Alternative HPLC-DAD Direct-Phase Approach to Measurement of Enantiopurity of Lactic Acid Derivatives
Abstract
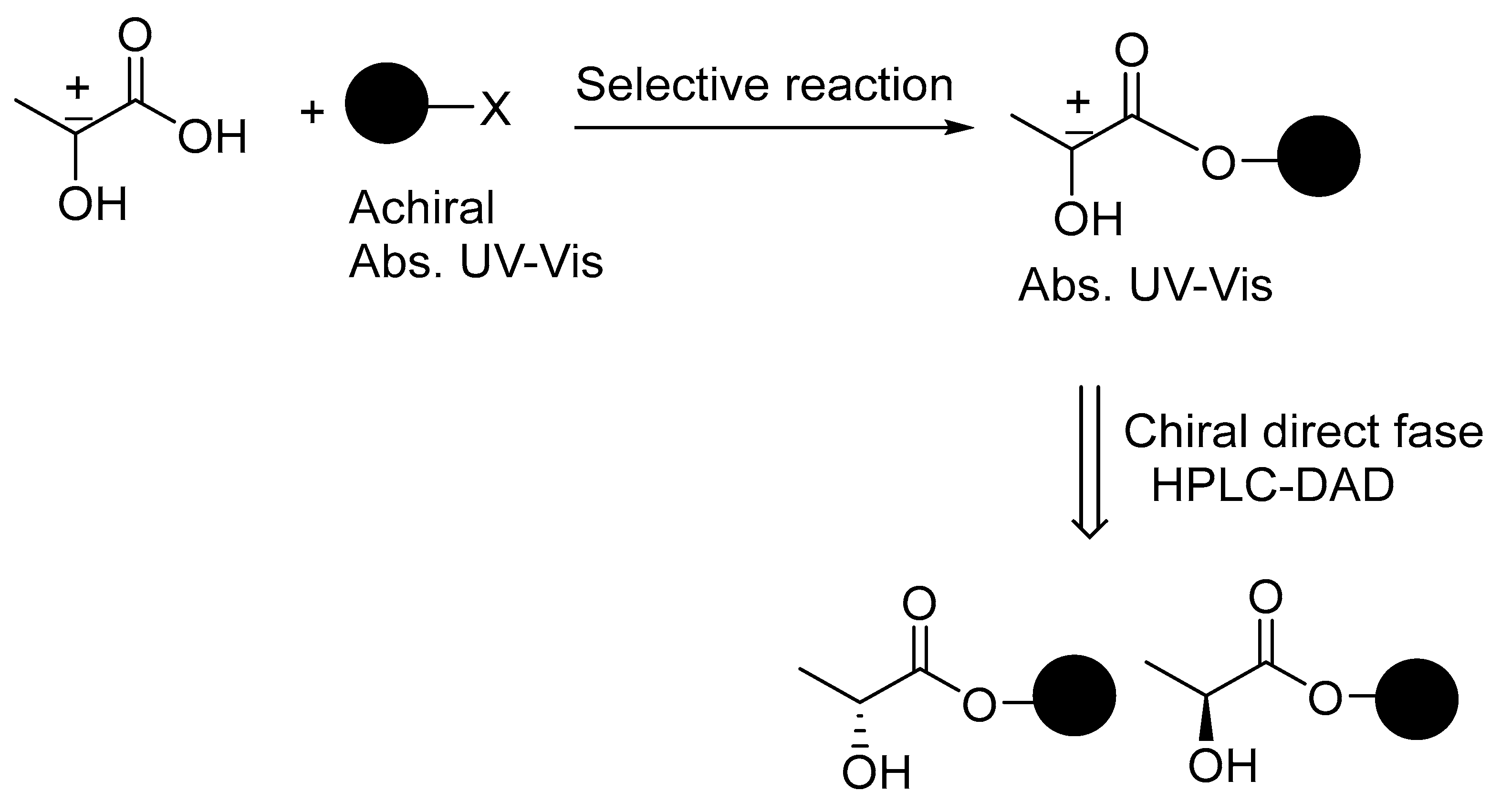
1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Instruments
2.3. Chiral Chromatography
3. Results and Discussion
3.1. Screening of Reaction Conditions
3.2. Chiral HPLC-DAD Analysis of Lactates
4. Experimental Part
Synthesis of Lactate Derivatives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Groot, W.; Van Krieken, J.; Sliekersl, O.; De Vos, S. Production and purification of lactic acid and lactide. In Poly (Lactic Acid) Synthesis, Structures, Properties, Processing, Applications, and End of Life, 2nd ed.; Auras, R.A., Lim, L.-T., Selke, S.E.M., Tsuji, H., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2022; pp. 1–18. [Google Scholar] [CrossRef]
- Meng, K.; Zhang, G.; Ding, C.; Zhang, T.; Yan, H.; Zhang, D.; Fang, T.; Liu, M.; You, Z.; Yang, C.; et al. Recent advances on purification of lactic acid. Chem. Rec. 2020, 20, 1236–1256. [Google Scholar] [CrossRef] [PubMed]
- DeStefano, V.; Khan, S.; Tabada, A. Applications of PLA in modern medicine. Eng. Regen. 2020, 1, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Dana, H.R.; Ebrahimi, F. Synthesis, properties, and applications of polylactic acid-based polymers. Polym. Eng. Sci. 2022, 63, 22–43. [Google Scholar] [CrossRef]
- Khouri, N.G.; Bahú, J.O.; Blanco-Llamero, C.; Severino, P.; Concha, V.O.C.; Souto, E.B. Polylactic acid (PLA): Properties, synthesis, and biomedical applications—A review of the literature. J. Mol. Struct. 2024, 1309, 13824–13840. [Google Scholar] [CrossRef]
- Chile, L.-E.; Mehrkhodavandi, P.; Hatzikiriakos, S.G. A Comparison of the Rheological and Mechanical Properties of Isotactic, Syndiotactic and Hetherotactic Poly(lactide). Macromolecules 2016, 49, 909–919. [Google Scholar] [CrossRef]
- Taib, N.A.A.B.; Rahman, M.R.; Huda, D.; Kuok, K.K.; Hamdan, S.; Bakri, M.K.B.; Julaihi, M.R.M.B.; Khan, A. A review on poly lactic acid (PLA) as a biodegradable polymer. Polym. Bull. 2023, 80, 1179–1213. [Google Scholar] [CrossRef]
- Song, L.; Yang, D.; Liu, R.; Liu, S.; Dai, L.; Dai, X. Microbial production of lactic acid from food waste: Latest advances, limits, and perspectives. Bioresour. Technol. 2022, 345, 126052. [Google Scholar] [CrossRef]
- Mora-Villalobos, J.A.; Montero-Zamora, J.; Natalia Barboza, N.; Rojas-Garbanzo, C.; Jessie Usaga, J.; Redondo-Solano, M.; Schroedter, L.; Olszewska-Widdrat, A.; López-Gómez, J.P. Multi-Product Lactic Acid Bacteria Fermentations: A Review. Fermentation 2020, 6, 23. [Google Scholar] [CrossRef]
- Duprat, F.; Coyard, V. Partial separation of lactic acid enantiomers by HPLC using (S)-(+) mandelic acid as chiral mobile-phase additive. Chromatographia 1992, 34, 31–34. [Google Scholar] [CrossRef]
- Ding, X.; Lin, S.; Weng, H.; Liang, J. Separation and determination of the enantiomers of lactic acid and 2-hydroxyglutaric acid by chiral derivatization combined with gas chromatography and mass spectrometry. J. Sep. Sci. 2018, 41, 2576–2584. [Google Scholar] [CrossRef]
- Omole, O.O.; Brocks, D.R.; Nappert, G.; Naylor, J.M.; Zello, G.A. High-performance liquid chromatographic assay of (±)-lactic acid and its enantiomers in calf serum. J. Chromatogr. B Biomed. Sci. Appl. 1999, 727, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Norton, D.; Crow, B.; Bishop, M.; Kovalcik, K.; George, J.; Bralley, J.A. High performance liquid chromatography–tandem mass spectrometry (HPLC/MS/MS) assay for chiral separation of lactic acid enantiomers in urine using a teicoplanin based stationary phase. J. Chromatogr. B 2007, 850, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Henry, H.; Marmy Conus, N.; Steenhout, P.; Béguin, A.; Boulat, O. Sensitive determination of d-lactic acid and l-lactic acid in urine by high-performance liquid chromatography–tandem mass spectrometry. Biomed. Chromatogr. 2012, 26, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Qiu, S.; Dai, Y.; Wu, Y.; Zeng, X. Distribution and Quantification of Lactic Acid Enantiomers in Baijiu. Foods 2022, 11, 2607. [Google Scholar] [CrossRef]
- Jánovová, L.; Hroboňová, K. Stationary phase type and temperature effect on HPLC separation of lactic acid enantiomers. Acta Chim. Slovaca 2021, 14, 60–65. [Google Scholar] [CrossRef]
- Berardozzi, R.; Guido, C.A.; Capozzi, M.A.M.; Cardellicchio, C.; Di Bari, L.; Pescitelli, G. Circular Dichroism and TDDFT Investigation of Chiral Fluorinated Aryl Benzyl Sulfoxides. Eur. J. Org. Chem. 2015, 2015, 5554–5562. [Google Scholar] [CrossRef]
- Capozzi, M.A.M.; Cardellicchio, C. Stereoselection in the Betti reaction of valine methyl esters. Tetrahedron Asymmetry 2017, 28, 1792–1796. [Google Scholar] [CrossRef]
- Capozzi, M.A.M.; Capitelli, F.; Cardellicchio, C. Structural Motifs in Enantiopure Halogenated Aryl Benzyl Sulfoxides: Effect of Fluorine Substitution. Cryst. Growth Des. 2014, 14, 5442–5451. [Google Scholar] [CrossRef]
- Górecki, M.; Capozzi, M.A.M.; Albano, G.; Cardellicchio, C.; Di Bari, L.; Pescitelli, G. Stereochemical analysis of β-keto sulfoxides by circular dichroism. Chirality 2018, 30, 29–42. [Google Scholar] [CrossRef]
- Poryvai, A.; Vojtylová-Jurkovi, T.; Šmahel, M.; Kolderová, N.; Tomášková, P.; David Sýkora, D.; Kohout, M. Determination of Optical Purity of Lactic Acid-Based Chiral Liquid Crystals and Corresponding Building Blocks by Chiral High-Performance Liquid Chromatography and Supercritical Fluid Chromatography. Molecules 2019, 24, 1099. [Google Scholar] [CrossRef]
- Gallo, E.A.; Gellman, S.H. Hydrogen-bond-mediated folding in depsipeptide models of.beta.-turns and.alpha.-helical turns. J. Am. Chem. Soc. 1993, 115, 9774–9788. [Google Scholar] [CrossRef]


| Entry | Benzyl Halide | Product | Base a | Solvent | Temperature | t (h) | Yield (%) |
|---|---|---|---|---|---|---|---|
| 1 | 1a | (±)-1b | DBU c | Toluene | 90 °C | 12 | 50 |
| 2 | 2a | (±)-2b | K2CO3 | CH3CN | 60 °C | 12 | 67 |
| 3 | 2a | (±)-2b | K2CO3 | EtOH | Rt | 12 | Nr b |
| 4 | 2a | (±)-2b | KOH | EtOH | Rt | 12 | Nr b |
| 5 | 2a | (±)-2b | NaOCH3 | Acetone | Rt | 12 | Nr b |
| 6 | 2a | (±)-2b | NaOH | Acetone | 50 °C | 2 | Nr b |
| 7 | 1a | (±)-1b | NEt3 | CH3CN | 60 °C | 4 | 72 |
| 8 | 2a | (±)-2b | NEt3 | CH3CN | 60 °C | 4 | 89 |
| 9 | 3a | (±)-3b | NEt3 | CH3CN | 60 °C | 4 | 56 |
| 10 | 2a | (−)-2b | NEt3 | CH3CN | 60 °C | 4 | 82 |
| 11 | 2a | (±)-2b | NEt3 | no solvent | 60 °C | 1 | 50 |
| 12 | 2a | (±)-2b | NEt3 | H2O | 60 °C | 1 | 38 |
| Entries | Compound | Column | α 1 | Rs 2 | Elution Conditions (n-Hexane:i-Propanol) | λobs |
|---|---|---|---|---|---|---|
| 1 | 1b | Chiralcel OD-H | 1.05 | 1.77 | 98:2 | 210 nm |
| 2 | Chiralpak IA | 1.04 | 1.98 | 98:2 | 210 nm | |
| 3 | (R,R)-Whelk O2 | - 3 | - 3 | 95:5 | 210 nm | |
| 4 | 2b | Chiralcel OD-H | 1.08 | 3.24 | 90:10 | 230, 254 nm |
| 5 | Chiralpak IA | 1.13 | 3.57 | 80:20 | 230, 254 nm | |
| 6 | (R,R)-Whelk O2 | 1.04 | 1.17 | 90:10 | 230, 254 nm | |
| 7 | 3b | Chiralcel OD-H | 1.09 | 2.03 | 70:30 | 230, 254 nm |
| 8 | Chiralpak IA | 1.08 | 2.12 | 70:30 | 230, 254 nm | |
| 9 | (R,R)-Whelk O2 | - 3 | - 3 | 80:20 | 230, 254 nm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montrone, M.; Cardellicchio, C.; Capozzi, M.A.M. Alternative HPLC-DAD Direct-Phase Approach to Measurement of Enantiopurity of Lactic Acid Derivatives. Appl. Sci. 2025, 15, 6433. https://doi.org/10.3390/app15126433
Montrone M, Cardellicchio C, Capozzi MAM. Alternative HPLC-DAD Direct-Phase Approach to Measurement of Enantiopurity of Lactic Acid Derivatives. Applied Sciences. 2025; 15(12):6433. https://doi.org/10.3390/app15126433
Chicago/Turabian StyleMontrone, Maria, Cosimo Cardellicchio, and Maria Annunziata M. Capozzi. 2025. "Alternative HPLC-DAD Direct-Phase Approach to Measurement of Enantiopurity of Lactic Acid Derivatives" Applied Sciences 15, no. 12: 6433. https://doi.org/10.3390/app15126433
APA StyleMontrone, M., Cardellicchio, C., & Capozzi, M. A. M. (2025). Alternative HPLC-DAD Direct-Phase Approach to Measurement of Enantiopurity of Lactic Acid Derivatives. Applied Sciences, 15(12), 6433. https://doi.org/10.3390/app15126433






