Gut–Brain–Microbiota Axis in Irritable Bowel Syndrome: A Narrative Review of Pathophysiology and Current Approaches
Abstract
1. Introduction
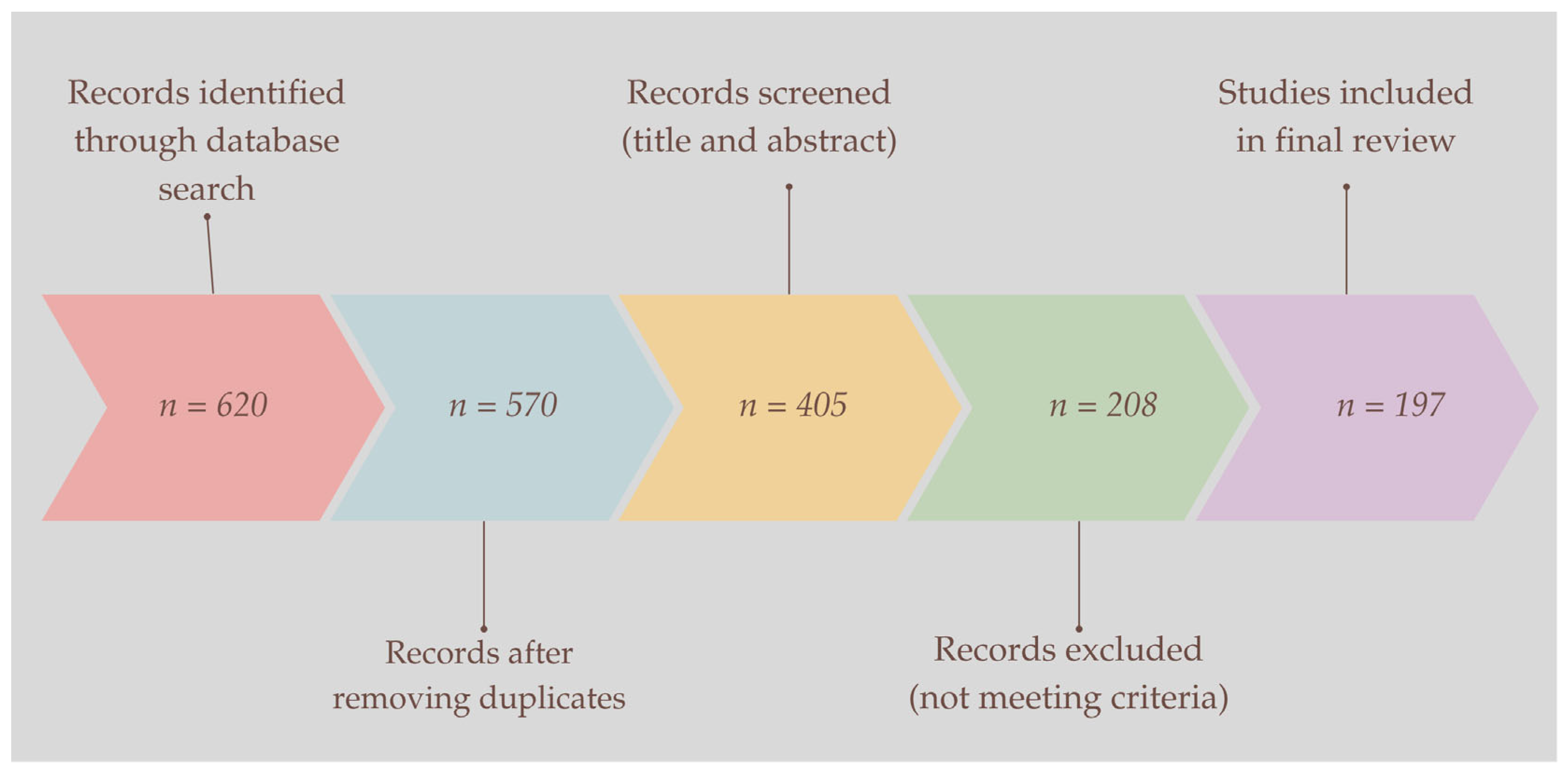
2. Materials and Methods
3. Results
3.1. Characteristics of IBS
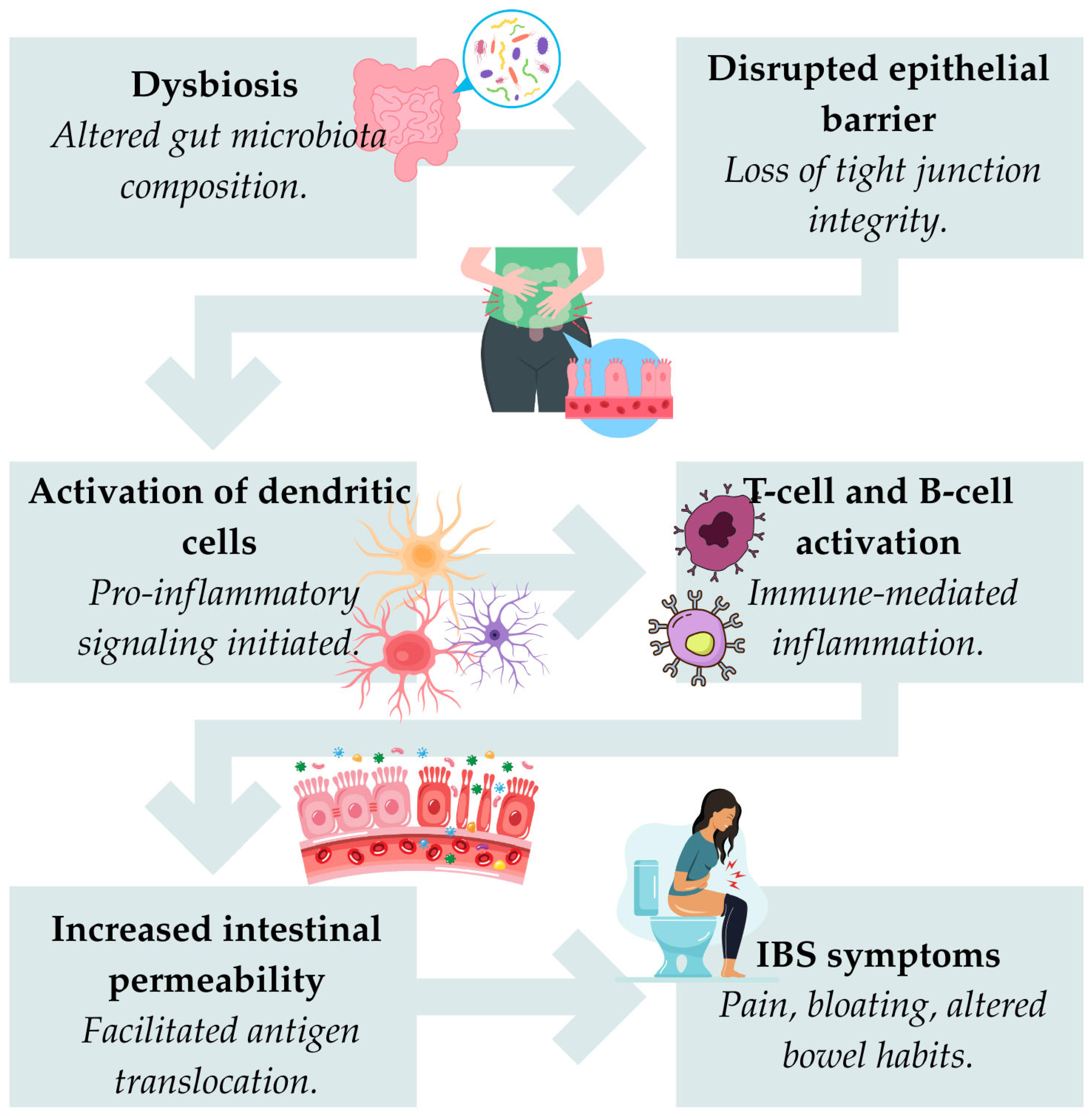
3.2. The Role of Microbiomes
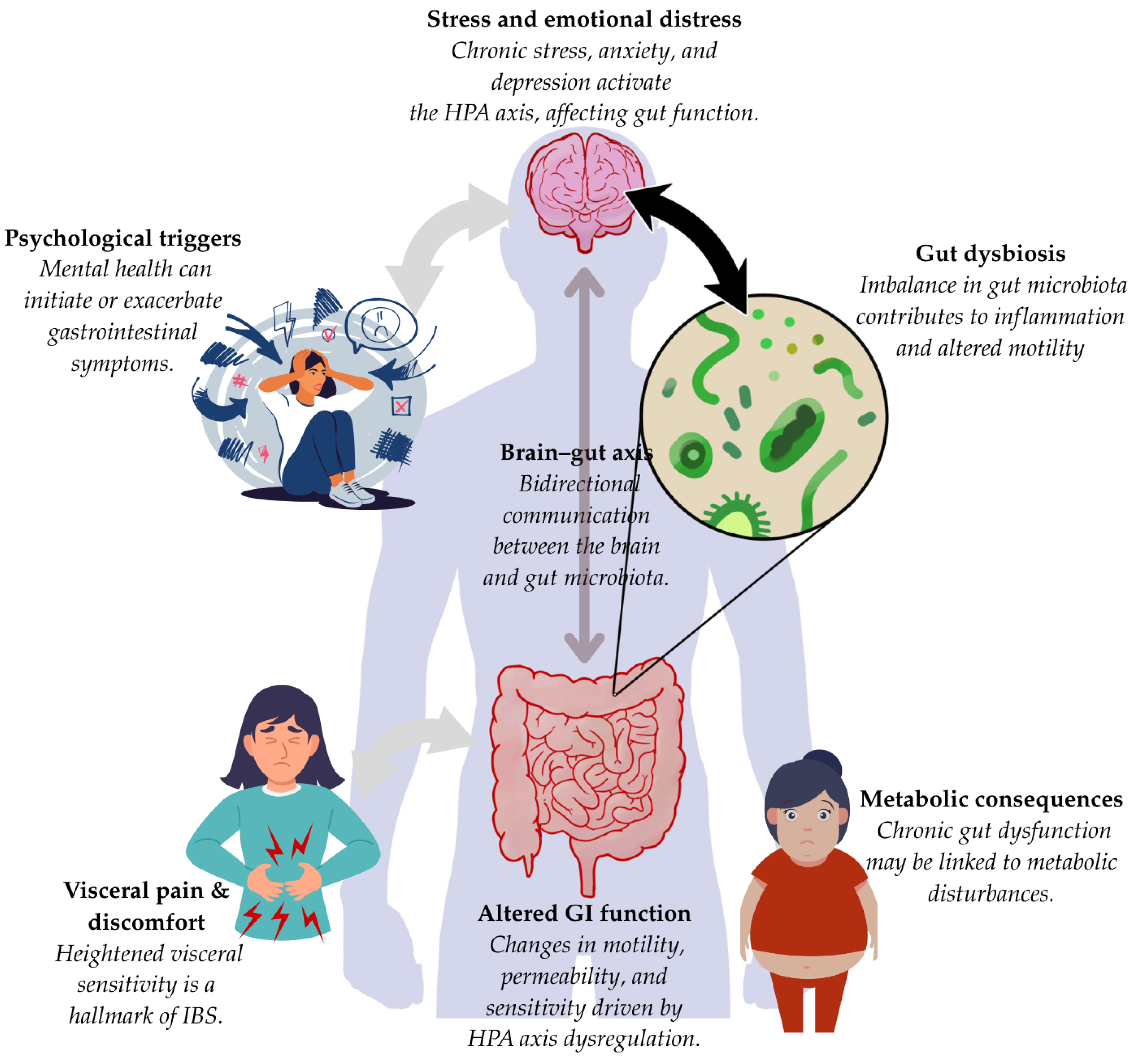
3.3. Gut–Brain Communication
3.4. The Role of the Enteric Nervous System in IBS
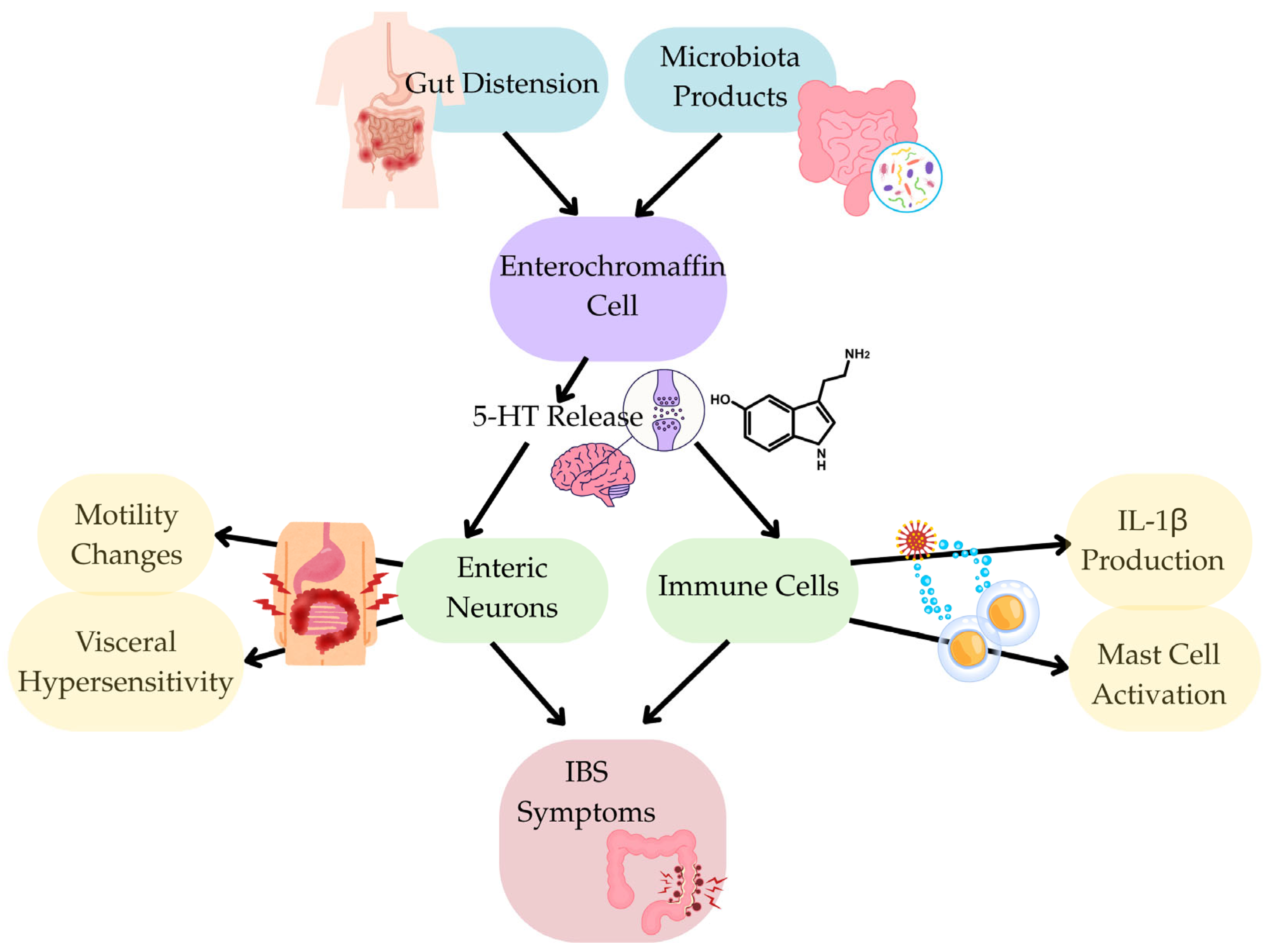
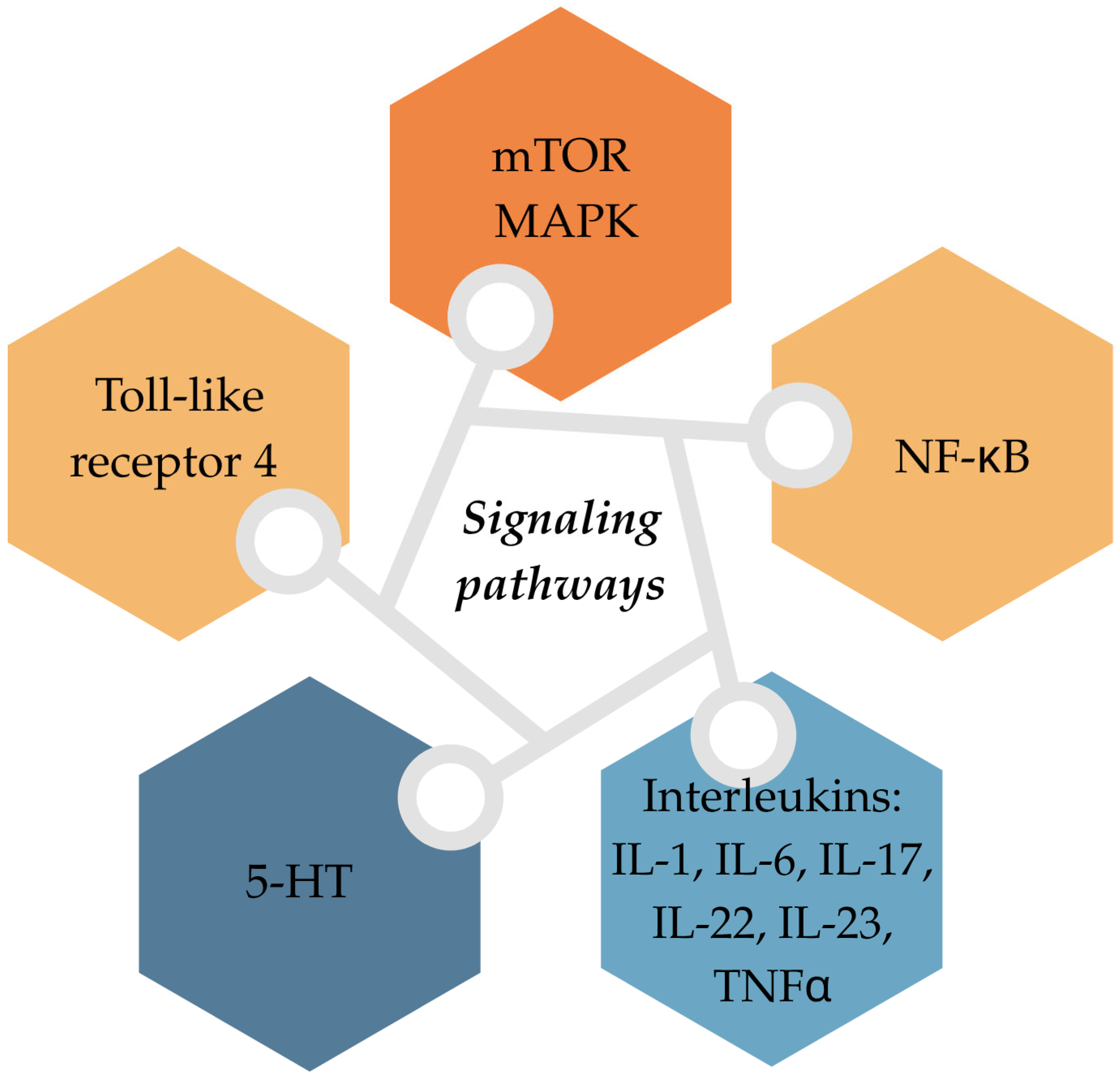
3.5. Signalling Pathway
| Amino Acids | Signaling Pathways | Functions | References |
| Glycine | NF-κB | Strengthen the intestinal mucosal barrier and reduce oxidative stress and TNF-a, IL-1, and IL6 levels | [156,157,158,159] |
| Alanine | Unclear | Enhance intestinal defense and protection function | [147,160] |
| Glutamine | NF-κB, mTORMAPK/ERK | Enhance the intestinal barrier, reduce proinflammatory cytokines, and have anti-inflammatory effect | [161,162,163,164,165] |
| Glutamate | Unclear | Strengthen the intestinal mucosal barrier, alleviates heat stress-induced impairment of intestinal morphology, and reduce oxidative stress and TNF-a, and IL-1 levels | [166,167,168,169] |
| Cysteine | NF-κB, Nrf2mTOR | Enhance intestinal barrier function, tight junctions, and homeostasis while lowering oxidative stress and decreasing TNF-α, IL-1β, IL-6, and IL-8 | [170,171,172,173,174,175,176] |
| Proline | Unclear | Increase levels of superoxide dismutase, tight junction proteins | [177,178,179] |
| Aspartate and asparagine | NF-κB and MAPK | Enhance intestinal barrier function and lower the levels of proinflammatory cytokines | [180,181,182,183,184,185,186] |
| Tyrosine | Calcium-sensing receptors | Improve intestinal health and immune response | [147,187,188,189,190] |
| Serine | Unclear | Increase colonic protection, mucosal healing and gut microbiota | [191,192,193,194] |
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Abbreviation | Meaning |
| 5-ASA | 5-aminosalicylic acid |
| 5-HT | Serotonin, 5-hydroxytryptamine |
| FBD | Functional bowel disorders |
| HPA | Hypothalamic–pituitary–adrenal |
| IBD | Inflammatory bowel disease |
| IBS | Irritable bowel syndrome |
| IL | Interleukin |
| mTOR | Mechanistic target of rapamycin |
| MRI | Magnetic resonance imaging |
| mRNA | Messenger RNA |
| NDDSs | Nanomaterial drug delivery systems |
| NF-κB | Nuclear factor-kappa-B |
| TNF | Tumour necrosis factor |
References
- Carvalho, G.B.; Damasio, A. Interoception and the Origin of Feelings: A New Synthesis. BioEssays 2021, 43, 2000261. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhang, X.; Li, Y.; Liu, C.; Yang, H.; Yang, C. The Role of Psychological Factors in Functional Gastrointestinal Disorders: A Systematic Review and Meta-Analysis. Int. J. Colorectal Dis. 2023, 38, 65. [Google Scholar] [CrossRef] [PubMed]
- Vasant, D.H.; Ford, A.C. Functional Gastrointestinal Disorders in Inflammatory Bowel Disease: Time for a Paradigm Shift? World J. Gastroenterol. 2020, 26, 3712–3719. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Sperber, A.D.; Corsetti, M.; Camilleri, M. Irritable Bowel Syndrome. Lancet 2020, 396, 1675–1688. [Google Scholar] [CrossRef]
- Frändemark, Å.; Törnblom, H.; Jakobsson, S.; Simrén, M. Work Productivity and Activity Impairment in Irritable Bowel Syndrome (IBS): A Multifaceted Problem. Am. J. Gastroenterol. 2018, 113, 1540–1549. [Google Scholar] [CrossRef]
- Porcari, S.; Ingrosso, M.R.; Maida, M.; Eusebi, L.H.; Black, C.; Gasbarrini, A.; Cammarota, G.; Ford, A.C.; Ianiro, G. Prevalence of Irritable Bowel Syndrome and Functional Dyspepsia after Acute Gastroenteritis: Systematic Review and Meta-Analysis. Gut 2024, 73, 1431–1440. [Google Scholar] [CrossRef]
- Tang, H.-Y.; Jiang, A.-J.; Wang, X.-Y.; Wang, H.; Guan, Y.-Y.; Li, F.; Shen, G.-M. Uncovering the Pathophysiology of Irritable Bowel Syndrome by Exploring the Gut-Brain Axis: A Narrative Review. Ann. Trans. Med. 2021, 9, 1187. [Google Scholar] [CrossRef]
- Mayer, E.A.; Ryu, H.J.; Bhatt, R.R. The Neurobiology of Irritable Bowel Syndrome. Mol. Psychiatry 2023, 28, 1451–1465. [Google Scholar] [CrossRef]
- Lacy, B.E.; Mearin, F.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel Disorders. Gastroenterology 2016, 150, 1393–1407.e5. [Google Scholar] [CrossRef]
- Moayyedi, P.; Mearin, F.; Azpiroz, F.; Andresen, V.; Barbara, G.; Corsetti, M.; Emmanuel, A.; Hungin, A.P.S.; Layer, P.; Stanghellini, V.; et al. Irritable Bowel Syndrome Diagnosis and Management: A Simplified Algorithm for Clinical Practice. United Eur. Gastroenterol. J. 2017, 5, 773–788. [Google Scholar] [CrossRef]
- Talley, N.J.; Holtmann, G.; Walker, M.M. Therapeutic Strategies for Functional Dyspepsia and Irritable Bowel Syndrome Based on Pathophysiology. J. Gastroenterol. 2015, 50, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Sarvepalli, S.S.; Vemula, S.L.; Aramadaka, S.; Mannam, R.; Narayanan, R.S.; Bansal, A.; Yanamaladoddi, V.R.; Sarvepalli, S.S.; Vemula, S.L.; Aramadaka, S.; et al. Digesting the Impact of Diet on Irritable Bowel Syndrome (IBS): Exploring Solutions for Controlling IBS. Cureus 2023, 15, 601–613. [Google Scholar] [CrossRef]
- Inadomi, J.M.; Fennerty, M.B.; Bjorkman, D. The Economic Impact of Irritable Bowel Syndrome. Aliment. Pharmacol. Ther. 2003, 18, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Staller, K.; Olén, O.; Söderling, J.; Roelstraete, B.; Törnblom, H.; Khalili, H.; Joshi, A.D.; Nguyen, L.H.; Song, M.; Kuo, B.; et al. Mortality Risk in Irritable Bowel Syndrome: Results from a Nationwide Prospective Cohort Study. Am. J. Gastroenterol. 2020, 115, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Han, Y.; Du, J.; Liu, R.; Jin, K.; Yi, W. Microbiota-Gut-Brain Axis and the Central Nervous System. Oncotarget 2017, 8, 53829–53838. [Google Scholar] [CrossRef]
- Guinane, C.M.; Cotter, P.D. Role of the Gut Microbiota in Health and Chronic Gastrointestinal Disease: Understanding a Hidden Metabolic Organ. Ther. Adv. Gastroenterol. 2013, 6, 295–308. [Google Scholar] [CrossRef]
- Lane, M.; Yadav, V. Multiple Sclerosis; Churchill Livingstone: London, UK, 2020; Volume 2, pp. 1587–1599.e3. ISBN 9780323523424. [Google Scholar] [CrossRef]
- Quagliariello, A.; Modi, A.; Innocenti, G.; Zaro, V.; Conati Barbaro, C.; Ronchitelli, A.; Boschin, F.; Cavazzuti, C.; Dellù, E.; Radina, F.; et al. Ancient Oral Microbiomes Support Gradual Neolithic Dietary Shifts towards Agriculture. Nat. Commun. 2022, 13, 6927. [Google Scholar] [CrossRef]
- Suzuki, T.A.; Fitzstevens, J.L.; Schmidt, V.T.; Enav, H.; Huus, K.E.; Mbong Ngwese, M.; Grießhammer, A.; Pfleiderer, A.; Adegbite, B.R.; Zinsou, J.F.; et al. Codiversification of Gut Microbiota with Humans. Science 2022, 377, 1328–1332. [Google Scholar] [CrossRef]
- Moeller, A.H.; Caro-Quintero, A.; Mjungu, D.; Georgiev, A.V.; Lonsdorf, E.V.; Muller, M.N.; Pusey, A.E.; Peeters, M.; Hahn, B.H.; Ochman, H. Cospeciation of Gut Microbiota with Hominids. Science 2016, 353, 380–382. [Google Scholar] [CrossRef]
- Hector, T.E.; Hoang, K.L.; Li, J.; King, K.C. Symbiosis and Host Responses to Heating. Trends Ecol. Evol. 2022, 37, 611–624. [Google Scholar] [CrossRef]
- Frazão, N.; Konrad, A.; Amicone, M.; Seixas, E.; Güleresi, D.; Lässig, M.; Gordo, I. Two Modes of Evolution Shape Bacterial Strain Diversity in the Mammalian Gut for Thousands of Generations. Nat. Commun. 2022, 13, 5604. [Google Scholar] [CrossRef] [PubMed]
- Barreto, H.C.; Gordo, I. Intrahost Evolution of the Gut Microbiota. Nat. Rev. Microbiol. 2023, 21, 590–603. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, Stability and Resilience of the Human Gut Microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef]
- Huang, K.-Y.; Wang, F.-Y.; Lv, M.; Ma, X.-X.; Tang, X.-D.; Lv, L. Irritable Bowel Syndrome: Epidemiology, Overlap Disorders, Pathophysiology and Treatment. World J. Gastroenterol. 2023, 29, 4120–4135. [Google Scholar] [CrossRef]
- Kogut, M.H.; Lee, A.; Santin, E. Microbiome and Pathogen Interaction with the Immune System. Poult. Sci. 2020, 99, 1906–1913. [Google Scholar] [CrossRef]
- Shaikh, S.D.; Sun, N.; Canakis, A.; Park, W.Y.; Weber, H.C. Irritable Bowel Syndrome and the Gut Microbiome: A Comprehensive Review. J. Clin. Med. 2023, 12, 2558. [Google Scholar] [CrossRef]
- Black, C.J.; Ford, A.C. Chronic Idiopathic Constipation in Adults: Epidemiology, Pathophysiology, Diagnosis and Clinical Management. Med. J. Aust. 2018, 209, 86–91. [Google Scholar] [CrossRef]
- Mou, Y.; Du, Y.; Zhou, L.; Yue, J.; Hu, X.; Liu, Y.; Chen, S.; Lin, X.; Zhang, G.; Xiao, H.; et al. Gut Microbiota Interact with the Brain through Systemic Chronic Inflammation: Implications on Neuroinflammation, Neurodegeneration, and Aging. Front. Immunol. 2022, 13, 796288. [Google Scholar] [CrossRef]
- Markey, L.; Shaban, L.; Green, E.R.; Lemon, K.P.; Mecsas, J.; Kumamoto, C.A. Pre-Colonization with the Commensal Fungus Candida Albicans Reduces Murine Susceptibility to Clostridium Difficile Infection. Gut Microbes 2018, 9, 497–509. [Google Scholar] [CrossRef]
- Zhang, F.; Aschenbrenner, D.; Yoo, J.Y.; Zuo, T. The Gut Mycobiome in Health, Disease, and Clinical Applications in Association with the Gut Bacterial Microbiome Assembly. Lancet Microbe 2022, 3, e969–e983. [Google Scholar] [CrossRef]
- Frey-Klett, P.; Burlinson, P.; Deveau, A.; Barret, M.; Tarkka, M.; Sarniguet, A. Bacterial-Fungal Interactions: Hyphens between Agricultural, Clinical, Environmental, and Food Microbiologists. Microbiol. Mol. Biol. Rev. 2011, 75, 583–609. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.; Coyte, K.Z.; Bainter, W.; Geha, R.S.; Martin, C.R.; Rakoff-Nahoum, S. Multi-Kingdom Ecological Drivers of Microbiota Assembly in Preterm Infants. Nature 2021, 591, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.; Wong, S.H.; Cheung, C.P.; Lam, K.; Lui, R.; Cheung, K.; Zhang, F.; Tang, W.; Ching, J.Y.L.; Wu, J.C.Y.; et al. Gut Fungal Dysbiosis Correlates with Reduced Efficacy of Fecal Microbiota Transplantation in Clostridium Difficile Infection. Nat. Commun. 2018, 9, 3663. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.T. Pathophysiology of Inflammatory Bowel Diseases. N. Eng. J. Med. 2020, 383, 2652–2664. [Google Scholar] [CrossRef]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef]
- Robbins, N.; Caplan, T.; Cowen, L.E. Molecular Evolution of Antifungal Drug Resistance. Annu. Rev. Microbiol. 2017, 71, 753–775. [Google Scholar] [CrossRef]
- Thambugala, K.M.; Daranagama, D.A.; Tennakoon, D.S.; Pamoda, D.; Hongsanan, S.; Xie, N. Humans vs. Fungi: An Overview of Fungal Pathogens against Humans. Pathogens 2024, 13, 426. [Google Scholar] [CrossRef]
- Roy, M.; Karhana, S.; Shamsuzzaman, M.; Khan, M.A. Recent Drug Development and Treatments for Fungal Infections. Braz. J. Microbiol. 2023, 54, 1695–1716. [Google Scholar] [CrossRef]
- Rabaan, A.A.; Sulaiman, T.; Al-Ahmed, S.H.; Buhaliqah, Z.A.; Buhaliqah, A.A.; AlYuosof, B.; Alfaresi, M.; Al Fares, M.A.; Alwarthan, S.; Alkathlan, M.S.; et al. Potential Strategies to Control the Risk of Antifungal Resistance in Humans: A Comprehensive Review. Antibiotics 2023, 12, 608. [Google Scholar] [CrossRef]
- Roemer, T.; Krysan, D.J. Antifungal Drug Development: Challenges, Unmet Clinical Needs, and New Approaches. Cold Spring Harb. Perspect. Med. 2014, 4, a019703. [Google Scholar] [CrossRef]
- Lee, Y.; Robbins, N.; Cowen, L.E. Molecular Mechanisms Governing Antifungal Drug Resistance. NPJ Antimicrob. Resist. 2023, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Beatty, J.K. Post-Infectious Irritable Bowel Syndrome: Mechanistic Insights into Chronic Disturbances Following Enteric Infection. World J. Gastroenterol. 2014, 20, 3976. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Verne, G.N. New Insights into Visceral Hypersensitivity —Clinical Implications in IBS. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 349–355. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Bahramsoltani, R.; Abdollahi, M.; Rahimi, R. The Role of Visceral Hypersensitivity in Irritable Bowel Syndrome: Pharmacological Targets and Novel Treatments. J. Neurogastroenterol. Motil. 2016, 22, 558–574. [Google Scholar] [CrossRef]
- Shukla, R.; Ghoshal, U.; Ranjan, P.; Ghoshal, U.C. Expression of Toll-like Receptors, Pro-, and Anti-Inflammatory Cytokines in Relation to Gut Microbiota in Irritable Bowel Syndrome: The Evidence for Its Micro-Organic Basis. J. Neurogastroenterol. Motil. 2018, 24, 628–642. [Google Scholar] [CrossRef]
- Ma, Q.; Xing, C.; Long, W.; Wang, H.Y.; Liu, Q.; Wang, R.-F. Impact of Microbiota on Central Nervous System and Neurological Diseases: The Gut-Brain Axis. J. Neuroinflamm. 2019, 16, 53. [Google Scholar] [CrossRef]
- Nicoletti, A.; Ponziani, F.R.; Biolato, M.; Valenza, V.; Marrone, G.; Sganga, G.; Gasbarrini, A.; Miele, L.; Grieco, A. Intestinal Permeability in the Pathogenesis of Liver Damage: From Non-Alcoholic Fatty Liver Disease to Liver Transplantation. World J. Gastroenterol. 2019, 25, 4814–4834. [Google Scholar] [CrossRef]
- Ford, A.C.; Spiegel, B.M.R.; Talley, N.J.; Moayyedi, P. Small Intestinal Bacterial Overgrowth in Irritable Bowel Syndrome: Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2009, 7, 1279–1286. [Google Scholar] [CrossRef]
- Goodoory, V.C.; Ford, A.C. Antibiotics and Probiotics for Irritable Bowel Syndrome. Drugs 2023, 83, 687–699. [Google Scholar] [CrossRef]
- Mamieva, Z.; Poluektova, E.; Svistushkin, V.; Sobolev, V.; Shifrin, O.; Guarner, F.; Ivashkin, V. Antibiotics, Gut Microbiota, and Irritable Bowel Syndrome: What Are the Relations? World J. Gastroenterol. 2022, 28, 1204–1219. [Google Scholar] [CrossRef]
- Almonajjed, M.B.; Wardeh, M.; Atlagh, A.; Ismaiel, A.; Popa, S.-L.; Rusu, F.; Dumitrascu, D.L. Impact of Microbiota on Irritable Bowel Syndrome Pathogenesis and Management: A Narrative Review. Medicina 2025, 61, 109. [Google Scholar] [CrossRef] [PubMed]
- Iribarren, C.; Maasfeh, L.; Öhman, L.; Simrén, M. Modulating the Gut Microenvironment as a Treatment Strategy for Irritable Bowel Syndrome: A Narrative Review. Gut Microbiome 2022, 3, e7. [Google Scholar] [CrossRef] [PubMed]
- Törnblom, H.; Holmvall, P.; Svenungsson, B.; Lindberg, G. Gastrointestinal Symptoms after Infectious Diarrhea: A Five-Year Follow-up in a Swedish Cohort of Adults. Clin. Gastroenterol. Hepatol. 2007, 5, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.M.; Yamanishi, S.; Sohn, J.; Alekseyenko, A.V.; Leung, J.M.; Cho, I.; Kim, S.G.; Li, H.; Gao, Z.; Mahana, D.; et al. Altering the Intestinal Microbiota during a Critical Developmental Window Has Lasting Metabolic Consequences. Cell 2014, 158, 705–721. [Google Scholar] [CrossRef]
- Yasmin, F.; Tun, H.M.; Konya, T.B.; Guttman, D.S.; Chari, R.S.; Field, C.J.; Becker, A.B.; Mandhane, P.J.; Turvey, S.E.; Subbarao, P.; et al. Cesarean Section, Formula Feeding, and Infant Antibiotic Exposure: Separate and Combined Impacts on Gut Microbial Changes in Later Infancy. Front. Pediatr. 2017, 5, 200. [Google Scholar] [CrossRef]
- Fallani, M.; Young, D.; Scott, J.; Norin, E.; Amarri, S.; Adam, R.; Aguilera, M.; Khanna, S.; Gil, A.; Edwards, C.A.; et al. Intestinal Microbiota of 6-Week-Old Infants across Europe: Geographic Influence beyond Delivery Mode, Breast-Feeding, and Antibiotics. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 77–84. [Google Scholar] [CrossRef]
- Krogsgaard, L.R.; Engsbro, A.L.; Bytzer, P. Antibiotics: A Risk Factor for Irritable Bowel Syndrome in a Population-Based Cohort. Scand. J. Gastroenterol. 2018, 53, 1027–1030. [Google Scholar] [CrossRef]
- Wang, L.; Alammar, N.; Singh, R.; Nanavati, J.; Song, Y.; Chaudhary, R.; Mullin, G.E. Gut Microbial Dysbiosis in the Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis of Case-Control Studies. J. Acad. Nutr. Diet. 2020, 120, 565–586. [Google Scholar] [CrossRef]
- Losurdo, G.; Leandro, G.; Ierardi, E.; Perri, F.; Barone, M.; Principi, M.; Leo, A.D. Breath Tests for the Non-Invasive Diagnosis of Small Intestinal Bacterial Overgrowth: A Systematic Review with Meta-Analysis. J. Neurogastroenterol. Motil. 2020, 26, 16–28. [Google Scholar] [CrossRef]
- Ducrotté, P. Clinical Trial:Lactobacillus Plantarum299v (DSM 9843) Improves Symptoms of Irritable Bowel Syndrome. World J. Gastroenterol. 2012, 18, 4012. [Google Scholar] [CrossRef]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and Prebiotics in Intestinal Health and Disease: From Biology to the Clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 1057–1073. [Google Scholar] [CrossRef] [PubMed]
- Suhr, M.J.; Hallen-Adams, H.E. The Human Gut Mycobiome: Pitfalls and Potentials—A Mycologists Perspective. Mycologia 2015, 107, 1057–1073. [Google Scholar] [CrossRef] [PubMed]
- Whelan, K.; Staudacher, H. Low FODMAP Diet in Irritable Bowel Syndrome: A Review of Recent Clinical Trials and Meta-Analyses. Curr. Opin. Clin. Nutr. Metab. Care 2022, 25, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Hallen-Adams, H.E.; Suhr, M.J. Fungi in the Healthy Human Gastrointestinal Tract. Virulence 2016, 8, 352–358. [Google Scholar] [CrossRef]
- Underhill, D.M.; Braun, J. Fungal Microbiome in Inflammatory Bowel Disease: A Critical Assessment. J. Clin. Investig. 2022, 132. [Google Scholar] [CrossRef]
- Ma, Z.; Zuo, T.; Frey, N.; Rangrez, A.Y. A Systematic Framework for Understanding the Microbiome in Human Health and Disease: From Basic Principles to Clinical Translation. Signal Transduct. Target. Ther. 2024, 9, 237. [Google Scholar] [CrossRef]
- Didari, T.; Mozaffari, S.; Nikfar, S.; Abdollahi, M. Effectiveness of Probiotics in Irritable Bowel Syndrome: Updated Systematic Review with Meta-Analysis. World J. Gastroenterol. 2015, 21, 3072–3084. [Google Scholar] [CrossRef]
- Liang, D.; Longgui, N.; Guoqiang, X. Efficacy of Different Probiotic Protocols in Irritable Bowel Syndrome. Medicine 2019, 98, e16068. [Google Scholar] [CrossRef]
- Simrén, M.; Barbara, G.; Flint, H.J.; Spiegel, B.M.R.; Spiller, R.C.; Vanner, S.; Verdu, E.F.; Whorwell, P.J.; Zoetendal, E.G. Intestinal Microbiota in Functional Bowel Disorders: A Rome Foundation Report. Gut 2012, 62, 159–176. [Google Scholar] [CrossRef]
- Ford, A.C.; Quigley, E.M.M.; Lacy, B.E.; Lembo, A.J.; Saito, Y.A.; Schiller, L.R.; Soffer, E.E.; Spiegel, B.M.R.; Moayyedi, P. Efficacy of Prebiotics, Probiotics, and Synbiotics in Irritable Bowel Syndrome and Chronic Idiopathic Constipation: Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2014, 109, 1547–1561. [Google Scholar] [CrossRef]
- Hungin, A.P.S.; Mitchell, C.R.; Whorwell, P.; Mulligan, C.; Cole, O.; Agréus, L.; Fracasso, P.; Lionis, C.; Mendive, J.; Philippart de Foy, J. -M.; et al. Systematic Review: Probiotics in the Management of Lower Gastrointestinal Symptoms—An Updated Evidence-Based International Consensus. Aliment. Pharmacol. Ther. 2018, 47, 1054–1070. [Google Scholar] [CrossRef] [PubMed]
- Quigley, E.M.M. Probiotics in Irritable Bowel Syndrome. J. Clin. Gastroenterol. 2015, 49, S60–S64. [Google Scholar] [CrossRef] [PubMed]
- Acosta, A.; Camilleri, M. Prokinetics in Gastroparesis. Gastroenterol. Clin. N. Am. 2015, 44, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Atieh, J. New Developments in Prokinetic Therapy for Gastric Motility Disorders. Front. Pharmacol. 2021, 12, 711500. [Google Scholar] [CrossRef]
- Simrén, M.; Tack, J. New Treatments and Therapeutic Targets for IBS and Other Functional Bowel Disorders. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 589–605. [Google Scholar] [CrossRef]
- Pinto-Sanchez, M.I.; Hall, G.B.; Ghajar, K.; Nardelli, A.; Bolino, C.; Lau, J.T.; Martin, F.-P.; Cominetti, O.; Welsh, C.; Rieder, A.; et al. Probiotic Bifidobacterium Longum NCC3001 Reduces Depression Scores and Alters Brain Activity: A Pilot Study in Patients with Irritable Bowel Syndrome. Gastroenterology 2017, 153, 448–459.e8. [Google Scholar] [CrossRef]
- Tillisch, K.; Labus, J.; Kilpatrick, L.; Jiang, Z.; Stains, J.; Ebrat, B.; Guyonnet, D.; Legrain–Raspaud, S.; Trotin, B.; Naliboff, B.; et al. Consumption of Fermented Milk Product with Probiotic Modulates Brain Activity. Gastroenterology 2013, 144, 1394–1401.e4. [Google Scholar] [CrossRef]
- Van Oudenhove, L.; Levy, R.L.; Crowell, M.D.; Drossman, D.A.; Halpert, A.D.; Keefer, L.; Lackner, J.M.; Murphy, T.B.; Naliboff, B.D. Biopsychosocial Aspects of Functional Gastrointestinal Disorders: How Central and Environmental Processes Contribute to the Development and Expression of Functional Gastrointestinal Disorders. Gastroenterology 2016, 150, 1355–1367.e2. [Google Scholar] [CrossRef]
- Drossman, D.A. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features, and Rome IV. Gastroenterology 2016, 150, 1262–1279.e2. [Google Scholar] [CrossRef]
- Al-Haddad, B.J.S.; Jacobsson, B.; Chabra, S.; Modzelewska, D.; Olson, E.M.; Bernier, R.; Enquobahrie, D.A.; Hagberg, H.; Östling, S.; Rajagopal, L.; et al. Long-Term Risk of Neuropsychiatric Disease after Exposure to Infection in Utero. JAMA Psychiatry 2019, 76, 594–602. [Google Scholar] [CrossRef]
- Yu, L.; Li, Y. Involvement of Intestinal Enteroendocrine Cells in Neurological and Psychiatric Disorders. Biomedicines 2022, 10, 2577. [Google Scholar] [CrossRef] [PubMed]
- Rojas, O.; Prö, A.-K.; Porfilio, E.; Robbins, C.; Baranzini, S.; Gommerman, J. Recirculating Intestinal IgA-Producing Cells Regulate Neuroinflammation via IL-10. Cell 2019, 176, 610–624. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, Z.; Frazer, G.; Ferro, A.; Clare, S.; Bouladoux, N.; Ferdinand, J.; Tuong, Z.K.; Negro-Demontel, M.L.; Kumar, N.; Suchanek, O.; et al. Gut-Educated IgA Plasma Cells Defend the Meningeal Venous Sinuses. Nature 2020, 587, 472–476. [Google Scholar] [CrossRef]
- Galley, J.D.; Bailey, M.T. Impact of Stressor Exposure on the Interplay between Commensal Microbiota and Host Inflammation. Gut Microbes 2014, 5, 390–396. [Google Scholar] [CrossRef]
- Hemmings, S.M.J.; Malan-Müller, S.; van den Heuvel, L.L.; Demmitt, B.A.; Stanislawski, M.A.; Smith, D.G.; Bohr, A.D.; Stamper, C.E.; Hyde, E.R.; Morton, J.T.; et al. The Microbiome in Posttraumatic Stress Disorder and Trauma-Exposed Controls. Psychosom. Med. 2017, 79, 936–946. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The Gut-Brain Axis: Interactions between Enteric Microbiota, Central and Enteric Nervous Systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Borre, Y.E.; O’Keeffe, G.W.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Microbiota and Neurodevelopmental Windows: Implications for Brain Disorders. Trends Mol. Med. 2014, 20, 509–518. [Google Scholar] [CrossRef]
- Leserman, J.; Drossman, D.A. Relationship of Abuse History to Functional Gastrointestinal Disorders and Symptoms. Trauma Violence Abuse 2007, 8, 331–343. [Google Scholar] [CrossRef]
- Hanna-Jairala, I.; Drossman, D.A. Central Neuromodulators in Irritable Bowel Syndrome: Why, How, and When. Am. J. Gastroenterol. 2024, 119, 1272–1284. [Google Scholar] [CrossRef]
- Spiller, R.C. Increased Rectal Mucosal Enteroendocrine Cells, T Lymphocytes, and Increased Gut Permeability Following Acute Campylobacter Enteritis and in Post-Dysenteric Irritable Bowel Syndrome. Gut 2000, 47, 804–811. [Google Scholar] [CrossRef]
- Tao, E.; Zhu, Z.; Hu, C.; Long, G.; Chen, B.; Guo, R.; Fang, M.; Jiang, M. Potential Roles of Enterochromaffin Cells in Early Life Stress-Induced Irritable Bowel Syndrome. Front. Cell. Neurosci. 2022, 16, 837166. [Google Scholar] [CrossRef] [PubMed]
- Hall, G.B.C.; Kamath, M.V.; Collins, S.; Ganguli, S.; Spaziani, R.; Miranda, K.L.; Bayati, A.; Bienenstock, J. Heightened Central Affective Response to Visceral Sensations of Pain and Discomfort in IBS. Neurogastroenterol. Motil. 2010, 22, 276-e80. [Google Scholar] [CrossRef] [PubMed]
- Elsenbruch, S.; Rosenberger, C.; Enck, P.; Forsting, M.; Schedlowski, M.; Gizewski, E.R. Affective Disturbances Modulate the Neural Processing of Visceral Pain Stimuli in Irritable Bowel Syndrome: An FMRI Study. Gut 2009, 59, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Strandwitz, P. Neurotransmitter Modulation by the Gut Microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef]
- Gros, M.; Gros, B.; Mesonero, J.E.; Latorre, E. Neurotransmitter Dysfunction in Irritable Bowel Syndrome: Emerging Approaches for Management. J. Clin. Med. 2021, 10, 3429. [Google Scholar] [CrossRef]
- Mishima, Y.; Ishihara, S. Molecular Mechanisms of Microbiota-Mediated Pathology in Irritable Bowel Syndrome. Int. J. Mol. Sci. 2020, 21, 8664. [Google Scholar] [CrossRef]
- Hellström, P.M. Pathophysiology of the Irritable Bowel Syndrome—Reflections of Today. Best Pract. Res. Clin. Gastroenterol. 2019, 40–41, 101620. [Google Scholar] [CrossRef]
- Bruta, K.; Vanshika; Bhasin, K.; Bhawana. The Role of Serotonin and Diet in the Prevalence of Irritable Bowel Syndrome: A Systematic Review. Transl. Med. Commun. 2021, 6, 1. [Google Scholar] [CrossRef]
- Camilleri, M.; Boeckxstaens, G. Dietary and Pharmacological Treatment of Abdominal Pain in IBS. Gut 2017, 66, 966–974. [Google Scholar] [CrossRef]
- Manocha, M.; Khan, W.I. Serotonin and GI Disorders: An Update on Clinical and Experimental Studies. Clin. Transl. Gastroenterol. 2012, 3, e13. [Google Scholar] [CrossRef]
- Ionescu, V.A.; Gheorghe, G.; Georgescu, T.F.; Bacalbasa, N.; Gheorghe, F.; Diaconu, C.C. The Latest Data Concerning the Etiology and Pathogenesis of Irritable Bowel Syndrome. J. Clin. Med. 2024, 13, 5124. [Google Scholar] [CrossRef] [PubMed]
- Gershon, M.D.; Tack, J. The Serotonin Signaling System: From Basic Understanding to Drug Development for Functional GI Disorders. Gastroenterology 2007, 132, 397–414. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, S.P.; Coleman, N.S.; Blackshaw, E.; Perkins, A.C.; Singh, G.; Marsden, C.A.; Spiller, R.C. Abnormalities of 5-Hydroxytryptamine Metabolism in Irritable Bowel Syndrome. Clin. Gastroenterol. Hepatol. 2005, 3, 349–357. [Google Scholar] [CrossRef]
- Atkinson, W.; Lockhart, S.; Whorwell, P.J.; Keevil, B.; Houghton, L.A. Altered 5-Hydroxytryptamine Signaling in Patients with Constipation- and Diarrhea-Predominant Irritable Bowel Syndrome. Gastroenterology 2006, 130, 34–43. [Google Scholar] [CrossRef]
- Cremon, C.; Carini, G.; Wang, B.; Vasina, V.; Cogliandro, R.F.; De Giorgio, R.; Stanghellini, V.; Grundy, D.; Tonini, M.; De Ponti, F.; et al. Intestinal Serotonin Release, Sensory Neuron Activation, and Abdominal Pain in Irritable Bowel Syndrome. Am. J. Gastroenterol. 2011, 106, 1290–1298. [Google Scholar] [CrossRef]
- Rokkas, T. Comparative Effectiveness of 5-Hydroxytryptamine 3 Receptor Antagonists in Irritable Bowel Syndrome: A Network Meta-Analysis of Randomized Controlled Studies. Ann. Gastroenterol. 2021, 34, 535–546. [Google Scholar] [CrossRef]
- Stasi, C.; Rosselli, M.; Bellini, M.; Laffi, G.; Milani, S. Altered Neuro-Endocrine–Immune Pathways in the Irritable Bowel Syndrome: The Top-down and the Bottom-up Model. J. Gastroenterol. 2012, 47, 1177–1185. [Google Scholar] [CrossRef]
- Gershon, M.D. Serotonin and Its Implication for the Management of Irritable Bowel Syndrome. Rev. Gastroenterol. Disord. 2003, 3 (Suppl S2), S25–S34. [Google Scholar]
- Dürk, T.; Panther, E.; Müller, T.; Sorichter, S.; Ferrari, D.; Pizzirani, C.; Di Virgilio, F.; Myrtek, D.; Norgauer, J.; Idzko, M. 5-Hydroxytryptamine Modulates Cytokine and Chemokine Production in LPS-Primed Human Monocytes via Stimulation of Different 5-HTR Subtypes. Int. Immunol. 2005, 17, 599–606. [Google Scholar] [CrossRef]
- Dinarello, C.A. Biologic Basis for Interleukin-1 in Disease. Blood 1996, 87, 2095–2147. [Google Scholar] [CrossRef]
- Vezza, T.; Rodríguez-Nogales, A.; Algieri, F.; Utrilla, M.; Rodriguez-Cabezas, M.; Galvez, J. Flavonoids in Inflammatory Bowel Disease: A Review. Nutrients 2016, 8, 211. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Xie, B.; Li, Y.; Shi, L.; Wan, J.; Chen, X.; Wang, H. Orally Deliverable Nanotherapeutics for the Synergistic Treatment of Colitis-Associated Colorectal Cancer. Theranostics 2019, 9, 7458–7473. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Dong, L.; Jia, A.; Chen, X.; Yang, Q.; Wang, Y.; Wang, Y.; Liu, R.; Cao, Y.; He, Y.; et al. Glucocorticoids Promote the Onset of Acute Experimental Colitis and Cancer by Upregulating MTOR Signaling in Intestinal Epithelial Cells. Cancers 2020, 12, 945. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; Zhou, X.; Liu, J.; Hou, P.; Ji, M. Therapeutic Potential and Deleterious Effect of Glucocorticoids on Azoxymethane/Dextran Sulfate Sodium-Induced Colorectal Cancer in Mice. Am. J. Cancer Res. 2021, 11, 4866–4883. [Google Scholar]
- Ma, H.-Q.; Yu, T.-T.; Zhao, X.-J.; Zhang, Y.; Zhang, H.-J. Fecal Microbial Dysbiosis in Chinese Patients with Inflammatory Bowel Disease. World J. Gastroenterol. 2018, 24, 1464–1477. [Google Scholar] [CrossRef]
- Raza, H.; John, A.; Benedict, S. Acetylsalicylic Acid-Induced Oxidative Stress, Cell Cycle Arrest, Apoptosis and Mitochondrial Dysfunction in Human Hepatoma HepG2 Cells. Eur. J. Pharmacol. 2011, 668, 15–24. [Google Scholar] [CrossRef]
- Gao, J.; Li, J.; Luo, Z.; Wang, H.; Ma, Z. Nanoparticle-Based Drug Delivery Systems for Inflammatory Bowel Disease Treatment. Drug Des. Devel. Ther. 2024, 18, 2921–2949. [Google Scholar] [CrossRef]
- Wang, D.; DuBois, R.N. The Role of Anti-Inflammatory Drugs in Colorectal Cancer. Annu. Rev. Med. 2013, 64, 131–144. [Google Scholar] [CrossRef]
- Ensign, L.M.; Cone, R.; Hanes, J. Oral Drug Delivery with Polymeric Nanoparticles: The Gastrointestinal Mucus Barriers. Adv. Drug Deliv. Rev. 2012, 64, 557–570. [Google Scholar] [CrossRef]
- Dulai, P.S.; Siegel, C.A.; Colombel, J.-F.; Sandborn, W.J.; Peyrin-Biroulet, L. Systematic Review: Monotherapy with Antitumour Necrosis Factor α Agents versus Combination Therapy with an Immunosuppressive for IBD. Gut 2014, 63, 1843–1853. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Hanauer, S.B. Antitumor Necrosis Factor Therapy for Inflammatory Bowel Disease: A Review of Agents, Pharmacology, Clinical Results, and Safety. Inflamm. Bowel Dis. 1999, 5, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Navaneethan, U.; Lashner, B.A. Effects of Immunosuppression and Liver Transplantation on Inflammatory Bowel Disease in Patients with Primary Sclerosing Cholangitis. Clin. Gastroenterol. Hepatol. 2013, 11, 524–525. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Guo, S.; Lin, C.M.; Liu, Q.; Huang, L. Nanoformulations for Combination or Cascade Anticancer Therapy. Adv. Drug Deliv. Rev. 2017, 115, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Qiu, N.; Gao, J.; Liu, Q.; Wang, J.; Shen, Y. Enzyme-Responsive Charge-Reversal Polymer-Mediated Effective Gene Therapy for Intraperitoneal Tumors. Biomacromolecules 2018, 19, 2308–2319. [Google Scholar] [CrossRef]
- Chen, Y.; Song, W.; Shen, L.; Qiu, N.; Hu, M.; Liu, Y.; Liu, Q.; Huang, L. Vasodilator Hydralazine Promotes Nanoparticle Penetration in Advanced Desmoplastic Tumors. ACS Nano 2019, 13, 1751–1763. [Google Scholar] [CrossRef]
- Li, X.; Lu, C.; Yang, Y.; Yu, C.; Rao, Y. Site-Specific Targeted Drug Delivery Systems for the Treatment of Inflammatory Bowel Disease. Biomed. Pharmacother. 2020, 129, 110486. [Google Scholar] [CrossRef]
- Di Sabatino, A.; Biancheri, P.; Rovedatti, L.; MacDonald, T.T.; Corazza, G.R. Recent Advances in Understanding Ulcerative Colitis. Intern. Emerg. Med. 2011, 7, 103–111. [Google Scholar] [CrossRef]
- Xiao, B.; Merlin, D. Oral Colon-Specific Therapeutic Approaches toward Treatment of Inflammatory Bowel Disease. Expert Opin. Drug Deliv. 2012, 9, 1393–1407. [Google Scholar] [CrossRef]
- Sun, T.; Kwong, C.H.; Gao, C.; Wei, J.; Yue, L.; Zhang, J.; Ye, R.D.; Wang, R. Amelioration of Ulcerative Colitis Via Inflammatory Regulation by Macrophage-Biomimetic Nanomedicine. Theranostics 2020, 10, 10106–10119. [Google Scholar] [CrossRef]
- Chung, C.H.; Jung, W.; Keum, H.; Kim, T.W.; Jon, S. Nanoparticles Derived from the Natural Antioxidant Rosmarinic Acid Ameliorate Acute Inflammatory Bowel Disease. ACS Nano 2020, 14, 6887–6896. [Google Scholar] [CrossRef]
- Xu, B.; Watkins, R.; Wu, L.; Zhang, C.; Davis, R. Natural Product-Based Nanomedicine: Recent Advances and Issues. Int. J. Nanomed. 2015, 10, 6055. [Google Scholar] [CrossRef] [PubMed]
- Khafaji, M.; Zamani, M.; Golizadeh, M.; Bavi, O. Inorganic Nanomaterials for Chemo/Photothermal Therapy: A Promising Horizon on Effective Cancer Treatment. Biophys. Rev. 2019, 11, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Sowmya, C.; Reddy, C.S.; Sandhya, R.; Keerthi, K. Colon Specific Drug Delivery Systems: A review of pharmaceutical approaches with Cur-Rent Trends. Int. Res. J. Pharm. 2012, 3, 45–57. [Google Scholar]
- Pan, Q.; Lu, Y.; Xie, L.; Wu, D.; Liu, R.; Gao, W.; Luo, K.; He, B.; Pu, Y. Recent Advances in Boosting EGFR Tyrosine Kinase Inhibitors-Based Cancer Therapy. Mol. Pharm. 2023, 20, 829–852. [Google Scholar] [CrossRef]
- Zhang, M.; Wen, Y.; Huang, Z.; Qin, X.; Zhou, M.; Xiao, D.; Cui, W.; Liu, Z.; Lin, Y. Targeted Therapy for Autoimmune Diseases Based on Multi-functional Frame Nucleic Acid System: Blocking TNF-α-NF-κB Signaling and Mediating Macrophage Polarization. Chem. Eng. J. 2023, 454, 140399. [Google Scholar] [CrossRef]
- Collins, S.M.; Surette, M.; Bercik, P. The Interplay between the Intestinal Microbiota and the Brain. Nat. Rev. Microbiol. 2012, 10, 735–742. [Google Scholar] [CrossRef]
- Cox, L.M.; Weiner, H.L. Microbiota Signaling Pathways That Influence Neurologic Disease. Neurotherapeutics 2018, 15, 135–145. [Google Scholar] [CrossRef]
- Cryan, J.F.; Dinan, T.G. Mind-Altering Microorganisms: The Impact of the Gut Microbiota on Brain and Behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, J.; Chen, Y. Regulation of Neurotransmitters by the Gut Microbiota and Effects on Cognition in Neurological Disorders. Nutrients 2021, 13, 2099. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Sandhu, K.; Peterson, V.; Dinan, T.G. The Gut Microbiome in Neurological Disorders. Lancet Neurol. 2019, 19. [Google Scholar] [CrossRef]
- Tang, H.-L.; Zhang, G.; Ji, N.-N.; Du, L.; Chen, B.-B.; Hua, R.; Zhang, Y.-M. Toll-like Receptor 4 in Paraventricular Nucleus Mediates Visceral Hypersensitivity Induced by Maternal Separation. Front. Pharmacol. 2017, 8, 309. [Google Scholar] [CrossRef] [PubMed]
- Xi, M.; Zhao, P.; Li, F.; Bao, H.; Ding, S.; Ji, L.; Yan, J. MicroRNA-16 Inhibits the TLR4/NF-ΚB Pathway and Maintains Tight Junction Integrity in Irritable Bowel Syndrome with Diarrhea. J. Biol. Chem. 2022, 298, 102461. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Wang, L.; Wang, Z.; Wan, C. Toll-like Receptor 4 Plays a Vital Role in Irritable Bowel Syndrome: A Scoping Review. Front. Immunol. 2024, 15, 1490653. [Google Scholar] [CrossRef] [PubMed]
- Giambra, V.; Pagliari, D.; Rio, P.; Totti, B.; Di Nunzio, C.; Bosi, A.; Giaroni, C.; Gasbarrini, A.; Gambassi, G.; Cianci, R. Gut Microbiota, Inflammatory Bowel Disease, and Cancer: The Role of Guardians of Innate Immunity. Cells 2023, 12, 2654. [Google Scholar] [CrossRef]
- Martin-Subero, M.; Anderson, G.; Kanchanatawan, B.; Berk, M.; Maes, M. Comorbidity between Depression and Inflammatory Bowel Disease Explained by Immune-Inflammatory, Oxidative, and Nitrosative Stress; Tryptophan Catabolite; and Gut–Brain Pathways. CNS Spectr. 2015, 21, 184–198. [Google Scholar] [CrossRef]
- He, F.; Wu, C.; Li, P.; Li, N.; Zhang, D.; Zhu, Q.; Ren, W.; Peng, Y. Functions and Signaling Pathways of Amino Acids in Intestinal Inflammation. BioMed Res. Int. 2018, 2018, 9171905. [Google Scholar] [CrossRef]
- Gershon, M.D. 5-Hydroxytryptamine (Serotonin) in the Gastrointestinal Tract. Curr. Opin. Endocrinol. Diabetes Obes. 2013, 20, 14–21. [Google Scholar] [CrossRef]
- Lamas, B.; Richard, M.L.; Leducq, V.; Pham, H.-P.; Michel, M.-L.; Da Costa, G.; Bridonneau, C.; Jegou, S.; Hoffmann, T.W.; Natividad, J.M.; et al. CARD9 Impacts Colitis by Altering Gut Microbiota Metabolism of Tryptophan into Aryl Hydrocarbon Receptor Ligands. Nat. Med. 2016, 22, 598–605. [Google Scholar] [CrossRef]
- Etienne-Mesmin, L.; Chassaing, B.; Gewirtz, A.T. Tryptophan: A Gut Microbiota-Derived Metabolites Regulating Inflammation. World J. Gastrointest. Pharmacol. Ther. 2017, 8, 7. [Google Scholar] [CrossRef]
- Yu, F.-Y.; Huang, S.-G.; Zhang, H.-Y.; Ye, H.; Chi, H.-G.; Zou, Y.; Lv, R.-X.; Zheng, X.-B. Comparison of 5-Hydroxytryptophan Signaling Pathway Characteristics in Diarrhea-Predominant Irritable Bowel Syndrome and Ulcerative Colitis. World J. Gastroenterol. 2016, 22, 3451–3459. [Google Scholar] [CrossRef]
- Guseva, D.; Holst, K.; Kaune, B.; Meier, M.; Keubler, L.; Glage, S.; Buettner, M.; Bleich, A.; Pabst, O.; Bachmann, O.; et al. Serotonin 5-HT7 Receptor Is Critically Involved in Acute and Chronic Inflammation of the Gastrointestinal Tract. Inflamm. Bowel Dis. 2014, 20, 1516–1529. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wu, G.; Zhou, Z.; Dai, Z.; Sun, Y.; Ji, Y.; Li, W.; Wang, W.; Liu, C.; Han, F.; et al. Glutamine and Intestinal Barrier Function. Amino Acids 2015, 47, 2143–2154. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Lin, G.; Dai, Z.; Zhou, T.; Li, T.; Yuan, T.; Wu, Z.; Wu, G.; Wang, J. L-Glutamine Deprivation Induces Autophagy and Alters the MTOR and MAPK Signaling Pathways in Porcine Intestinal Epithelial Cells. Amino Acids 2014, 47, 2185–2197. [Google Scholar] [CrossRef]
- Hou, Y.-C.; Chu, C.-C.; Ko, T.-L.; Yeh, C.-L.; Yeh, S.-L. Effects of Alanyl-Glutamine Dipeptide on the Expression of Colon-Inflammatory Mediators during the Recovery Phase of Colitis Induced by Dextran Sulfate Sodium. Eur. J. Nutr. 2012, 52, 1089–1098. [Google Scholar] [CrossRef]
- Chen, J.; Yang, Y.; Yang, Y.; Dai, Z.; Kim, I.H.; Wu, G.; Wu, Z. Dietary Supplementation with Glycine Enhances Intestinal Mucosal Integrity and Ameliorates Inflammation in C57BL/6J Mice with High-Fat Diet-Induced Obesity. J. Nutr. 2021, 151, 1769–1778. [Google Scholar] [CrossRef]
- Muro, P.; Zhang, L.; Li, S.; Zhao, Z.; Jin, T.; Mao, F.; Mao, Z. The Emerging Role of Oxidative Stress in Inflammatory Bowel Disease. Front. Endocrino. 2024, 15, 1390351. [Google Scholar] [CrossRef]
- Li, P.; Wu, G. Roles of Dietary Glycine, Proline, and Hydroxyproline in Collagen Synthesis and Animal Growth. Amino Acids 2017, 50, 29–38. [Google Scholar] [CrossRef]
- Bruns, H.; Kazanavicius, D.; Schultze, D.; Saeedi, M.A.; Yamanaka, K.; Strupas, K.; Schemmer, P. Glycine Inhibits Angiogenesis in Colorectal Cancer: Role of Endothelial Cells. Amino Acids 2016, 48, 2549–2558. [Google Scholar] [CrossRef]
- Chen, L.; Zhong, Y.; Ouyang, X.; Wang, C.; Yin, L.; Huang, J.; Li, Y.; Wang, Q.; Xie, J.; Huang, P.; et al. Effects of β-Alanine on Intestinal Development and Immune Performance of Weaned Piglets. Anim. Nutr. 2022, 12, 398–408. [Google Scholar] [CrossRef]
- Wischmeyer, P.E. Glutamine: Role in Gut Protection in Critical Illness. Curr. Opin. Clin. Nutr. Metab. Care 2006, 9, 607–612. [Google Scholar] [CrossRef]
- Achamrah, N.; Déchelotte, P.; Coëffier, M. Glutamine and the Regulation of Intestinal Permeability: From Bench to Bedside. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Ren, W.; Fang, J.; Hu, C.-A.A.; Guan, G.; Al-Dhabi, N.A.; Yin, J.; Duraipandiyan, V.; Chen, S.; Peng, Y.; et al. L-Glutamine and L-Arginine Protect against Enterotoxigenic Escherichia Coli Infection via Intestinal Innate Immunity in Mice. Amino Acids 2017, 49, 1945–1954. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-H.; Kim, H. The Roles of Glutamine in the Intestine and Its Implication in Intestinal Diseases. Int. J. Mol. Sci. 2017, 18, 1051. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; He, Y.; Liu, Z.; Liu, X.; Jing, Y. Glutamine Peptides: Preparation, Analysis, Applications, and Their Role in Intestinal Barrier Protection. Nutrients 2025, 17, 1017. [Google Scholar] [CrossRef]
- Hissen, K.L.; He, W.; Wu, G.; Criscitiello, M.F. Dietary L-Glutamate Modulates Intestinal Mucosal Immunity of Juvenile Hybrid Striped Bass (Morone saxatilis ♀ × Morone chrysops ♂). Front. Immunol. 2025, 16, 1575644. [Google Scholar] [CrossRef]
- Wu, Q.J.; Liu, N.; Wu, X.H.; Wang, G.Y.; Lin, L. Glutamine Alleviates Heat Stress-Induced Impairment of Intestinal Morphology, Intestinal Inflammatory Response, and Barrier Integrity in Broilers. Poult. Sci. 2018, 97, 2675–2683. [Google Scholar] [CrossRef]
- Guo, J.; Liang, T.; Chen, H.; Li, X.; Ren, X.; Wang, X.; Xiao, K.; Zhao, J.; Zhu, H.; Liu, Y. Glutamate Attenuates Lipopolysaccharide Induced Intestinal Barrier Injury by Regulating Corticotropin-Releasing Factor Pathway in Weaned Pigs. Anim. Biosci. 2022, 35, 1235–1249. [Google Scholar] [CrossRef]
- Jiao, N.; Wu, Z.; Ji, Y.; Wang, B.; Dai, Z.; Wu, G. L-Glutamate Enhances Barrier and Antioxidative Functions in Intestinal Porcine Epithelial Cells. J. Nutr. 2015, 145, 2258–2264. [Google Scholar] [CrossRef]
- Hasegawa, T.; Mizugaki, A.; Inoue, Y.; Kato, H.; Murakami, H. Cystine Reduces Tight Junction Permeability and Intestinal Inflammation Induced by Oxidative Stress in Caco-2 Cells. Amino Acids 2021, 53, 1021–1032. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Zhang, X.; Lu, Y.; Chen, H. New Insights in Intestinal Oxidative Stress Damage and the Health Intervention Effects of Nutrients: A Review. J. Funct. Foods 2020, 75, 104248. [Google Scholar] [CrossRef]
- Di Vincenzo, F.; Del Gaudio, A.; Petito, V.; Lopetuso, L.R.; Scaldaferri, F. Gut Microbiota, Intestinal Permeability, and Systemic Inflammation: A Narrative Review. Intern. Emerg. Med. 2023, 19, 275–293. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F.; Artis, D.; Becker, C. The Intestinal Barrier: A Pivotal Role in Health, Inflammation, and Cancer. Lancet Gastroenterol. Hepatol. 2025, 10, 573–592. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.H.; Tong, G.; Xiao, K.; Jiao, L.F.; Ke, Y.L.; Hu, C.H. L-Cysteine Protects Intestinal Integrity, Attenuates Intestinal Inflammation and Oxidant Stress, and Modulates NF-ΚB and Nrf2 Pathways in Weaned Piglets after LPS Challenge. Innate Immun. 2016, 22, 152–161. [Google Scholar] [CrossRef]
- Yi, D.; Hou, Y.; Xiao, H.; Wang, L.; Zhang, Y.; Chen, H.; Wu, T.; Ding, B.; Hu, C.-A.A.; Wu, G. N-Acetylcysteine Improves Intestinal Function in Lipopolysaccharides-Challenged Piglets through Multiple Signaling Pathways. Amino Acids 2017, 49, 1915–1929. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, J.; Hou, Y.; Yi, D.; Ding, B.; Xie, J.; Zhang, Y.; Chen, H.; Wu, T.; Zhao, D.; et al. N-Acetylcysteine Supplementation Alleviates Intestinal Injury in Piglets Infected by Porcine Epidemic Diarrhea Virus. Amino Acids 2017, 49, 1931–1943. [Google Scholar] [CrossRef]
- Renzetti, M.; Funck, D.; Trovato, M. Proline and ROS: A Unified Mechanism in Plant Development and Stress Response? Plants 2024, 14, 2. [Google Scholar] [CrossRef]
- Trovato, M.; Forlani, G.; Signorelli, S.; Funck, D. Proline Metabolism and Its Functions in Development and Stress Tolerance. In Osmoprotectant-Mediated Abiotic Stress Tolerance in Plants; Springer: Berlin/Heidelberg, Germany, 2019; pp. 41–72. [Google Scholar] [CrossRef]
- Kang, P.; Zhang, L.; Hou, Y.; Ding, B.; Yi, D.; Wang, L.; Zhu, H.; Liu, Y.; Yin, Y.; Wu, G. Effects of L-Proline on the Growth Performance, and Blood Parameters in Weaned Lipopolysaccharide (LPS)-Challenged Pigs. Asian-Australas. J. Anim. Sci. 2014, 27, 1150–1156. [Google Scholar] [CrossRef]
- Chen, S.; Liu, Y.; Wang, X.; Wang, H.; Li, S.; Shi, H.; Zhu, H.; Zhang, J.; Pi, D.; Hu, C.-A.A.; et al. Asparagine Improves Intestinal Integrity, Inhibits TLR4 and NOD Signaling, and Differently Regulates P38 and ERK1/2 Signaling in Weanling Piglets after LPS Challenge. Innate Immun. 2016, 22, 577–587. [Google Scholar] [CrossRef]
- Zhu, H.; Pi, D.; Leng, W.; Wang, X.; Hu, C.-A.A.; Hou, Y.; Xiong, J.; Wang, C.; Qin, Q.; Liu, Y. Asparagine Preserves Intestinal Barrier Function from LPS-Induced Injury and Regulates CRF/CRFR Signaling Pathway. Innate Immun. 2017, 23, 546–556. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Hu, C.-A. Therapeutic Potential of Amino Acids in Inflammatory Bowel Disease. Nutrients 2017, 9, 920. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Hou, Y.; Yin, Y.; Qiu, Y.; Wu, G.; Hu, C.-A.A. Roles of Amino Acids in Preventing and Treating Intestinal Diseases: Recent Studies with Pig Models. Amino Acids 2017, 49, 1277–1291. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, Y.; Shi, H.; Wang, X.; Zhu, H.; Pi, D.; Leng, W.; Li, S. Aspartate Attenuates Intestinal Injury and Inhibits TLR4 and NODs/NF-ΚB and P38 Signaling in Weaned Pigs after LPS Challenge. Eur. J. Nutr. 2016, 56, 1433–1443. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Liu, S.; Chen, S.; Zeng, Z.; Huang, R.; Yin, Y.; Liu, G. The Effect of Aspartate Supplementation on the Microbial Composition and Innate Immunity on Mice. Amino Acids 2017, 49, 2045–2051. [Google Scholar] [CrossRef]
- Duan, J.; Yin, J.; Ren, W.; Liu, T.; Cui, Z.; Huang, X.; Wu, L.; Sung, W.K.; Liu, G.; Wu, X.; et al. Dietary Supplementation with L-Glutamate and L-Aspartate Alleviates Oxidative Stress in Weaned Piglets Challenged with Hydrogen Peroxide. Amino Acids 2015, 48, 53–64. [Google Scholar] [CrossRef]
- Ji, Y.; Hou, Y.; Blachier, F.; Wu, Z. Editorial: Amino Acids in Intestinal Growth and Health. Front. Nutr. 2023, 10, 1172548. [Google Scholar] [CrossRef]
- Chen, M.; Chen, X.-Q.; Tian, L.-X.; Liu, Y.-J.; Niu, J. Enhanced Intestinal Health, Immune Responses and Ammonia Resistance in Pacific White Shrimp (Litopenaeus vannamei) Fed Dietary Hydrolyzed Yeast (Rhodotorula mucilaginosa) and Bacillus Licheniformis. Aquac. Rep. 2020, 17, 100385. [Google Scholar] [CrossRef]
- Marchelletta, R.R.; Krishnan, M.; Spalinger, M.R.; Placone, T.; Alvarez, R.; Sayoc-Becerra, A.; Canale, V.; Shawki, A.; Park, Y.-S.; Bernts, L.H.P.; et al. T Cell Protein Tyrosine Phosphatase Protects Intestinal Barrier Function by Restricting Epithelial Tight Junction Remodeling. J. Clin. Investig. 2021, 131, e138230. [Google Scholar] [CrossRef]
- Li, X.; Lin, Y.; Li, X.; Xu, X.; Zhao, Y.; Xu, L.; Gao, Y.; Li, Y.; Tan, Y.; Qian, P.; et al. Tyrosine Supplement Ameliorates Murine AGVHD by Modulation of Gut Microbiome and Metabolome. eBioMedicine 2020, 61, 103048. [Google Scholar] [CrossRef]
- Faure, M.; Mettraux, C.; Moënnoz, D.; Godin, J.; Vuichoud, J.; Rochat, F.; Breuillé, D.; Obled, C.; Corthésy-Theulaz, I. Specific Amino Acids Increase Mucin Synthesis and Microbiota in Dextran Sulfate Sodium–Treated Rats. J. Nutr. 2006, 136, 1558–1564. [Google Scholar] [CrossRef]
- Wu, J.; Li, M.; Zhou, C.; Rong, J.; Zhang, F.; Wen, Y.; Qu, J.; Wu, R.; Miao, Y.; Niu, J. Changes in Amino Acid Concentrations and the Gut Microbiota Composition Are Implicated in the Mucosal Healing of Ulcerative Colitis and Can Be Used as Noninvasive Diagnostic Biomarkers. Clin. Proteom. 2024, 21, 62. [Google Scholar] [CrossRef]
- Jablaoui, A.; Kriaa, A.; Mkaouar, H.; Akermi, N.; Soussou, S.; Wysocka, M.; Wołoszyn, D.; Amouri, A.; Gargouri, A.; Maguin, E.; et al. Fecal Serine Protease Profiling in Inflammatory Bowel Diseases. Front. Cell. Infect. Microbiol. 2020, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Ma, E.H.; Bantug, G.; Griss, T.; Condotta, S.; Johnson, R.M.; Samborska, B.; Mainolfi, N.; Suri, V.; Guak, H.; Balmer, M.L.; et al. Serine Is an Essential Metabolite for Effector T Cell Expansion. Cell Metab. 2017, 25, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Kasti, A.; Katsas, K.; Nikolaki, M.D.; Triantafyllou, K. The Role and the Regulation of NLRP3 Inflammasome in Irritable Bowel Syndrome: A Narrative Review. Microorganisms 2025, 13, 171. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, Z.; Zhang, G.-H. Involvement of NF-ΚB Signal Pathway in Acupuncture Treatment of Patients with Rheumatoid Arthritis. Zhen Ci Yan Jiu 2020, 45, 914–919. [Google Scholar] [CrossRef]
- He, X.; Cui, L.-H.; Wang, X.-H.; Yan, Z.-H.; Li, C.; Gong, S.-D.; Zheng, Y.; Luo, Z.; Wang, Y. Modulation of Inflammation by Toll-like Receptor 4/Nuclear Factor-Kappa B in Diarrhea-Predominant Irritable Bowel Syndrome. Oncotarget 2017, 8, 113957–113965. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoyanova, M.; Gledacheva, V.; Nikolova, S. Gut–Brain–Microbiota Axis in Irritable Bowel Syndrome: A Narrative Review of Pathophysiology and Current Approaches. Appl. Sci. 2025, 15, 6441. https://doi.org/10.3390/app15126441
Stoyanova M, Gledacheva V, Nikolova S. Gut–Brain–Microbiota Axis in Irritable Bowel Syndrome: A Narrative Review of Pathophysiology and Current Approaches. Applied Sciences. 2025; 15(12):6441. https://doi.org/10.3390/app15126441
Chicago/Turabian StyleStoyanova, Mihaela, Vera Gledacheva, and Stoyanka Nikolova. 2025. "Gut–Brain–Microbiota Axis in Irritable Bowel Syndrome: A Narrative Review of Pathophysiology and Current Approaches" Applied Sciences 15, no. 12: 6441. https://doi.org/10.3390/app15126441
APA StyleStoyanova, M., Gledacheva, V., & Nikolova, S. (2025). Gut–Brain–Microbiota Axis in Irritable Bowel Syndrome: A Narrative Review of Pathophysiology and Current Approaches. Applied Sciences, 15(12), 6441. https://doi.org/10.3390/app15126441








