Novel Design of Expandable Spinal Cage for Efficient Lumbar Spine Fusion Operation
Abstract
1. Introduction
2. Design of an Expandable Cage
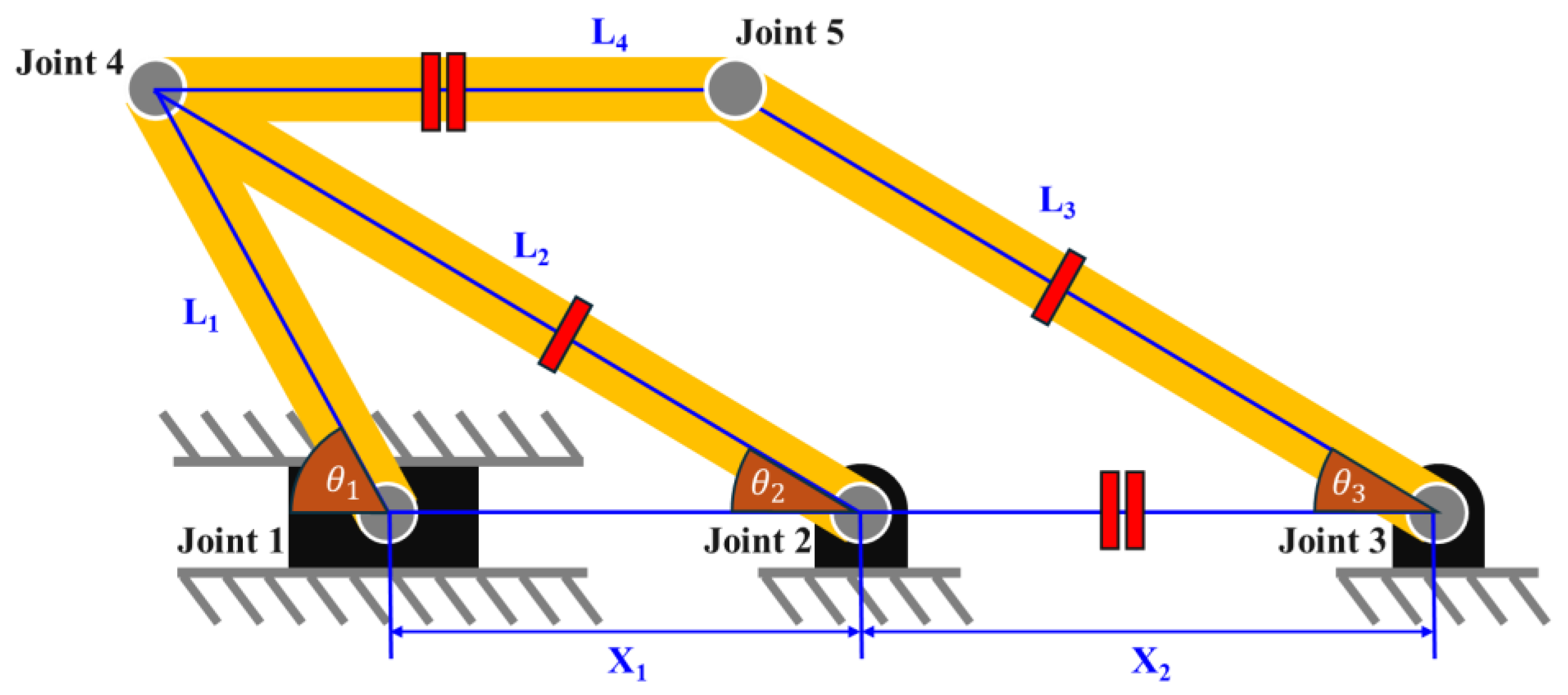
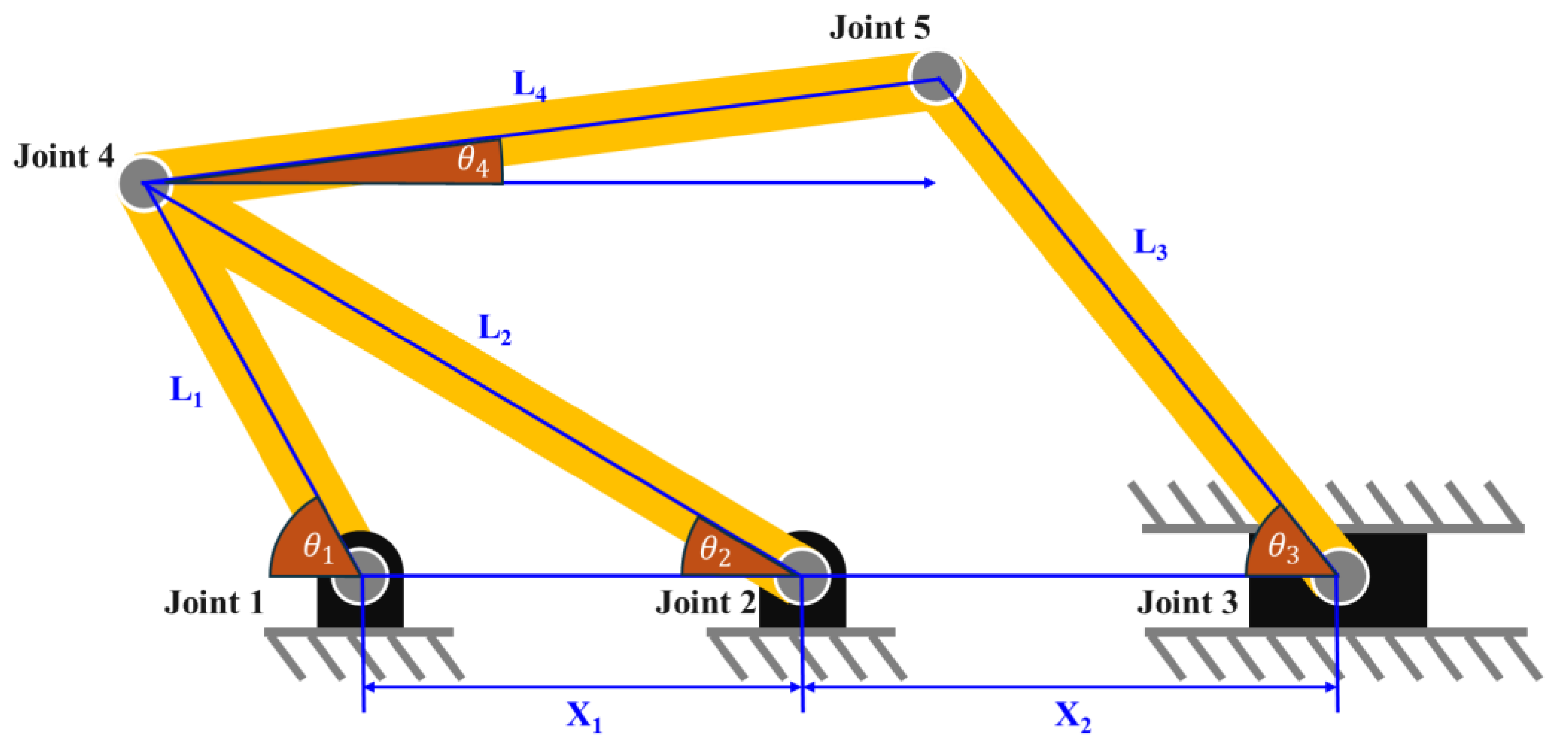
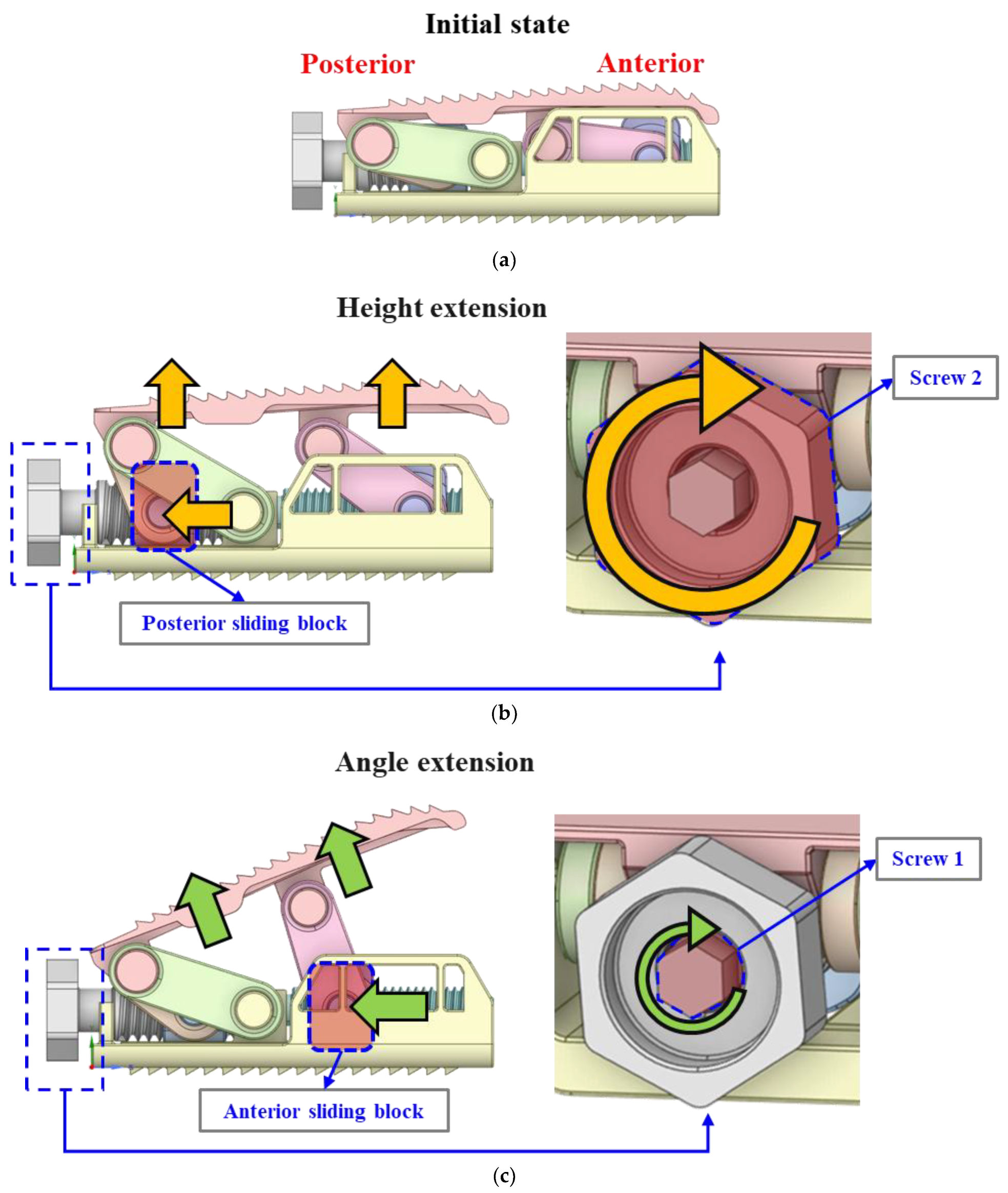
2.1. Link Mechanism for Height Extension
2.2. Link Mechanism for Angle Extension
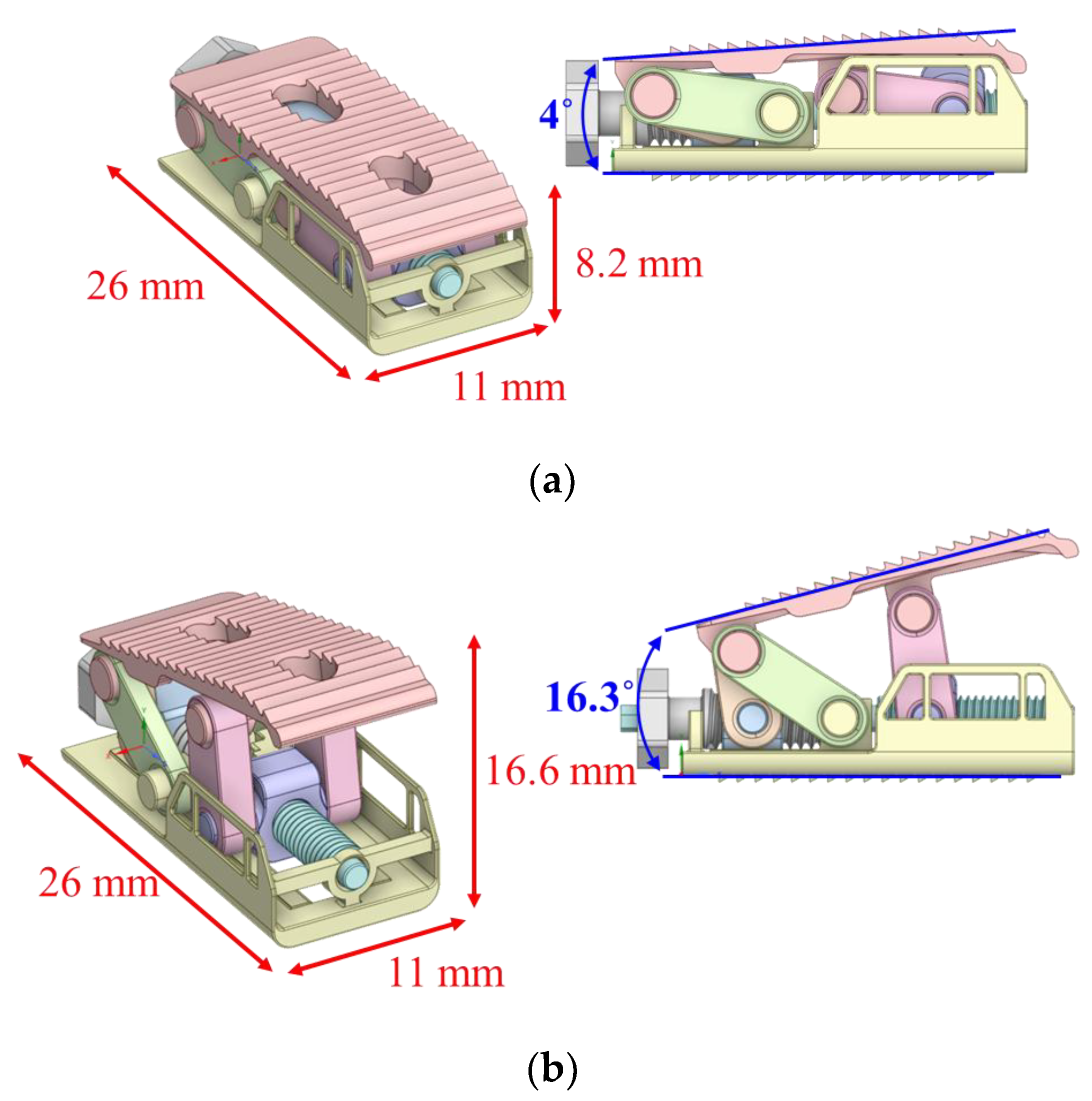
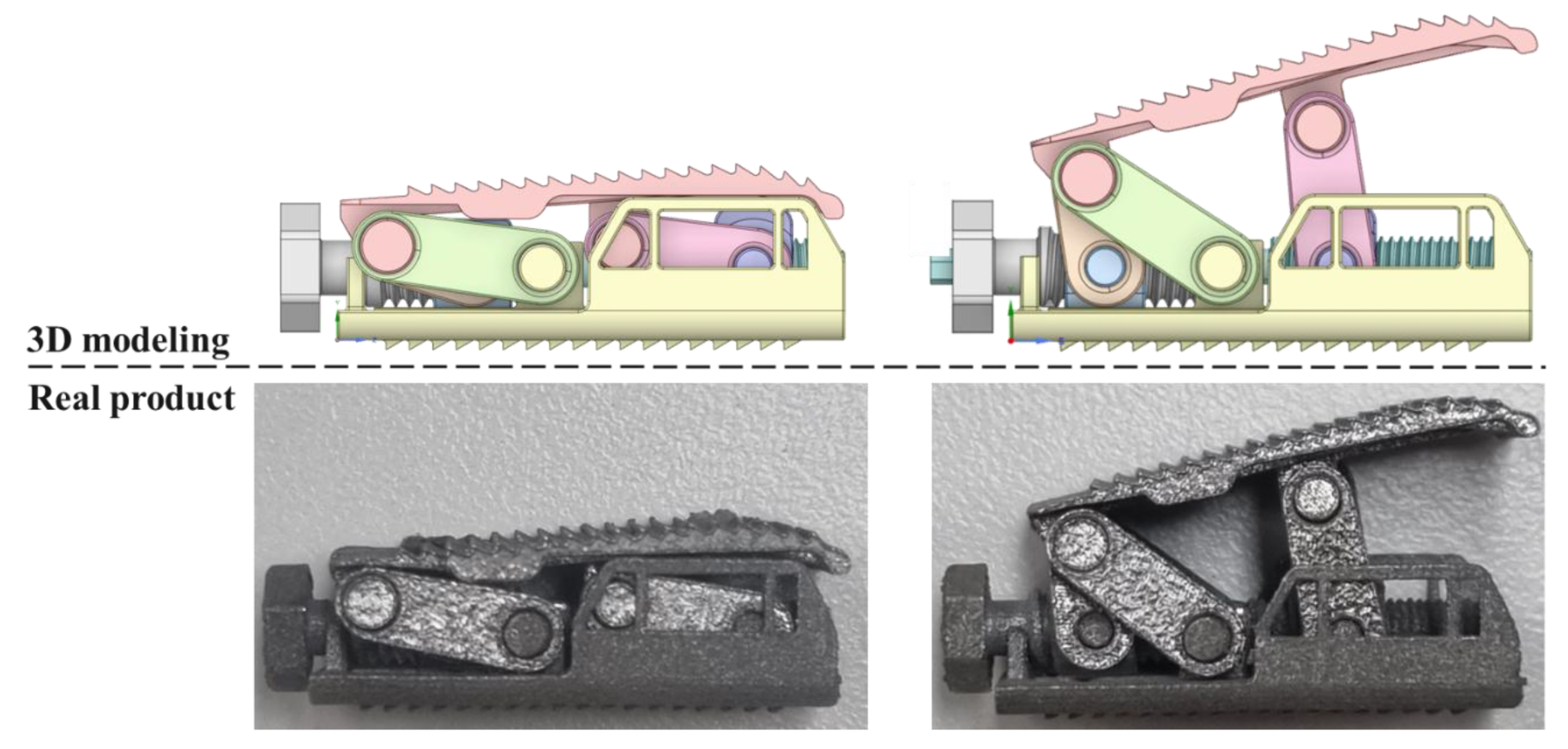
2.3. Final Modeling
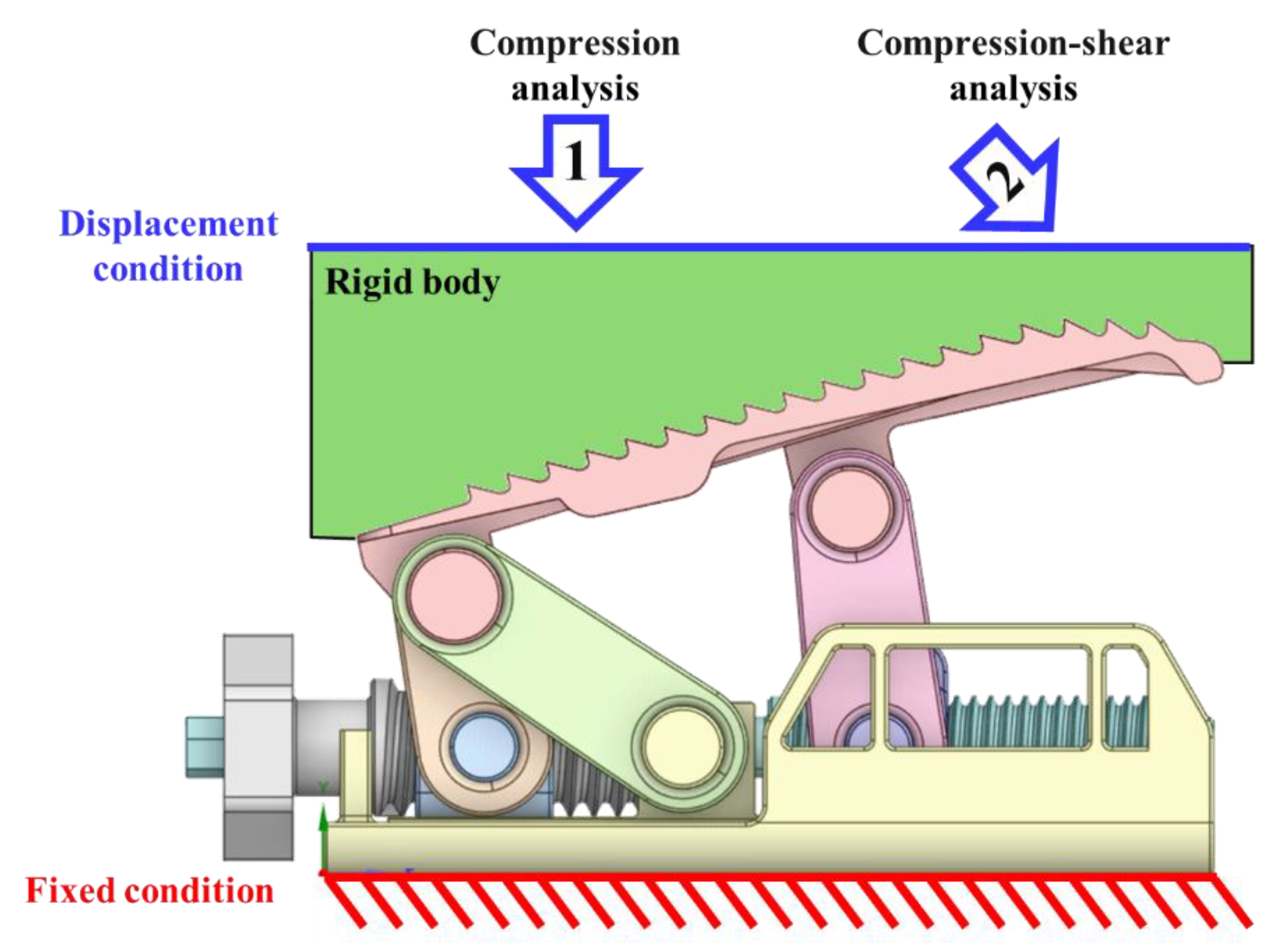
3. FEA for Mechanical Performance Verification
3.1. Verification Criteria
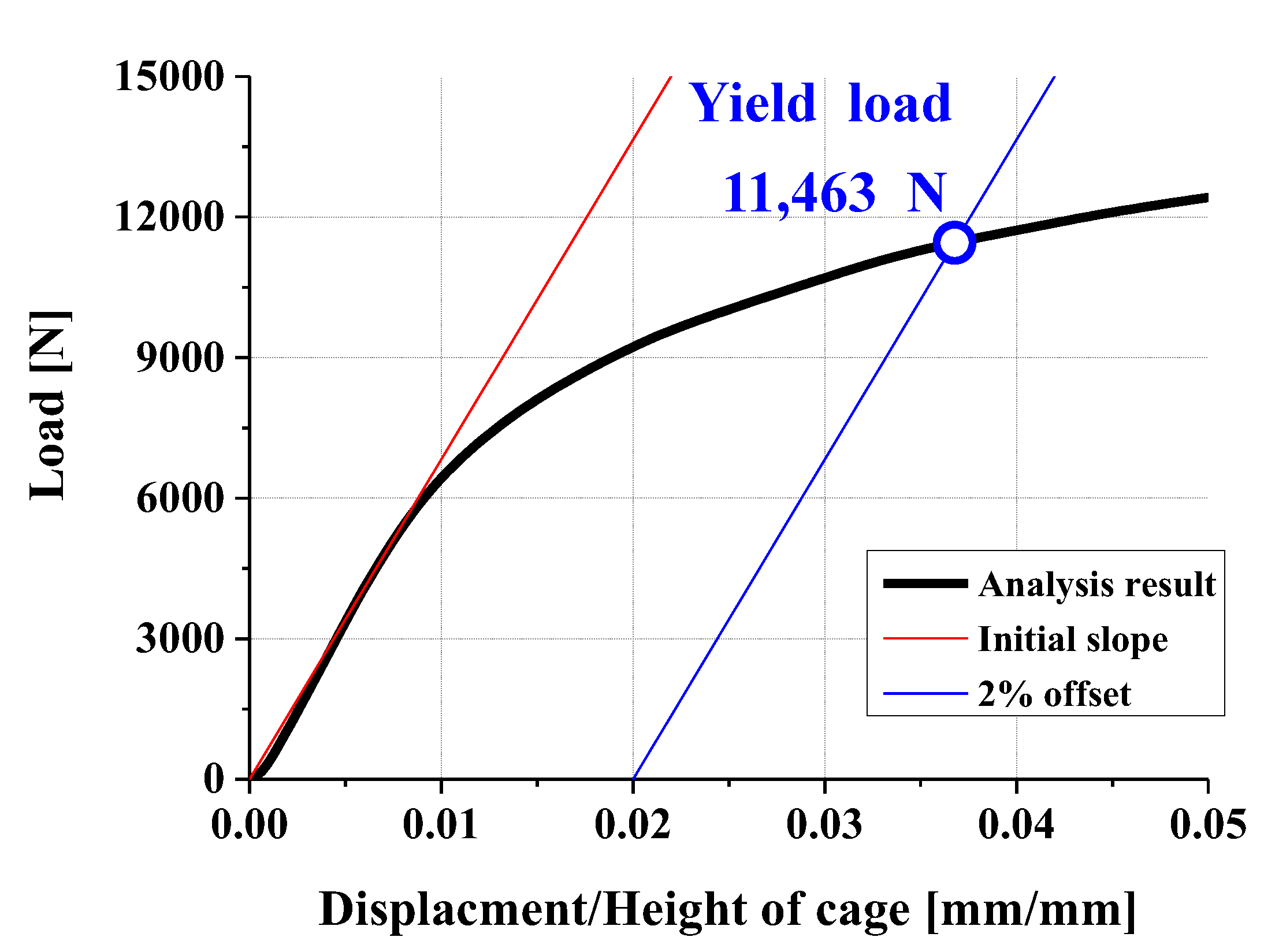
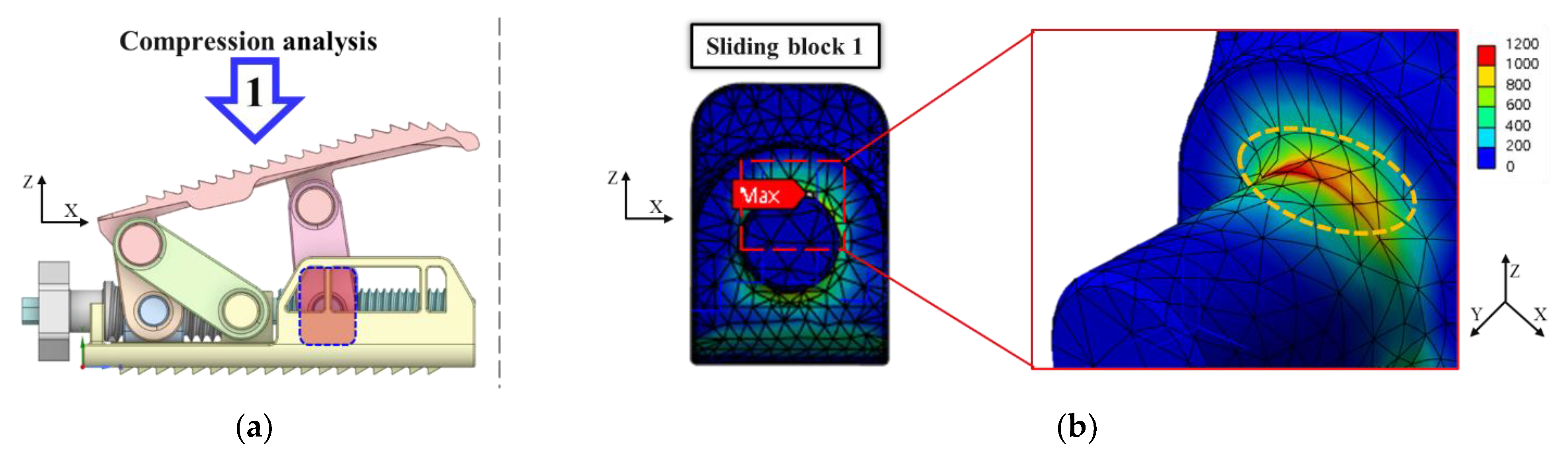
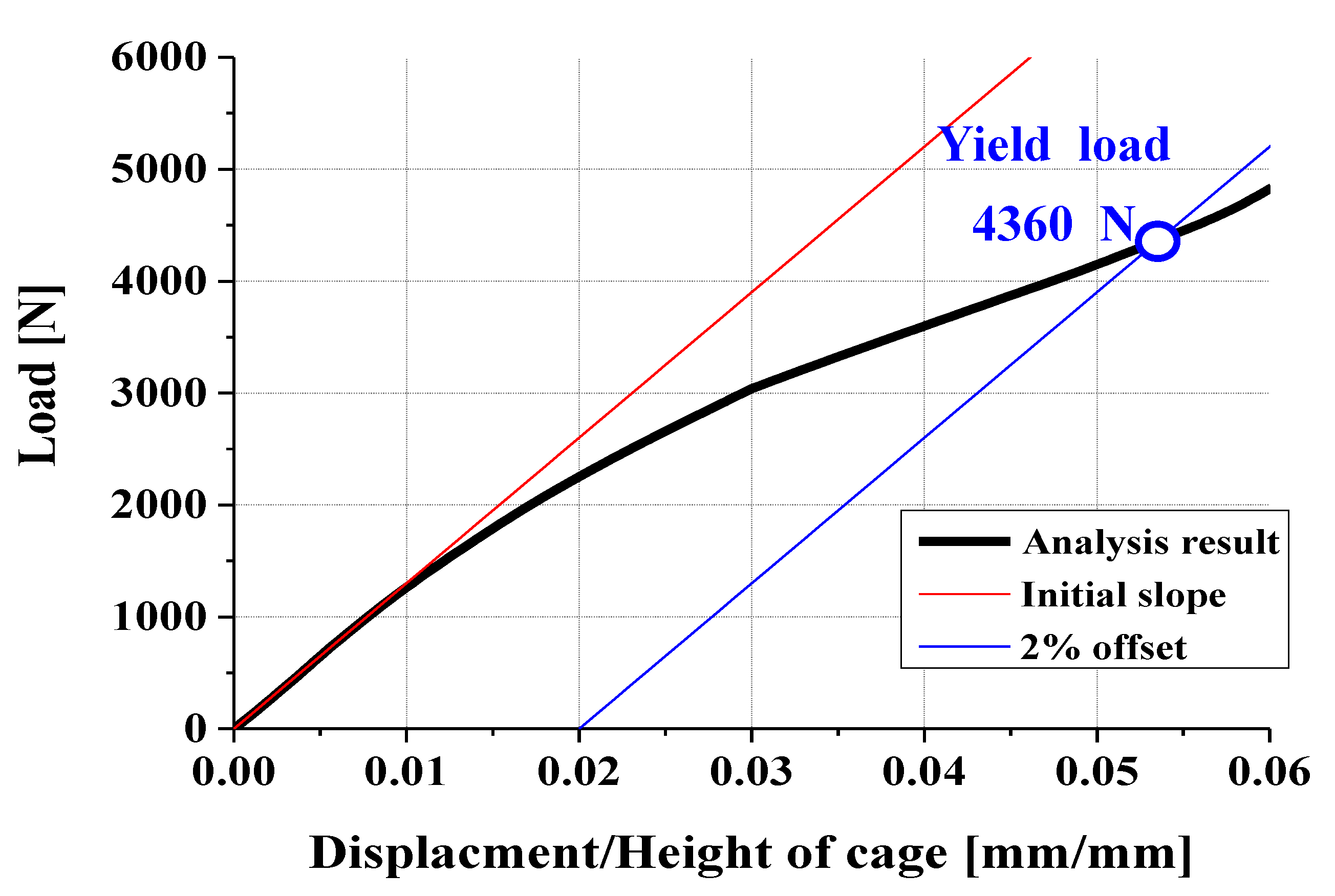
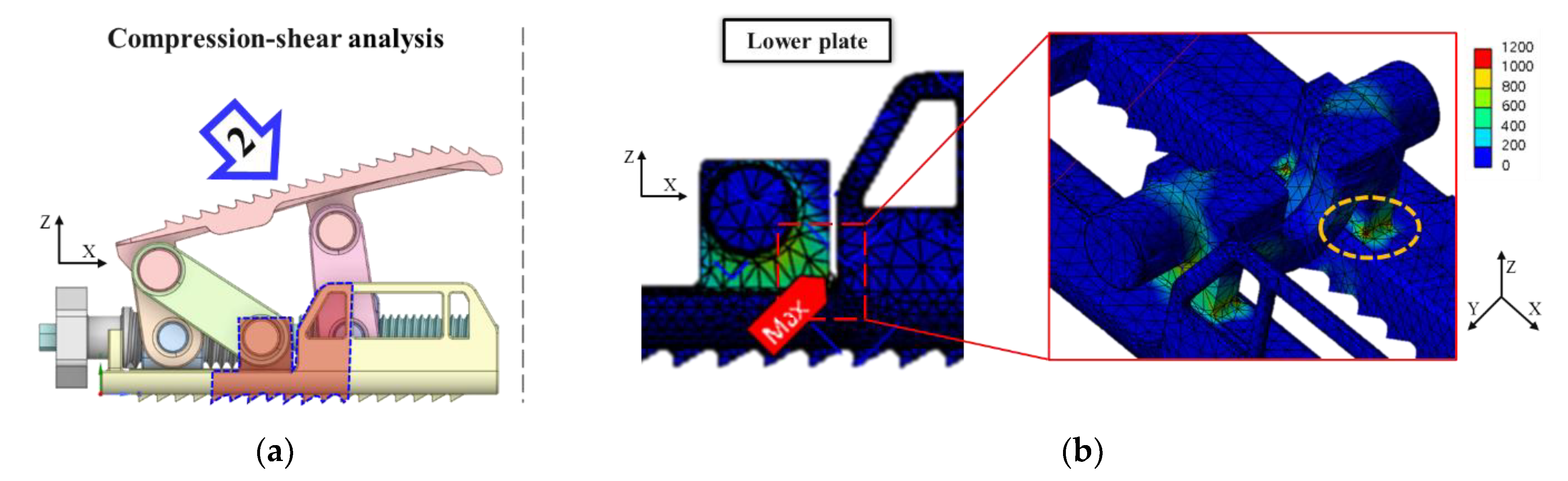
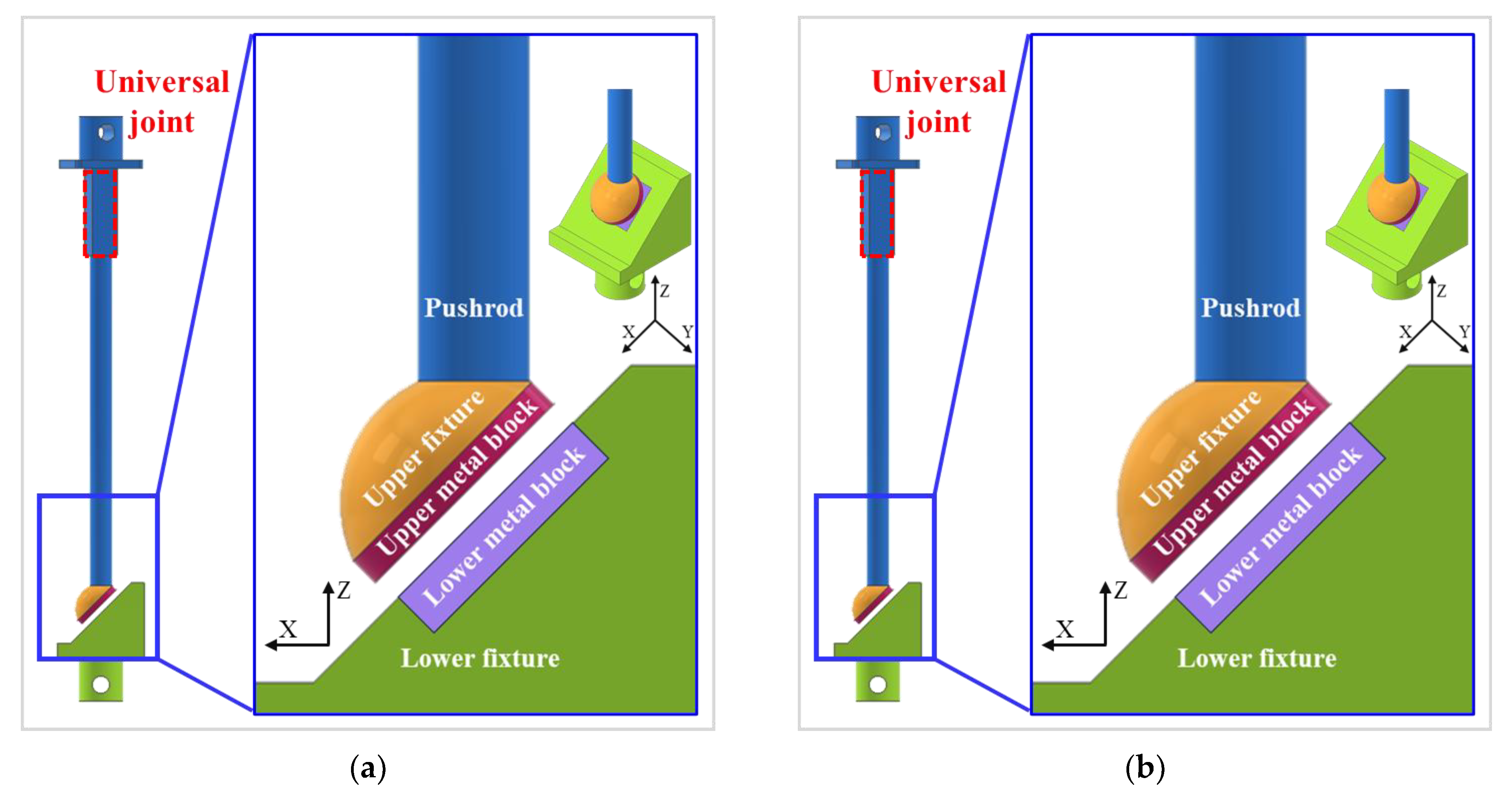
3.2. FEA Model and Results
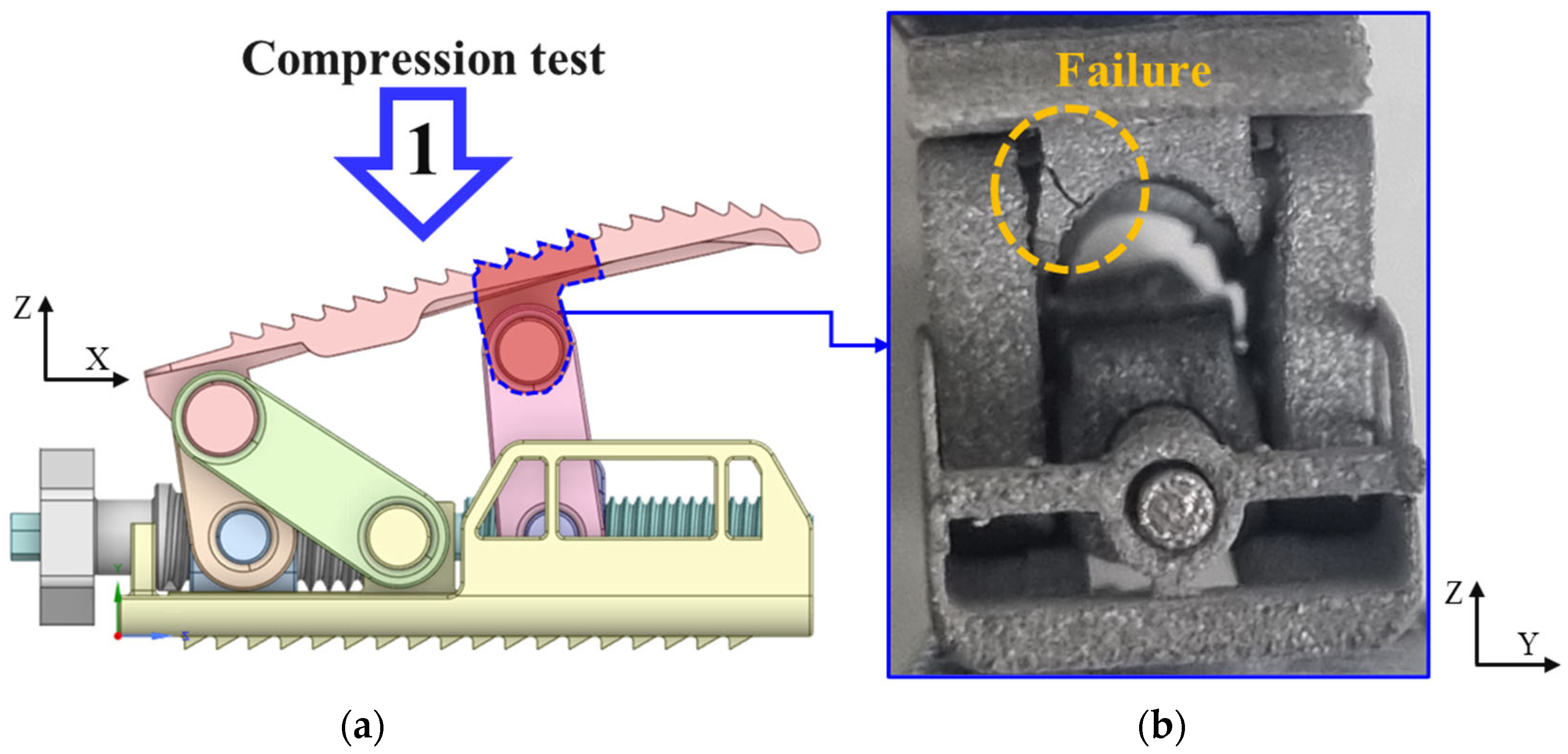
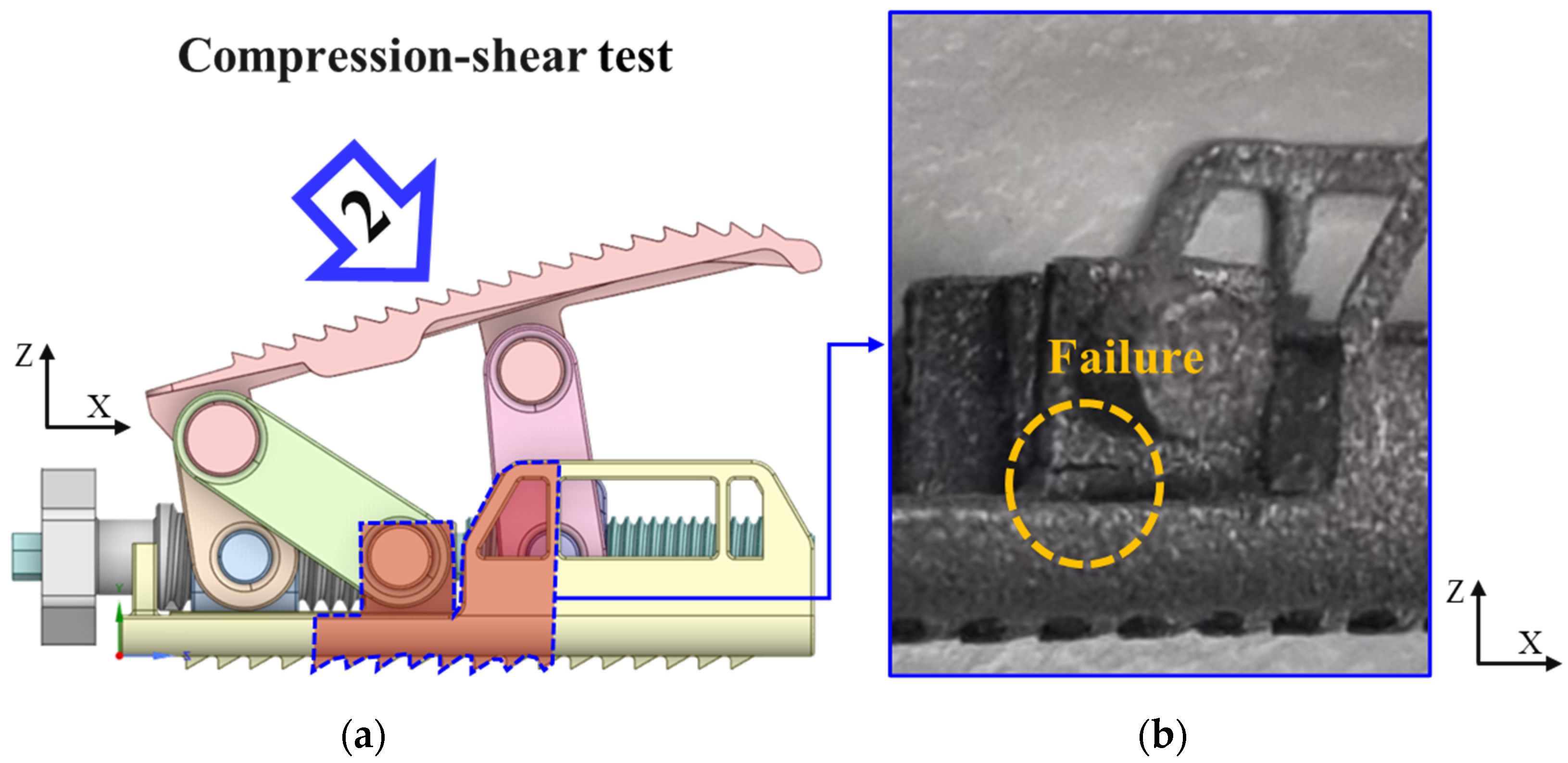
4. Compression and Compression–Shear Test
4.1. Three-Dimensional Printing
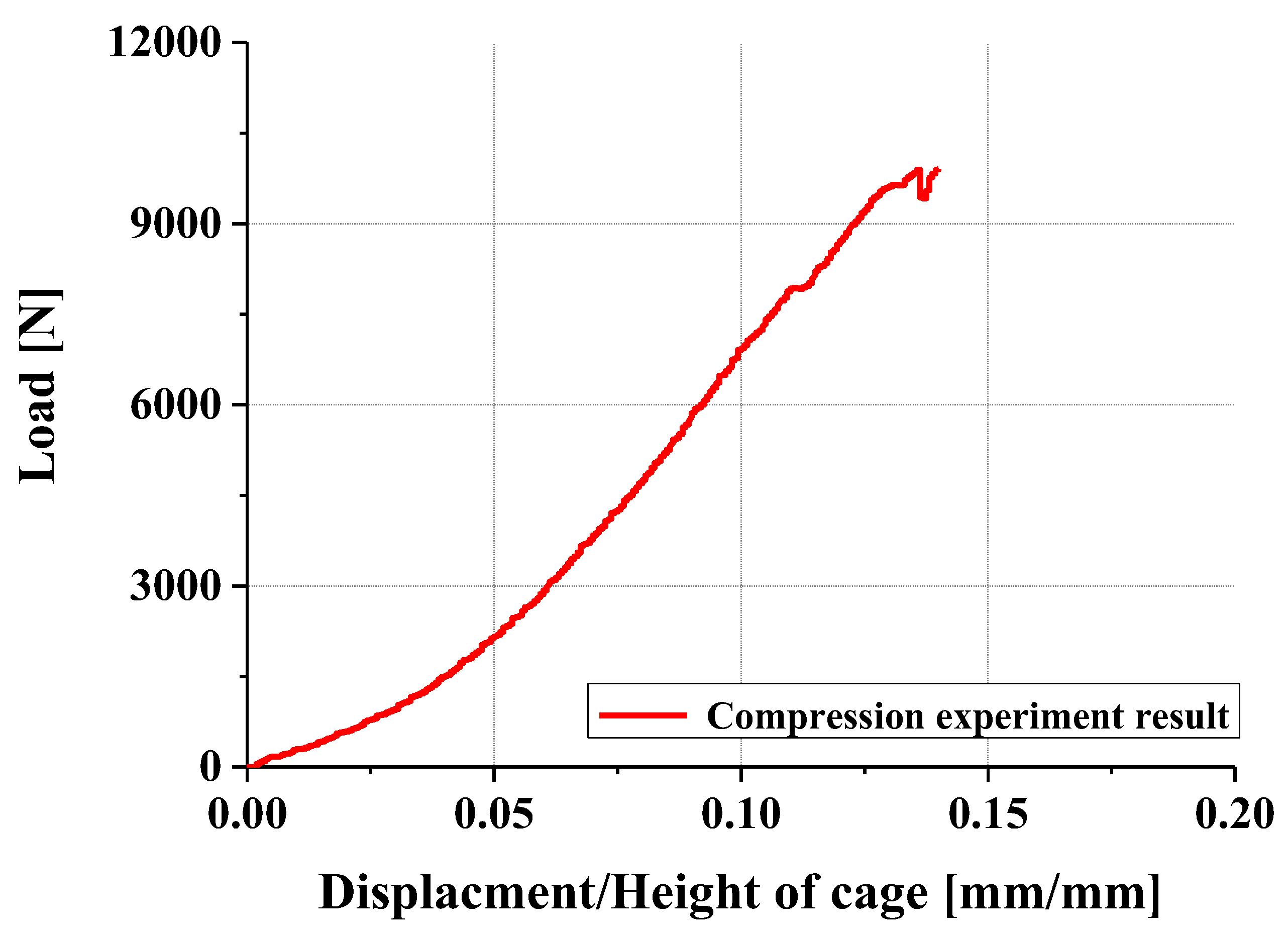
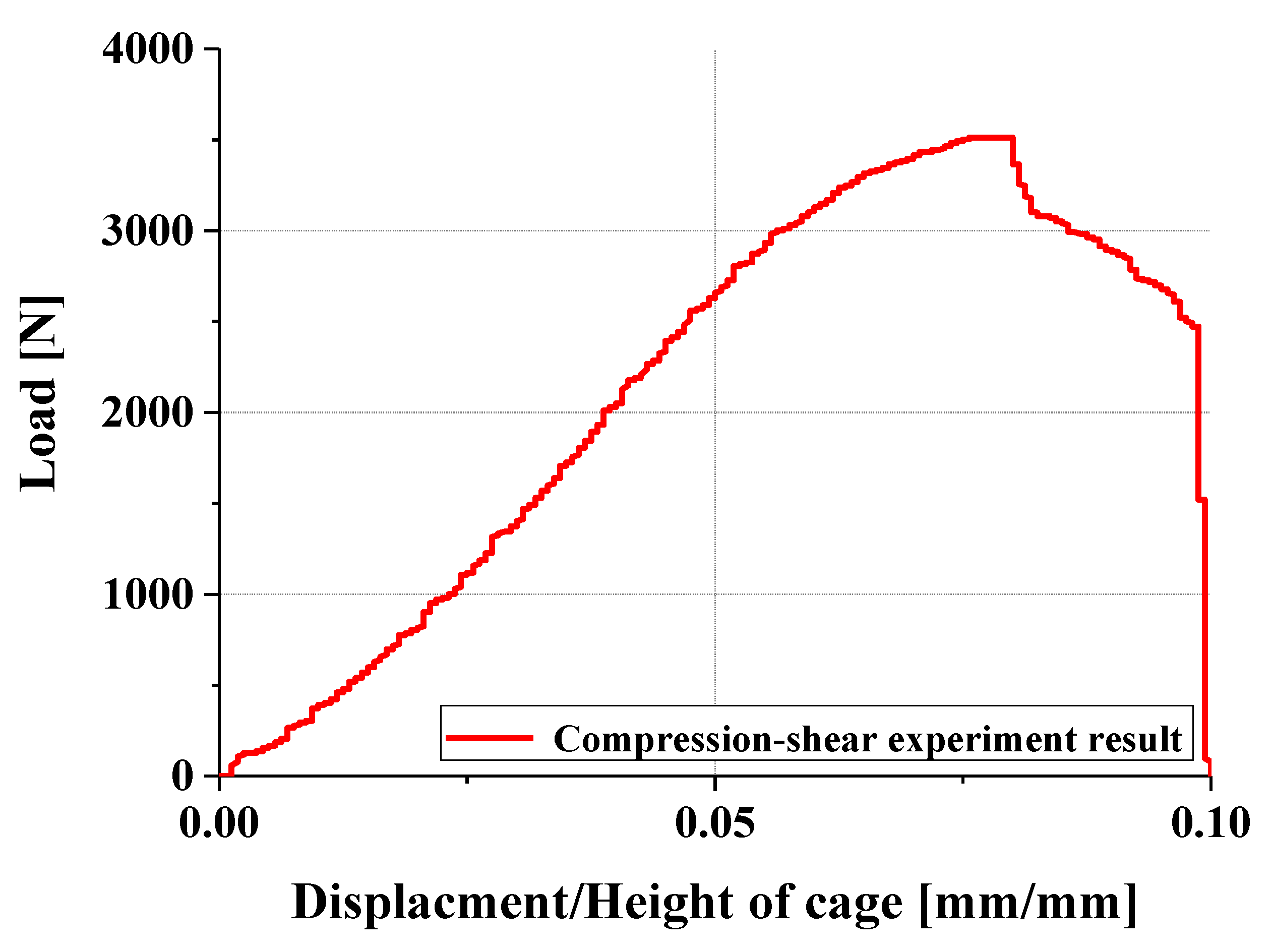
4.2. Compression and Compression–Shear Tests and Results
5. Results and Discussions
6. Conclusions
- The new linkage mechanism applied in this design enables the independent control of height and angle, a functionality that is difficult to achieve with traditional rack-and-pinion or wedge-type designs. Additionally, continuous fine-tuning facilitated by sliders allows surgeons to achieve precise alignment tailored to the anatomical characteristics of individual patients during surgery.
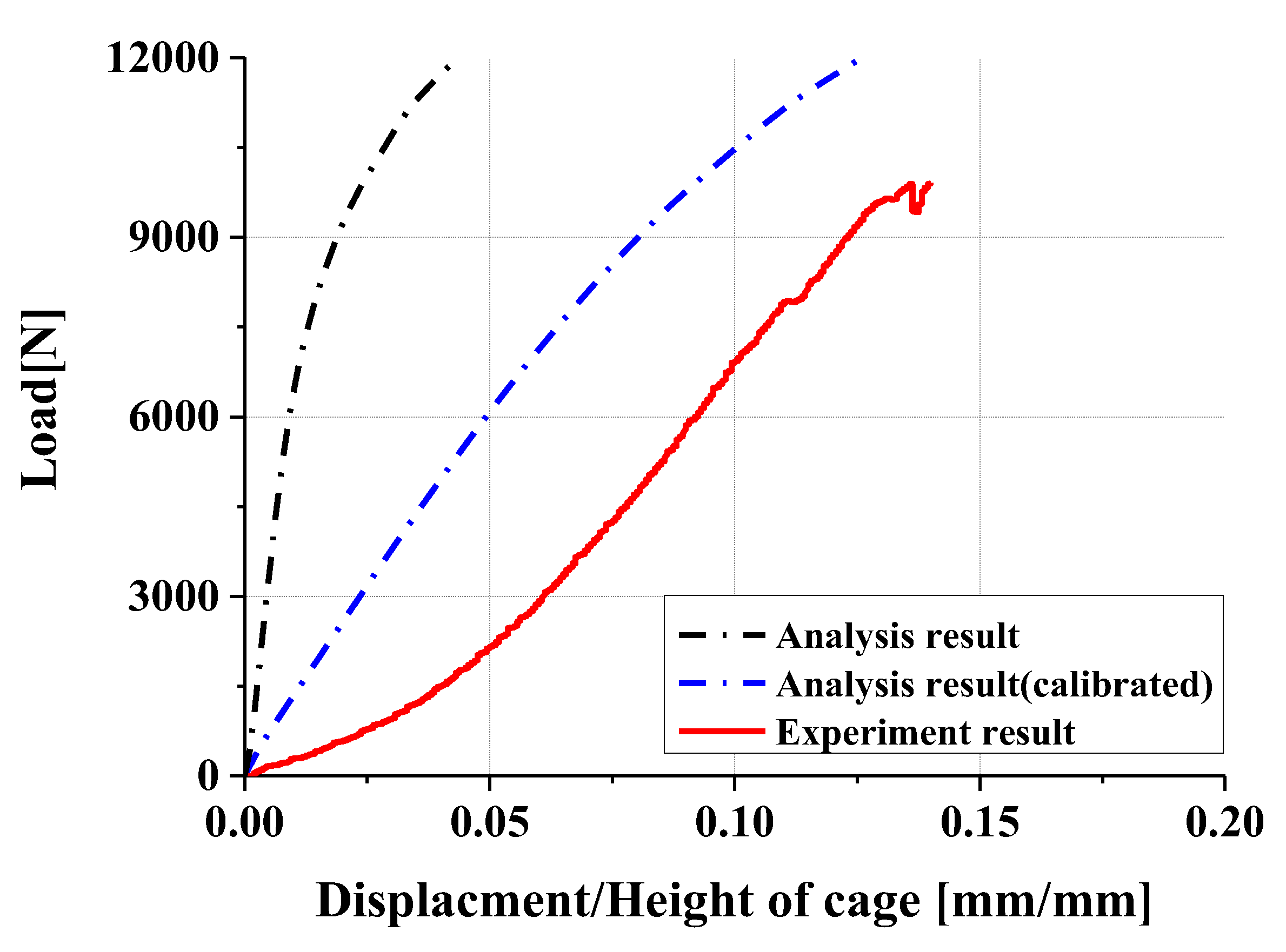
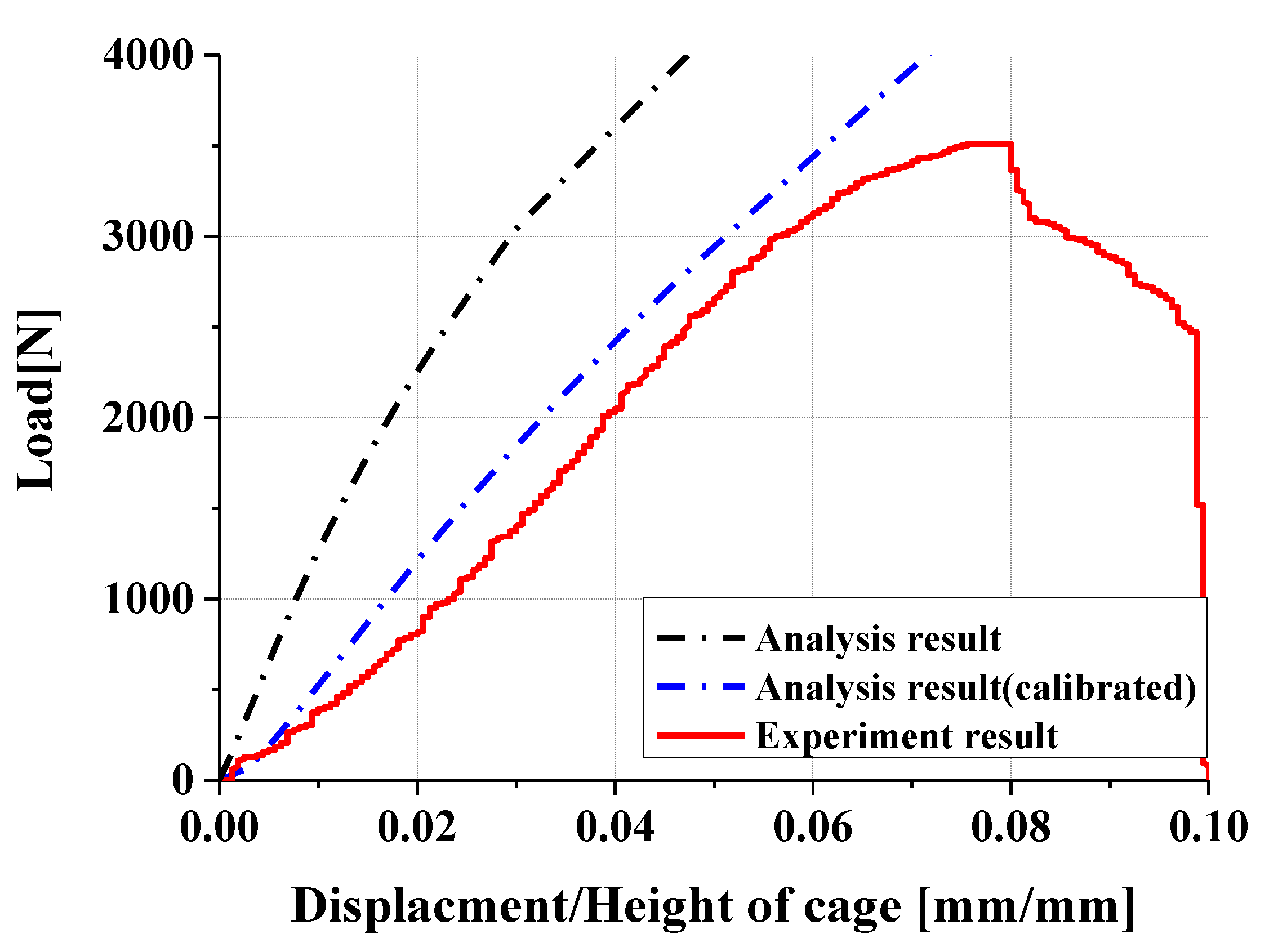
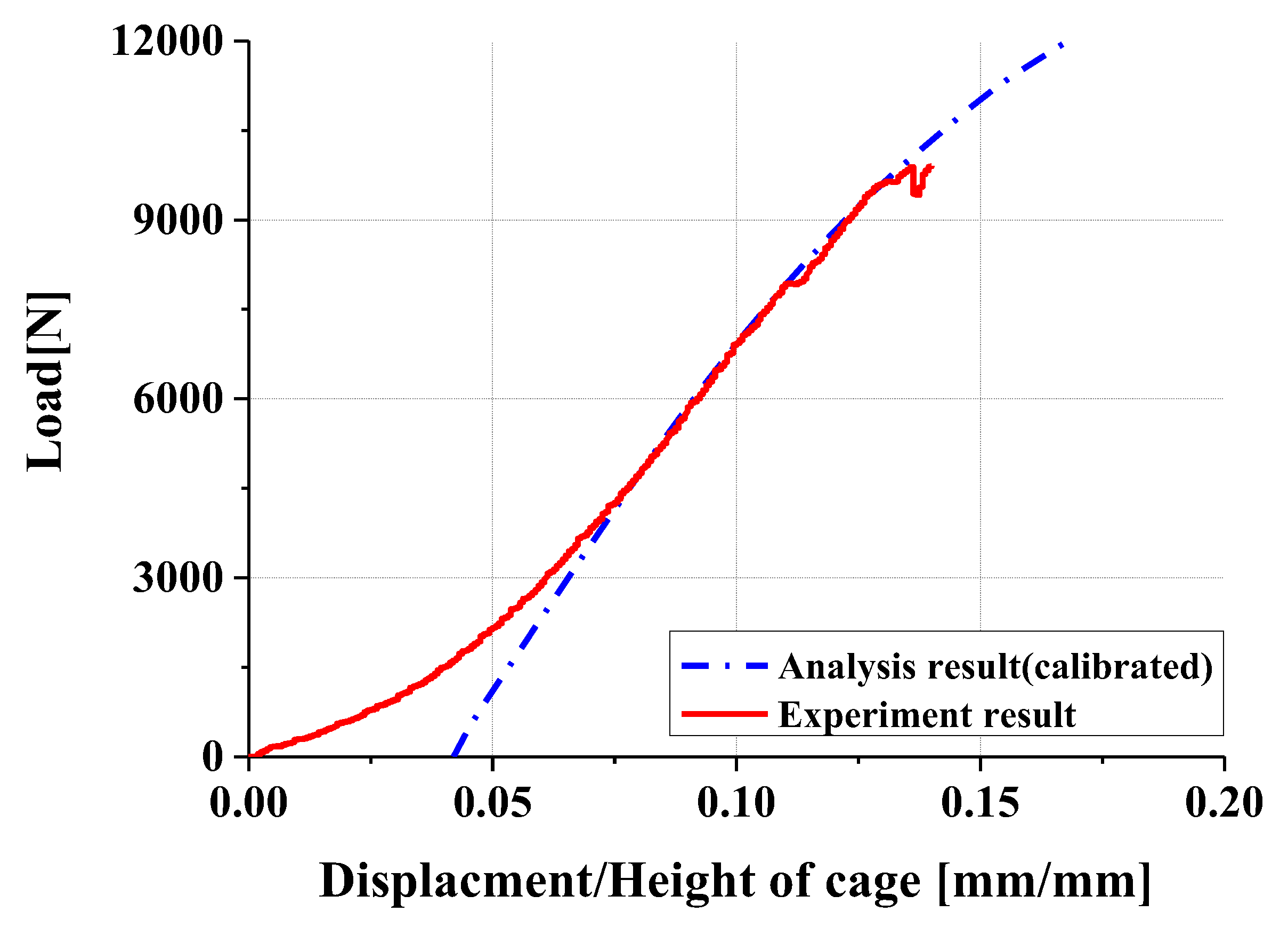
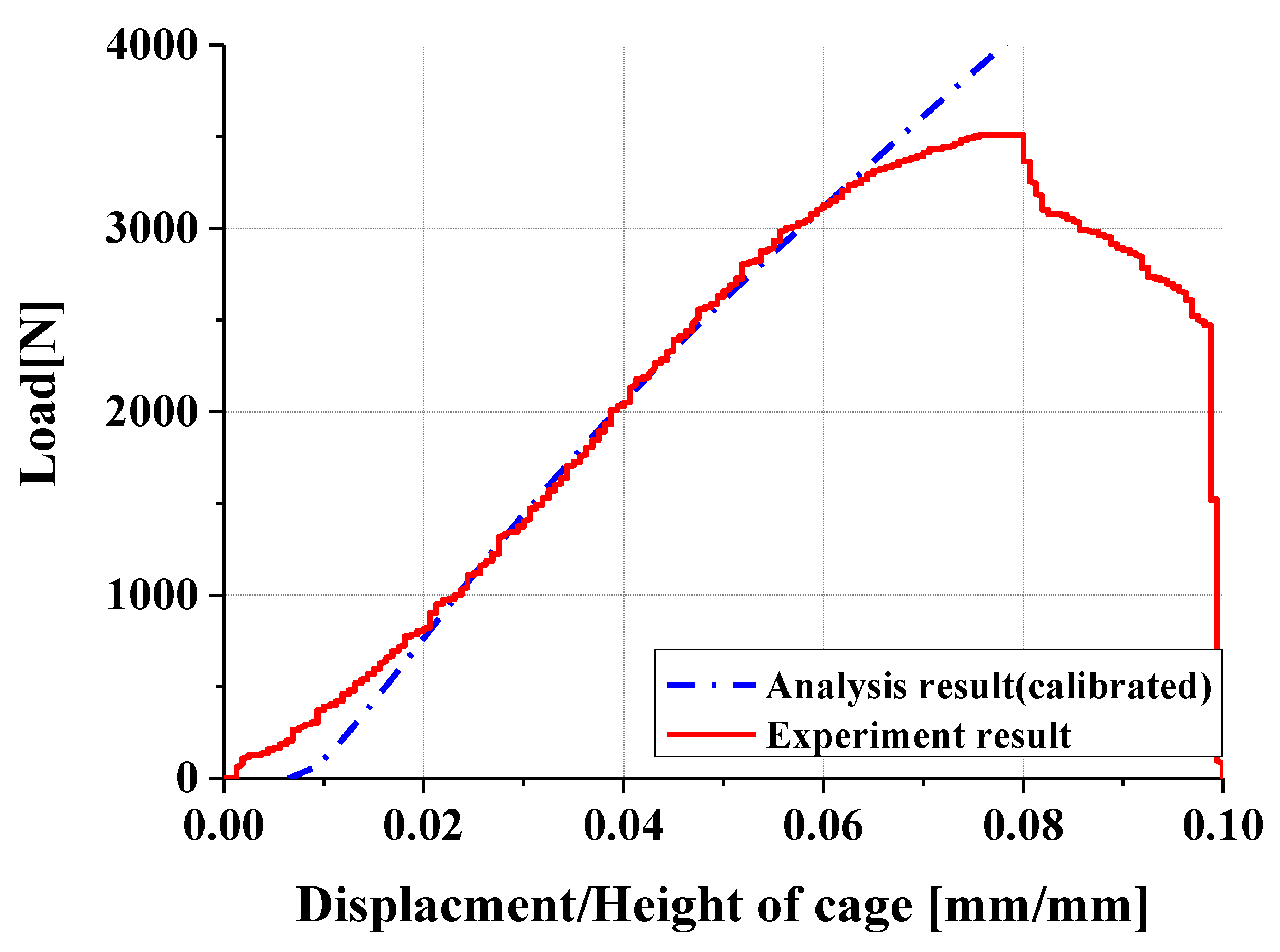
- Experimental results showed trends similar to calibrated FEM analysis, particularly demonstrating a high correlation between two evaluation methods in predicting yield locations at anterior sliding block and lower plate shaft regions. FEM analysis was calibrated considering elastic deformation of testing apparatus, enhancing the reliability of the FEM model.
- In compression and compression–shear tests, the designed spinal cage exhibited an ultimate load exceeding the lower fifth percentile of FDA-approved products, thereby satisfying clinical requirements. These findings confirm that proposed design offers significant improvements over existing products and demonstrates high potential for clinical application as a patient-specific spinal fusion device.
- However, this study has several limitations. Firstly, the evaluation was limited to static load testing, without performing dynamic fatigue testing, leaving durability under prolonged cyclic loading inadequately assessed. Since real clinical environments involve tens of millions of repetitive loads, fatigue testing is necessary to confirm long-term mechanical stability. Moreover, as this research was confined to in vitro testing, clinical trials or animal studies evaluating actual in vivo applicability have not yet been conducted. Future research should include such validation processes to establish clinical efficacy.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roberts, S.; Evans, H.; Trivedi, J.; Menage, J. Histology and pathology of human intervertebral disc. J. Bone Jt. Surg. 2006, 88 (Suppl. S2), 10–14. [Google Scholar]
- Mirza, S.K.; White, A.A. Anatomy of intervertebral disc and pathophysiology of herniated disc disease. J. Clin. Laser Med. Surg. 1995, 13, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, J.D.; Loback, D.; Yong-Hing, K.; Tchang, S. Intervertebral disc herniation. Spine 1992, 17, 570–574. [Google Scholar]
- Deyo, R.A.; Mirza, S.K. Clinical practice. herniated lumbar intervertebral disk. N. Engl. J. Med. 2016, 374, 1763–1772. [Google Scholar] [CrossRef]
- Hasan, M.; Alam, S.; Jahan, S.; Chowdhury, A.A.M.; Junayed, S.A.; Khan, M.R.; Hasan, Z. Outcome of prolapse lumbar intervertebral disc (PLID) surgery: A study at a district-level private hospital in Bangladesh. J. Spine Res. Surg. 2024, 6, 109–113. [Google Scholar] [CrossRef]
- Purushotham, S.; Hodson, N.; Greig, C.; Gardner, A.; Falla, D. Microscopic changes in the multifidus muscle in people with low back pain associated with lumbar disc herniation. Sci. Rep. 2024, 14, 1–13. [Google Scholar] [CrossRef]
- Lee, J.S.; Lee, S.-B.; Kang, K.-Y.; Oh, S.H.; Chae, D.-S. Review of recent treatment strategies for lumbar disc herniation (LDH) focusing on nonsurgical and regenerative therapies. J. Clin. Med. 2025, 14, 1196. [Google Scholar] [CrossRef]
- Alam, K.Q.; Sahu, N.; Gulia, A.; Kumar, R.; Kohli, G.; Kumar, S. Assessment of pain and neurological involvement in patients with lumbar intervertebral disc prolapse who underwent fenestration discectomy. Int. J. Life Sci. 2024, 10, 6481–6488. [Google Scholar] [CrossRef]
- Gopi, J.; Mammu, S.; Jayan, A.; Jamshad, O.P.; Fijad, N.R.; Vaisakh, V.K. Transformative spinal surgery: Lateral position unilateral biportal endoscopic discectomy. J. Orthop. Case Rep. 2025, 15, 67–72. [Google Scholar] [CrossRef]
- Blumenthal, S.L.; Ohnmeiss, D.D. Intervertebral cages for degenerative spinal diseases. Spine J. 2003, 3, 301–309. [Google Scholar] [CrossRef]
- Laubach, M.; Kobbe, P.; Hutmacher, D.W. Biodegradable interbody cages for lumbar spine fusion: Current concepts and future directions. Biomaterials 2022, 288, 121699. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Liu, S.-S.; Zou, D.; Ni, R.-H.; Wei, C.-B.; Wang, H.; Li, W.-S. A novel porous interbody fusion cage modified by microarc oxidation and hydrothermal treatment technology accelerate osseointegration and spinal fusion in sheep. RSC Adv. 2024, 14, 31966–31978. [Google Scholar] [CrossRef] [PubMed]
- Delfini, R.; Landi, A.; Mancarella, C.; Gregori, F. (Eds.) Modern Thoraco-Lumbar Implants for Spinal Fusion; Springer International Publishing: Vienna, Austria, 2018. [Google Scholar]
- Dave, B.R.; Marathe, N.; Mayi, S.; Degulmadi, D.; Rai, R.R.; Patil, S.; Jadav, K.; Bali, S.K.; Kumar, A.; Meena, U.; et al. Does conventional open TLIF cause more muscle injury when compared to minimally invasive TLIF?—A prospective single center analysis. Glob. Spine J. 2024, 14, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, M.C.; Jacob, K.C.; Patel, M.R.; Pawlowski, H.; Vanjani, N.N.; Singh, K. History and evolution of the minimally invasive transforaminal lumbar interbody fusion. Neurospine 2022, 19, 479–491. [Google Scholar] [CrossRef]
- Chang, S.Y.; Kang, D.-H.; Cho, S.K. Innovative developments in lumbar interbody cage materials and design: A comprehensive narrative review. Asian Spine J. 2023, 18, 444–457. [Google Scholar] [CrossRef]
- Lewandrowski, K.-U.; Vira, S.; Elfar, J.C.; Lorio, M.P. Advancements in custom 3D-printed titanium interbody spinal fusion cages and their relevance in personalized spine care. J. Pers. Med. 2024, 14, 809. [Google Scholar] [CrossRef]
- Iqbal, J.; Zafar, Z.; Skandalakis, G.; Kuruba, V.; Madan, S.; Kazim, S.F.; Bowers, C.A. Recent advances of 3D-printing in spine surgery. Surg. Neurol. Int. 2024, 15, 297. [Google Scholar] [CrossRef]
- Alimi, M.; Shin, B.; Macielak, M.; Hofstetter, C.P.; Njoku, I.; Tsiouris, A.J.; Elowitz, E.; Härtl, R. Expandable polyaryl-ether-ether-ketone spacers for interbody distraction in the lumbar spine. Glob. Spine J. 2015, 5, 169–178. [Google Scholar] [CrossRef]
- Kim, J.W.; Park, H.C.; Yoon, S.H.; Oh, S.H.; Roh, S.W.; Rim, D.C.; Kim, T.S. A multi-center clinical study of posterior lumbar interbody fusion with the expandable stand-alone cage (Tyche® Cage) for degenerative lumbar spinal disorders. J. Korean Neurosurg. Soc. 2007, 42, 251–257. [Google Scholar] [CrossRef]
- Jitpakdee, K.; Sommer, F.; Gouveia, E.; Mykolajtchuk, C.; Boadi, B.; Berger, J.; Hussain, I.; Härtl, R. Expandable cages that expand both height and lordosis provide improved immediate effect on sagittal alignment and short-term clinical outcomes following minimally invasive transforaminal lumbar interbody fusion (MIS TLIF). J. Spine Surg. 2024, 10, 55–67. [Google Scholar] [CrossRef]
- Moon, E.-S.; Kim, N.-H.; Park, J.-O.; Park, S.-Y.; Kim, H.-J.; Choi, W.-J.; Han, C.-W.; Kim, H.-S.; Lee, H.-M.; Moon, S.-H. Radiographic morphometry of lumbar intervertebral disc space in normal Korean. J. Korean Soc. Spine Surg. 2007, 14, 129–136. [Google Scholar] [CrossRef]
- Kim, D.-S.; Kim, Y.-M.; Choi, E.-S.; Shon, H.-C.; Park, K.-J.; Park, G.-K.; Lee, E.-M.; Cui, H.-S. Shape and motion of each lumbar segment in normal Korean Adults. J. Korean Orthop. Assoc. 2008, 43, 595–600. [Google Scholar] [CrossRef]
- Park, D.Y.; Heo, D.H. The use of dual direction expandable titanium cage with biportal endoscopic transforaminal lumbar interbody fusion: A technical consideration with preliminary results. Neurospine 2023, 20, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Emstad, E.; Del Monaco, D.C.; Fielding, L.C.; Block, J.E. VariLift® interbody fusion system: Expandable, standalone interbody fusion. Med. Devices Evid. Res. 2015, 8, 219–230. [Google Scholar]
- Neely, W.F.; Fichtel, F.; Del Monaco, D.C.; Block, J.E. Treatment of symptomatic lumbar disc degeneration with VariLift-L interbody fusion system: Retro-spective review of 470 cases. Int. J. Spine Surgery 2016, 10, 15. [Google Scholar] [CrossRef]
- Orr, D.J.; Payne, C.; Jones, H.; Anderson, J.; Sperry, A.; Sargent, B.; Frankel, B.M.; Howell, L.L.; Bowden, A.E. Leveraging compliance to design a minimally invasive, expandable interbody cage capable of customized anatomical fit for spinal fusion surgery. J. Med. Devices 2024, 18, 1–30. [Google Scholar] [CrossRef]
- ASTM F2077-20; Standard Test Methods for Intervertebral Body Fusion Devices. ASTM International: West Conshohocken, PA, USA, 2020.
- Warburton, A.; Girdler, S.J.; Mikhail, C.M.; Ahn, A.; Cho, S.K. Biomaterials in spinal implants: A review. Neurospine 2019, 17, 101–110. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- ISO 23089–2:2021; Implants for Surgery—Preclinical Mechanical Assessment of Spinal Implants and Particular Requirements—Part 2: Spinal in-Tervertebral Body Fusion Devices. International Organization for Standardization: Geneva, Switzerland, 2021.
| Test | Parameter | Value |
|---|---|---|
| Static axial compression | Stiffness (N/mm) | 5914 |
| Yield load (N) | 6371 | |
| Ultimate load (N) | 6989 | |
| Static compression–shear | Stiffness (N/mm) | 1435 |
| Yield load (N) | 1996 | |
| Ultimate load (N) | 2147 |
| Model | Height Adjustable Range | Angle Adjustable Range | Independent Adjustment | Continuous Adjustment |
|---|---|---|---|---|
| Our study | 8.4 mm | 12.3° | Possible | Possible |
| Tyche [20] | 2 mm | 4° | Impossible | Impossible |
| Rise [21] | 7 mm | 0° | Impossible | Possible |
| Sable [21] | 8 mm | 22° | Impossible | Possible |
| Orr et al. [27] | 4 mm | 10° | Impossible | Impossible |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, C.; Trong Khanh Dat, T.; Park, S.-J.; Chae, D.-S.; Choi, S.H.; Yoon, J. Novel Design of Expandable Spinal Cage for Efficient Lumbar Spine Fusion Operation. Appl. Sci. 2025, 15, 6323. https://doi.org/10.3390/app15116323
Park C, Trong Khanh Dat T, Park S-J, Chae D-S, Choi SH, Yoon J. Novel Design of Expandable Spinal Cage for Efficient Lumbar Spine Fusion Operation. Applied Sciences. 2025; 15(11):6323. https://doi.org/10.3390/app15116323
Chicago/Turabian StylePark, Chanwoo, Than Trong Khanh Dat, Sung-Jun Park, Dong-Sik Chae, Sung Hoon Choi, and Jonghun Yoon. 2025. "Novel Design of Expandable Spinal Cage for Efficient Lumbar Spine Fusion Operation" Applied Sciences 15, no. 11: 6323. https://doi.org/10.3390/app15116323
APA StylePark, C., Trong Khanh Dat, T., Park, S.-J., Chae, D.-S., Choi, S. H., & Yoon, J. (2025). Novel Design of Expandable Spinal Cage for Efficient Lumbar Spine Fusion Operation. Applied Sciences, 15(11), 6323. https://doi.org/10.3390/app15116323







