Prati, C.; Gandolfi, M.G. Calcium Silicate Bioactive Cements: Biological Perspectives and Clinical Applications. Dent. Mater. 2015, 31, 351–370. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z. Bioceramic Materials in Endodontics. Endod. Top. 2015, 32, 3–30. [Google Scholar] [CrossRef]
- Jitaru, S.; Hodisan, I.; Timis, L.; Lucian, A.; Bud, M. The Use of Bioceramics in Endodontics-Literature Review. Clujul. Med. 2016, 89, 470. [Google Scholar] [CrossRef]
- Dong, X.; Xu, X. Bioceramics in Endodontics: Updates and Future Perspectives. Bioengineering 2023, 10, 354. [Google Scholar] [CrossRef]
- Torabinejad, M.; Watson, T.; Ford, T.P. Sealing Ability of a Mineral Trioxide Aggregate When Used as a Root End Filling Material. J. Endod. 1993, 19, 591–595. [Google Scholar] [CrossRef]
- Schwartz, R.S.; Mauger, M.; Clement, D.J.; WALKER III, W.A. Mineral Trioxide Aggregate: A New Material for Endodontics. J. Am. Dent. Assoc. 1999, 130, 967–975. [Google Scholar] [CrossRef]
- Ribeiro, D.A.; Duarte, M.A.H.; Matsumoto, M.A.; Marques, M.E.A.; Salvadori, D.M.F. Biocompatibility in Vitro Tests of Mineral Trioxide Aggregate and Regular and White Portland Cements. J. Endod. 2005, 31, 605–607. [Google Scholar] [CrossRef]
- Parirokh, M.; Torabinejad, M. Mineral Trioxide Aggregate: A Comprehensive Literature Review-Part I: Chemical, Physical, and Antibacterial Properties. J. Endod. 2010, 36, 16–27. [Google Scholar] [CrossRef]
- Kogan, P.; He, J.; Glickman, G.N.; Watanabe, I. The Effects of Various Additives on Setting Properties of MTA. J. Endod. 2006, 32, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Dawood, A.E.; Parashos, P.; Wong, R.H.; Reynolds, E.C.; Manton, D.J. Calcium Silicate-based Cements: Composition, Properties, and Clinical Applications. J. Investig. Clin. Dent. 2017, 8, e12195. [Google Scholar] [CrossRef] [PubMed]
- Tay, F.R. Bioactivity of Mineral Trioxide Aggregate and Mechanism of Action. Miner. Trioxide Aggreg. Dent. Prep. Appl. 2014, 61–85. [Google Scholar]
- Neeraj Malhotra, M.; Antara Agarwal, M.; Kundabala Mala, M. Mineral Trioxide Aggregate: A Review of Physical Properties. Compendium 2013, 34, e25-32. [Google Scholar]
- Jiang, Y.; Zheng, Q.; Zhou, X.; Gao, Y.; Huang, D. A Comparative Study on Root Canal Repair Materials: A Cytocompatibility Assessment in L929 and MG63 Cells. Sci. World J. 2014, 2014, 463826. [Google Scholar] [CrossRef]
- Baek, S.-H.; Plenk Jr, H.; Kim, S. Periapical Tissue Responses and Cementum Regeneration with Amalgam, SuperEBA, and MTA as Root-End Filling Materials. J. Endod. 2005, 31, 444–449. [Google Scholar] [CrossRef]
- Salehimehr, G.; Nobahar, S.; Hosseini-Zijoud, S.-M.; Yari, S. Comparison of Physical & Chemical Properties of Angelus MTA and New Endodontic Restorative Material. J. Appl. Pharm. Sci. 2014, 4, 105–109. [Google Scholar]
- Tilakchand, M.; Pandey, P.; Shetty, P.; Naik, B.; Shetti, S.; Nirmala, C. The Comparative Evaluation of Various Additives on Setting Time and Compressive Strength of MTA Plus: An: In Vitro: Study. Endodontology 2021, 33, 36–42. [Google Scholar] [CrossRef]
- Tang, Y.; Li, X.; Yin, S. Outcomes of MTA as Root-End Filling in Endodontic Surgery: A Systematic Review. Quintessence Int. 2010, 41, 557–566. [Google Scholar]
- Harrison, J.W.; Johnson, S.A. Excisional Wound Healing Following the Use of IRM as a Root-End Filling Material. J. Endod. 1997, 23, 19–27. [Google Scholar] [CrossRef]
- Hung, C.-J.; Kao, C.-T.; Shie, M.-Y.; Huang, T.-H. Comparison of Host Inflammatory Responses between Calcium-Silicate Base Material and IRM. J. Dent. Sci. 2014, 9, 158–164. [Google Scholar] [CrossRef]
- Rud, J.; Munksgaard, E.; Andreasen, J.; Rud, V.; Asmussen, E. Retrograde Root Filling with Composite and a Dentin-bonding Agent. 1. Dent. Traumatol. 1991, 7, 118–125. [Google Scholar] [CrossRef]
- Dorn, S.O.; Gartner, A.H. Retrograde Filling Materials: A Retrospective Success-Failure Study of Amalgam, EBA, and IRM. J. Endod. 1990, 16, 391–393. [Google Scholar] [CrossRef] [PubMed]
- Kararia, N.; Yadav, A.; Adyanthaya, B.; Kararia, V.; Poonia, S.; Jain, S. Comparison of Sealing Ability of MTA and Retroplast as Root End Filling Materials Evaluated under a Stereomicroscope Using Rhodamine B Dye: An: In Vitro: Study. Indian J. Dent. Res. 2022, 33, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Niederman, R.; Theodosopoulou, J. A Systematic Review of in Vivo Retrograde Obturation Materials. Int. Endod. J. 2003, 36, 577–585. [Google Scholar] [CrossRef]
- Walsh, R.M.; He, J.; Schweitzer, J.; Opperman, L.A.; Woodmansey, K.F. Bioactive Endodontic Materials for Everyday Use: A Review. Gen. Dent. 2018, 66, 48–51. [Google Scholar]
- Al-Haddad, A.; Che Ab Aziz, Z.A. Bioceramic-based Root Canal Sealers: A Review. Int. J. Biomater. 2016, 2016, 9753210. [Google Scholar] [CrossRef]
- Maria, N.L.; Andreea-Simona, B.-P.; Mihai, H.R.; Mona, K.; Elena, C.L. Comparison of Three Calcium Silicate Cements Used as Retrograde Filling Materials. An In Vitro Study. Res. Clin. Med. 2020, 4, 32–36. [Google Scholar]
- Rodríguez-Lozano, F.J.; López-García, S.; García-Bernal, D.; Tomás-Catalá, C.; Santos, J.; Llena, C.; Lozano, A.; Murcia, L.; Forner, L. Chemical Composition and Bioactivity Potential of the New Endosequence BC Sealer Formulation HiFlow. Int. Endod. J. 2020, 53, 1216–1228. [Google Scholar] [CrossRef]
- Li, H.; Guo, Z.; Li, C.; Ma, X.; Wang, Y.; Zhou, X.; Johnson, T.M.; Huang, D. Materials for Retrograde Filling in Root Canal Therapy. Cochrane Database Syst. Rev. 2021, 10, CD005517. [Google Scholar] [CrossRef]
- Shokouhinejad, N.; Nekoofar, M.H.; Razmi, H.; Sajadi, S.; Davies, T.E.; Saghiri, M.; Gorjestani, H.; Dummer, P.M.H. Bioactivity of EndoSequence Root Repair Material and Bioaggregate. Int. Endod. J. 2012, 45, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Edanami, N.; Takenaka, S.; Ibn Belal, R.S.; Yoshiba, K.; Takahara, S.; Yoshiba, N.; Ohkura, N.; Noiri, Y. In Vivo Assessment of the Apatite-Forming Ability of New-Generation Hydraulic Calcium Silicate Cements Using a Rat Subcutaneous Implantation Model. J. Funct. Biomater. 2023, 14, 213. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, M.G.; Siboni, F.; Botero, T.; Bossù, M.; Riccitiello, F.; Prati, C. Calcium Silicate and Calcium Hydroxide Materials for Pulp Capping: Biointeractivity, Porosity, Solubility and Bioactivity of Current Formulations. J. Appl. Biomater. Funct. Mater. 2015, 13, 43–60. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.; Karabucak, B.; Wang, C.; Wang, H.-G.; Koyama, E.; Kohli, M.R.; Nah, H.-D.; Kim, S. Healing after Root-End Microsurgery by Using Mineral Trioxide Aggregate and a New Calcium Silicate–Based Bioceramic Material as Root-End Filling Materials in Dogs. J. Endod. 2015, 41, 389–399. [Google Scholar] [CrossRef]
- Mahgoub, N.; Alqadasi, B.; Aldhorae, K.; Assiry, A.; Altawili, Z.M.; Hong, T. Comparison between iRoot BP Plus (EndoSequence Root Repair Material) and Mineral Trioxide Aggregate as Pulp-Capping Agents: A Systematic Review. J. Int. Soc. Prev. Community Dent. 2019, 9, 542–552. [Google Scholar]
- Benetti, F.; Queiroz, Í.O.D.A.; Cosme-Silva, L.; Conti, L.C.; Oliveira, S.H.P.D.; Cintra, L.T.A. Cytotoxicity, Biocompatibility and Biomineralization of a New Ready-for-Use Bioceramic Repair Material. Braz. Dent. J. 2019, 30, 325–332. [Google Scholar] [CrossRef]
- Abusrewil, S.M.; McLean, W.; Scott, J.A. The Use of Bioceramics as Root-End Filling Materials in Periradicular Surgery: A Literature Review. Saudi Dent. J. 2018, 30, 273–282. [Google Scholar] [CrossRef]
- Kim, S.; Kratchman, S. Modern endodontic surgery concepts and practice: A review. J. Endo. 2006, 32, 601–623. [Google Scholar] [CrossRef]
| Search | Terms |
|---|---|
| #1 | (Apicectomy OR endodontic surgery OR apicoectomy OR root-end resection OR root end resection OR root-end surgery OR surgical endodontic treatment OR microapical surgery OR periradicular surgery OR apical microsurgery) |
| #2 | (Pulpectomy OR pulpotomy OR root canal therapy OR root filled tooth OR root filled teeth OR endodontically treated tooth OR endodontically treated teeth OR root canal treatment OR root filling OR root canal therapy OR endodontic treatment) |
| #3 | (Bioceramic OR Biodentine OR mineral trioxide aggregate OR repair material) |
| #4 | #1 and #2 and #3 |
| Study and Year | Type of Study | Number of Patients Included (Number of Teeth Treated) | Retrograde Root Filling Used | Follow-Up | Main Results |
|---|---|---|---|---|---|
| Chong, 2003 [26] | Randomized clinical trial | 108 patients (108 teeth) | Intermediate Restorative Material (IRM) (Dentsply) Mineral trioxide aggregate (MTA) (Loma Linda University, CA, USA) | 24 months | No statistically significant differences were found between the materials. |
| Lindeboom, 2005 [27] | Randomized clinical trial | 100 patients (100 teeth) | IRM (Dentsply, Konstanz, Germany) MTA (Dentsply) | 1 year | No statistically significant differences were found between the retrofilling materials. |
| Christiansen, 2008 [28] | Randomized clinical trial | 44 patients (52 teeth) | MTA (Dentsply) Gutta-percha | 12 months | Teeth treated with MTA showed significantly higher healing rates. |
| Kim, 2008 [29] | Randomized clinical trial | 227 patients (263 teeth) | IRM (Dentsply) Super Ethoxybenzoic Acid (Super EBA) (Harry J. Bosworth, Skokie, IL, USA) ProRoot MTA (Dentsply) | 24 months | All three filling materials showed successful outcomes. |
| Song, 2012 [30] | Randomized clinical trial | 388 patients (260 teeth) | Super EBA (Harry J. Bosworth Co.) ProRoot MTA (Dentsply) | 1 year | No significant difference was observed. |
| Kruse, 2016 [31] | Randomized clinical trial | 44 patients (52 teeth) | ProRoot MTA White (Dentsply) Gutta-percha | 6 years | The study found a success rate for surgical endodontic retreatment in the MTA group. |
| da Silva, 2016 [32] | Randomized clinical trial | 12 patients (30 teeth) | Pozzolana Biologic Silva cement (experimental material) MTA white (Angelus, Londrina, Brazil) | 6 months | Both materials showed significant periradicular tissue regeneration. |
| Öğütlü, 2018 [33] | Randomized clinical trial | 112 patients (112 teeth) | MTA (Angelus) Super EBA (Harry J. Bosworth Co.) | 6 months | Apical surgery using SuperEBA and MTA achieved an 88.4% success rate. |
| Parmar, 2019 [34] | Randomized clinical trial | 32 patients (52 teeth) | MTA (Dentsply) | 1 year | Collagen membranes did not significantly enhance healing periapical lesions. |
| Bharathi, 2021 [35] | Randomized clinical trial | 40 patients (74 teeth) | MTA (Dentsply) | Not specified | Piezoelectric surgery reduced pain and swelling. |
| Salah, 2024 [36] | Randomized clinical trial | 56 patients (56 teeth) | MTA (Dentsply) TotalFill (FKG Dentaire SA, La Chaux-de-Fonds, Switzerland) | 12 months | Both MTA and TotalFill root-end filling materials demonstrated high success rates. |
| Gulsever, 2024 [37] | Randomized clinical trial | 64 patients (64 teeth) | MTA (Angelus) | 9 months | Retrograde MTA obturation showed high success rates. |
| Dong, 2024 [38] | Randomized clinical trial | 240 teeth | iRoot BP Plus (ENP) iRoot SP (ENP) | 12 months | Both materials demonstrated good clinical success. |
| Dhamija, 2024 [39] | Randomized clinical trial | 32 patients (59 teeth) | MTA (Dentsply) | 5 years | MTA showed high clinical success. |
| Von Arx, 2007 [40] | Non-randomized clinical trial | 194 patients (194 teeth) | Super EBA (Harry J. Bosworth Co.) MTA (Dentsply) | 12 months | Material used for retrograde filling was not significant. |
| Von Arx, 2010 [41] | Non-randomized clinical trial | 353 patients (353 teeth) | ProRoot (Dentsply) Retroplast (Retroplast Trading, Rorvig, Denmark) | 12 months | MTA had statistically significant higher success. |
| Song, 2013 [42] | Non-randomized clinical trial | 135 patients (199 teeth) | Super EBA (Harry J. Bosworth) ProRoot MTA (Dentsply) | 7 years | Both materials achieved higher success rates. |
| Von Arx, 2014 [43] | Non-randomized clinical trial | 271 patients (271 teeth) | ProRoot (Dentsply) Retroplast (Retroplast Trading, Skaidiškės, Lithuania ) | 5 years | The overall rate of healed cases was significantly higher for MTA. |
| Tortorici, 2014 [44] | Non-randomized clinical trial | 843 patients (938 teeth) | MTA (ProRoot, Charlotte, NC, USA) | 5 years | The use of MTA demonstrated significantly higher clinical success rates. |
| Von Arx, 2020 [45] | Non-randomized clinical trial | 146 patients (170 teeth) | TotalFill® (Brasseler, Georgetown, Georgia ) | 1 year | Retrograde healing achieved a 94.1% success rate. |
| Kaur, 2024 [46] | Non-randomized clinical trial | 45 patients (45 teeth) | Gutta-percha Retroplast (Endoplast) DiaRoot BioAggregate (Dia Dent, Cheongju-si, Republic of Korea) | 16 months | There was no significant difference in the clinical outcome after endodontic surgery. |
| Wang, 2017 [47] | Cohort | 81 patients (74 teeth) | ProRoot MTA (Dentsply) | 12 to 30 months | The study found a 90.5% rate of complete healing in teeth. |
| Ramis-Alario, 2022 [48] | Cohort | 298 patients (57 teeth) | MTA (Dentsply) | 2 years | The study found a 93% success rate 2 years after endodontic microsurgery. |
| Yoo, 2024 [49] | Retrospective cohort | 362 patients (309 teeth) | ProRoot MTA (Dentsply Sirona) | 10–17.5 years | Long-term healing and survival after endodontic microsurgery were significantly influenced by the preoperative status of the tooth. |
| Liu, 2024 [50] | Retrospective cohort | 74 patients (74 teeth) | iRoot BP Plus (Innovative Bioceramix, Burnaby, BC, Canada) | 6 years | The apical barrier technique using premixed calcium silicate-based putty showed high long-term success. |
| Saunders, 2008 [51] | Cross-sectional | 321 patients (321 teeth) | Proroot MTA White (Dentsply) | 12 months | The overall success rate for MTA was 88.8%. |
| Pantchev, 2009 [52] | Cross-sectional | 131 patients (186 teeth) | Super EBA (Staident International, Tokyo, Japan) | 4 years | Super EBA cement achieved high success rates. |
| Shinbori, 2015 [53] | Cross-sectional | 94 patients (113 teeth) | EndoSequence BC Root Repair (Brasseler) | 1 year | Microsurgery resulted in a 92% overall success rate. |
| Shen, 2016 [54] | Cross-sectional | 97 patients (128 teeth) | MTA (Dentsply) | 1 year | Microsurgical techniques and MTA resulted in a 57.7% success rate. |
| Sutter, 2019 [55] | Cross-sectional | 81 patients (81 teeth) | Super EBA (Staident International) MTA (Dentsply) | 1 year | A statistically reasonable evaluation of the impact of the filling material was not feasible. |
| Truschnegg, 2019 [56] | Cross-sectional | 73 patients (87 teeth) | IRM (Dentsply) | 13 years | IRM as a filling material demonstrates good performance after 10–13 years. |
| Van, 2020 [57] | Cross-sectional | 24 patients (22 teeth) | MTA (Angelus) | 1 year | After treatment, bone defect volume reduction was observed. |
| Stueland, 2022 [58] | Cross-sectional | 1157 patients (351 teeth with non-surgical retreatment and 107 teeth with endodontic microsurgery) | Most retrograde fillings were with bioceramic materials, only two cases had IRM (Dentsply) | >36 months | Microsurgical apicectomy and retrograde filling outperformed non-surgical retreatment for apical periodontitis. |
| Yamada, 2024 [59] | Cross-sectional | 46 patients (46 teeth) | ProRoot MTA White (Dentsply) | 12 months | Endodontic microsurgery showed a 93.5% success rate after 1 year. |
| Flórez, 2024 [60] | Cross-sectional | 52 patients (52 teeth) | MTA (Dentsply) Super EBA (Staident International) Biodentine (Septodont, Saint-Maur-des-Fossés, France) | 12 months | The overall success rate was 78.84%. |
| Study | Randomization | Deviations from the Intended Interventions | Missing Outcome Data | Measurement of the Outcome | Selection of the Reported Result |
|---|---|---|---|---|---|
| Chong, 2003 [26] | Low | Low | Low | Low | Low |
| Lindeboom, 2005 [27] | High | Low | Low | Low | Low |
| Christiansen, 2008 [28] | Low | Low | Low | Low | Low |
| Kim, 2008 [29] | Low | High | High | High | High |
| Song, 2012 [30] | Low | Low | High | Low | Low |
| da Silva, 2016 [32] | Low | Low | High | High | High |
| Kruse, 2016 [31] | Low | Low | Low | Low | Low |
| Öğütlü, 2018 [33] | Low | Low | Low | Low | Low |
| Parmar, 2019 [34] | Low | Low | Low | Low | Low |
| Bharathi, 2021 [35] | Low | Low | Low | Low | Low |
| Salah, 2024 [36] | Low | Low | Low | Low | Low |
| Gulsever, 2024 [37] | High | Low | Low | Low | Low |
| Dong, 2024 [38] | Low | Low | Low | Low | Low |
| Dhamija, 2024 [39] | High | Low | Low | Low | Low |
| Study | Confounding | Selection of Participants for the Study | Classification of Interventions | Deviations from Intended Interventions | Missing Data | Measurement of Outcomes | Selection of the Reported Result |
|---|---|---|---|---|---|---|---|
| Von Arx, 2007 [40] | Low | Low | Low | Low | Low | Low | Low |
| Von Arx, 2010 [41] | Low | Low | Low | Low | Low | Low | Low |
| Song, 2013 [42] | Low | Low | Low | Low | Low | Low | Moderate |
| Von Arx, 2014 [43] | Low | Low | Low | Low | Low | Low | Low |
| Tortorici, 2014 [44] | Moderate | Low | Low | Moderate | Low | Low | Low |
| Von Arx, 2020 [45] | Low | Low | Low | Low | Low | Low | Low |
| Kaur, 2024 [46] | Low | Low | Low | Low | Low | Low | Moderate |
| Study | Selection | Comparability | Outcome | Total | |||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the Exposed Cohort | Selection of the Non-Exposed Cohort | Ascertainment of Exposure | Outcome of Interest Not Present at the Start of the Study | Comparability of Cohorts on the Basis of the Design or Analysis | Assessment of Outcome | Duration of Follow-Up | Adequacy of Follow-Up | ||
| Wang, 2017 [47] | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 6 |
| Ramis-Alario, 2022 [48] | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 4 |
| Yoo, 2024 [49] | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 5 |
| Liu, 2024 [50] | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 4 |
| Study | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Score |
|---|---|---|---|---|---|---|---|---|---|
| Saunders, 2008 [51] | Y | Y | Y | Y | N | N | Y | Y | 6 |
| Pantchev, 2009 [52] | Y | Y | Y | Y | N | N | Y | Y | 6 |
| Shinbori, 2015 [53] | N | Y | Y | Y | N | N | Y | N | 4 |
| Shen, 2016 [54] | Y | Y | Y | Y | N | N | Y | Y | 6 |
| Sutter, 2019 [55] | Y | Y | Y | Y | N | N | Y | Y | 6 |
| Truschnegg, 2019 [56] | U | U | Y | Y | N | N | Y | Y | 4 |
| Van, 2020 [57] | Y | Y | Y | Y | N | N | Y | N | 5 |
| Stueland, 2022 [58] | Y | Y | Y | Y | N | N | Y | N | 5 |
| Yamada, 2024 [59] | N | Y | Y | Y | N | N | Y | N | 4 |
| Florez, 2024 [60] | Y | Y | Y | Y | N | N | Y | Y | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashi, T.; Bourgi, R.; Cuevas-Suárez, C.E.; Hardan, L.; Nahat, C.; Altaqi, Z.; Kharouf, N.; Haikel, Y. Healing Ability of Endodontic Filling Materials in Retrograde Treatment: A Systematic Review of Clinical Studies. Appl. Sci. 2025, 15, 6461. https://doi.org/10.3390/app15126461
Ashi T, Bourgi R, Cuevas-Suárez CE, Hardan L, Nahat C, Altaqi Z, Kharouf N, Haikel Y. Healing Ability of Endodontic Filling Materials in Retrograde Treatment: A Systematic Review of Clinical Studies. Applied Sciences. 2025; 15(12):6461. https://doi.org/10.3390/app15126461
Chicago/Turabian StyleAshi, Tarek, Rim Bourgi, Carlos Enrique Cuevas-Suárez, Louis Hardan, Carmen Nahat, Zaher Altaqi, Naji Kharouf, and Youssef Haikel. 2025. "Healing Ability of Endodontic Filling Materials in Retrograde Treatment: A Systematic Review of Clinical Studies" Applied Sciences 15, no. 12: 6461. https://doi.org/10.3390/app15126461
APA StyleAshi, T., Bourgi, R., Cuevas-Suárez, C. E., Hardan, L., Nahat, C., Altaqi, Z., Kharouf, N., & Haikel, Y. (2025). Healing Ability of Endodontic Filling Materials in Retrograde Treatment: A Systematic Review of Clinical Studies. Applied Sciences, 15(12), 6461. https://doi.org/10.3390/app15126461











