Comparative Assessment of an IMU-Based Wearable Device and a Marker-Based Optoelectronic System in Trunk Motion Analysis: A Cross-Sectional Investigation
Abstract
1. Introduction
2. Materials and Methods
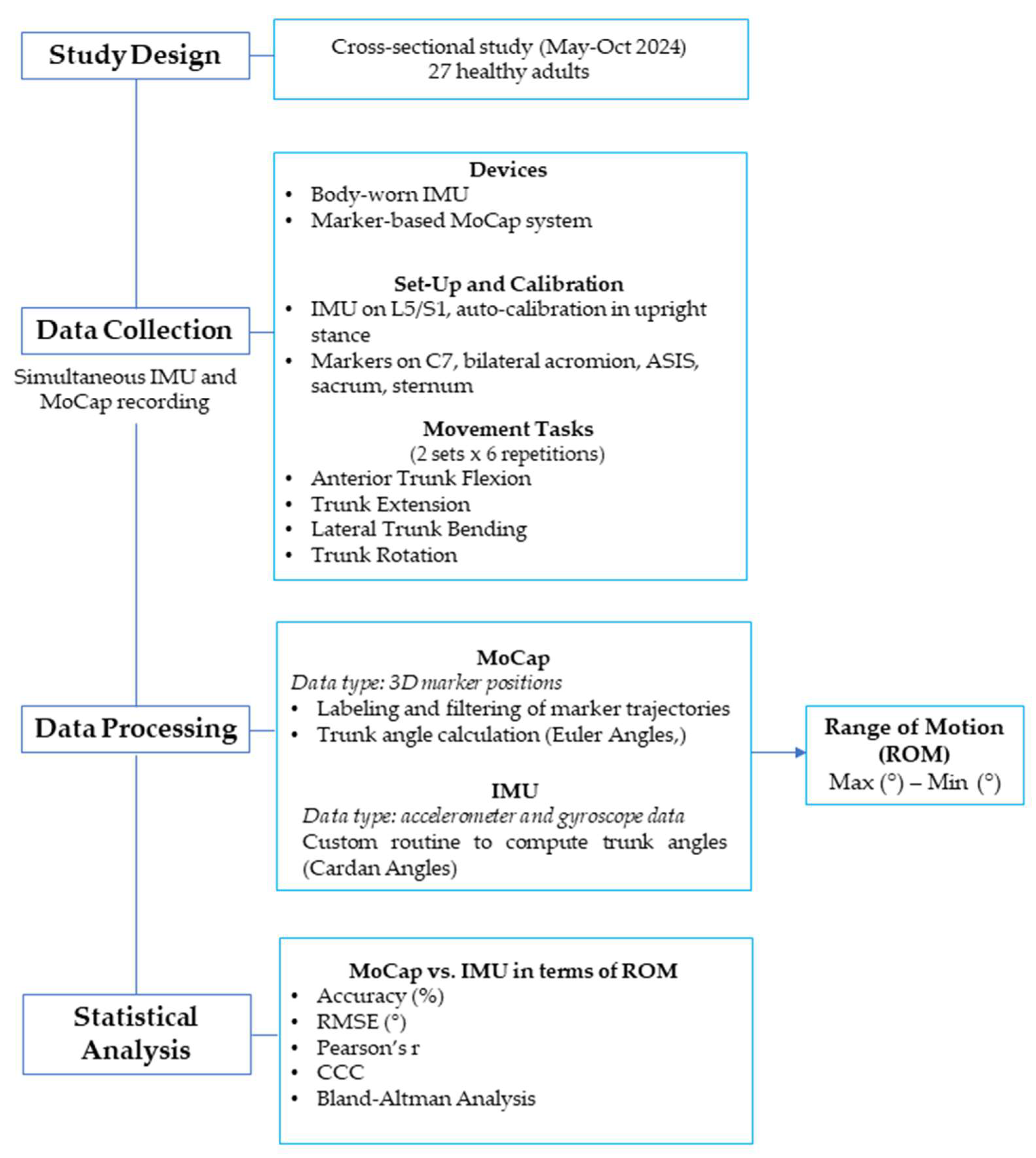
2.1. Study Design
2.2. Participants
2.3. Sample Size Calculation
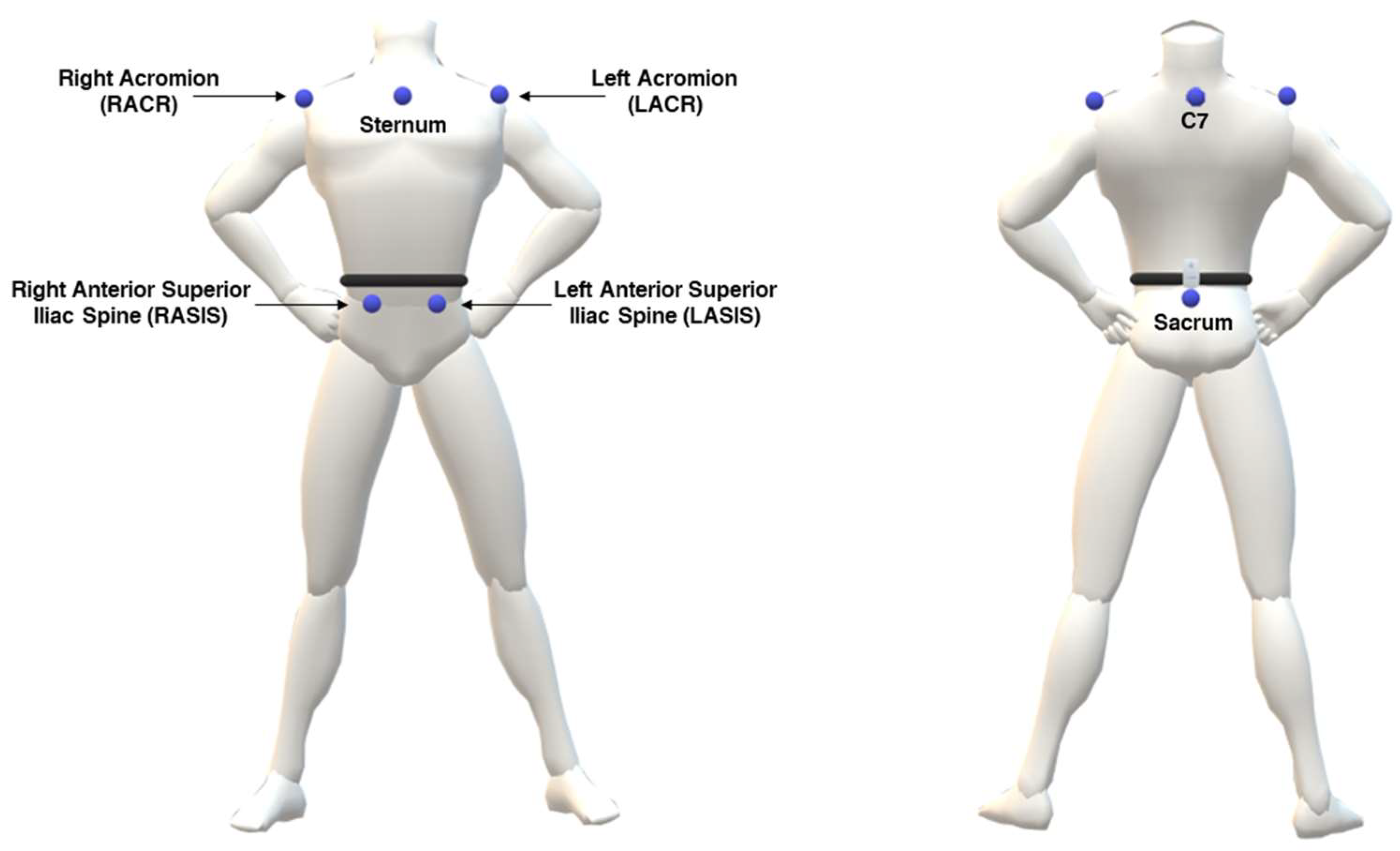
2.4. MoCap System
2.5. IMU-Based System
2.6. Testing Procedures
- Trunk flexion (Figure 2a): participants bent forward at the waist level while maintaining a neutral spine, aiming to reach toward the floor without knee flexion;
- Trunk extension (Figure 2b): participants extended the trunk backward while ensuring hip stability;
- Lateral bending toward right/left (Figure 2c): participants performed a lateral bending movement at the waist, lowering one arm toward the corresponding leg while maintaining pelvic stability;
- Trunk rotation toward right/left (Figure 2d): participants rotated the upper body to one side while keeping the hips oriented forward.
2.7. Data Analysis and Processing
2.8. Statistical Analysis
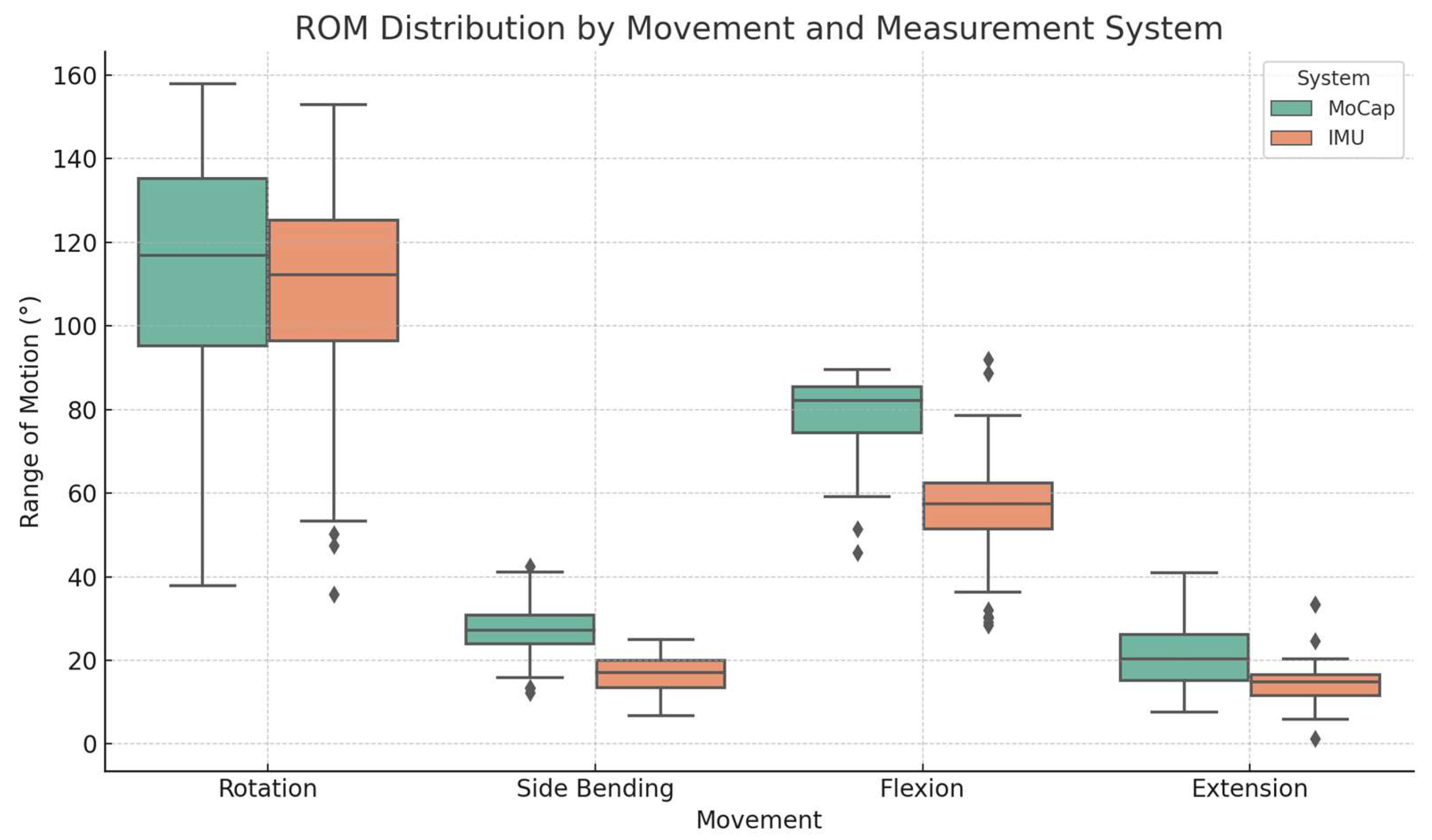
3. Results
3.1. Accuracy and RMSE
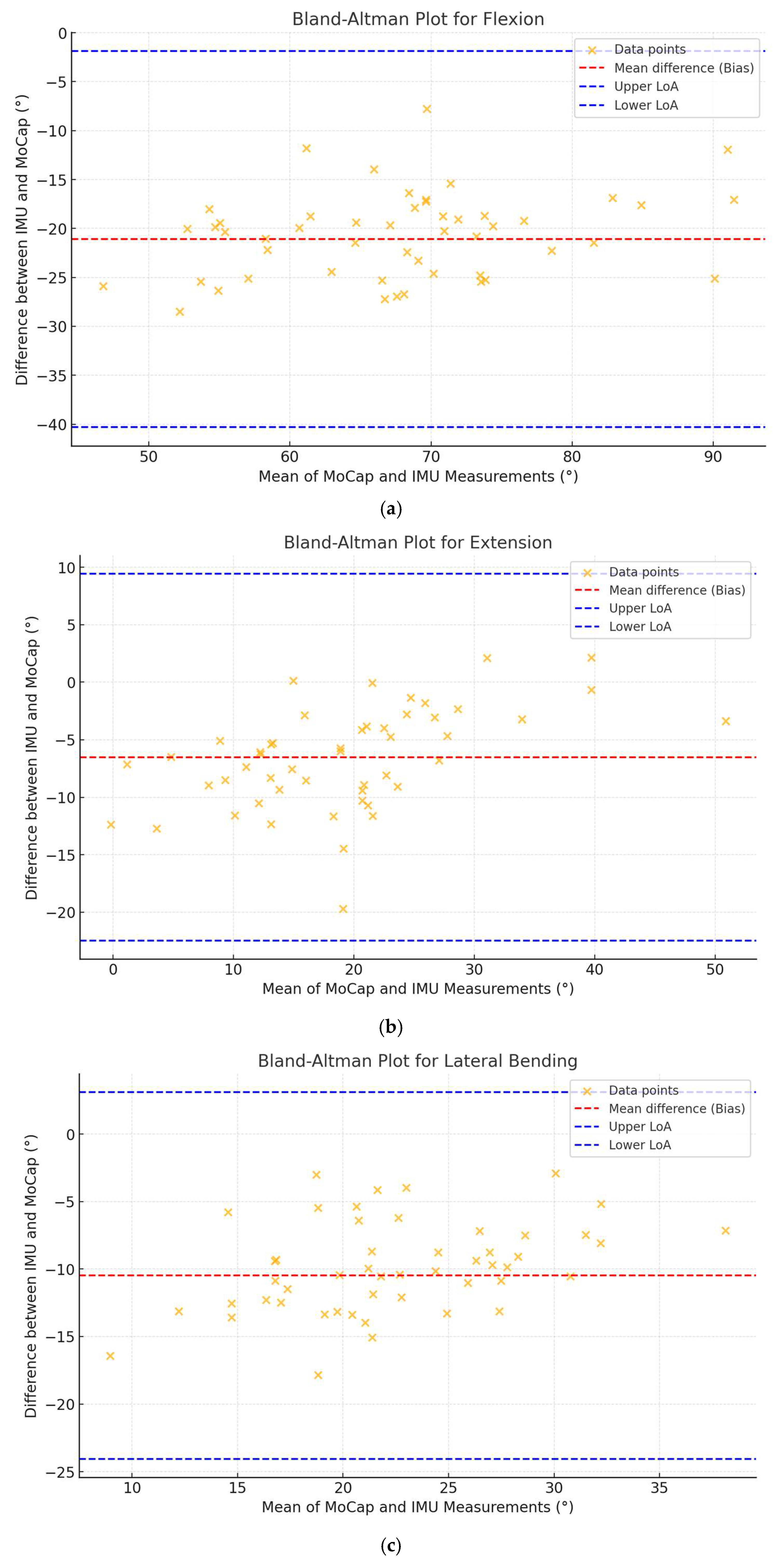
3.2. Correlation and Agreement Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ROM | Range of Motion |
| MoCap | Motion Capture |
| IMU | Inertial Measurement Unit |
| LBP | Low Back Pain |
References
- Benedetti, M.G.; Negrini, S. Instrumental motion analysis: From the research laboratory to the rehabilitation clinic. Eur. J. Phys. Rehabil. Med. 2016, 52, 557–559. [Google Scholar] [PubMed]
- Quinn, L.; Riley, N.; Tyrell, C.M.; Judd, D.L.; Gill-Body, K.M.; Hedman, L.D.; Packel, A.; Brown, D.A.; Nabar, N.; Scheets, P. A Framework for Movement Analysis of Tasks: Recommendations From the Academy of Neurologic Physical Therapy’s Movement System Task Force. Phys. Ther. 2021, 101, pzab154. [Google Scholar] [CrossRef]
- Martin, C.; Phillips, B.A.; Kilpatrick, T.J.; Butzkueven, H.; Tubridy, N.; McDonald, E.; Galea, M.P. Gait and balance impairment in early multiple sclerosis in the absence of clinical disability. Mult. Scler. J. 2006, 12, 620–628. [Google Scholar] [CrossRef]
- Stolze, H.; Klebe, S.; Baecker, C.; Zechlin, C.; Friege, L.; Pohle, S.; Deuschl, G. Prevalence of gait disorders in hospitalized neurological patients. Mov. Disord. 2004, 20, 89–94. [Google Scholar] [CrossRef]
- Dal Farra, F.; Arippa, F.; Carta, G.; Segreto, M.; Porcu, E.; Monticone, M. Sport and non-specific low back pain in athletes: A scoping review. BMC Sports Sci. Med. Rehabil. 2022, 14, 216. [Google Scholar] [CrossRef] [PubMed]
- Reis, F.J.J.d.; Macedo, A.R.d. Influence of hamstring tightness in pelvic, lumbar and trunk range of motion in low back pain and asymptomatic volunteers during forward bending. Asian Spine J. 2015, 9, 535. [Google Scholar] [CrossRef]
- Dal Farra, F.; Arippa, F.; Arru, M.; Cocco, M.; Porcu, E.; Tramontano, M.; Monticone, M. Effects of exercise on balance in patients with non-specific low back pain: A systematic review and meta-analysis. Eur. J. Phys. Rehabil. Med. 2022, 58, 423–434. [Google Scholar] [CrossRef]
- VanDijk, M.; Smorenburg, N.; Visser, B.; Heerkens, Y.F.; Der Sanden, M.W.G.N.-V. How clinicians analyze movement quality in patients with non-specific low back pain: A cross-sectional survey study with Dutch allied health care professionals. BMC Musculoskelet. Disord. 2017, 18, 288. [Google Scholar]
- Schlager, A.; Ahlqvist, K.; Rasmussen-Barr, E.; Bjelland, E.K.; Pingel, R.; Olsson, C.; Nilsson-Wikmar, L.; Kristiansson, P. Inter- and intra-rater reliability for measurement of range of motion in joints included in three hypermobility assessment methods. BMC Musculoskelet. Disord. 2018, 19, 376. [Google Scholar] [CrossRef]
- Cano-de-la-Cuerda, R.; Vela, L.; Moreno-Verdú, M.; Ferreira-Sánchez, M.d.R.; Macías-Macías, Y.; Miangolarra-Page, J.C. Trunk range of motion is related to axial rigidity, functional mobility and quality of life in parkinson’s disease: An exploratory study. Sensors 2020, 20, 2482. [Google Scholar] [CrossRef]
- Shamsi, M.; Mirzaei, M.; Khabiri, S. Universal goniometer and electro-goniometer intra-examiner reliability in measuring the knee range of motion during active knee extension test in patients with chronic low back pain with short hamstring muscle. BMC Sports Sci. Med. Rehabil. 2019, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, E. Test-retest reliability of an active range of motion test for the shoulder and hip joints by unskilled examiners using a manual goniometer. J. Phys. Ther. Sci. 2016, 28, 722–724. [Google Scholar] [CrossRef]
- McGinley, J.L.; Baker, R.; Wolfe, R.; Morris, M.E. The reliability of three-dimensional kinematic gait measurements: A systematic review. Gait Posture 2009, 29, 360–369. [Google Scholar] [CrossRef]
- Alarcón-Aldana, A.C.; Callejas-Cuervo, M.; Bó, A.P.L. Upper limb physical rehabilitation using serious videogames and motion capture systems: A systematic review. Sensors 2020, 20, 5989. [Google Scholar] [CrossRef]
- Moro, M.; Marchesi, G.; Hesse, F.; Odone, F.; Casadio, M. Markerless vs. marker-based gait analysis: A proof of concept study. Sensors 2022, 22, 2011. [Google Scholar] [CrossRef] [PubMed]
- Poitras, I.; Dupuis, F.; Bielmann, M.; Campeau-Lecours, A.; Mercier, C.; Bouyer, L.J.; Roy, J. Validity and reliability of wearable sensors for joint angle estimation: A systematic review. Sensors 2019, 19, 1555. [Google Scholar] [CrossRef] [PubMed]
- Fong, D.T.; Chan, Y.M. The use of wearable inertial motion sensors in human lower limb biomechanics studies: A systematic review. Sensors 2010, 10, 11556–11565. [Google Scholar] [CrossRef]
- Cerfoglio, S.; Capodaglio, P.; Rossi, P.; Conforti, I.; D’Angeli, V.; Milani, E.; Galli, M.; Cimolin, V. Evaluation of upper body and lower limbs kinematics through an imu-based medical system: A comparative study with the optoelectronic system. Sensors 2023, 23, 6156. [Google Scholar] [CrossRef]
- Tadano, S.; Takeda, R.; Miyagawa, H. Three dimensional gait analysis using wearable acceleration and gyro sensors based on quaternion calculations. Sensors 2013, 13, 9321–9343. [Google Scholar] [CrossRef]
- Li, H.; Khoo, S.; Yap, H.J. Differences in motion accuracy of baduanjin between novice and senior students on inertial sensor measurement systems. Sensors 2020, 20, 6258. [Google Scholar] [CrossRef]
- Manupibul, U.; Tanthuwapathom, R.; Jarumethitanont, W.; Kaimuk, P.; Limroongreungrat, W.; Charoensuk, W. Integration of force and imu sensors for developing low-cost portable gait measurement system in lower extremities. Sci. Rep. 2023, 13, 10653. [Google Scholar] [CrossRef] [PubMed]
- Schall, M.C.; Fethke, N.B.; Chen, H.; Oyama, S.; Douphrate, D.I. Accuracy and repeatability of an inertial measurement unit system for field-based occupational studies. Ergonomics 2015, 59, 591–602. [Google Scholar] [CrossRef]
- Zhang, H.; Tao, Y.; Shi, K.; Li, J.; Shi, J.; Xu, S.; Guo, Y. Multi-Object Recognition and Motion Detection Based on Flexible Pressure Sensor Array and Deep Learning. Appl. Sci. 2025, 15, 3302. [Google Scholar] [CrossRef]
- Zhu, P.; Niu, M.; Liang, S.; Yang, W.; Zhang, Y.; Chen, K.; Pan, Z.; Mao, Y. Non-hand-worn, load-free VR hand rehabilitation system assisted by deep learning based on ionic hydrogel. Nano Res. 2025, 18, 94907301. [Google Scholar] [CrossRef]
- Lu, L.; Hu, G.; Liu, J.; Yang, B. 5G NB-IoT System Integrated with High-Performance Fiber Sensor Inspired by Cirrus and Spider Structures. Adv. Sci. (Weinh. Baden-Wurtt. Ger.) 2024, 11, e2309894. [Google Scholar] [CrossRef] [PubMed]
- Liengswangwong, W.; Lertviboonluk, N.; Yuksen, C.; Jamkrajang, P.; Limroongreungrat, W.; Mongkolpichayaruk, A.; Jenpanitpong, C.; Watcharakitpaisan, S.; Palee, C.; Thaipasong, S.; et al. Validity of inertial measurement unit (imu sensor) for measurement of cervical spine motion, compared with eight optoelectronic 3d cameras under spinal immobilization devices. Med. Devices Evid. Res. 2024, 17, 261–269. [Google Scholar] [CrossRef]
- Ali, F.; Hogen, C.A.; Miller, E.J.; Kaufman, K.R. Validation of Pelvis and Trunk Range of Motion as Assessed Using Inertial Measurement Units. Bioengineering 2024, 11, 659. [Google Scholar] [CrossRef]
- Parrington, L.; Jehu, D.A.; Fino, P.C.; Pearson, S.; El-Gohary, M.; King, L.A. Validation of an inertial sensor algorithm to quantify head and trunk movement in healthy young adults and individuals with mild traumatic brain injury. Sensors 2018, 18, 4501. [Google Scholar] [CrossRef]
- Abdollahi, M.; Ashouri, S.; Abedi, M.; Azadeh-Fard, N.; Parnianpour, M.; Khalaf, K.; Rashedi, E. Using a motion sensor to categorize nonspecific low back pain patients: A machine learning approach. Sensors 2020, 20, 3600. [Google Scholar] [CrossRef]
- Bossuyt, P.M.; Reitsma, J.B.; Bruns, D.E.; Gatsonis, C.A.; Glasziou, P.P.; Irwig, L.; Lijmer, J.G.; Moher, D.; Rennie, D.; de Vet, H.C.W.; et al. STARD 2015: An Updated List of Essential Items for Reporting Diagnostic Accuracy Studies. Radiology 2015, 277, 826–832. [Google Scholar] [CrossRef]
- Davis, R.B.; Õunpuu, S.; Tybursky, D.; Gage, J.R. A gait analysis data collection and reduction technique. Hum. Mov. Sci. 1991, 10, 575–587. [Google Scholar] [CrossRef]
- Cole, G.K.; Nigg, B.M.; Ronsky, J.L.; Yeadon, M.R. Application of the joint coordinate system to three-dimensional joint attitude and movement representation: A standardization proposal. J. Biomech. Eng. 1993, 115, 344–349. [Google Scholar] [CrossRef]
- Akoglu, H. User’s guide to correlation coefficients. Turk. J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef]
- Hiriote, S.; Chinchilli, V.M. Matrix-based concordance correlation coefficient for repeated measures. Biometrics 2011, 67, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Zaki, R.A.; Bulgiba, A.; Ismail, R.; Ismail, N.A. Statistical methods used to test for agreement of medical instruments measuring continuous variables in method comparison studies: A systematic review. PLoS ONE 2012, 7, e37908. [Google Scholar] [CrossRef] [PubMed]
- Al-Amri, M.; Nicholas, K.; Button, K.; Sparkes, V.; Sheeran, L.; Davies, J. Inertial measurement units for clinical movement analysis: Reliability and concurrent validity. Sensors 2018, 18, 719. [Google Scholar] [CrossRef] [PubMed]
- Cerfoglio, S.; Lopomo, N.F.; Capodaglio, P.; Scalona, E.; Monfrini, R.; Verme, F.; Galli, M.; Cimolin, V. Assessment of an IMU-Based Experimental Set-Up for Upper Limb Motion in Obese Subjects. Sensors 2023, 23, 9264. [Google Scholar] [CrossRef]
- Brouwer, N.P.; Yeung, T.; Bobbert, M.F.; Besier, T.F. 3D trunk orientation measured using inertial measurement units during anatomical and dynamic sports motions. Scand. J. Med. Sci. Sports. 2021, 31, 358–370. [Google Scholar] [CrossRef]
- Lee, R.; Akhundov, R.; James, C.; Edwards, S.; Snodgrass, S.J. Variations in concurrent validity of two independent inertial measurement units compared to gold standard for upper body posture during computerised device use. Sensors 2023, 23, 6761. [Google Scholar] [CrossRef]
- Khobkhun, F.; Hollands, M.A.; Richards, J.; Ajjimaporn, A. Can we accurately measure axial segment coordination during turning using inertial measurement units (imus)? Sensors 2020, 20, 2518. [Google Scholar] [CrossRef]
- Suvorkin, V.; Garcia-Fernandez, M.; González-Casado, G.; Li, M.; Rovira-Garcia, A. Assessment of noise of mems imu sensors of different grades for gnss/imu navigation. Sensors 2024, 24, 1953. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Li, J.; Li, D.; Fan, B.; Shull, P.B. Imu shoulder angle estimation: Effects of sensor-to-segment misalignment and sensor orientation error. IEEE Trans. Neural Syst. Rehabil. Eng. 2023, 31, 4481–4491. [Google Scholar] [CrossRef]
- Lebleu, J.; Gosseye, T.; Detrembleur, C.; Mahaudens, P.; Cartiaux, O.; Penta, M. Lower limb kinematics using inertial sensors during locomotion: Accuracy and reproducibility of joint angle calculations with different sensor-to-segment calibrations. Sensors 2020, 20, 715. [Google Scholar] [CrossRef]
- Fang, Z.; Woodford, S.C.; Senanayake, D.; Ackland, D.C. Conversion of upper-limb inertial measurement unit data to joint angles: A systematic review. Sensors 2023, 23, 6535. [Google Scholar] [CrossRef] [PubMed]
- Pasciuto, I.; Ligorio, G.; Bergamini, E.; Vannozzi, G.; Sabatini, A.; Cappozzo, A. How angular velocity features and different gyroscope noise types interact and determine orientation estimation accuracy. Sensors 2015, 15, 23983–24001. [Google Scholar] [CrossRef] [PubMed]
- Almassri, A.M.M.; Shirasawa, N.; Purev, A.; Uehara, K.; Oshiumi, W.; Mishima, S.; Wagatsuma, H. Artificial neural network approach to guarantee the positioning accuracy of moving robots by using the integration of imu/uwb with motion capture system data fusion. Sensors 2022, 22, 5737. [Google Scholar] [CrossRef]
- Adans-Dester, C.; Hankov, N.; O’Brien, A.; Vergara-Diaz, G.; Black-Schaffer, R.M.; Zafonte, R.; Dy, J.; Lee, S.I.; Bonato, P. Enabling precision rehabilitation interventions using wearable sensors and machine learning to track motor recovery. NPJ Digit. Med. 2020, 3, 121. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Yoon, S. Validity evaluation of an inertial measurement unit (imu) in gait analysis using statistical parametric mapping (spm). Sensors 2021, 21, 3667. [Google Scholar] [CrossRef]
- Jiménez-Olmedo, J.M.; Pueo, B.; Mossi, J.M.; Villalón-Gasch, L. Concurrent validity of the inertial measurement unit vmaxpro in vertical jump estimation. Appl. Sci. 2023, 13, 959. [Google Scholar] [CrossRef]
- Komaris, D.; Tarfali, G.; O’Flynn, B.; Tedesco, S. Unsupervised imu-based evaluation of at-home exercise programmes: A feasibility study. BMC Sports Sci. Med. Rehabil. 2022, 14, 28. [Google Scholar] [CrossRef]
- Lee, S.I.; Adans-Dester, C.; O’Brien, A.; Vergara-Diaz, G.; Black-Schaffer, R.M.; Zafonte, R.; Dy, J.G.; Bonato, P. Predicting and monitoring upper-limb rehabilitation outcomes using clinical and wearable sensor data in brain injury survivors. IEEE Trans. Biomed. Eng. 2021, 68, 1871–1881. [Google Scholar] [CrossRef] [PubMed]
- Dal Farra, F.; Arippa, F.; Arru, M.; Cocco, M.; Porcu, E.; Solla, F.; Monticone, M. Is dynamic balance impaired in people with non-specific low back pain when compared to healthy people? A systematic review. Eur. J. Phys. Rehabil. Med. 2025, 61, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Liu, L.; Mei, F.; Yang, C. Joint constraints based dynamic calibration of imu position on lower limbs in imu-mocap. Sensors 2021, 21, 7161. [Google Scholar] [CrossRef]
- Tramontano, M.; Orejel Bustos, A.S.; Montemurro, R.; Vasta, S.; Marangon, G.; Belluscio, V.; Morone, G.; Modugno, N.; Buzzi, M.G.; Formisano, R.; et al. Dynamic Stability, Symmetry, and Smoothness of Gait in People with Neurological Health Conditions. Sensors 2024, 24, 2451. [Google Scholar] [CrossRef] [PubMed]
- Castiglia, S.F.; Dal Farra, F.; Trabassi, D.; Turolla, A.; Serrao, M.; Nocentini, U.; Brasiliano, P.; Bergamini, E.; Tramontano, M. Discriminative ability, responsiveness, and interpretability of smoothness index of gait in people with multiple sclerosis. Arch. Physiother. 2025, 15, 9–18. [Google Scholar] [CrossRef]


| Gender N (M/F) | 27 (11/16) |
| Age (years) | 31.1 (11.0) |
| Body mass (kg) | 64.9 (9.68) |
| Height (cm) | 171 (8.46) |
| BMI (kg/m2) | 22.1 (2.16) |
| Analyzed Movement | MoCap (°) | IMU (°) | Accuracy (%) | RMSE (°) |
|---|---|---|---|---|
| Flexion | 78.5 (9.8) | 57.4 (14.4) | 72.1 (12.7) | 3.01 (1.32) |
| Extension | 21.2 (8.14) | 14.7 (5.92) | 64.1 (23.5) | 1.15 (0.83) |
| Lateral bending | 27.2 (6.93) | 16.7 (4.76) | 61.4 (16.8) | 1.59 (0.84) |
| Rotation | 113.0 (28.3) | 108.0 (27.0) | 92.4 (7.61) | 1.09 (1.01) |
| Motion | Pearson “r” | p-Value | CCC (95%CI) | Bias° | LoA Lower° | LoA Upper° |
|---|---|---|---|---|---|---|
| Flexion | 0.703 | <0.001 | 0.262 (0.156–0.363) | −21.09 | −41.18 | −1.01 |
| Extension | 0.564 | <0.001 | 0.375 (0.187–0.537) | −6.53 | −19.96 | 6.91 |
| Lat. Bending | 0.430 | 0.003 | 0.155 (0.004–0.260) | −10.48 | −23.23 | 2.26 |
| Rotation | 0.944 | <0.001 | 0.927 (0.877–0.957) | −5.12 | −23.56 | 13.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dal Farra, F.; Cerfoglio, S.; Porta, M.; Pau, M.; Galli, M.; Lopomo, N.F.; Cimolin, V. Comparative Assessment of an IMU-Based Wearable Device and a Marker-Based Optoelectronic System in Trunk Motion Analysis: A Cross-Sectional Investigation. Appl. Sci. 2025, 15, 5931. https://doi.org/10.3390/app15115931
Dal Farra F, Cerfoglio S, Porta M, Pau M, Galli M, Lopomo NF, Cimolin V. Comparative Assessment of an IMU-Based Wearable Device and a Marker-Based Optoelectronic System in Trunk Motion Analysis: A Cross-Sectional Investigation. Applied Sciences. 2025; 15(11):5931. https://doi.org/10.3390/app15115931
Chicago/Turabian StyleDal Farra, Fulvio, Serena Cerfoglio, Micaela Porta, Massimiliano Pau, Manuela Galli, Nicola Francesco Lopomo, and Veronica Cimolin. 2025. "Comparative Assessment of an IMU-Based Wearable Device and a Marker-Based Optoelectronic System in Trunk Motion Analysis: A Cross-Sectional Investigation" Applied Sciences 15, no. 11: 5931. https://doi.org/10.3390/app15115931
APA StyleDal Farra, F., Cerfoglio, S., Porta, M., Pau, M., Galli, M., Lopomo, N. F., & Cimolin, V. (2025). Comparative Assessment of an IMU-Based Wearable Device and a Marker-Based Optoelectronic System in Trunk Motion Analysis: A Cross-Sectional Investigation. Applied Sciences, 15(11), 5931. https://doi.org/10.3390/app15115931













