A Novel Approach for Acute Mental Stress Mitigation Through Adapted Binaural Beats: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
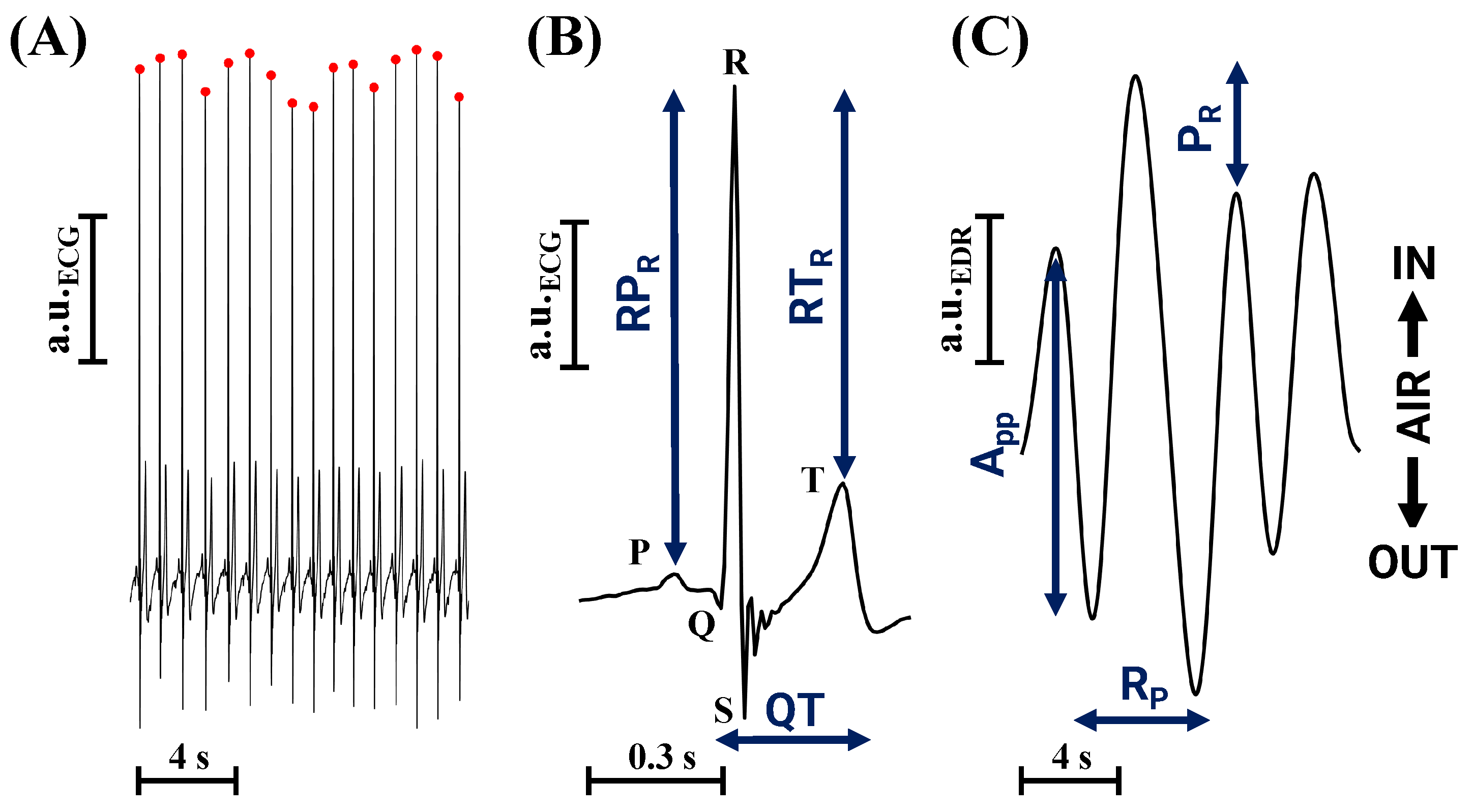
2.2. Instruments
2.3. Experimental Protocol
2.4. Stress Index Estimation
2.5. Adaptive Stimulation
2.6. Statistical Analysis
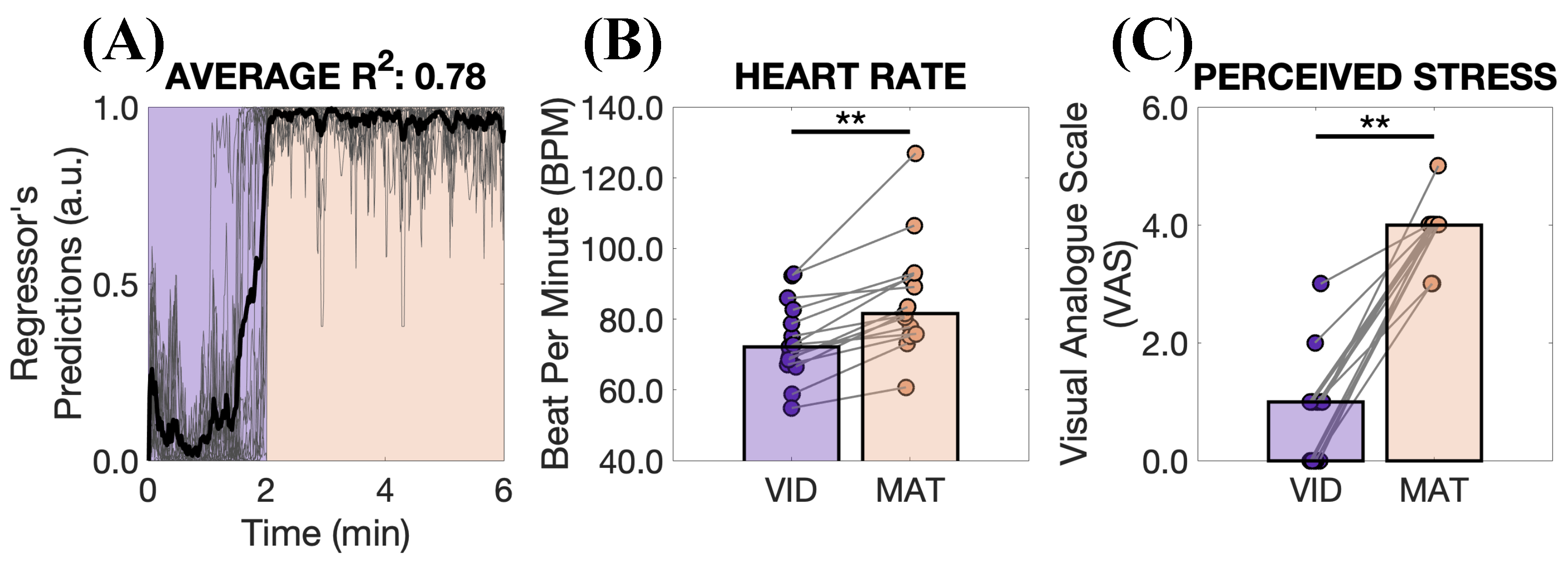
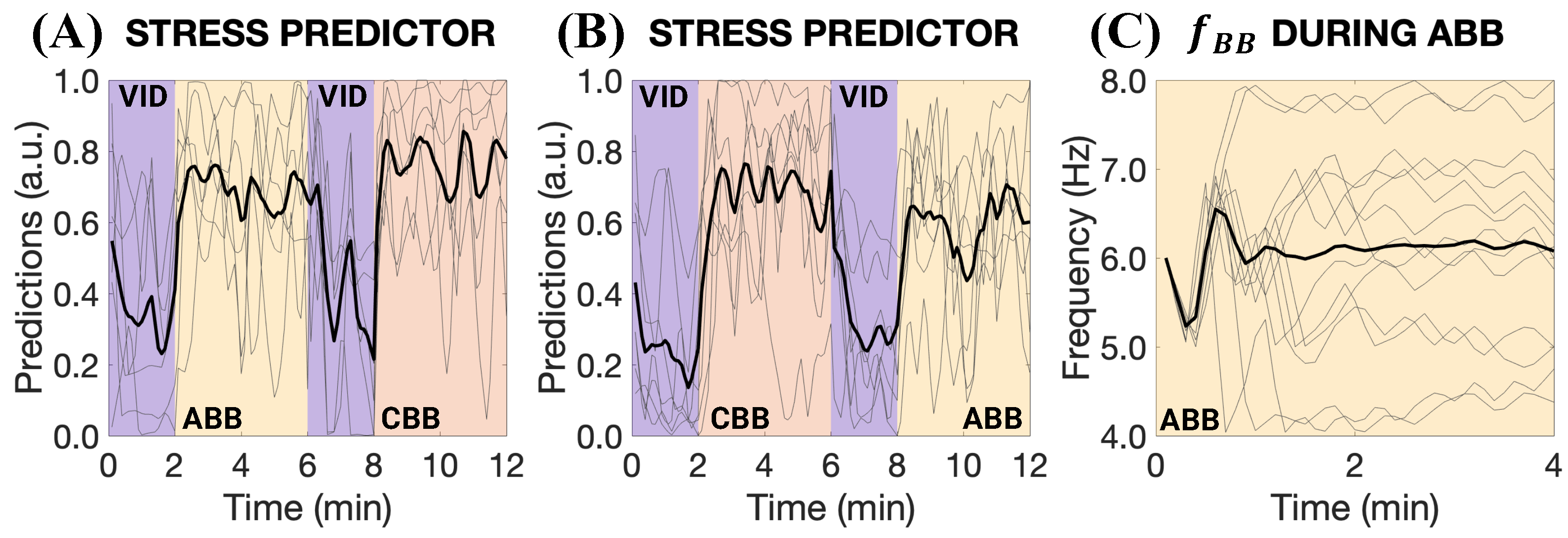
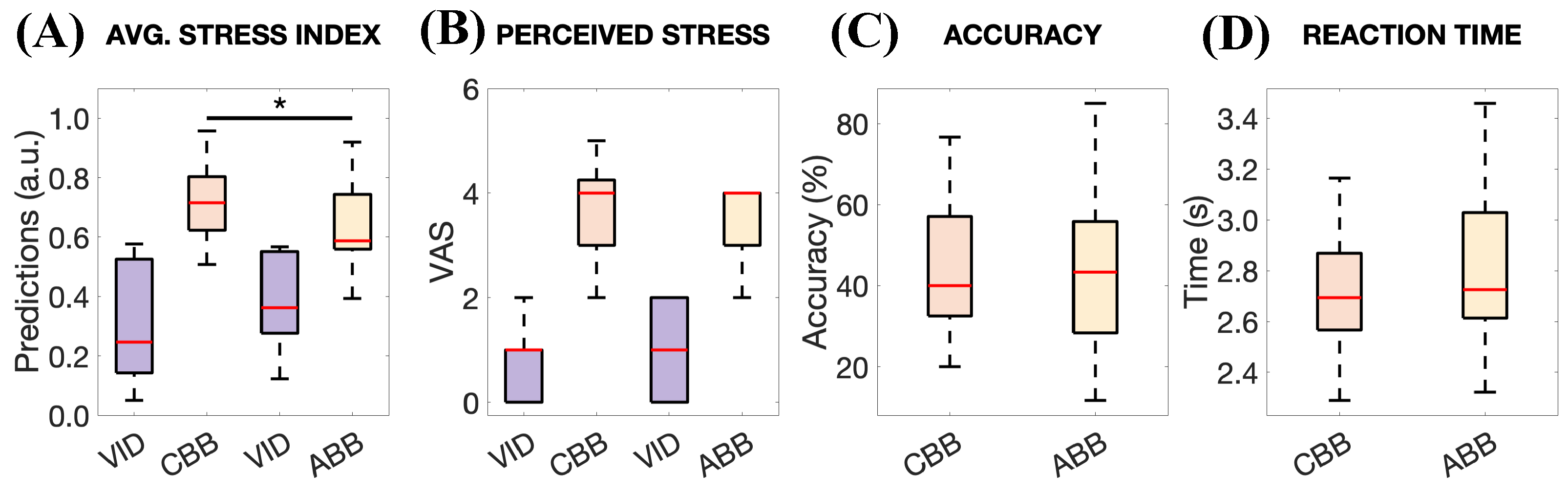
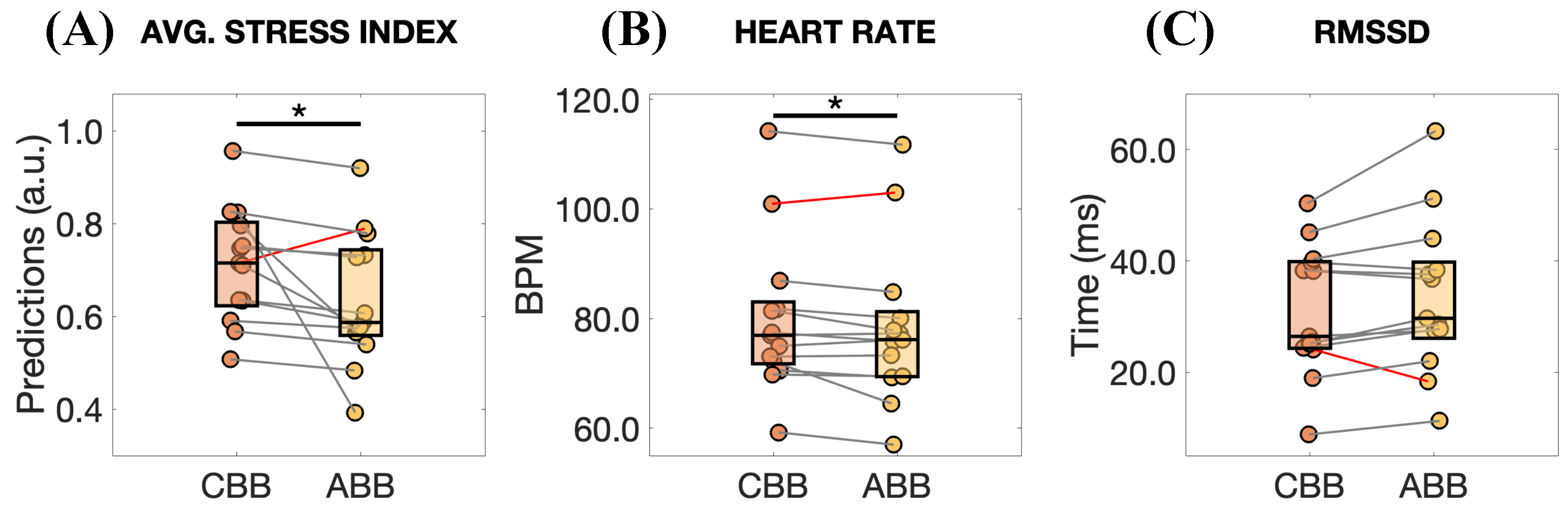
3. Results
4. Discussion
Limitations and Future Directions
- Number of participants and their mental health condition: The small number of participants limits the trustworthiness of our approach. For this reason, it is necessary to consider a wider sample and structure the experiments to account for multiple factors influencing cortisol levels [17]. However, additional consideration should be given. Despite the interesting results of this study, the effects of this stimulation are modest, especially in the HR and RMSSD changes (see Figure 7). We believe this result is largely attributable to the fact that our participants were healthy individuals. Accordingly, we anticipate that a more pronounced effect might be observed in individuals experiencing chronic stress, as they may have greater potential for improvement.
- Task: Given the intrinsic variability in the subject’s response to stress [52,53,54], recreating a stressful event for all the participants is still a challenge [17]. In this regard, technologies like virtual and augmented reality can be utilized to design laboratory experiments that replicate the stress levels encountered in real-world situations [55].
- Sensors: For this pilot study, a commercial chest strap (PolarH10) was used to collect the ECG. This device does not ensure that the signal quality meets medical standards. Therefore, future studies could consider using higher-quality sensors while simultaneously ensuring user comfort, such as integrating electrodes into clothes [56]. In our work, we decided to minimize the number of sensors, but future experiments could consider the acquisition of additional signals like electrodermal activity, which is well established for stress detection [57,58], and the EEG, which could permit a better understanding of the physiological and neural response of the participants during the stimulation. However, it should be pointed out that increasing the number of sensors can significantly increase the complexity of the system and discomfort on the part of the user, which could also impact stress perception.
- Algorithm for modulation: The proposed algorithm can certainly be optimized. For instance, it does not currently include a “reset” to restart the search for the optimal stimulation frequency (e.g., from the initial one at 6.0 Hz). Implementing this feature would enhance adaptability, allowing the algorithm to better accommodate variations in the subject’s optimal stimulation frequency throughout the task or under stressful conditions.
- Loudness of the BBs: In this study, we chose to focus solely on the frequency of the BBs, keeping their loudness constant. Indeed, our choice was to work on one degree of freedom at a time, leaving the combination of more stimulation parameters (e.g., beat frequency and amplitude) for future works, to assess if better results than the ones presented here can be obtained.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UK Mental Health. The Burnout Report; Report; Mental Health UK: London, UK, 2024. [Google Scholar]
- Iqbal, T.; Elahi, A.; Wijns, W.; Shahzad, A. Cortisol detection methods for stress monitoring in connected health. Health Sci. Rev. 2023, 6, 100079. [Google Scholar] [CrossRef]
- Benchekroun, M.; Istrate, D.; Zalc, V.; Lenne, D. Mmsd: A Multi-modal Dataset for Real-time, Continuous Stress Detection from Physiological Signals. In Proceedings of the HEALTHINF: 15th International Conference on Health Informatics, Virtual, 9–11 February 2022; pp. 240–248. [Google Scholar]
- Giannakakis, G.; Grigoriadis, D.; Giannakaki, K.; Simantiraki, O.; Roniotis, A.; Tsiknakis, M. Review on psychological stress detection using biosignals. IEEE Trans. Affect. Comput. 2019, 13, 440–460. [Google Scholar] [CrossRef]
- Taskasaplidis, G.; Fotiadis, D.A.; Bamidis, P. Review of Stress Detection Methods Using Wearable Sensors. IEEE Access 2024, 12, 38219–38246. [Google Scholar] [CrossRef]
- Gedam, S.; Paul, S. A review on mental stress detection using wearable sensors and machine learning techniques. IEEE Access 2021, 9, 84045–84066. [Google Scholar] [CrossRef]
- Nath, R.K.; Thapliyal, H.; Caban-Holt, A.; Mohanty, S.P. Machine learning based solutions for real-time stress monitoring. IEEE Consum. Electron. Mag. 2020, 9, 34–41. [Google Scholar] [CrossRef]
- Robles, D.; Benchekroun, M.; Zalc, V.; Istrate, D.; Taramasco, C. Stress detection from surface electromyography using convolutional neural networks. In Proceedings of the 2022 44th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Glasgow, UK, 11–15 July 2022; pp. 3235–3238. [Google Scholar]
- Gil-Martin, M.; San-Segundo, R.; Mateos, A.; Ferreiros-Lopez, J. Human stress detection with wearable sensors using convolutional neural networks. IEEE Aerosp. Electron. Syst. Mag. 2022, 37, 60–70. [Google Scholar] [CrossRef]
- Puzi, N.M.; Jailani, R.; Norhazman, H.; Zaini, N.M. Alpha and Beta brainwave characteristics to binaural beat treatment. In Proceedings of the 2013 IEEE 9th International Colloquium on Signal Processing and its Applications, Kuala Lumpur, Malaysia, 8–10 March 2013; pp. 344–348. [Google Scholar]
- On, F.R.; Jailani, R.; Norhazman, H.; Zaini, N.M. Binaural beat effect on brainwaves based on EEG. In Proceedings of the 2013 IEEE 9th International Colloquium on Signal Processing and its Applications, Kuala Lumpur, Malaysia, 8–10 March 2013; pp. 339–343. [Google Scholar]
- Vernon, D.; Peryer, G.; Louch, J.; Shaw, M. Tracking EEG changes in response to alpha and beta binaural beats. Int. J. Psychophysiol. 2014, 93, 134–139. [Google Scholar] [CrossRef]
- Ala, T.S.; Ahmadi-Pajouh, M.A.; Nasrabadi, A.M. Cumulative effects of theta binaural beats on brain power and functional connectivity. Biomed. Signal Process. Control 2018, 42, 242–252. [Google Scholar]
- Beauchene, C.; Abaid, N.; Moran, R.; Diana, R.A.; Leonessa, A. The effect of binaural beats on visuospatial working memory and cortical connectivity. PLoS ONE 2016, 11, e0166630. [Google Scholar] [CrossRef]
- Lane, J.D.; Kasian, S.J.; Owens, J.E.; Marsh, G.R. Binaural auditory beats affect vigilance performance and mood. Physiol. Behav. 1998, 63, 249–252. [Google Scholar] [CrossRef]
- Katmah, R.; Al-Shargie, F.; Tariq, U.; Babiloni, F.; Al-Mughairbi, F.; Al-Nashash, H. Mental stress management using fnirs directed connectivity and audio stimulation. IEEE Trans. Neural Syst. Rehabil. Eng. 2023, 31, 1086–1096. [Google Scholar] [CrossRef]
- Raggi, M.; Moore, L.J.; Mesin, L. Inducing and Assessing Acute Mental Stress in Controlled Conditions: Topical Review and Guidelines for Effective Experimental Protocols. IEEE Access 2025, 13, 10022–10042. [Google Scholar] [CrossRef]
- Melnichuk, A.; Cooper, R.K., Jr.; Hawk, L.W., Jr. A parametric investigation of binaural beats for brain entrainment and enhancing sustained attention. Sci. Rep. 2025, 15, 4308. [Google Scholar] [CrossRef] [PubMed]
- Engelbregt, H.; Barmentlo, M.; Keeser, D.; Pogarell, O.; Deijen, J.B. Effects of binaural and monaural beat stimulation on attention and EEG. Exp. Brain Res. 2021, 239, 2781–2791. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, W.; Li, X.; Yang, S. The effect of 40 Hz binaural beats on working memory. IEEE Access 2022, 10, 81556–81567. [Google Scholar] [CrossRef]
- Beauchene, C.; Abaid, N.; Moran, R.; Diana, R.A.; Leonessa, A. The effect of binaural beats on verbal working memory and cortical connectivity. J. Neural Eng. 2017, 14, 026014. [Google Scholar] [CrossRef]
- Kelton, K.; Weaver, T.L.; Willoughby, L.; Kaufman, D.; Santowski, A. The Efficacy of Binaural Beats as a Stress-buffering Technique. Altern. Ther. Health Med. 2021, 27, 28. [Google Scholar]
- Al-Shargie, F.; Katmah, R.; Tariq, U.; Babiloni, F.; Al-Mughairbi, F.; Al-Nashash, H. Stress management using fNIRS and binaural beats stimulation. Biomed. Opt. Express 2022, 13, 3552–3575. [Google Scholar] [CrossRef]
- Carrington, P.; Collings, G.H., Jr.; Benson, H.; Robinson, H.; Wood, L.W.; Lehrer, P.M.; Woolfolk, R.L.; Cole, J.W. The use of meditation–relaxation techniques for the management of stress in a working population. J. Occup. Environ. Med. 1980, 22, 221–231. [Google Scholar]
- Grossman, P.; Niemann, L.; Schmidt, S.; Walach, H. Mindfulness-based stress reduction and health benefits: A meta-analysis. J. Psychosom. Res. 2004, 57, 35–43. [Google Scholar] [CrossRef]
- Baseanu, I.C.C.; Roman, N.A.; Minzatanu, D.; Manaila, A.; Tuchel, V.I.; Basalic, E.B.; Miclaus, R.S. The Efficiency of Binaural Beats on Anxiety and Depression—A Systematic Review. Appl. Sci. 2024, 14, 5675. [Google Scholar] [CrossRef]
- Klichowski, M.; Wicher, A.; Kruszwicka, A.; Golebiewski, R. Reverse effect of home-use binaural beats brain stimulation. Sci. Rep. 2023, 13, 11079. [Google Scholar] [CrossRef]
- López-Caballero, F.; Escera, C. Binaural beat: A failure to enhance EEG power and emotional arousal. Front. Hum. Neurosci. 2017, 11, 557. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Cao, H.; Ming, D.; Qi, H.; Wang, X.; Wang, X.; Chen, R.; Zhou, P. Analysis of EEG activity in response to binaural beats with different frequencies. Int. J. Psychophysiol. 2014, 94, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Ingendoh, R.M.; Posny, E.S.; Heine, A. Binaural beats to entrain the brain? A systematic review of the effects of binaural beat stimulation on brain oscillatory activity, and the implications for psychological research and intervention. PLoS ONE 2023, 18, e0286023. [Google Scholar] [CrossRef]
- Corona-González, C.E.; Alonso-Valerdi, L.M.; Ibarra-Zarate, D.I. Personalized theta and beta binaural beats for brain entrainment: An electroencephalographic analysis. Front. Psychol. 2021, 12, 764068. [Google Scholar] [CrossRef]
- Klimesch, W. The frequency architecture of brain and brain body oscillations: An analysis. Eur. J. Neurosci. 2018, 48, 2431–2453. [Google Scholar] [CrossRef] [PubMed]
- Musicoterapia. Musicoterapia Musica Rilassante + Musica Zen + Da Massaggio + Per Yoga + Musica New Age. 2024. Available online: https://www.youtube.com/watch?v=kADG8l20Yuc (accessed on 30 September 2024).
- Wahbeh, H.; Calabrese, C.; Zwickey, H.; Zajdel, D. Binaural beat technology in humans: A pilot study to assess neuropsychologic, physiologic, and electroencephalographic effects. J. Altern. Complement. Med. 2007, 13, 199–206. [Google Scholar] [CrossRef]
- Kraus, J.; Porubanová, M. The effect of binaural beats on working memory capacity. Stud. Psychol. 2015, 57, 135. [Google Scholar] [CrossRef]
- Linares, N.N.; Charron, V.; Ouimet, A.J.; Labelle, P.R.; Plamondon, H. A systematic review of the Trier Social Stress Test methodology: Issues in promoting study comparison and replicable research. Neurobiol. Stress 2020, 13, 100235. [Google Scholar]
- Grassini, S.; Segurini, G.V.; Koivisto, M. Watching nature videos promotes physiological restoration: Evidence from the modulation of alpha waves in electroencephalography. Front. Psychol. 2022, 13, 871143. [Google Scholar] [CrossRef]
- Huang, M.; Ebey, J.; Wolf, S. Responses of the QT interval of the electrocardiogram during emotional stress. Psychosom. Med. 1989, 51, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Haapalahti, P.; Mäkijärvi, M.; Montonen, J.; Korhonen, P.; Salorinne, Y.; Oikarinen, L.; Viitasalo, M.; Toivonen, L. Effects of cardiovascular autonomic function tests on QT dispersion in the 12-lead electrocardiogram of healthy patients. J. Electrocardiol. 2000, 33, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Andrássy, G.; Szabo, A.; Ferencz, G.; Trummer, Z.; Simon, E.; Tahy, Á. Mental stress may induce QT-interval prolongation and T-wave notching. Ann. Noninvasive Electrocardiol. 2007, 12, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Heslegrave, R.J.; Furedy, J.J. Sensitivities of HR and T-wave amplitude for detecting cognitive and anticipatory stress. Physiol. Behav. 1979, 22, 17–23. [Google Scholar] [CrossRef]
- Widjaja, D.; Orini, M.; Vlemincx, E.; Van Huffel, S. Cardiorespiratory Dynamic Response to Mental Stress: A Multivariate Time-Frequency Analysis. Comput. Math. Methods Med. 2013, 2013, 451857. [Google Scholar] [CrossRef]
- Grossman, P. Respiration, stress, and cardiovascular function. Psychophysiology 1983, 20, 284–300. [Google Scholar] [CrossRef]
- Scheirer, C.J.; Ray, W.S.; Hare, N. The analysis of ranked data derived from completely randomized factorial designs. Biometrics 1976, 32, 429–434. [Google Scholar] [CrossRef]
- Gambetta-Tessini, K.; Mariño, R.; Morgan, M.; Evans, W.; Anderson, V. Stress and health-promoting attributes in Australian, New Zealand, and Chilean dental students. J. Dent. Educ. 2013, 77, 801–809. [Google Scholar] [CrossRef]
- Kayikcioglu, O.; Bilgin, S.; Seymenoglu, G.; Deveci, A. State and trait anxiety scores of patients receiving intravitreal injections. Biomed. Hub 2017, 2, 1–5. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An overview of heart rate variability metrics and norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef] [PubMed]
- Paiva, J.S.; Rodrigues, S.; Cunha, J.P.S. Changes in ST, QT and RR ECG intervals during acute stress in firefighters: A pilot study. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 3378–3381. [Google Scholar]
- Latkin, C.A.; Edwards, C.; Davey-Rothwell, M.A.; Tobin, K.E. The relationship between social desirability bias and self-reports of health, substance use, and social network factors among urban substance users in Baltimore, Maryland. Addict. Behav. 2017, 73, 133–136. [Google Scholar] [CrossRef]
- Seipäjärvi, S.M.; Tuomola, A.; Juurakko, J.; Rottensteiner, M.; Rissanen, A.P.E.; Kurkela, J.L.; Kujala, U.M.; Laukkanen, J.A.; Wikgren, J. Measuring psychosocial stress with heart rate variability-based methods in different health and age groups. Physiol. Meas. 2022, 43, 055002. [Google Scholar] [CrossRef] [PubMed]
- Caravati, E.; Barbeni, F.; Chiarion, G.; Raggi, M.; Mesin, L. Closed-Loop Transcranial Electrical Neurostimulation for Sustained Attention Enhancement: A Pilot Study towards Personalized Intervention Strategies. Bioengineering 2024, 11, 467. [Google Scholar] [CrossRef]
- Lazarus, R.S. Stress and Emotion: A New Synthesis; Springer Publishing Company, Inc.: New York, NY, USA, 1999. [Google Scholar]
- Thatcher, J.; Day, M.C. Re-appraising stress appraisals: The underlying properties of stress in sport. Psychol. Sport Exerc. 2008, 9, 318–335. [Google Scholar] [CrossRef]
- Lazarus, R.S. Emotions and interpersonal relationships: Toward a person-centered conceptualization of emotions and coping. J. Personal. 2006, 74, 9–46. [Google Scholar] [CrossRef]
- Ladakis, I.; Filos, D.; Chouvarda, I. Virtual reality environments for stress reduction and management: A scoping review. Virtual Real. 2024, 28, 50. [Google Scholar] [CrossRef]
- Li, M.; Xiong, W.; Li, Y. Wearable measurement of ECG signals based on smart clothing. Int. J. Telemed. Appl. 2020, 2020, 6329360. [Google Scholar] [CrossRef]
- Raju, A.R.; Ramadevi, R.; Babu, P.R.; Vetriselvi, D. Galvanic Skin Response based Stress Detection System using Machine Learning and IoT. In Proceedings of the 2023 Second International Conference on Augmented Intelligence and Sustainable Systems (ICAISS), Trichy, India, 23–25 August 2023; pp. 709–714. [Google Scholar]
- Navea, R.F.; Buenvenida, P.J.; Cruz, C.D. Stress detection using galvanic skin response: An android application. In Proceedings of the International Conference on Biomedical Engineering, Penang Island, Malaysia, 26–27 August 2019. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raggi, M.; Chiri, S.; Roatta, S.; Rabbito, R.; Mesin, L. A Novel Approach for Acute Mental Stress Mitigation Through Adapted Binaural Beats: A Pilot Study. Appl. Sci. 2025, 15, 5742. https://doi.org/10.3390/app15105742
Raggi M, Chiri S, Roatta S, Rabbito R, Mesin L. A Novel Approach for Acute Mental Stress Mitigation Through Adapted Binaural Beats: A Pilot Study. Applied Sciences. 2025; 15(10):5742. https://doi.org/10.3390/app15105742
Chicago/Turabian StyleRaggi, Matteo, Stefania Chiri, Silvestro Roatta, Rosita Rabbito, and Luca Mesin. 2025. "A Novel Approach for Acute Mental Stress Mitigation Through Adapted Binaural Beats: A Pilot Study" Applied Sciences 15, no. 10: 5742. https://doi.org/10.3390/app15105742
APA StyleRaggi, M., Chiri, S., Roatta, S., Rabbito, R., & Mesin, L. (2025). A Novel Approach for Acute Mental Stress Mitigation Through Adapted Binaural Beats: A Pilot Study. Applied Sciences, 15(10), 5742. https://doi.org/10.3390/app15105742






