From Ocean to Market: Technical Applications of Fish Protein Hydrolysates in Human Functional Food, Pet Wellness, Aquaculture and Agricultural Bio-Stimulant Product Sectors
Abstract
1. Introduction
2. FPHs for Human Functional Benefits
2.1. FPH with Anti-Inflammatory Activities
2.2. FPH with Anti-Hypertensive Activities
2.3. FPH with Anti-Diabetes Type 2 (T2D) and Satiety Activities
2.4. FPH with Bone and Skin Health Implications
2.5. FPH with Antioxidant and Antimicrobial Activities
| Product Name | Manufacturer | Fish Source and Active Ingredient | Health Benefit Claimed | References |
|---|---|---|---|---|
| Jellice (hydrolysate) found in CollametaTM | Jellice Pioneer Europe PV, (7821 BG, Emmen, The Netherlands); supplied into product CollaMeta produced by Glanbia Nutritionals Ltd. (Global) | Skin from Tilapia and Pangasius sp./Collagen tripeptide | Skin health, possible prevention of arthritis in humans | [63] |
| Collagen HMTM | Copalis, France (Le Portel, Hauts, France) | Different fish sources including white fish species and salmon/Collagen HMTM polypeptides mean molecular weight is 3600 Da, making it soluble in aqueous phase and fully digestible | Skin and joint health | [64] |
| NutripeptinTM | Copalis, France (Le Portel, Hauts, France) | Bioactive marine peptide | Reduces the glycaemic index of foods and thus helps to reduce fat storage in weight-control formulas | [65] |
| ProtizenTM | Copalis, France (Le Portel, Hauts, France) | Pollock and coalfish digest produced by enzymatic hydrolysis/anti-stress peptide that promotes well-being, found in Serenlider product—possess anxiolytic properties and is considered as beneficial for mental health/stress | Anti-stress, mood food, promotes restful sleep | [66] |
| Molval® | Dielen Laboratoire, France (Cherbourg-en-Cotentin, Normandy, France) | Extracted from cod and mackerel/Gabolysat PC60. | Beneficial effects on dyslipidaemia and cardiovascular risk, contains omega-3 fatty acids and Gabolysat PC60/anti-stress effects | [67] |
| Curcumega® | Dielen Laboratoire, France (Cherbourg-en-Cotentin, Normandy, France) | Fish oil rich in omega-3 fatty acids combined with turmeric | Nutritional impact and antioxidative effects | [67] |
| Seagest® | Trimedica, USA (Santa Monica, CA, USA) | Deep ocean within fish species | none found | |
| PeptACE® | Natural factors, USA (14224 167TH Ave SE Monroe, WA, USA) | Bonito fish hydrolysate/nine small peptides derived from Bonito fish | Maintains normal blood pressure | [68] |
| Stabilium® 200 | Nutricology, Canada (Salt Lake City, UT, USA) | Derived hydrolysate from Molva dypterygia | Improves resilience to stress, may reduce fatigue and supports normal psychological functions, such as memory, concentration, and cognitive abilities | [69] |
| Amizate® | Zymtech Production AS, Norway (Oppland, Lesja) | Enzymatic process extracts amino acids, short peptides, and micronutrients from Atlantic salmon (Salmo salar) | Excellent nutritive value; mood improving agent | [70] |
| WhiteCal® | BioMarine Ingredients Ireland (BII), Ltd. (Ballybay, Monaghan, Ireland) | Blue whiting fish/WhiteCal is a marine mineral complex, naturally rich in collagen peptides and high in calcium, phosphorus, and magnesium. It can be easily used in a wide range of human nutrition applications | Growth and repair of the body and maintenance of good health, bone, and joint health. | [71] |
| ProAtlantic® | BioMarine Ingredients Ireland (BII), Ltd. (Ballybay, Monaghan, Ireland) | Premium grade hydrolysed Fish Protein Isolates (FPI) containing 95% protein and 0.5% fat for human products | Glycaemic control, diabetes prevention, satiety | [72] |
3. FPHs—As a Pet Health Food Ingredient
3.1. Cardiovascular and Hypertensive Health Issues
3.2. Arthritis
3.3. Hair and Coat Issues
3.4. Digestive, Renal, and Metabolic Health Issues
3.5. Cognitive and Emotional Health in Ageing Pets
4. FPHs as Pet Palatants
4.1. Palatant Form
4.2. The Difference Between Cats and Dogs
4.3. Marine Hydrolysates as Pet Palatants
5. FPHs—Based Aquaculture Feeds
6. FPHs as Biostimulants
6.1. Mechanisms of Action of FPH Biostimulants
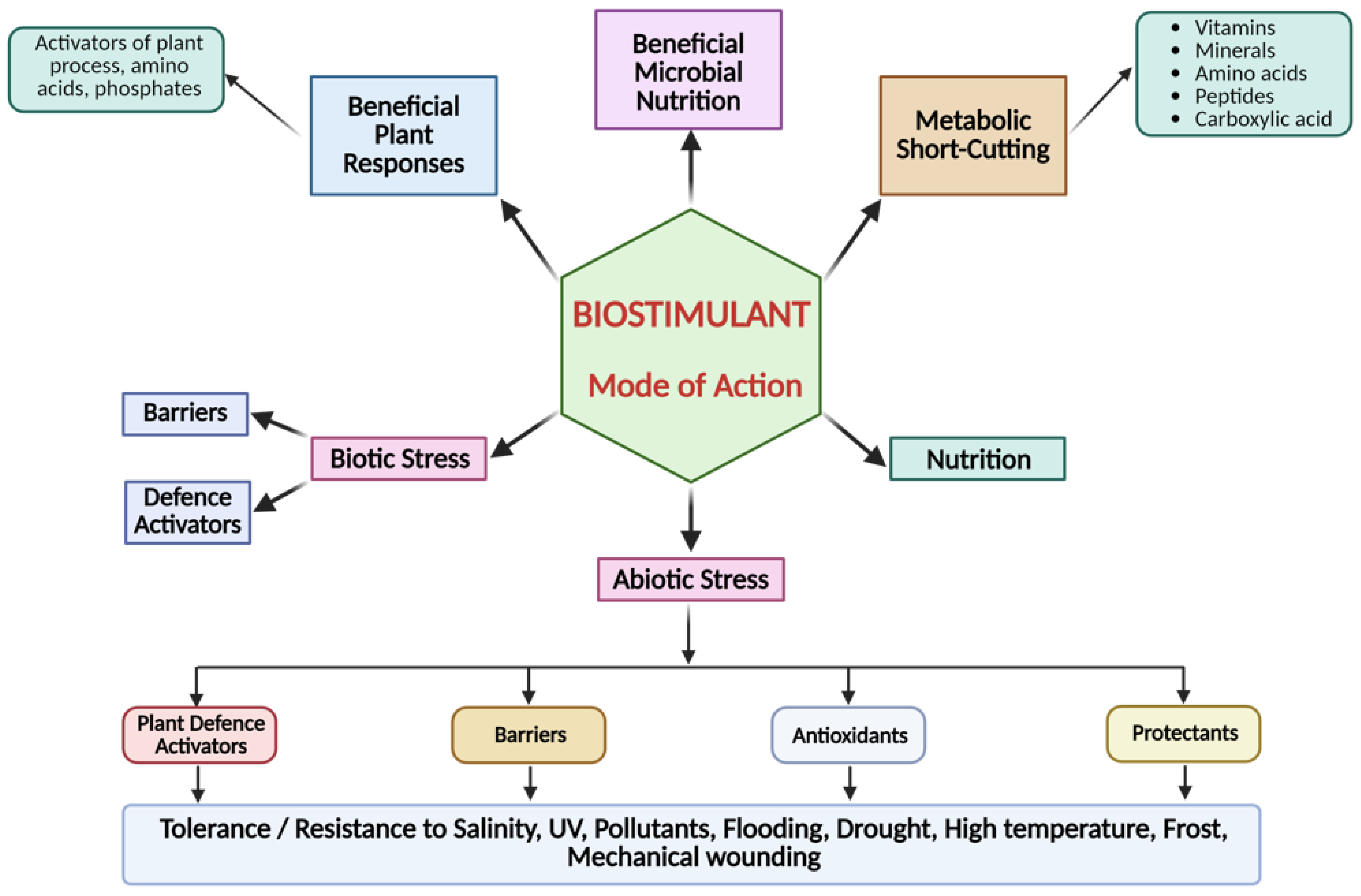
6.2. Modes of Action of FPH as Biostimulants
6.3. Product Development
6.4. Current Research Gaps
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mora, L.; Toldrá, F. Advanced enzymatic hydrolysis of food proteins for the production of bioactive peptides. Curr. Opin. Food Sci. 2023, 49, 100973. [Google Scholar] [CrossRef]
- de Carvalho Oliveira, L.; Martinez-Villaluenga, C.; Frias, J.; Cartea, M.E.; Francisco, M.; Cristianini, M.; Peñas, E. High pressure-assisted enzymatic hydrolysis potentiates the production of quinoa protein hydrolysates with antioxidant and ACE-inhibitory activities. Food Chem. 2024, 447, 138887. [Google Scholar] [CrossRef] [PubMed]
- Fadimu, G.J.; Le, T.T.; Gill, H.; Farahnaky, A.; Olatunde, O.O.; Truong, T. Enhancing the Biological Activities of Food Protein-Derived Peptides Using Non-Thermal Technologies: A Review. Foods 2022, 11, 1823. [Google Scholar] [CrossRef] [PubMed]
- Suryaningtyas, I.T.; Je, J.Y. Bioactive peptides from food proteins as potential anti-obesity agents: Mechanisms of action and future perspectives. Trends Food Sci. Technol. 2023, 138, 141–152. [Google Scholar] [CrossRef]
- Zaky, A.A.; Simal-Gandara, J.; Eun, J.B.; Shim, J.H.; Abd El-Aty, A.M. Bioactivities, Applications, Safety, and Health Benefits of Bioactive Peptides from Food and By-Products: A Review. Front. Nutr. 2022, 8, 815640. [Google Scholar] [CrossRef]
- Du, Z.; Li, Y. Review and perspective on bioactive peptides: A roadmap for research, development, and future opportunities. J. Agric. Food Res. 2022, 9, 100353. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Ghosh, D.; Williams, R.O., 3rd. Just how prevalent are peptide therapeutic products? A critical review. Int. J. Pharm. 2020, 587, 119491. [Google Scholar] [CrossRef]
- Ribeiro, T.B.; Maia, M.R.G.; Fonseca, A.J.M.; Marques, B.; Caleja, C.; Rosa, A.; Martins, R.; Almeida, A.; Mota, M.J.; Aires, T.; et al. A Comprehensive Review of Fish Protein Hydrolysates Targeting Pet Food Formulations. Food Rev. Int. 2024, 1–39. [Google Scholar] [CrossRef]
- Hayes, M. Maximizing Use of Pelagic Capture Fisheries for Global Protein Supply: Potential and Caveats Associated with Fish and Co-Product Conversion into Value-Add Ingredients. Glob. Chall. 2023, 7, 2200098. [Google Scholar] [CrossRef]
- Kurnianto, M.A.; Defri, I.; Syahbanu, F.; Aulia, S.S. Fish-derived bioactive peptide: Bioactivity potency, structural characteristics, and conventional and bioinformatics approaches for identification. Future Foods 2024, 9, 100386. [Google Scholar] [CrossRef]
- Hasler, C.M. Functional foods: Benefits, concerns and challenges—A position paper from the American Council on Science and Health. J. Nutr. 2002, 132, 3772–3781. [Google Scholar] [CrossRef] [PubMed]
- Venkatakrishnan, K.; Chiu, H.F.; Wang, C.K. Impact of functional foods and nutraceuticals on high blood pressure with a special focus on meta-analysis: Review from a public health perspective. Food Funct. 2020, 11, 2792–2804. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.C.; Yen, G.C. Antioxidative and anti-inflammatory activity of functional foods. Curr. Opin. Food Sci. 2015, 2, 1–8. [Google Scholar] [CrossRef]
- Faienza, M.F.; Giardinelli, S.; Annicchiarico, A.; Chiarito, M.; Barile, B.; Corbo, F.; Brunetti, G. Nutraceuticals and Functional Foods: A Comprehensive Review of Their Role in Bone Health. Int. J. Mol. Sci. 2024, 25, 5873. [Google Scholar] [CrossRef]
- Mohamed, S. Functional foods against metabolic syndrome (obesity, diabetes, hypertension and dyslipidemia) and cardiovascular disease. Trends Food Sci. Technol. 2014, 35, 114–128. [Google Scholar] [CrossRef]
- Vignesh, A.; Amal, T.C.; Sarvalingam, A.; Vasanth, K. A review on the influence of nutraceuticals and functional foods on health. Food Chem. Adv. 2024, 5, 100749. [Google Scholar] [CrossRef]
- Henriques, A.; Vázquez, J.A.; Valcarcel, J.; Mendes, R.; Bandarra, N.M.; Pires, C. Characterization of Protein Hydrolysates from Fish Discards and By-Products from the North-West Spain Fishing Fleet as Potential Sources of Bioactive Peptides. Mar. Drugs 2021, 19, 338. [Google Scholar] [CrossRef]
- Aziz, T.; Hussain, N.; Hameed, Z.; Lin, L. Elucidating the role of diet in maintaining gut health to reduce the risk of obesity, cardiovascular and other age-related inflammatory diseases: Recent challenges and future recommendations. Gut Microbes 2024, 16, 2297864. [Google Scholar] [CrossRef]
- Whitaker, R.D.; Altintzoglou, T.; Lian, K.; Fernandez, E.N. Marine bioactive peptides in supplements and functional foods—A commercial perspective. Curr. Pharm. Des. 2021, 27, 1353–1364. [Google Scholar] [CrossRef]
- Gevaert, B.; Veryser, L.; Verbeke, F.; Wynendaele, E.; De Spiegeleer, B. Fish hydrolysates: A regulatory perspective of bioactive peptides. Protein Pept. Lett. 2016, 23, 1052–1060. [Google Scholar] [CrossRef]
- Rivero-Pino, F.; Villanueva, Á.; Montserrat-de-la-Paz, S.; Sanchez-Fidalgo, S.; Millán-Linares, M.C. Evidence of Immunomodulatory Food-Protein Derived Peptides in Human Nutritional Interventions: Review on the Outcomes and Potential Limitations. Nutrients 2023, 15, 2681. [Google Scholar] [CrossRef] [PubMed]
- Arias, L.; Sánchez-Henao, C.P.; Zapata, J.E. Optimization of the separation conditions of antioxidant peptides from red tilapia (Oreochromis spp.) viscera on cross-flow filtration ceramic membranes. S. Afr. J. Chem. Eng. 2023, 45, 100–110. [Google Scholar] [CrossRef]
- La Manna, S.; Di Natale, C.; Florio, D.; Marasco, D. Peptides as therapeutic agents for inflammatory-related diseases. Int. J. Mol. Sci. 2018, 19, 2714. [Google Scholar] [CrossRef] [PubMed]
- Kemp, D.C.; Kwon, J.Y. Fish and Shellfish-Derived Anti-Inflammatory Protein Products: Properties and Mechanisms. Molecules 2021, 26, 3225. [Google Scholar] [CrossRef] [PubMed]
- Lessa, R.C.; Ebrahimi, B.; Li, H.; Guan, X.; Li, Y.; Lu, J. Investigation of the In Vitro Immunomodulatory Effects of Extracts from Green-Lipped Mussels (Perna canaliculus). Nutraceuticals 2024, 4, 127–146. [Google Scholar] [CrossRef]
- Phadke, G.G.; Rathod, N.B.; Ozogul, F.; Elavarasan, K.; Karthikeyan, M.; Shin, K.H.; Kim, S.K. Exploiting of Secondary Raw Materials from Fish. Processing Industry as a Source of Bioactive Peptide-Rich Protein Hydrolysates. Mar. Drugs 2021, 19, 480. [Google Scholar] [CrossRef]
- Hayes, M.; Gargan, C.; Milovanovic, I. Antihypertensive Food Ingredients for Companion Animal Applications. US Patent Application 18/250/822, 5 May 2022. [Google Scholar]
- Kawasaki, T.; Seki, E.; Osajima, K.; Yoshida, M.; Asada, K.; Matsui, T.; Osajima, Y. Antihypertensive effect of valyl-tyrosine, a short chain peptide derived from sardine muscle hydrolyzate, on mild hypertensive subjects. J. Hum. Hypertens. 2000, 14, 519–523. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, G.X.; Zhao, Y.Q.; Qiu, Y.T.; Chi, C.F.; Wang, B. Identification and Active Evaluation of Antioxidant Peptides from Protein Hydrolysates of Skipjack Tuna (Katsuwonus pelamis) Head. Antioxidants 2019, 8, 318. [Google Scholar] [CrossRef]
- Abdelhedi, O.; Nasri, M. Basic and recent advances in marine antihypertensive peptides: Production, structure-activity relationship and bioavailability. Trends Food Sci. Technol. 2019, 88, 543–557. [Google Scholar] [CrossRef]
- Wang, X.; Wu, S.; Xu, D.; Xie, D.; Guo, H. Inhibitor and substrate binding by angiotensin-converting enzyme: Quantum mechanical/molecular mechanical molecular dynamics studies. J. Chem. Inf. Model. 2011, 51, 1074–1082. [Google Scholar] [CrossRef]
- Naik, A.S.; Mora, L.; Hayes, M. Characterisation of Seasonal Mytilus edulis By-Products and Generation of Bioactive Hydrolysates. Appl. Sci. 2020, 10, 6892. [Google Scholar] [CrossRef]
- Girgih, A.T.; Nwachukwu, I.D.; Hasan, F.; Fagbemi, T.N.; Gill, T.; Aluko, R.E. Kinetics of the inhibition of renin and angiotensin I-converting enzyme by cod (Gadus morhua) protein hydrolysates and their antihypertensive effects in spontaneously hypertensive rats. Food Nutr. Res. 2015, 59, 29788. [Google Scholar] [CrossRef] [PubMed]
- Power, O.; Nongonierma, A.B.; Jakeman, P.; FitzGerald, R.J. Food protein hydrolysates as a source of dipeptidyl peptidase IV inhibitory peptides for the management of type 2 diabetes. Proc. Nutr. Soc. 2014, 73, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chai, L.; Wu, Q.; Wang, Y.; Li, S.; Chen, J. Anti-diabetic properties of bioactive components from fish and milk. J. Funct. Foods 2021, 85, 104669. [Google Scholar] [CrossRef]
- Cudennec, B.; Fouchereau-Peron, M.; Ferry, F.; Duclos, E.; Ravallec, R. In vitro and in vivo evidence for a satiating effect of fish protein hydrolysate obtained from blue whiting (Micromesistius poutassou) muscle. J. Funct. Foods 2012, 4, 271–277. [Google Scholar] [CrossRef]
- Lees, M.J.; Carson, B.P. The potential role of fish-derived protein hydrolysates on metabolic health, skeletal muscle mass and function in ageing. Nutrients 2020, 12, 2434. [Google Scholar] [CrossRef]
- Pilon, G.; Ruzzin, J.; Rioux, L.E.; Lavigne, C.; White, P.J.; Frøyland, L.; Jacques, H.; Bryl, P.; Beaulieu, L.; Marette, A. Differential effects of various fish proteins in altering body weight, adiposity, inflammatory status, and insulin sensitivity in high-fat–fed rats. Metabolism 2011, 60, 1122–1130. [Google Scholar] [CrossRef]
- Li-Chan, E.C.; Hunag, S.L.; Jao, C.L.; Ho, K.P.; Hsu, K.C. Peptides derived from Atlantic salmon skin gelatin as dipeptidyl-peptidase IV inhibitors. J. Agric. Food Chem. 2012, 60, 973–978. [Google Scholar] [CrossRef]
- Rivero-Pino, F.; Espejo-Carpio, F.J.; Guadix, E.M. Production and identification of dipeptidyl peptidase IV (DPP-IV) inhibitory peptides from discarded Sardine pilchardus protein. Food Chem. 2020, 328, 127096. [Google Scholar] [CrossRef]
- Liu, D.; Ren, Y.; Zhong, S.; Xu, B. New Insight into Utilization of Fish By-Product Proteins and Their Skin Health Promoting Effects. Mar. Drugs 2024, 22, 215. [Google Scholar] [CrossRef]
- Zhang, X.; Zhuang, H.; Wu, S.; Mao, C.; Dai, Y.; Yan, H. Marine Bioactive Peptides: Anti-Photoaging Mechanisms and Potential Skin Protective Effects. Curr. Issues Mol. Biol. 2024, 46, 990–1009. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wan, F.; Su, W.; Xie, W. Research Progress on Skin Aging and Active Ingredients. Molecules 2023, 28, 5556. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Hou, H.; Fan, Y.; Wang, S.; Chen, Q.; Si, L.; Li, B. Protective effect of gelatin peptides from pacific cod skin against photoaging by inhibiting the expression of MMPs via MAPK signaling pathway. J. Photochem. Photobiol. 2016, 165, 34–41. [Google Scholar] [CrossRef]
- Li, H.-L.; Li, M.-J.; Xiong, G.-Q.; Cai, J.; Liao, T.; Zu, X.-Y. Silver Carp (Hypophthalmichthys molitrix) Scale Collagen Peptides-1 (SCPs1) Inhibit Melanogenesis through Downregulation of the cAMP-CREB Signaling Pathway. Nutrients 2023, 15, 2449. [Google Scholar] [CrossRef]
- Iosageanu, A.; Ilie, D.; Craciunescu, O.; Seciu-Grama, A.-M.; Oancea, A.; Zarnescu, O.; Moraru, I.; Oancea, F. Effect of Fish Bone Bioactive Peptides on Oxidative, Inflammatory and Pigmentation Processes Triggered by UVB Irradiation in Skin Cells. Molecules 2021, 26, 2691. [Google Scholar] [CrossRef]
- Kong, J.; Hu, X.-M.; Cai, W.-W.; Wang, Y.-M.; Chi, C.-F.; Wang, B. Bioactive Peptides from Skipjack Tuna Cardiac Arterial Bulbs (II): Protective Function on UVB-Irradiated HaCaT Cells through Antioxidant and Anti-Apoptotic Mechanisms. Mar. Drugs 2023, 21, 105. [Google Scholar] [CrossRef]
- Azizi, M.; Sharifan, A.; Hoseini, E.; Ghavami, A. Enzymatic Hydrolysis of Whitefish Viscera of Caspian Sea (Caspian kutum) and Evaluation of Antioxidant Properties of hydrolyzed protein. Iran. J. Biosyst. Eng. 2021, 52, 379–390. [Google Scholar] [CrossRef]
- Shahidi, F.; Saeid, A. Bioactivity of Marine-Derived Peptides and Proteins: A Review. Mar. Drugs 2025, 23, 157. [Google Scholar] [CrossRef]
- Halim, N.R.; Yusof, H.M.; Sarbon, N.M. Functional and bioactive properties of fish protein hydolysates and peptides: A comprehensive review. Trends Food Sci. Technol. 2016, 51, 24–33. [Google Scholar] [CrossRef]
- Hasani, K.; Ariaii, P.; Ahmadi, M. Antimicrobial, antioxidant and anti-cancer properties of protein hydrolysates from indian mackerel (Rastrelliger kanagurta) waste prepared using commercial enzyme. Int. J. Pept. Res. Ther. 2022, 28, 86. [Google Scholar] [CrossRef]
- Pezeshk, S.; Ojagh, S.M.; Rezaei, M.; Shabanpour, B. Fractionation of Protein Hydrolysates of Fish Waste Using Membrane Ultrafiltration: Investigation of Antibacterial and Antioxidant Activities. Probiotics Antimicrob. Proteins 2019, 11, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Tejpal, C.S.; Vijayagopal, P.; Elavarasan, K.; Linga Prabu, D.; Lekshmi, R.G.K.; Asha, K.K.; Anandan, R.; Chatterjee, S.; Mathew, S. Antioxidant, functional properties and amino acid composition of pepsin-derived protein hydrolysates from whole tilapia waste as influenced by pre-processing ice storage. J. Food Sci. Technol. 2017, 54, 4257–4267. [Google Scholar] [CrossRef] [PubMed]
- Hamzeh, A.; Rezaei, M.; Khodabandeh, S.; Motamedzadegan, A.; Noruzinia, M.; Regenstein, J.M. Optimization of Antioxidant Peptides Production from the Mantle of Cuttlefish (Sepia pharaonis) Using RSM and Fractionation. J. Aquat. Food Product. Technol. 2019, 28, 392–401. [Google Scholar] [CrossRef]
- Tadesse, S.A.; Emire, S.A. Production and processing of antioxidant bioactive peptides: A driving force for the functional food market. Heliyon 2020, 6, e04765. [Google Scholar] [CrossRef] [PubMed]
- Daniele, F.; Marasco, D.; La Manna, S. Approaches for developing peptide-and metal complexes-or chelators-based leads for anti-amyloid drugs. Inorganica Chim. Acta 2024, 577, 122474. [Google Scholar] [CrossRef]
- Liao, X.; Zhu, Z.; Wu, S.; Chen, M.; Huang, R.; Wang, J.; Wu, Q.; Ding, Y. Preparation of Antioxidant Protein Hydrolysates from Pleurotus geesteranus and Their Protective Effects on H2O2 Oxidative Damaged PC12 Cells. Molecules 2020, 25, 5408. [Google Scholar] [CrossRef]
- Sohaib, M.; Anjum, F.M.; Sahar, A.; Arshad, M.S.; Rahman, U.U.; Imran, A.; Hussain, S. Antioxidant proteins and peptides to enhance the oxidative stability of meat and meat products: A comprehensive review. Int. J. Food Prop. 2017, 20, 2581–2593. [Google Scholar] [CrossRef]
- Brogden, N.K.; Mehalick, L.; Fischer, C.L.; Wertz, P.W.; Brogden, K.A. The emerging role of peptides and lipids as antimicrobial epidermal barriers and modulators of local inflammation. Skin Pharmacol. Physiol. 2012, 25, 167–181. [Google Scholar] [CrossRef]
- Bi, J.; Tian, C.; Jiang, J.; Zhang, G.L.; Hao, H.; Hou, H.M. Antibacterial Activity and Potential Application in Food Packaging of Peptides Derived from Turbot Viscera Hydrolysate. J. Agric. Food Chem. 2020, 68, 9968–9977. [Google Scholar] [CrossRef]
- Perez Espitia, P.J.; de Fátima Ferreira Soares, N.; Dos Reis Coimbra, J.S.; de Andrade, N.J.; Souza Cruz, R.; Alves Medeiros, E.A. Bioactive Peptides: Synthesis, Properties, and Applications in the Packaging and Preservation of Food. Compr. Rev. Food Sci. Food Saf. 2012, 11, 187–204. [Google Scholar] [CrossRef]
- Da Rocha, M.; Alemán, A.; Romani, V.P.; López-Caballero, M.; Gómez-Guillén, M.; Montero, P.; Prentice, C. Effects of agar films incorporated with fish protein hydrolysate or clove essential oil on flounder (Paralichthys orbignyanus) fillets shelf-life. Food Hydrocoll. 2018, 81, 351–363. [Google Scholar] [CrossRef]
- Chen, M.F.; Gong, F.; Zhang, Y.Y.; Li, C.; Zhou, C.; Hong, P.; Sun, S.; Qian, Z.J. Preventive Effect of YGDEY from Tilapia Fish Skin Gelatin Hydrolysates against Alcohol-Induced Damage in HepG2 Cells through ROS-Mediated Signaling Pathways. Nutrients 2019, 11, 392. [Google Scholar] [CrossRef] [PubMed]
- Choi, F.D.; Sung, C.T.; Juhasz, M.L.; Mesinkovsk, N.A. Oral Collagen Supplementation: A Systematic Review of Dermatological Applications. J. Drugs Dermatol. 2019, 18, 9–16. [Google Scholar]
- Jahandideh, F.; Bourque, S.L.; Wu, J. A comprehensive review on the glucoregulatory properties of food-derived bioactive peptides. Food Chem. 2022, 13, 100222. [Google Scholar] [CrossRef]
- Borges, S.; Odila, J.; Voss, G.; Martins, R.; Rosa, A.; Couto, J.A.; Almeida, A.; Pintado, M. Fish By-Products: A Source of Enzymes to Generate Circular Bioactive Hydrolysates. Molecules 2023, 28, 1155. [Google Scholar] [CrossRef]
- Jensen, C.; Fjeldheim Dale, H.; Hausken, T.; Hatlebakk, J.G.; Brønstad, I.; Lied, G.A.; Hoff, D.A.L. Supplementation with Low Doses of a Cod Protein Hydrolysate on Glucose Regulation and Lipid Metabolism in Adults with Metabolic Syndrome: A Randomized, Double-Blind Study. Nutrients 2020, 12, 1991. [Google Scholar] [CrossRef] [PubMed]
- Guénard, F.; Jacques, H.; Gagnon, C.; Marette, A.; Vohl, M.C. Acute Effects of Single Doses of Bonito Fish Peptides and Vitamin D on Whole Blood Gene Expression Levels: A Randomized Controlled Trial. Int. J. Mol. Sci. 2019, 20, 1944. [Google Scholar] [CrossRef]
- Le Poncin, M.; Lamproglou, I. Experimental study: Stress and memory. Eur. Neuropsychopharmacol. 1996, 6, 110. [Google Scholar] [CrossRef]
- Nesse, K.O.; Nagalakshmi, A.P.; Marimuthu, P.; Singh, M. Efficacy of a fish protein hydrolysate in malnourished children. Indian J. Clin. Biochem. 2011, 26, 360–365. [Google Scholar] [CrossRef]
- Heffernan, S.; Nunn, L.; Harnedy-Rothwell, P.A.; Gite, S.; Whooley, J.; Giblin, L.; FitzGerald, R.J.; O’Brien, N.M. Blue Whiting (Micromesistius poutassou) Protein Hydrolysates Increase GLP-1 Secretion and Proglucagon Production in STC-1 Cells Whilst Maintaining Caco-2/HT29-MTX Co-Culture Integrity. Mar. Drugs 2022, 20, 112. [Google Scholar] [CrossRef]
- Harnedy, P.A.; Parthsarathy, V.; McLaughlin, C.M.; O’Keeffe, M.B.; Allsopp, P.J.; McSorley, E.M.; O’Harte, F.P.M.; FitzGerald, R.J. Blue whiting (Micromesistius poutassou) muscle protein hydrolysate with in vitro and in vivo antidiabetic properties. J. Funct. Foods 2018, 40, 137–145. [Google Scholar] [CrossRef]
- Available online: https://www.futuremarketinsights.com/reports/fish-based-pet-food-market (accessed on 3 September 2024).
- Zhang, W.; Cao, H.; Lin, L. Analysis of the future development trend of the pet industry. In Proceedings of the 2022 7th International Conference on Financial Innovation and Economic Development (ICFIED 2022), Harbin, China, 21–23 January 2022; Atlantis Press: Zhengzhou, China, 2022; pp. 1682–1689. [Google Scholar] [CrossRef]
- Di Cerbo, A.; Morales-Medina, J.C.; Palmieri, B.; Pezzuto, F.; Cocco, R.; Flores, G.; Iannitti, T. Functional foods in pet nutrition: Focus on dogs and cats. Res. Vet. Sci. 2017, 112, 161–166. [Google Scholar] [CrossRef]
- Swanson, K.S.; Carter, R.A.; Yount, T.P.; Aretz, J.; Buff, P.R. Nutritional sustainability of pet foods. Adv. Nutr. 2013, 4, 141–150. [Google Scholar] [CrossRef]
- Gasco, L.; Acuti, G.; Bani, P.; Dalle Zotte, A.; Danieli, P.P.; De Angelis, A.; Fortina, R.; Marino, R.; Parisi, G.; Piccolo, G.; et al. Insect and fish by-products as sustainable alternatives to conventional animal proteins in animal nutrition. Ital. J. Anim. Sci. 2020, 19, 360–372. [Google Scholar] [CrossRef]
- Meeker, D.L.; Meisinger, J.L. Companion Animals Symposium: Rendered ingredients significantly influence sustainability, quality, and safety of pet food. J. Anim. Sci. 2015, 93, 835–847. [Google Scholar] [CrossRef]
- Ashraf, S.A.; Adnan, M.; Patel, M.; Siddiqui, A.J.; Sachidanandan, M.; Snoussi, M.; Hadi, S. Fish-Based Bioactives as Potent Nutraceuticals: Exploring the Therapeutic Perspective of Sustainable Food from the Sea. Mar. Drugs 2020, 18, 265. [Google Scholar] [CrossRef] [PubMed]
- Naik, A.S.; Whitaker, R.D.; Albrektsen, S.; Solstad, R.G.; Thoresen, L.; Hayes, M. Mesopelagic fish protein hydrolysates and extracts: A source of novel anti-hypertensive and anti-diabetic peptides. Front. Mar. Sci. 2021, 8, 719608. [Google Scholar] [CrossRef]
- Geirsdottir, M.; Sigurgisladottir, S.; Hamaguchi, P.Y.; Thorkelsson, G.; Johannsson, R.; Kristinsson, H.G.; Kristjansson, M.M. Enzymatic hydrolysis of blue whiting (Micromesistius poutassou); functional and bioactive properties. J. Food Sci. 2011, 76, C14–C20. [Google Scholar] [CrossRef]
- de Oliveira Coelho, L.; Pinto, R. Amino Acids as Key Mediators of Immune Status and Nutritional Condition in Fish; University of Porto: Porto, Portugal, 2020. [Google Scholar]
- Cave, N.J. Hydrolyzed protein diets for dogs and cats. Vet. Clin. Small Anim. Pract. 2006, 36, 1251–1268. [Google Scholar] [CrossRef]
- Noli, C.; Varina, A.; Barbieri, C.; Pirola, A.; Olivero, D. Analysis of Intestinal Microbiota and Metabolic Pathways before and after a 2-Month-Long Hydrolyzed Fish and Rice Starch Hypoallergenic Diet Trial in Pruritic Dogs. Vet. Sci. 2023, 10, 478. [Google Scholar] [CrossRef]
- Martínez-Alvarez, O.; Chamorro, S.; Brenes, A. Protein hydrolysates from animal processing by-products as a source of bioactive molecules with interest in animal feeding: A review. Food Res. Int. 2015, 73, 204–212. [Google Scholar] [CrossRef]
- Baco, N.; Oslan, S.N.H.; Shapawi, R.; Mohhtar, R.A.M.; Noordin, W.N.M.; Huda, N. Antibacterial Activity of Functional Bioactive Peptides Derived from Fish Protein Hydrolysate; IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2022; Volume 967, p. 012019. [Google Scholar] [CrossRef]
- Da Rocha, M.; Alemán, A.; Baccan, G.C.; López-Caballero, M.E.; Gómez-Guillén, C.; Montero, P.; Prentice, C. Anti-inflammatory, antioxidant, and antimicrobial effects of underutilized fish protein hydrolysate. J. Aquat. Food Prod. Technol. 2018, 27, 592–608. [Google Scholar] [CrossRef]
- Hatab, S.; Chen, M.-L.; Miao, W.; Lin, J.; Wu, D.; Wang, C.; Yuan, P.; Deng, S. Protease hydrolysates of filefish (Thamnaconus modestus) byproducts effectively inhibit foodborne pathogens. Foodborne Pathog. Dis. 2017, 14, 656–666. [Google Scholar] [CrossRef]
- Trang, H.T.H.; Pasuwan, P. Screening antimicrobial activity against pathogens from protein hydrolysate of rice bran and Nile Tilapia by-products. Int. Food Res. J. 2018, 25, 2157–2163. [Google Scholar]
- Lima, K.O.; da Costa de Quadros, C.; Rocha, M.D.; Jocelino Gomes de Lacerda, J.T.; Juliano, M.A.; Dias, M.; Mendes, M.A.; Prentice, C. Bioactivity and bioaccessibility of protein hydrolyzates from industrial byproducts of Stripped weakfish (Cynoscion guatucupa). LWT-Food Sci. Technol. 2019, 111, 408–413. [Google Scholar] [CrossRef]
- Hou, Y.; Wu, Z.; Dai, Z.; Wang, G.; Wu, G. Protein hydrolysates in animal nutrition: Industrial production, bioactive peptides, and functional significance. J. Anim. Sci. Biotechnol. 2022, 8, 209–232. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Liang, C.H.; Wu, H.T.; Pang, H.Y.; Chen, C.; Wang, G.H.; Chan, L.P. Antioxidant and anti-inflammatory capacities of collagen peptides from milkfish (Chanos chanos) scales. J. Food Sci. Technol. 2018, 55, 2310–2317. [Google Scholar] [CrossRef]
- Giannetto, A.; Esposito, E.; Lanza, M.; Oliva, S.; Riolo, K.; Di Pietro, S.; Abbate, J.M.; Briguglio, G.; Cassata, G.; Cicero, L.; et al. Protein Hydrolysates from Anchovy (Engraulis encrasicolus) Waste: In Vitro and In Vivo Biological Activities. Mar. Drugs 2020, 18, 86. [Google Scholar] [CrossRef]
- Narayanasamy, A.; Balde, A.; Raghavender, P.; Shashanth, D.; Abraham, J.; Joshi, I.; Nazeer, R.A. Isolation of marine crab (Charybdis natator) leg muscle peptide and its anti-inflammatory effects on macrophage cells. Biocatal. Agric. Biotechnol. 2020, 25, 101577. [Google Scholar] [CrossRef]
- Zhu, W.; Ren, L.; Zhang, L.; Qiao, Q.; Farooq, M.Z.; Xu, Q. The Potential of Food Protein-Derived Bioactive Peptides against Chronic Intestinal Inflammation. Mediat. Inflamm. 2020, 2020, 6817156. [Google Scholar] [CrossRef]
- Zamora-Sillero, J.; Gharsallaoui, A.; Prentice, C. Peptides from Fish By-product Protein Hydrolysates and Its Functional Properties: An Overview. Mar. Biotechnol. 2018, 20, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Larder, C.E.; Iskandar, M.M.; Kubow, S. Collagen Hydrolysates: A Source of Bioactive Peptides Derived from Food Sources for the Treatment of Osteoarthritis. Medicines 2023, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Yu, Q.; Shen, Y.; Chu, Q.; Chen, G.; Fen, S.; Yang, M.; Yuan, L.; Mcclements, D.J.; Sun, Q. Production, bioactive properties, and potential applications of fish protein hydrolysates: Developments and challenges. Trends Food Sci. Technol. 2021, 110, 687–699. [Google Scholar] [CrossRef]
- Cabrita, A.R.J.; Maia, M.R.G.; Alves, A.P.; Aires, T.; Rosa, A.; Almeida, A.; Martins, R.; Fonseca, A.J.M. Protein hydrolysate and oil from fish waste reveal potential as dog food ingredients. Front. Vet. Sci. 2024, 11, 1372023. [Google Scholar] [CrossRef]
- Ortizo, R.G.G.; Sharma, V.; Tsai, M.L.; Wang, J.X.; Sun, P.P.; Nargotra, P.; Kuo, C.H.; Chen, C.W.; Dong, C.D. Extraction of Novel Bioactive Peptides from Fish Protein Hydrolysates by Enzymatic Reactions. Appl. Sci. 2023, 13, 5768. [Google Scholar] [CrossRef]
- Hayes, M.; Naik, A.; Mora, L.; Iñarra, B.; Ibarruri, J.; Bald, C.; Cariou, T.; Reid, D.; Gallagher, M.; Dragøy, R.; et al. Generation, Characterisation and Identification of Bioactive Peptides from Mesopelagic Fish Protein Hydrolysates Using in Silico and in Vitro Approaches. Mar. Drugs 2024, 22, 297. [Google Scholar] [CrossRef] [PubMed]
- Yathisha, U.G.; Bhat, I.; Karunasagar, I.; Mamatha, B.S. Antihypertensive activity of fish protein hydrolysates and its peptides. Crit. Rev. Food Sci. Nutr. 2019, 59, 2363–2374. [Google Scholar] [CrossRef]
- Kasran, M.; Mohamed, N.; Ishak, Z. Extraction of protein and antioxidant and antihypertensive properties of protein hydrolysates derived from black tilapia (Oreochromis placidus). J. Trop. Agric. Food Sci. 2023, 51, 51–60. [Google Scholar]
- Kubinyi, E.; Bel Rhali, S.; Sándor, S.; Szabó, A.; Felföldi, T. Gut Microbiome Composition is Associated with Age and Memory Performance in Pet Dogs. Animals 2020, 10, 1488. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Hemalatha, R.; Jyothirmayi, T. Fish protein hydrolysates: Proximate composition, amino acid composition, antioxidant activities and applications: A review. Food Chem. 2012, 135, 3020–3038. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Santivarangkna, C.; Benjakul, S.; Maqsood, S. Fish protein hydrolysates as a health-promoting ingredient-recent update. Nutr. Rev. 2022, 80, 1013–1026. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Hasegawa, A.; Hosoi, Y.; Sugiura, K. Association between breed, gender and age in relation to cardiovascular disorders in insured dogs in Japan. J. Vet. Med. Sci. 2016, 78, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Svensson, M.; Selling, J.; Dirven, M. Myxomatous Mitral Valve Disease in Large Breed Dogs: Survival Characteristics and Prognostic Variables. Vet. Sci. 2024, 11, 136. [Google Scholar] [CrossRef]
- Druzhaeva, N.; Nemec Svete, A.; Tavčar-Kalcher, G.; Babič, J.; Ihan, A.; Pohar, K.; Krapež, U.; Domanjko Petrič, A. Effects of Coenzyme Q10 Supplementation on Oxidative Stress Markers, Inflammatory Markers, Lymphocyte Subpopulations, and Clinical Status in Dogs with Myxomatous Mitral Valve Disease. Antioxidants 2022, 11, 1427. [Google Scholar] [CrossRef]
- Larsen, J.A.; Fascetti, A.J. The role of taurine in cardiac health in dogs and cats. Adv. Small Anim. Care 2020, 1, 227–238. [Google Scholar] [CrossRef]
- Lordan, S.; Ross, R.P.; Stanton, C. Marine bioactives as functional food ingredients: Potential to reduce the incidence of chronic diseases. Mar. Drugs 2011, 9, 1056–1100. [Google Scholar] [CrossRef] [PubMed]
- Pasławski, R.; Kurosad, A.; Ząbek, A.; Pasławska, U.; Noszczyk-Nowak, A.; Michałek, M.; Młynarz, P. Effect of 6-Month Feeding with a Diet Enriched in EPA + DHA from Fish Meat on the Blood Metabolomic Profile of Dogs with Myxomatous Mitral Valve Disease. Animals 2021, 11, 3360. [Google Scholar] [CrossRef]
- Liu, H.; Yang, Y.; Liu, Y.; Cui, L.; Fu, L.; Li, B. Various bioactive peptides in collagen hydrolysate from salmo salar skin and the combined inhibitory effects on atherosclerosis in vitro and in vivo. Food Res. Int. 2022, 157, 111281. [Google Scholar] [CrossRef]
- Maneesai, P.; Wattanathorn, J.; Potue, P.; Khamseekaew, J.; Rattanakanokchai, S.; Thukham-Mee, W.; Muchimapura, S.; Pakdeechote, P. Cardiovascular complications are resolved by tuna protein hydrolysate supplementation in rats fed with a high-fat diet. Sci. Rep. 2023, 13, 12280. [Google Scholar] [CrossRef]
- Brown, S.; Atkins, C.; Bagley, R.; Carr, A.; Cowgill, L.; Davidson, M.; Egner, B.; Elliott, J.; Henik, R.; Labato, M.; et al. Guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. J. Vet. Intern. Med. 2007, 21, 542–558. [Google Scholar] [CrossRef]
- Available online: https://todaysveterinarypractice.com/cardiology/systemic-hypertension-in-dogs-cats/ (accessed on 10 August 2024).
- Aspevik, T.; Oterhals, Å.; Rønning, S.B.; Altintzoglou, T.; Wubshet, S.G.; Gildberg, A.; Afseth, N.K.; Whitaker, R.D.; Lindberg, D. Valorization of Proteins from Co- and By-Products from the Fish and Meat Industry. Top. Curr. Chem. 2017, 375, 53. [Google Scholar] [CrossRef]
- Atanassova, M.R.; Stangeland, J.K.; Lausen, S.E.; Dahl, T.H.; Barnung, T.; Larssen, W.E. Antioxidants, ACE I Inhibitory Peptides, and Physicochemical Composition, with a Special Focus on Trace Elements and Pollutants, of SPRING Spawning Atlantic Herring (Clupea harengus) Milt and Hydrolysates for Functional Food Applications. Fishes 2024, 9, 456. [Google Scholar] [CrossRef]
- Aissaoui, N.; Abidi, F.; Marzouki, M.N. ACE inhibitory and antioxidant activities of red scorpionfish (Scorpaena notata) protein hydrolysates. J. Food Sci. Technol. 2015, 52, 7092–7102. [Google Scholar] [CrossRef]
- Taheri, A.; Bakhshizadeh, G.A. Antioxidant and ACE Inhibitory Activities of Kawakawa (Euthynnus affinis) Protein Hydrolysate Produced by Skipjack Tuna Pepsin. J. Aquat. Food Prod. Technol. 2019, 29, 148–166. [Google Scholar] [CrossRef]
- Johnson, K.A.; Lee, A.H.; Swanson, K.S. Nutrition and nutraceuticals in the changing management of osteoarthritis for dogs and cats. J. Am. Vet. Med. Assoc. 2020, 256, 1335–1341. [Google Scholar] [CrossRef]
- Barrouin-Melo, S.M.; Anturaniemi, J.; Sankari, S.; Griinari, M.; Atroshi, F.; Ounjaijean, S.; Hielm-Björkman, A.K. Evaluating oxidative stress, serological- and haematological status of dogs suffering from osteoarthritis, after supplementing their diet with fish or corn oil. Lipids Health Dis. 2016, 15, 139. [Google Scholar] [CrossRef]
- Manfredi, S.; Di Ianni, F.; Di Girolamo, N.; Canello, S.; Gnudi, G.; Guidetti, G.; Miduri, F.; Fabbi, M.; Daga, E.; Parmigiani, E.; et al. Effect of a commercially available fish-based dog food enriched with nutraceuticals on hip and elbow dysplasia in growing Labrador retrievers. Can. J. Vet. Res. 2018, 82, 154–158. [Google Scholar]
- Eckert, T.; Jährling-Butkus, M.; Louton, H.; Burg-Roderfeld, M.; Zhang, N.; Zhang, R.; Hesse, K.; Petridis, A.K.; Kožár, T.; Steinmeyer, J.; et al. Biophysical, Biochemical and Biomedical Evaluation of Collagen Hydrolysate in Comparison to Sulfated Glucosamine from Marine Organisms and Lipids of Fish Oil Origin Used as Chondroprotective Food Supplements. Preprints 2021. [Google Scholar]
- Heinrich, N.A.; Eisenschenk, M.; Harvey, R.G.; Nuttall, T. Skin Diseases of the Dog and Cat, 3rd. ed.; CRC Press: London, UK, 2018. [Google Scholar] [CrossRef]
- Hsu, C.; Marx, F.; Guldenpfennig, R.; Valizadegan, N.; de Godoy, M.R.C. The effects of hydrolyzed protein on macronutrient digestibility, fecal metabolites and microbiota, oxidative stress and inflammatory biomarkers, and skin and coat quality in adult dogs. J. Anim. Sci. 2024, 102, skae057. [Google Scholar] [CrossRef]
- Tresamol, P.V.; Nissar, B.J. Nutritional approaches for management of dermatological disorders in canine. Intas Polivet 2020, 21, 474–477. [Google Scholar]
- Fritsch, D.A.; Roudebush, P.; Allen, T.A.; Leventhal, P.S.; Brejda, J.; Hahn, K.A. Effect of Two Therapeutic Foods in Dogs with Chronic Nonseasonal Pruritic Dermatitis. Int. J. Appl. Res. Vet. Med. 2010, 8, 146–154. [Google Scholar]
- Szczepanik, M.P.; Gołyński, M.; Wilkołek, P.; Kalisz, G. Evaluation of a hydrolysed salmon and pea hypoallergenic diet application in dogs and cats with cutaneous adverse food reaction. Pol. J. Vet. Sci. 2022, 25, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Meineri, G.; Martello, E.; Radice, E.; Bruni, N.; Saettone, V.; Atuahene, D.; Armandi, A.; Testa, G.; Ribaldone, D.G. Chronic Intestinal Disorders in Humans and Pets: Current Management and the Potential of Nutraceutical Antioxidants as Alternatives. Animals 2022, 12, 812. [Google Scholar] [CrossRef] [PubMed]
- Dandrieux, J.R. Inflammatory bowel disease versus chronic enteropathy in dogs: Are they one and the same? J. Small Anim. Pract. 2016, 57, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Allenspach, K. Clinical immunology and immunopathology of the canine and feline intestine. Vet. Clin. N. Am. Small Anim. Pract. 2011, 41, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Awuchi, C.G.; Chukwu, C.N.; Iyiola, A.O.; Noreen, S.; Morya, S.; Adeleye, A.O.; Twinomuhwezi, H.; Leicht, K.; Mitaki, N.B.; Okpala, C.O.R. Bioactive Compounds and Therapeutics from Fish: Revisiting Their Suitability in Functional Foods to Enhance Human Wellbeing. BioMed Res. Int. 2022, 2022, 3661866. [Google Scholar] [CrossRef]
- Jergens, A.E.; Heilmann, R.M. Canine chronic enteropathy-Current state-of-the-art and emerging concepts. Front. Vet. Sci. 2022, 9, 923013. [Google Scholar] [CrossRef]
- Marks, S.L.; Laflamme, D.P.; McAloose, D. Dietary trial using a commercial hypoallergenic diet containing hydrolyzed protein for dogs with inflammatory bowel disease. Vet. Ther. Res. Appl. Vet. Med. 2002, 3, 109–118. [Google Scholar]
- Marchesi, M.C.; Timpano, C.C.; Busechian, S.; Pieramati, C.; Rueca, F. The role of diet in managing inflamatory bowel disease affected dogs: A retrospective cohort study on 76 cases. Vet. Ital. 2017, 53, 297–302. [Google Scholar] [CrossRef]
- Ontsouka, E.C.; Burgener, I.A.; Luckschander-Zeller, N.; Blum, J.W.; Albrecht, C. Fish-meal diet enriched with omega-3 PUFA and treatment of canine chronic enteropathies. Eur. J. Lipid Sci. Technol. 2012, 114, 412–422. [Google Scholar] [CrossRef]
- Simpson, K.W.; Miller, M.L.; Loftus, J.P.; Rishniw, M.; Frederick, C.E.; Wakshlag, J.J. Randomized controlled trial of hydrolyzed fish diets in dogs with chronic enteropathy. J. Vet. Intern. Med. 2023, 37, 2334–2343. [Google Scholar] [CrossRef]
- Zinn, K.E.; Hernot, D.C.; Fastinger, N.D.; Karr-Lilienthal, L.K.; Bechtel, P.J.; Swanson, K.S.; Fahey, G.C., Jr. Fish protein substrates can substitute effectively for poultry by-product meal when incorporated in high-quality senior dog diets. J. Anim. Physiol. Anim. Nutr. 2009, 93, 447–455. [Google Scholar] [CrossRef]
- Sechi, S.; Fiore, F.; Chiavolelli, F.; Dimauro, C.; Nudda, A.; Cocco, R. Oxidative stress and food supplementation with antioxidants in therapy dogs. Can. J. Vet. Res. 2017, 81, 206–216. [Google Scholar]
- Brown, S.A. Oxidative stress and chronic kidney disease. Vet. Clin. N. Am. Small Anim. Pract. 2008, 38, 157–166. [Google Scholar] [CrossRef]
- Nasri, R.; Abdelhedi, O.; Jemil, I.; Daoued, I.; Hamden, K.; Kallel, C.; Elfeki, A.; Lamri-Senhadji, M.; Boualga, A.; Nasri, M.; et al. Ameliorating effects of goby fish protein hydrolysates on high-fat-high-fructose diet-induced hyperglycemia, oxidative stress and deterioration of kidney function in rats. Chem.-Biol. Interact. 2015, 242, 71–80. [Google Scholar] [CrossRef]
- de Godoy, M.R.C.; Conway, C.E.; Mcleod, K.R.; Harmon, D.L. Influence of feeding a fish oil-containing diet to young, lean, adult dogs: Effects on lipid metabolites, postprandial glycaemia and body weight. Arch. Anim. Nutr. 2015, 69, 499–514. [Google Scholar] [CrossRef]
- Riyadi, P.H.; Atho’illah, M.F.; Tanod, W.A.; Rahmawati, I.S. Tilapia viscera hydrolysate extract alleviates oxidative stress and renal damage in deoxycorticosterone acetate-salt induced hypertension rats. Vet. World 2020, 13, 2477–2483. [Google Scholar] [CrossRef]
- Ravić, B.; Debeljak-Martacić, J.; Pokimica, B.; Vidović, N.; Ranković, S.; Glibetić, M.; Stepanović, P.; Popović, T. The Effect of Fish Oil-Based Foods on Lipid and Oxidative Status Parameters in Police Dogs. Biomolecules 2022, 12, 1092. [Google Scholar] [CrossRef]
- Theysgeur, S.; Cudennec, B.; Deracinois, B.; Perrin, C.; Guiller, I.; Lepoudère, A.; Flahaut, C.; Ravallec, R. New Bioactive Peptides Identified from a Tilapia Byproduct Hydrolysate Exerting Effects on DPP-IV Activity and Intestinal Hormones Regulation after Canine Gastrointestinal Simulated Digestion. Molecules 2020, 26, 136. [Google Scholar] [CrossRef] [PubMed]
- Sordo, L.; Gunn-Moore, D.A. Cognitive Dysfunction in Cats: Update on Neuropathological and Behavioural Changes Plus Clinical Management. Vet. Rec. 2021, 188, e3. [Google Scholar] [CrossRef] [PubMed]
- Zicker, S.C.; Jewell, D.E.; Yamka, R.M.; Milgram, N.W. Evaluation of cognitive learning, memory, psychomotor, immunologic, and retinal functions in healthy puppies fed foods fortified with docosahexaenoic acid-rich fish oil from 8 to 52 weeks of age. J. Am. Vet. Med. Assoc. 2012, 241, 583–594. [Google Scholar] [CrossRef]
- Pan, Y.; Araujo, J.A.; Burrows, J.; de Rivera, C.; Gore, A.; Bhatnagar, S.; Milgram, N.W. Cognitive enhancement in middle-aged and old cats with dietary supplementation with a nutrient blend containing fish oil, B vitamins, antioxidants and arginine. Br. J. Nutr. 2013, 110, 40–49. [Google Scholar] [CrossRef]
- Pan, Y.; Kennedy, A.D.; Jönsson, T.J.; Milgram, N.W. Cognitive enhancement in old dogs from dietary supplementation with a nutrient blend containing arginine, antioxidants, B vitamins and fish oil. Br. J. Nutr. 2018, 119, 349–358. [Google Scholar] [CrossRef]
- Chataigner, M.; Mortessagne, P.; Lucas, C.; Pallet, V.; Layé, S.; Mehaignerie, A.; Bouvret, E.; Dinel, A.L.; Joffre, C. Dietary fish hydrolysate supplementation containing n-3 LC-PUFAs and peptides prevents short-term memory and stress response deficits in aged mice. Brain Behav. Immun. 2021, 91, 716–730. [Google Scholar] [CrossRef]
- Landsberg, G.M.; Mougeot, I.; Kelly, S.; Milgram, N.W. Assessment of noise-induced fear and anxiety in dogs: Modification by a novel fish hydrolysate supplemented diet. J. Vet. Behav. 2015, 10, 391–398. [Google Scholar] [CrossRef]
- Bernet, F.; Montel, V.; Noël, B.; Dupouy, J.P. Diazepam-like effects of a fish protein hydrolysate (Gabolysat PC60) on stress responsiveness of the rat pituitary-adrenal system and sympathoadrenal activity. Psychopharmacology 2000, 149, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Freret, T.; Largilliere, S.; Nee, G.; Coolzaet, M.; Corvaisier, S.; Boulouard, M. Fast Anxiolytic-Like Effect Observed in the Rat Conditioned Defensive Burying Test, after a Single Oral Dose of Natural Protein Extract Products. Nutrients 2021, 13, 2445. [Google Scholar] [CrossRef]
- Titeux, E.; Padilla, S.; Paragon, B.M.; Gilbert, C. Effects of a new dietary supplement on behavioural responses of dogs exposed to mild stressors. Vet. Med. Sci. 2021, 7, 1469–1482. [Google Scholar] [CrossRef]
- Jeusette, I.C.; Tami, G.; Fernández, A.; Torre, C.; Tvarijonaviciute, A.; Cerón, J.J.; Salas-Mani, A.; Fatjó, J. Evaluation of a new prescription diet with lemon balm, fish peptides, oligofructose and L-tryptophan to reduce urinary cortisol, used as a marker of stress, in cats. J. Vet. Behav. 2021, 42, 30–36. [Google Scholar] [CrossRef]
- Porcheron, G.; Bodet, M.; Poiron, K.; Brault, J.; Massal, N.; Roy, O.; Fantini, O. Supplementation Effects of an Alpha-Casozepine and White Fish Muscle Hydrolyzed Complementary Feed on Canine Separation-Related Disorders and Quality of Life of Dogs and Their Pet Caregivers. Open J. Vet. Med. 2023, 13, 68–81. [Google Scholar] [CrossRef]
- Ephraim, E.; Brockman, J.A.; Jewell, D.E. A Diet Supplemented with Polyphenols, Prebiotics and Omega-3 Fatty Acids Modulates the Intestinal Microbiota and Improves the Profile of Metabolites Linked with Anxiety in Dogs. Biology 2022, 11, 976. [Google Scholar] [CrossRef] [PubMed]
- Dinel, A.-L.; Lucas, C.; Le Faouder, J.; Bouvret, E.; Pallet, V.; Layé, S.; Joffre, C. Supplementation with low molecular weight peptides from fish protein hydrolysate reduces acute mild stress-induced corticosterone secretion and modulates stress responsive gene expression in mice. J. Funct. Foods 2021, 76, 104292. [Google Scholar] [CrossRef]
- Venslauskas, K.; Navickas, K.; Nappa, M.; Kangas, P.; Mozūraitytė, R.; Šližytė, R.; Župerka, V. Energetic and Economic Evaluation of Zero-Waste Fish Co-Stream Processing. Int. J. Environ. Res. Public Health 2021, 18, 2358. [Google Scholar] [CrossRef]
- Available online: https://www.fortunebusinessinsights.com/industry-reports/pet-food-market-100554 (accessed on 23 September 2024).
- Nagodawithana, T.W.; Nelles, L.; Trivedi, N.B. Protein Hydrolysates as Hypoallergenic, Flavors and Palatants for Companion Animals. In Protein Hydrolysates in Biotechnology; Pasupuleti, V., Demain, A., Eds.; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar] [CrossRef]
- Watson, P.E.; Thomas, D.G.; Bermingham, E.N.; Schreurs, N.M.; Parker, M.E. Drivers of Palatability for Cats and Dogs—What It Means for Pet Food Development. Animals 2023, 13, 1134. [Google Scholar] [CrossRef]
- Samant, S.S.; Crandall, P.G.; Jarma Arroyo, S.E.; Seo, H.S. Dry Pet Food Flavor Enhancers and Their Impact on Palatability: A Review. Foods 2021, 10, 2599. [Google Scholar] [CrossRef]
- Available online: https://www.precisionbusinessinsights.com/market-reports/pet-food-palatants-market (accessed on 23 September 2024).
- Folador, J.F.; Karr-Lilienthal, L.K.; Parsons, C.M.; Bauer, L.L.; Utterback, P.L.; Schasteen, C.S.; Bechtel, P.J.; Fahey, G.C., Jr. Fish meals, fish components, and fish protein hydrolysates as potential ingredients in pet foods. J. Anim. Sci. 2006, 84, 2752–2765. [Google Scholar] [CrossRef]
- Aldrich, G.C.; Koppel, K. Pet Food Palatability Evaluation: A Review of Standard Assay Techniques and Interpretation of Results with a Primary Focus on Limitations. Animals 2015, 5, 43–55. [Google Scholar] [CrossRef] [PubMed]
- van Rooijen, C.; Bosch, G.; van der Poel, A.F.; Wierenga, P.A.; Alexander, L.; Hendriks, W.H. The Maillard reaction and pet food processing: Effects on nutritive value and pet health. Nutr. Res. Rev. 2013, 26, 130–148. [Google Scholar] [CrossRef]
- Pekel, A.Y.; Mülazımoğlu, S.B.; Acar, N. Taste preferences and diet palatability in cats. J. Appl. Anim. Res. 2020, 48, 281–292. [Google Scholar] [CrossRef]
- Salaun, F.; Blanchard, G.; Le Paih, L.; Roberti, F.; Niceron, C. Impact of macronutrient composition and palatability in wet diets on food selection in cats. J. Anim. Physiol. Anim. Nutr. 2017, 101, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Guilherme-Fernandes, J.; Aires, T.; Fonseca, A.J.M.; Yergaliyev, T.; Camarinha-Silva, A.; Lima, S.A.C.; Maia, M.R.G.; Cabrita, A.R.J. Squid meal and shrimp hydrolysate as novel protein sources for dog food. Front. Vet. Sci. 2024, 11, 1360939. [Google Scholar] [CrossRef] [PubMed]
- Cottrell, R.S.; Blanchard, J.L.; Halpern, B.S.; Metian, M.; Froehlich, H.E. Global adoption of novel aquaculture feeds could substantially reduce forage fish demand by 2030. Nat. Food 2020, 1, 301–308. [Google Scholar] [CrossRef]
- Sandström, V.; Chrysafi, A.; Lamminen, M.; Troell, M.; Jalava, M.; Piipponen, J.; Siebert, S.; van Hal, O.; Virkki, V.; Kummu, M. Food system by-products upcycled in livestock and aquaculture feeds can increase global food supply. Nat. Food 2022, 3, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Naylor, R.L.; Hardy, R.W.; Buschmann, A.H.; Bush, S.R.; Cao, L.; Klinger, D.H.; Little, D.C.; Lubchenco, J.; Shumway, S.E.; Troell, M. A 20-year retrospective review of global aquaculture. Nature 2021, 591, 551–563. [Google Scholar] [CrossRef]
- Miao, W.; Wang, W. Trends of aquaculture production and trade: Carp, tilapia, and shrimp. Asian Fish. Sci. 2020, 33 (Suppl. 1), 1–10. [Google Scholar] [CrossRef]
- Boyd, C.E.; D’Abramo, L.R.; Glencross, B.D.; Huyben, D.C.; Juarez, L.M.; Lockwood, G.S.; McNevin, A.A.; Tacon, A.G.J.; Teletchea, F.; Tomasso, J.R., Jr.; et al. Achieving sustainable aquaculture: Historical and current perspectives and future needs and challenges. J. World Aquac. Soc. 2020, 51, 578–633. [Google Scholar] [CrossRef]
- Shahzad, S.M. Fish as a Healthy Source of Human Nutrition: An Exploratory Study. J. Naut. Eye Strateg. Stud. 2024, 4, 1–22. [Google Scholar]
- Thazeem, B.; Umesh, M.; Sarojini, S.; Allwyn Vyas, G.; Adhithya Sankar, S.; Sapthami, K.; Suresh, S.; Merin Stanly, L. Developments in Feeds in Aquaculture Sector: Contemporary Aspects. In Aquaculture Science and Engineering; Balasubramanian, B., Liu, W.C., Sattanathan, G., Eds.; Springer: Singapore, 2022. [Google Scholar] [CrossRef]
- Abdullah, F.I.; Hamid, N.H.; Abd Karim, M.M.; Ismail, M.F.; Sin, N.L.W.W.; Kamaruddin, M.S. Fish protein hydrolysate for fish health. Biocatal. Agric. Biotechnol. 2024, 60, 103292. [Google Scholar] [CrossRef]
- Chaklader, M.R.; Fotedar, R.; Howieson, J.; Siddik, M.A.; Foysal, M.J. The ameliorative effects of various fish protein hydrolysates in poultry by-product meal based diets on muscle quality, serum biochemistry and immunity in juvenile barramundi, Lates calcarifer. Fish Shellfish. Immunol. 2020, 104, 567–578. [Google Scholar] [CrossRef]
- Sampathkumar, K.; Yu, H.; Loo, S.C.J. Valorisation of industrial food waste into sustainable aquaculture feeds. Future Foods 2023, 7, 100240. [Google Scholar] [CrossRef]
- Gunathilaka, B.E.; Kim, S.; Herault, M.; Fournier, V.; Lee, K.-J. Marine protein hydrolysates as a substitute of squid-liver powder in diets for Pacific white shrimp (Litopenaeus vannamei). Aquac. Res. 2022, 53, 4059–4068. [Google Scholar] [CrossRef]
- Sezu, N.H.; Nandi, S.K.; Suma, A.Y.; Kari, Z.A.; Wei, L.S.; Seguin, P.; Herault, M.; Hossain, M.S.; Irwan Khoo, M.; El-Sayed Hemdan, I.; et al. Ameliorative effects of different dietary levels of fish protein hydrolysate (FPH) on growth and reproductive performance, feed stability, tissues biochemical composition, haematobiochemical profile, liver histology, and economic analysis of Pabda (Ompok pabda) broodstock. Aquac. Res. 2024, 2024, 6044920. [Google Scholar] [CrossRef]
- Kabir, M.A.; Nandi, S.K.; Suma, A.Y.; Abdul Kari, Z.; Mohamad Sukri, S.A.; Wei, L.S.; Al Mamun, A.; Seguin, P.; Herault, M.; Khoo, M.I.; et al. The Potential of Fish Protein Hydrolysate Supplementation in Nile Tilapia Diets: Effects on Growth and Health Performance, Disease Resistance, and Farm Economic Analysis. Appl. Biochem. Biotechnol. 2024, 196, 7145–7167. [Google Scholar] [CrossRef]
- Kotzamanis, Y.P.; Gisbert, E.; Gatesoupe, F.J.; Zambonino Infante, J.; Cahu, C. Effects of different dietary levels of fish protein hydrolysates on growth, digestive enzymes, gut microbiota, and resistance to Vibrio anguillarum in European sea bass (Dicentrarchus labrax) larvae. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 147, 205–214. [Google Scholar] [CrossRef]
- Colombo, S.M.; Roy, K.; Mraz, J.; Wan, A.H.; Davies, S.J.; Tibbetts, S.M.; Øverland, S.M.; Francis, D.S.; Rocker, M.M.; Gasco, L.; et al. Towards achieving circularity and sustainability in feeds for farmed blue foods. Rev. Aquac. 2023, 15, 1115–1141. [Google Scholar] [CrossRef]
- Refstie, S.; Olli, J.J.; Standal, H. Feed intake, growth, and protein utilisation by post-smolt Atlantic salmon (Salmo salar) in response to graded levels of fish protein hydrolysate in the diet. Aquaculture 2004, 239, 331–349. [Google Scholar] [CrossRef]
- Tang, H.G.; Wu, T.X.; Zhao, Z.Y.; Pan, X.D. Effects of fish protein hydrolysate on growth performance and humoral immune response in large yellow croaker (Pseudosciaena crocea R.). J. Zhejiang Univ. Sci. B 2008, 9, 684–690. [Google Scholar] [CrossRef]
- Zheng, K.; Liang, M.; Yao, H.; Wang, J.; Chang, Q. Effect of dietary fish protein hydrolysate on growth, feed utilisation and IGF-I levels of Japanese flounder (Paralichthys olivaceus). Aquac. Nutr. 2012, 18, 297–303. [Google Scholar] [CrossRef]
- Siddik, M.A.; Pham, H.D.; Francis, D.S.; Vo, B.V.; Shahjahan, M. Dietary supplementation of fish protein hydrolysate in high plant protein diets modulates growth, liver and kidney health, and immunity of barramundi (Lates calcarifer). Aquac. Nutr. 2021, 27, 86–98. [Google Scholar] [CrossRef]
- Siddaiah, G.M.; Kumar, R.; Kumari, R.; Damle, D.K.; Rasal, K.D.; Manohar, V.; Sundaray, J.K.; Pillai, B.R. Dietary supplementation of fish protein hydrolysate improves growth, feed efficiency and immune response in freshwater carnivore fish, Channa striata fingerlings. Aquac. Res. 2022, 53, 3401–3415. [Google Scholar] [CrossRef]
- Suratip, N.; Charoenwattanasak, S.; Klahan, R.; Herault, M.; Yuangsoi, B. An investigation into the effects of using protein hydrolysate in low fish meal diets on growth performance, feed utilization and health status of snakehead fish (Channa striata) fingerling. Aquac. Rep. 2023, 30, 101623. [Google Scholar] [CrossRef]
- Tola, S.; Sommit, N.; Seel-audom, M.; Khamtavee, P.; Waiho, K.; Boonmee, T.; Yuangsoi, B.; Munpholsri, N. Effects of dietary tuna hydrolysate supplementation on feed intake, growth performance, feed utilization and health status of Asian sea bass (Lates calcarifer) fed a low fish meal soybean meal-based diet. Aquac. Res. 2022, 53, 3898–3912. [Google Scholar] [CrossRef]
- Suma, A.Y.; Nandi, S.K.; Abdul Kari, Z.; Goh, K.W.; Wei, L.S.; Tahiluddin, A.B.; Seguin, P.; Herault, M.; Al Mamun, A.; Téllez-Isaías, G.; et al. Beneficial Effects of Graded Levels of Fish Protein Hydrolysate (FPH) on the Growth Performance, Blood Biochemistry, Liver and Intestinal Health, Economics Efficiency, and Disease Resistance to Aeromonas hydrophila of Pabda (Ompok pabda) Fingerling. Fishes 2023, 8, 147. [Google Scholar] [CrossRef]
- Sandbakken, I.S.; Five, K.K.; Bardal, T.; Knapp, J.L.; Olsen, R.E. Salmon hydrolysate as a protein source for Atlantic salmon; prion content and effects on growth, digestibility and gut health. Aquaculture 2023, 576, 739863. [Google Scholar] [CrossRef]
- Jaies, I.; Qayoom, I.; Saba, F.; Khan, S. Fish wastes as sources of fertiliser and manure. In Fish Wastes to Valuable Products, 1st ed.; Mqsood, S., Naseer, M.N., Benjakul, S., Zaidi, A.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2024; pp. 329–338. [Google Scholar]
- du Jardin, P. Plant biostimulants: Definition, concept, main categories & regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar]
- Lindsey, A.J.; Thoms, A.W.; Christians, N.E. Evaluation of Humic Fertilizers on Kentucky Bluegrass Subjected to Simulated Traffic. Agronomy 2021, 11, 611. [Google Scholar] [CrossRef]
- Madende, M.; Hayes, M. Fish By-Product Use as Biostimulants: An Overview of the Current State of the Art, Including Relevant Legislation and Regulations within the EU and USA. Molecules 2020, 25, 1122. [Google Scholar] [CrossRef]
- Domínguez, H.; Iñarra, B.; Labidi, J.; Bald, C. Fish Viscera Hydrolysates and Their Use as Biostimulants for Plants as an Approach towards a Circular Economy in Europe: A Review. Sustainability 2024, 16, 8779. [Google Scholar] [CrossRef]
- Malécange, M.; Sergheraert, R.; Teulat, B.; Mounier, E.; Lothier, J.; Sakr, S. Biostimulant Properties of Protein Hydrolysates: Recent Advances and Future Challenges. Int. J. Mol. Sci. 2023, 24, 9714. [Google Scholar] [CrossRef]
- Takayama, S.; Sakagami, Y. Peptide signalling in plants. Curr. Opin. Plant Biol. 2002, 5, 382–387. [Google Scholar] [CrossRef]
- Bulgari, R.; Cocetta, G.; Trivellini, A.; Vernieri, P.; Ferrante, A. Biostimulants and crop responses: A review. Biol. Agric. Hortic. 2014, 31, 1–17. [Google Scholar] [CrossRef]
- Rajabi Hamedani, S.; Rouphael, Y.; Colla, G.; Colantoni, A.; Cardarelli, M. Biostimulants as a Tool for Improving Environmental Sustainability of Greenhouse Vegetable Crops. Sustainability 2020, 12, 5101. [Google Scholar] [CrossRef]
- Rouphael, Y.; Franken, P.; Schneider, C.; Schwarz, D.; Giovannetti, M.; Agnolucci, M.; De Pascale, S.; Bonini, P.; Colla, G. Arbuscular mycorrhizal fungi act as biostimulants in horticultural crops. Sci. Hortic. 2015, 196, 91–108. [Google Scholar] [CrossRef]
- Petrova, I.; Tolstorebrov, I.; Eikevik, T.M. Production of fish protein hydrolysates step by step: Technological aspects, equipment used, major energy costs and methods of their minimizing. Int. Aquat. Res. 2018, 10, 223–241. [Google Scholar] [CrossRef]
- Ruzzi, M.; Cola, G.; Rouphael, Y. Biostimulants in Agriculture II: Towards a sustainable future. Crop Prod. Physiol. 2024, 15, 1427283. [Google Scholar] [CrossRef]
| Product | Manufacturers | Ingredients/Raw Material | Product Description | Specific Features |
|---|---|---|---|---|
| Salmigo® Protect L60 | Biomega (Skogvåg, Vestland, Norway) [160] | Fresh, high-quality Atlantic salmon leftovers |
|
|
| Salmigo® Active | Fresh, high-quality Atlantic salmon leftovers |
|
| |
| Dog Adult Dermal Hydrolysed Fish | Trovet + Plus (De Vergert 4, 6681 Le Bemmel, Netherlands) [126] | White FPHs, rice, potato, apple, fish oil, linseed, minerals, borage oil |
|
|
| Cat Intestinal Fresh Hydrolysed White Fish | Extruded rice, rice protein, hydrolysed white fish, fish oil, sunflower oil, hydrolysed fish, krill Antarctic, apple fibre, vegetable fibres, psyllium, hydrolysed yeast cell wall, and inulin |
|
| |
| Multi-Purpose Treat for cats (Hydrolysed Protein) | Rice, salmon protein (hydrolysate), sodium chloride, sugar, collagen (hydrolysate), cellulose, poultry fat |
|
| |
| Sensitive Soft Chews Salmon | Optima Nova (ARGANDA DEL REY, Madrid, Spain) | Salmon and salmon by-products, tapioca and potato, and fats |
|
|
| Adult Sensitive Salmon and Potato | Fresh, ground, hydrolysed salmon, dehydrated potato, potato protein, oil, sugar beet meal, yeast, FOS, MOS |
|
| |
| Medica Hypoallergenic PLUS Salmon for dogs | Select Gold (Germany) | Potato flakes, salmon protein, hydrolysates, hydrolysed poultry liver fish oil, potato protein, sunflower oil, poultry fat |
|
|
| Medica Hypoallergenic PLUS Fish and rice for cats | Rice, potato protein, hydrolysed fish protein, poultry fat, potato starch, dried beet pulp, beef fat, cellulose, sardine oil, hydrolysed poultry liver, salmon oil, and linseed oil |
|
| |
| Functional treats—sensitive protein | Boxby (Berltsum, Friesland, Netherlands) | Hydrolysed salmon, potato flakes, rice meal, omega-3 fatty acids, propylene glycol, omega-6 fatty acids |
|
|
| SEACURE®—Daily protein supplement for dogs | Proper Nutrition (West Chester, PA, USA). | Made from Pacific whiting caught in the Pacific Northwest |
|
|
| HY Food Allergen Management | Specific Cat FDD (Dechra, Northwich, UK) | Rice, rice protein, hydrolysed salmon protein, pork fat, cellulose, protein hydrolysate, fish oil, psyllium husks, rosemary extract |
|
|
| Veterinary HPM® Hypoallergic dog foodwith hydrolysed fish protein | Virbac UK (Woolpit, Bury, Suffolk, UK) | Potato starch, hydrolysed fish protein, animal fats, lignocellulose, minerals, hydrolysed pork and poultry proteins, beet pulp, FOS, mono di and triglycerides of fatty acids, Lactobacillus acidophilus |
|
|
| Vet Life Cat Ultra hypo | Farmina Pet Foods (Essex, UK) [84] | Rice starch, hydrolysed fish protein, fish oil, potassium chloride, calcium carbonate, mono-di-calcium phosphate |
|
|
| Feline c/d stress urinary care—ocean fish | Hill’s® Prescription Diet® [124,128] | Brewer’s rice, ocean FPHs, chicken- and turkey meal, maize gluten meal, animal fats, tuna fish meal, soya bean oil, minerals, fish oil, linseeds |
|
|
| Canine skin support potato salmon formula | Salmon proteins, potato protein, potato starch, pork fat, soybean oil, pork flavour, fish oil, fish meal. |
|
| |
| Natural veterinary diet hf hydrolysed intolerance, salmon, dry cat food | Blue Buffalo (Wilton, CT, USA) | Salmon hydrolysate, peas, potatoes, pea starch, canola oil, pea protein, flaxseed, pea fibre, fish oil, pumpkin, dried kelp, oil of rosemary. |
|
|
| Pure mackerel hydrolysate | iQi—Trusted pet food ingredients (MG Amersfoort, The Netherlands) Fiordo Austral (Calbuco, Chile) & ORIVO (Molde, Norway) | Mackerel processing side streams |
|
|
| Pure salmon hydrolysate | Salmon processing side streams |
|
| |
| Pure sardine hydrolysate | Sardine processing side streams |
|
| |
| Pure tuna hydrolysate | Tuna processing side streams |
|
| |
| Veterinary diet—hypoallergenic | [129] | Dehydrated salmon, yellow peas, hydrolysed salmon protein, buckwheat, salmon oil, hydrolysed salmon gravy, minerals, dried Ascophyllum nodosum, MOS, FOS |
|
|
| Product | Manufacturers | Market Presence | Ingredients/Raw Material | Product Description | Specific Features | Average Price Point (per 200 kg) |
|---|---|---|---|---|---|---|
| PalasuranceTM | Kemin (palatants produced in (De Moines, IA, USA, (Cavriago RE), Italy, and Macuco, Valinhos, Brazil) | Europe, USA, Australia | Salmon, lamb, beef | Available in both dry and liquid forms |
| USD 6.29 (per kg) |
| PalivateTM | Europe, USA, Australia | Duck, chicken, salmon, soy, lamb | Palatant for wet pet foods meeting flavour, pH, and texture expectations |
| >USD 6.29 (per kg) | |
| PaltevaTM & Palteva PTM | Europe, USA, Australia | Duck, chicken, salmon, turkey, lamb, plant proteins | Natural flavour addition to pet diets |
| >USD 6.29 (per kg) | |
| Fish cream (cat food palatant) | Matchwell Nanjing Pet Products Supply Co., Ltd. (Nanjing, Jiangsu, China) | Available globally—online network | Ocean fish | Brown liquid, Crude protein (>15%), ash (<10%), moisture (<70%) |
| USD 2.00/barrel |
| Seafood liquid palatant | Available globally—online network | Seafood |
| USD 38.00 per barrel | ||
| CPSP® 90 and CPSP® G | Copalis Sea Solutions ® (Hauts, France, Symrise®, Teterboro, NJ, USA) | Europe and Asia | All fish—salmon and whitefish | Fine powder and liquid, soluble fish protein concentrates and hydrolysates, high value ingredient |
| Unknown |
| Range of palatants from fish, animal proteins | AFB International ® (St. Louis, MO, USA). | Global—US based company | Fish, chicken, pork, turkey, duck, lamb, and non-meat protein | Liquid and dry no-GMO, grain free, non-soy, non-wheat, gluten free and clean label palatant products |
| Depends on the form of the product |
| ProShore—Fish protein Concentrates (FPC) and Fish Protein Isolate (FPI) | BioMarine Ingredients Ireland (BII) (Ballybay, Monaghan, Ireland). | Western Europe; Eastern Europe; Middle East; Asia; Australia; North America; Africa; Central/South America | Unknown | High quality liquid and powdered ingredients for premium pet food formulations |
| Unknown |
| ProAtlantic—FPH | Unknown | Spray dried, free flowing with a neutral odour, and superior solubility |
| Unknown | ||
| PetSavio™ PPP 400 | Essentia Protein Solutions (Oak Tree Court Ankeny, IA 50021 USA) | Global—US based company | Plaice | Paste form with protein (39%), fat (0.6%), ash (<10%) |
| Unknown |
| PetSavio™ PCP 400 | Global—US-based company | Cod | Paste form with protein (40%), fat (1%), ash (9.5%) | Unknown | ||
| Seasoning Creame for cats (YCG-MT-915) | Jiangsu Yichong Biotechnology Company Limited (Suqian, Jiangsu, China) | Global—China-based company | Ocean fish, chicken liver, compound amino acids | Crude protein (>10%), ash (<10%) |
| Unknown |
| Seasoning powder for cats (YCF-MT-02) | Global—China-based company | Ocean fish, chicken liver, Brewer’s Yeast, acidity regulator | Crude protein (>30%), crude fat (<20%), Ash (<45%) |
| Unknown | |
| Fish cream for cats | Global—China-based company | Ocean fish, compound amino acids, Brewer’s Yeast, Protease | Deep brown colour product, crude protein (>10%), ash (<10%) |
| Unknown |
| Product | Manufacturers | Ingredients/Raw Material | Product Description | Specific Features | References |
|---|---|---|---|---|---|
| Actipal™ (Fish hydrolysate for fish and shrimp) | Diana Group S.r.L (Treviso, Italy) | Locally sourced, 100% fish side-stream |
|
| Gunathilaka et al. [182] Suma et al. [194] |
| Nutri™ Tuna (Tuna hydrolysates) | Locally sourced, 100% side-stream tuna |
|
| Tola et al., [193] | |
| Nutri™ Tuna Soluble extract (TSE) | Locally sourced, 100% side-stream tuna |
|
| Suratip et al. [192] | |
| Nutri™ Tuna Liver powder (TLP) | Locally sourced, 100% side-stream tuna |
|
| ||
| ProShore | Bio Marine Ingredients (Ballybay, Monaghan, Ireland) | Premium FPHs |
|
| |
| Fish Protein Hydrolysate Liquid | Janatha Fish Meal & Oil Products (Kota, India) | Liquid FPH with ≥40% hydrolysed protein |
| Siddaiah et al. [191] | |
| Fish Protein Hydrolysate Powder | Powder FPH with ≥80% hydrolysed protein |
| |||
| Fish Soluble Paste (Super attractant) | Liquid FPH with ≥60% hydrolysed protein |
| |||
| ScanPro™ | Scanbio Marine Group AS (Brattørkaia, Trondheim, Norway) | Norwegian salmon, pelagic and white fish | Enzymatically hydrolysed and concentrated fish protein is an excellent economical ingredient for feeds |
| |
| Natural Nautic® | Omega Protein Corporation (Reedville, VA, USA) | Menhaden | Fish meal is a high-quality protein made from menhaden and is naturally stabilised with mixed tocopherols |
| |
| Special Select® | Menhaden | Premium fish meal is a high-quality protein made from menhaden | |||
| SeaLac® | Menhaden | ||||
| Hydrolysed Salmon Protein | Sociedad Pesquera Landes Sa (Talcahuano, Chile) | By-products of the Chilean salmon industry | A perfect ingredient for fish nutrition with 71% protein, 20% lipids, and > 98% digestible |
|
| Products | Manufacturer | Origin | Bioactive | Application Method | Plant Trials | Bioactivity |
|---|---|---|---|---|---|---|
| CBio Biostimulant | C Fish (Charlie Vial) (Dunkineely, Co. Donegal, Ireland) | White fish/mixed fish composition autolysates and hydrolysates | Peptides, amino acids | Foliar, pre-planting, irrigation | Fruits and vegetables | Enhances plant resistance to biotic (insect, disease) and abiotic (heat, drought) stresses. |
| Radifarm | Valagro (Atessa, Chieti, Italy) | Commercial formulation | Amino acids, peptides, saponins, betaines, polysaccharides, vitamins, microelements | Irrigation, soil drench, foliar application | Fruits and vegetables | Stimulates extensive root architecture by accelerating lateral and adventitious root elongation. |
| Megafol | Syngenta Biologicals (Basel, Switzerland) | Commercial formulation | Amino acids, betaines, proteins, vitamins, auxin, gibberellin | Irrigation, soil drench, foliar application | Fruits and vegetables | Supports balanced vegetative growth, boosts overall productivity, and strengthens resilience to environmental stresses such as frost, root asphyxia, weeding, and hail. |
| BioRoot | DASA’s elfer® (Menàrguens, Lleida, Spain) | Plant and mineral derived organic humates, soybean meal | Plant derived protein hydrolysate | Irrigation, foliar, soil drench | Irrigation, foliar, soil drench of fruits and vegetables | Improves rooting efficiency while increasing chlorophyll and protein content in plant tissues. |
| Ergonfill | K—Adriatica (Loreo, Italy) | Animal derived protein hydrolysate | Animal protein hydrolysates, cysteine, folic acid, keratin derivatives | Foliar, pre-planting, irrigation | Fruits and vegetables | Promotes synthesis of indole-3-acetic acid (IAA) and chlorophyll, enhancing nutrient translocation and chelation of macro and micronutrients. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhati, D.; Hayes, M. From Ocean to Market: Technical Applications of Fish Protein Hydrolysates in Human Functional Food, Pet Wellness, Aquaculture and Agricultural Bio-Stimulant Product Sectors. Appl. Sci. 2025, 15, 5769. https://doi.org/10.3390/app15105769
Bhati D, Hayes M. From Ocean to Market: Technical Applications of Fish Protein Hydrolysates in Human Functional Food, Pet Wellness, Aquaculture and Agricultural Bio-Stimulant Product Sectors. Applied Sciences. 2025; 15(10):5769. https://doi.org/10.3390/app15105769
Chicago/Turabian StyleBhati, Dolly, and Maria Hayes. 2025. "From Ocean to Market: Technical Applications of Fish Protein Hydrolysates in Human Functional Food, Pet Wellness, Aquaculture and Agricultural Bio-Stimulant Product Sectors" Applied Sciences 15, no. 10: 5769. https://doi.org/10.3390/app15105769
APA StyleBhati, D., & Hayes, M. (2025). From Ocean to Market: Technical Applications of Fish Protein Hydrolysates in Human Functional Food, Pet Wellness, Aquaculture and Agricultural Bio-Stimulant Product Sectors. Applied Sciences, 15(10), 5769. https://doi.org/10.3390/app15105769





