Antihyperglycemic Potential of Mace Water Extract from Myristica fragrans Houtt
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample and Extract Preparation
2.2. Experimental Animals
2.3. Test of Acute Effects of Mace Water Extract
2.3.1. Oral Starch Tolerance Test
2.3.2. Oral Glucose Tolerance Test
2.4. Hyperglycemic Induction
2.5. Test of Long-Term Effects of Mace Water Extract
2.6. Estimation of Body Weight, Daily Water Consumption, and Relative Organ Weights
2.7. Statistical Analysis
3. Results and Discussion
3.1. Oral Starch Tolerance Test
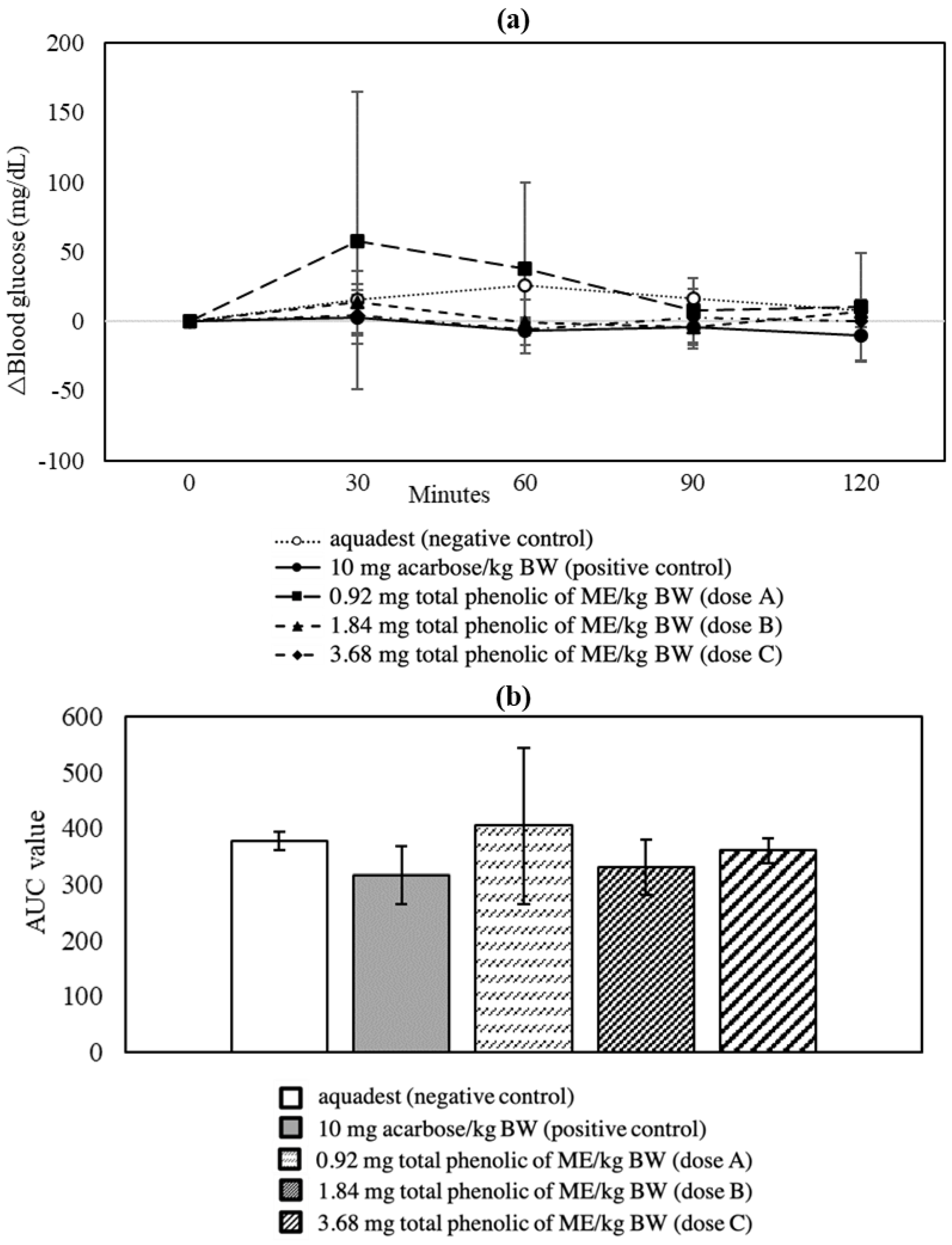
3.2. Oral Glucose Tolerance Test
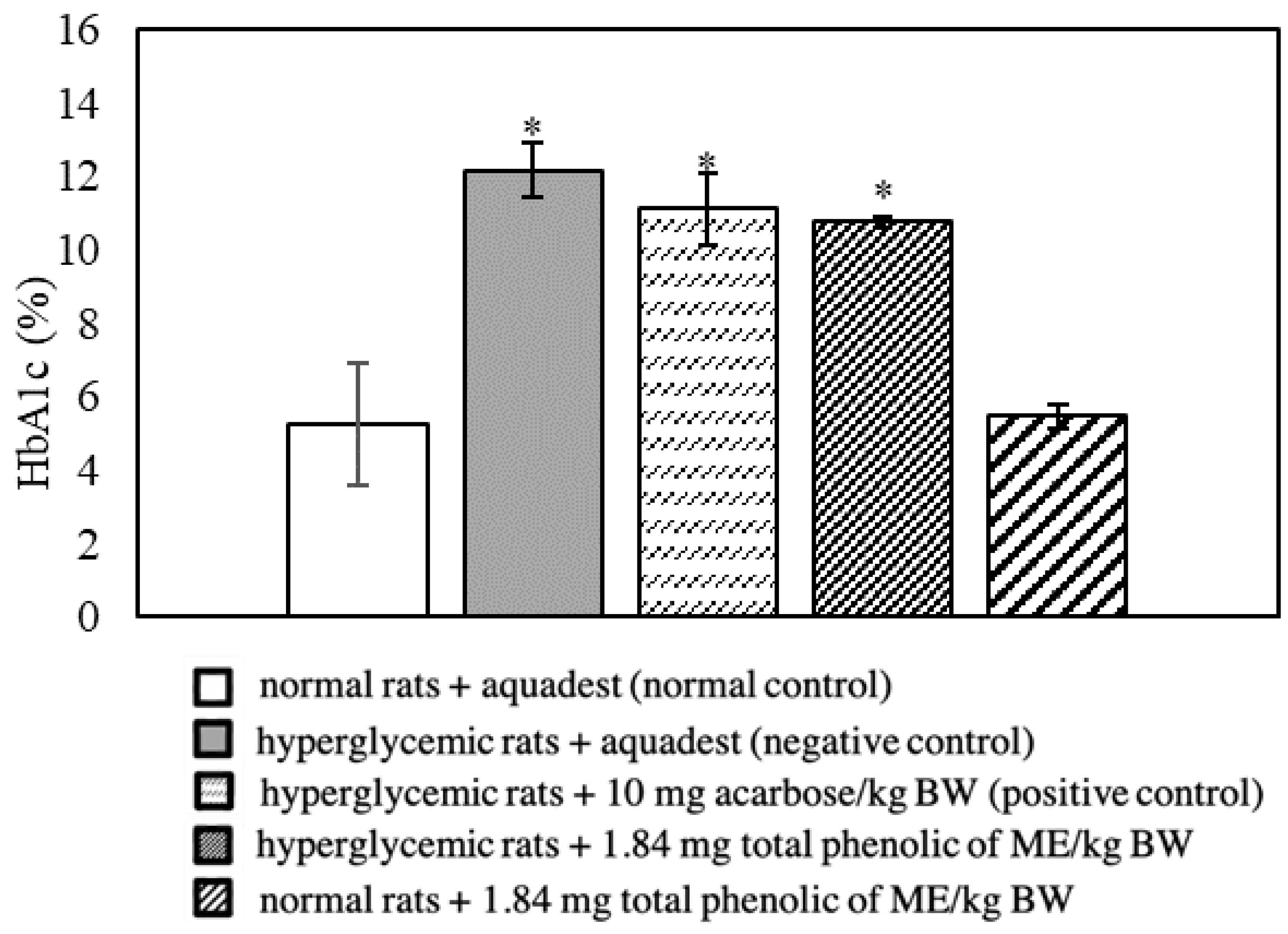
3.3. Effect of Mace Water Extract on Blood Glucose and HbA1c
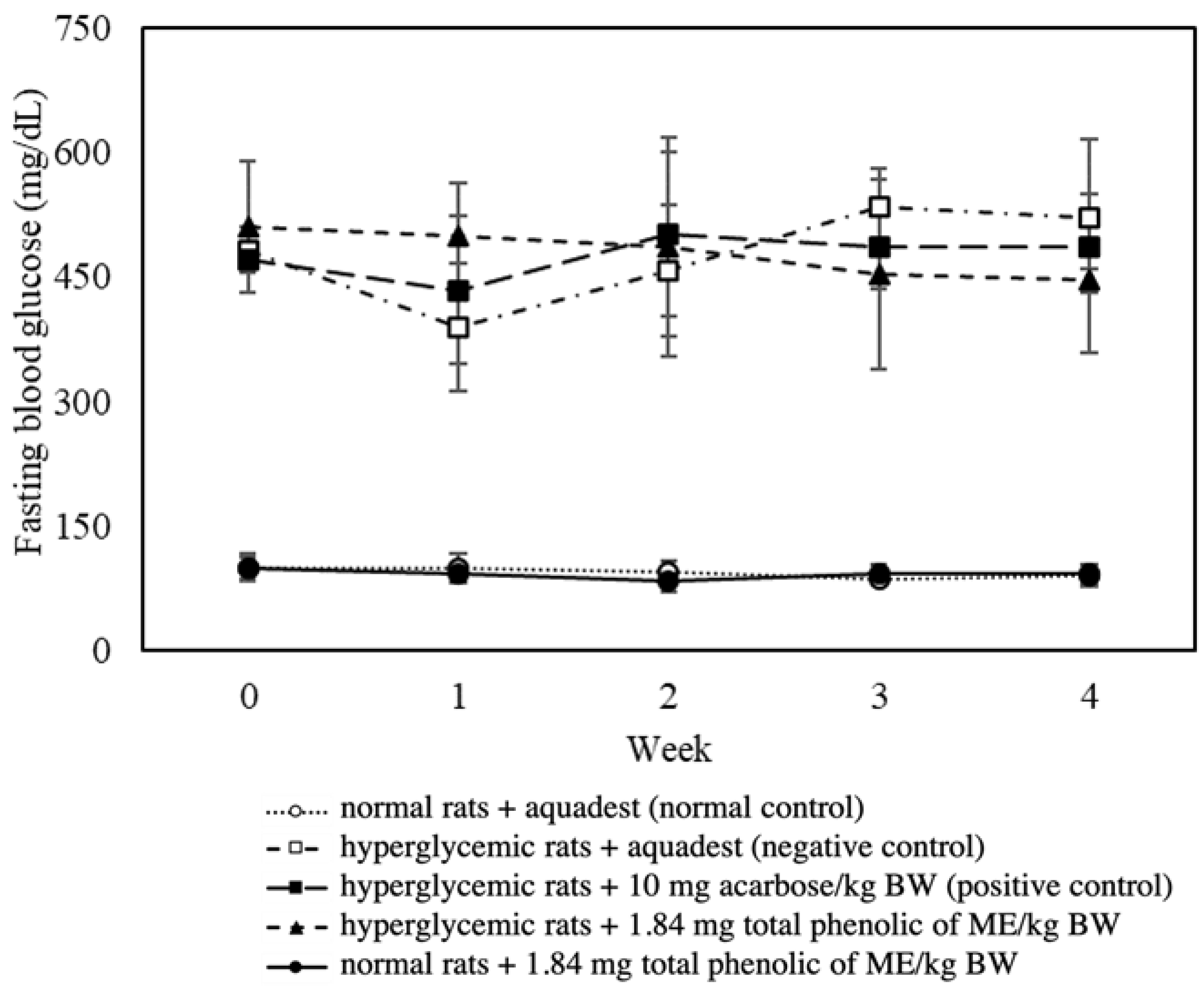
3.4. Effect of Mace Water Extract on the Glycemic Parameters of Hyperglycemic Rats
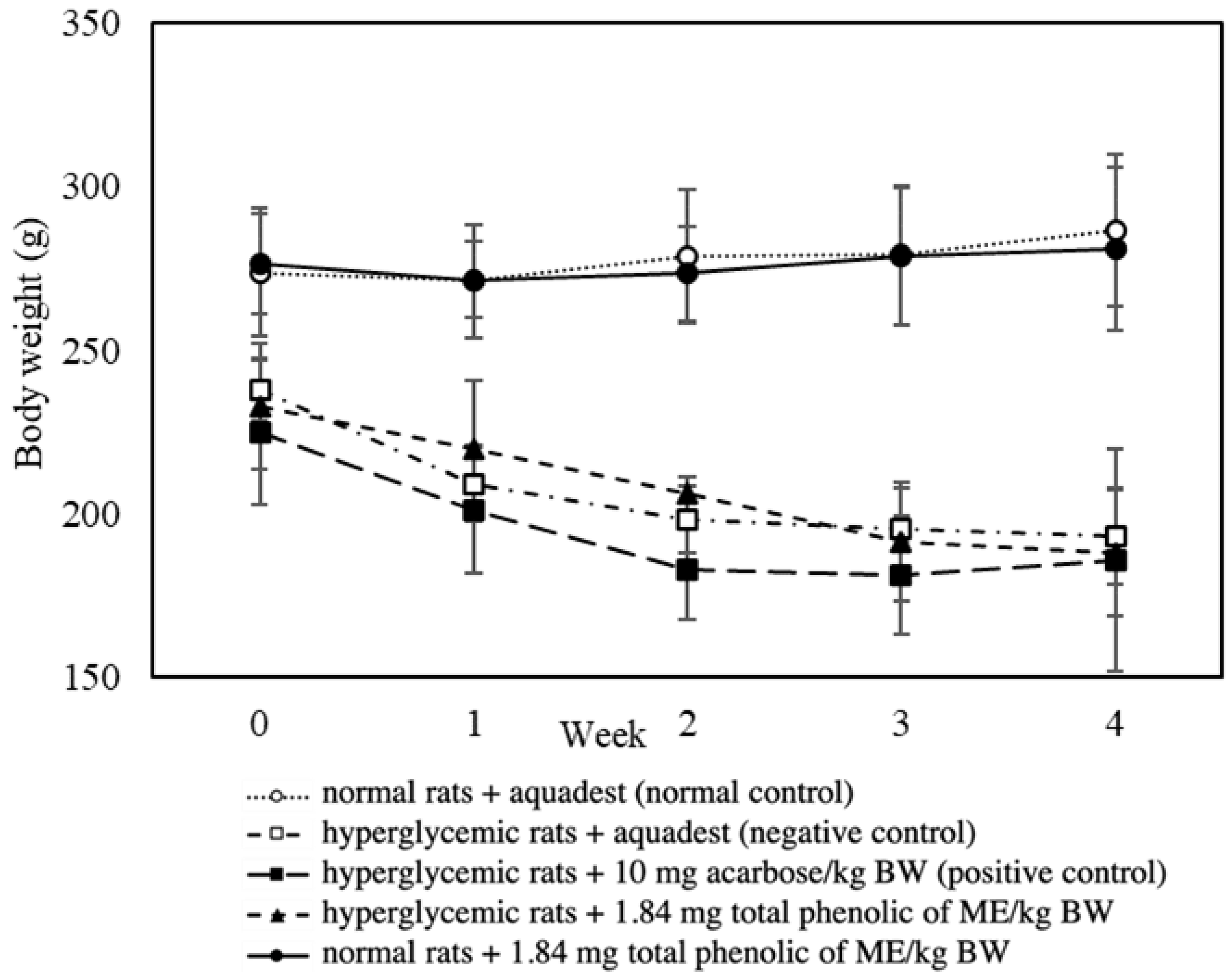
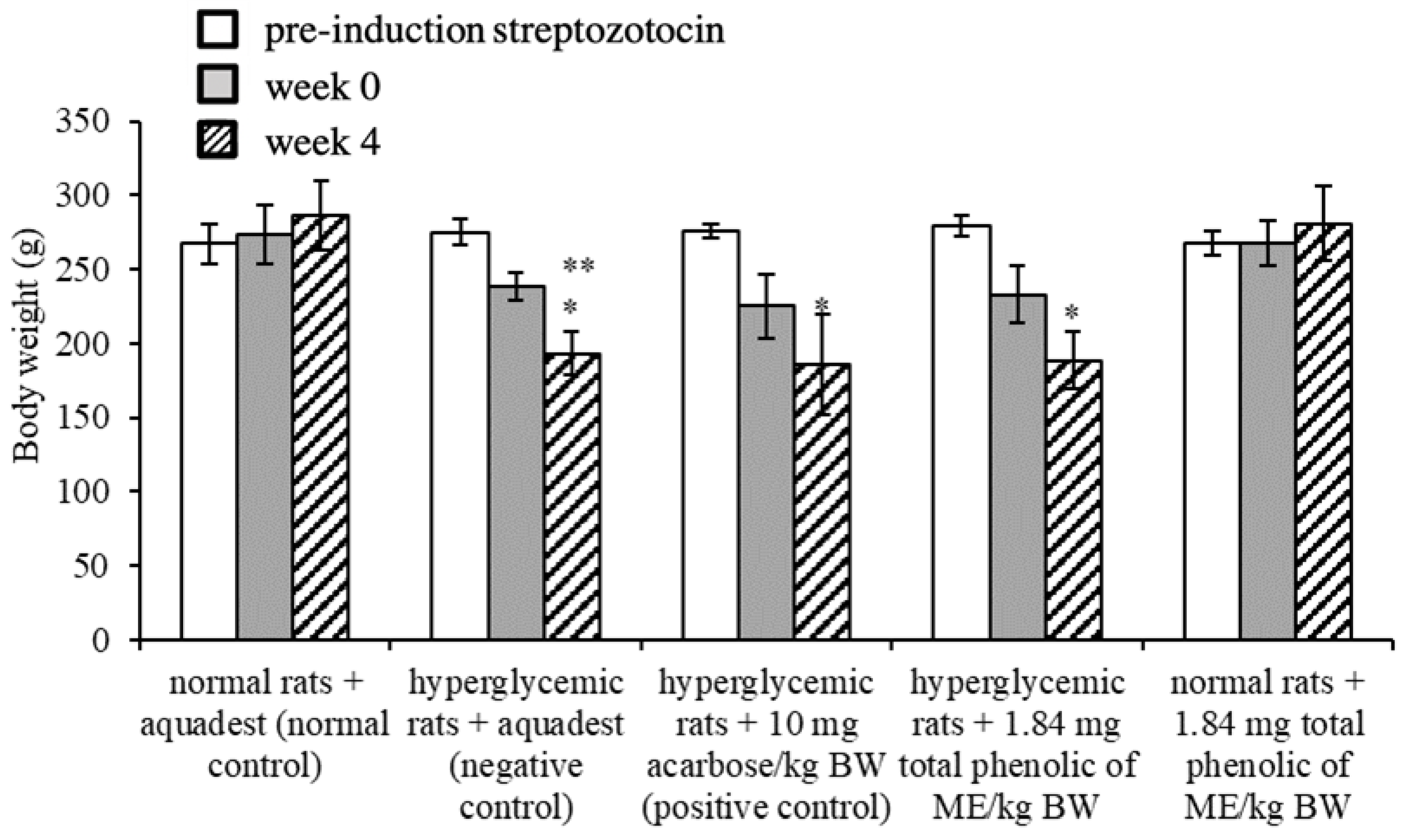
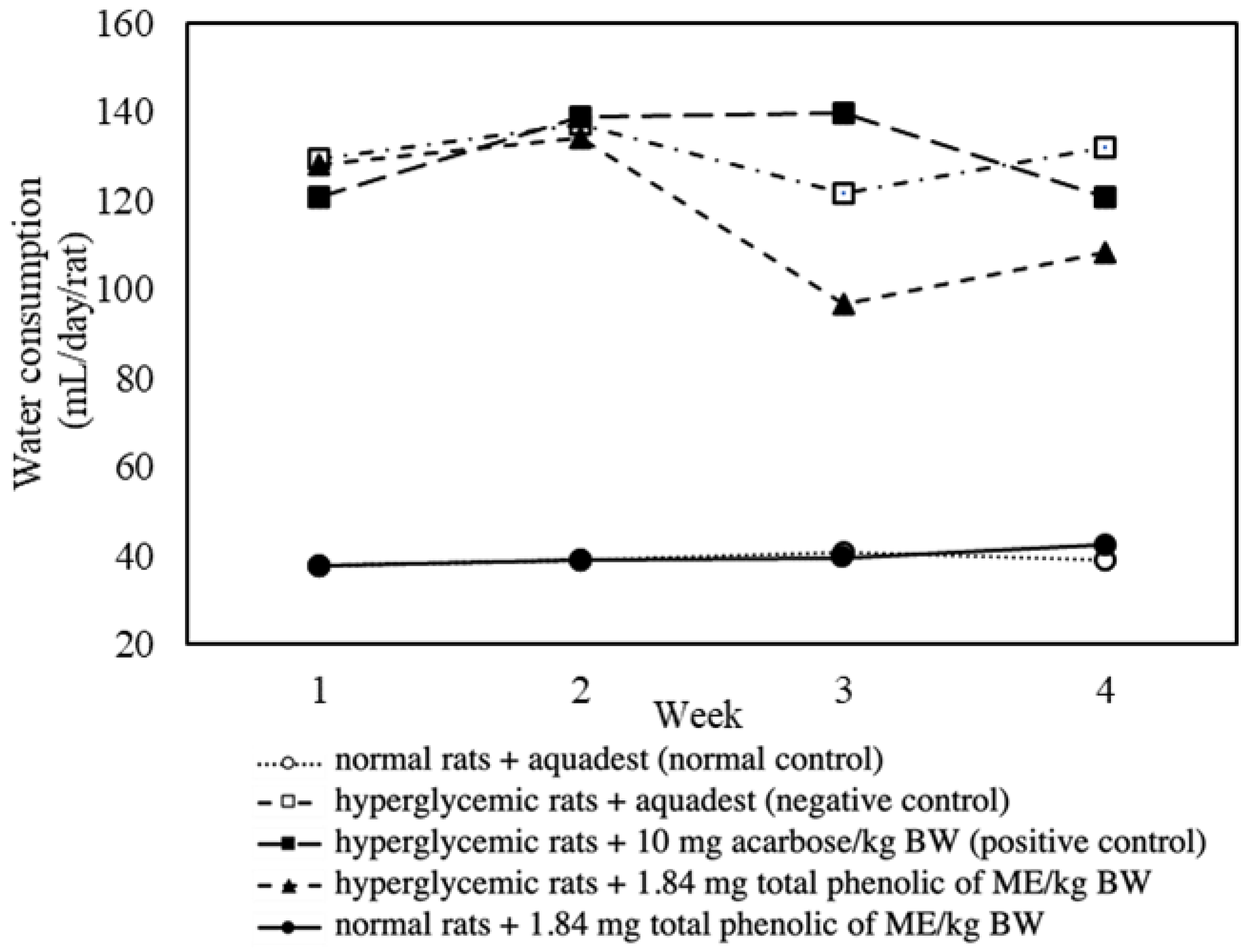
3.5. Effect of Mace Water Extract on Body Weight and Water Consumption
3.6. Effect of Mace Water Extract on the Relative Weight of the Pancreas, Kidney, and Liver
3.7. Correlation Between Clinical Parameters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gandhi, G.R.; Sasikumar, P. Antidiabetic Effect of Merremia Emarginata Burm. F. in Streptozotocin Induced Diabetic Rats. Asian Pac. J. Trop. Biomed. 2012, 2, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.R.; Shrivastava, P.S.; Ramasamy, J. Role of Self-Care in Management of Diabetes Mellitus. J. Diabetes Metab. Disord. 2013, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Hasbullah; Faridah, D.N.; Dewi, F.N.A.; Indrasti, D.; Andarwulan, N. Effect of Nutmeg on Glycemic Status in Rat and Mice: A Systematic Review. Food Sci. Technol. 2023, 43, e130122. [Google Scholar] [CrossRef]
- Alshammari, M.; Windle, R.; Bowskill, D.; Adams, G. Diabetes Treatment Guidelines and Nurses’ Adherence to Them: A Case of the UK and Kuwait. Nurs. Healthc. Int. J. 2020, 4, 1–12. [Google Scholar] [CrossRef]
- Tran, N.; Pham, B.; Le, L. Bioactive Compounds in Anti-Diabetic Plants: From Herbal Medicine to Modern Drug Discovery. Biology 2020, 9, 252. [Google Scholar] [CrossRef]
- Simanjuntak, H.A. The Utilization of Diabetes Mellitus Medicinal Plants in Simalungun Ethnic Society of Simalungun Regency (North Sumatera Province, Indonesia). BioLink 2018, 5, 59–71. [Google Scholar] [CrossRef][Green Version]
- Nagja, T.; Vimal, K.; Sanjeev, A. Myristica Fragrans: A Comprehensive Review. Int. J. Pharm. Pharm. Sci. 2016, 8, 9–12. [Google Scholar]
- Mendhekar, S.Y.; Balsaraf, C.D.; Bangar, M.S.; Jadhav, S.L.; Gaikwad, D.D. TLC Profile Study Mace (Aril) of Myristica Malabarica Lamk. (Myristicaceae). J. Phytopharm. 2017, 6, 329–334. [Google Scholar] [CrossRef]
- Ismiyarto; Ngadiwiyana; Mustika, R. Isolation, Identification of Essential Oil from Nutmeg Mace (Myristica fragrans) and Activity Test as a Larvicidal. J. Kim. Sains Dan Apl. 2009, 12, 23–30. [Google Scholar] [CrossRef]
- Arrizqiyani, T.; Sonjaya, N.; Asty, A. Optimizing the Potential of Nutmeg Plants as Antibacterial Escherichia Coli Using the Extraction Method. In Proceedings of the Seminar Nasional Publikasi Hasil-Hasil Penelitian dan Pengabdian Masyarakat; Universitas Muhammadiyah Semarang (UNIMUS): Semarang, Indonesia, 2017; pp. 375–382. [Google Scholar]
- Ha, M.T.; Vu, N.K.; Tran, T.H.; Kim, J.A.; Woo, M.H.; Min, B.S. Phytochemical and Pharmacological Properties of Myristica Fragrans Houtt.: An Updated Review. Arch. Pharmacal Res. 2020, 43, 1067–1092. [Google Scholar] [CrossRef]
- Pan, A.; Sun, J.; Chen, Y.; Ye, X.; Li, H.; Yu, Z.; Wang, Y.; Gu, W.; Zhang, X.; Chen, X.; et al. Effects of a Flaxseed-Derived Lignan Supplement in Type 2 Diabetic Patients: A Randomized, Double-Blind, Cross-over Trial. PLoS ONE 2007, 2, e1148. [Google Scholar] [CrossRef]
- Han, K.L.; Choi, J.S.; Lee, J.Y.; Song, J.; Joe, M.K.; Jung, M.H. Therapeutic Potential of Peroxisome Proliferators–Activated Receptor-α/ΓDual Agonist With Alleviation of Endoplasmic Reticulum Stress for the Treatment of Diabetes. Diabetes 2008, 57, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wang, X.; Zhou, M.; Ma, L.; Deng, Y.; Zhang, H.; Zhao, A.; Zhang, Y.; Jia, W. The Antidiabetic Activity of Total Lignan from Fructus Arctii against Alloxan-Induced Diabetes in Mice and Rats. Phytother. Res. 2008, 22, 97–101. [Google Scholar] [CrossRef]
- Katare, C.; Saxena, S.; Agrawal, S.; Prasad, G.; Bisen, P.S. Flax Seed: A Potential Medicinal Food Journal of Nutrition & Food Sciences. J. Nutr. Food Sci. 2012, 2, 1–8. [Google Scholar] [CrossRef]
- Meutia. Effect of Green Tea Extract Addition in Extruded Rice Process to Decrease Glycemic Index. Bachelor’s Thesis, Institut Pertanian Bogor, Bogor, Indonesia, 2013. [Google Scholar]
- Malik, A. Antidiabetic Activity and Isolation of Bioactive Compounds from Ethanol Extract of Piper betle L. Leaf. In Proceedings of the Seminar Nasional Kimia dan Pendidikan Kimia 2016, Universitas Negeri Medan (UNIMED), Medan, Indonesia, 30–31 May 2016; pp. 1–6. [Google Scholar]
- Topal, F. Antidiabetic Potential: Effect of Resorcinol on α-Glycosidase and α- Amylase Enzymes. Cumhur. Sci. J. 2018, 39, 828–832. [Google Scholar] [CrossRef]
- Lee, Y.G.; Rodriguez, I.; Nam, Y.H.; Gwag, J.E.; Woo, S.H.; Kim, H.G.; Ko, J.; Hong, B.N.; Kang, T.H.; Baek, N. Recovery Effect of Lignans and Fermented Extracts from Forsythia Koreana Flowers on Pancreatic Islets Damaged by Alloxan in Zebrafish (Danio Rerio). Appl. Biol. Chem. 2019, 62, 7. [Google Scholar] [CrossRef]
- Worawalai, W.; Phuwapraisirisan, P. Samin-Derived Flavonolignans, a New Series of Antidiabetic Agents Having Dual Inhibition against α -Glucosidase and Free Radicals. Nat. Prod. Res. 2019, 34, 3169–3175. [Google Scholar] [CrossRef] [PubMed]
- Afandi, F.A. Meta-Analysis of Factors Affecting the Glycemic Index of Starch-Based Foods and Its Verification Using Food Model. Ph.D. Thesis, Institut Pertanian Bogor, Bogor, Indonesia, 2020. [Google Scholar]
- Barre, D.E.; Mizier-Barre, K.A. Lignans’ Potential in Pre and Post-Onset Type 2 Diabetes Management. Curr. Diabetes Rev. 2020, 16, 2–11. [Google Scholar] [CrossRef]
- Zhao, C.C.; Shao, J.H.; Chen, J.; Zhang, X.H.; Gu, W.Y.; Shen, J.; Liu, Y. Lignan Constituents from the Fruits of Viburnum Macrocephalum f. Keteleeri and Their α-Amylase, α-Glucosidase, and Protein Tyrosine Phosphatase 1b Inhibitory Activities. J. Agric. Food Chem. 2020, 68, 11151–11160. [Google Scholar] [CrossRef]
- Afandi, F.A.; Wijaya, C.H.; Faridah, D.N.; Suyatma, N.E.; Jayanegara, A. Evaluation of Various Starchy Foods: A Systematic Review and Meta-Analysis on Chemical Properties Affecting the Glycemic Index Values Based on In Vitro and In Vivo Experiments. Foods 2021, 10, 364. [Google Scholar] [CrossRef]
- Draganescu, D.; Andritoiu, C.; Hritcu, D.; Dodi, G.; Popa, M.I. Flaxseed Lignans and Polyphenols Enhanced Activity in Streptozotocin-Induced Diabetic Rats. Biology 2021, 10, 43. [Google Scholar] [CrossRef]
- Vangoori, Y.; Dakshinamoorthi, A.; Kavimani, S. Effect of Myristica Fragrans Extract on Lipid Profile, Glucose, Body Weight, Food Intake, Liver and Renal Functions in Experimental Obese Rats. Biomed. Pharmacol. J. 2019, 12, 669–676. [Google Scholar] [CrossRef]
- Patil, S.B.; Ghadyale, V.A.; Taklikar, S.S.; Kulkarni, C.R.; Arvindekar, A.U. Insulin Secretagogue, Alpha-Glucosidase and Antioxidant Activity of Some Selected Spices in Streptozotocin-Induced Diabetic Rats. Plant Foods Hum. Nutr. 2011, 66, 85–90. [Google Scholar] [CrossRef]
- Hasbullah; Faridah, D.N.; Indrasti, D.; Dewi, F.N.A.; Andarwulan, N. In Vitro Antioxidant and Antidiabetic Activity of Mace from Myristica Fragrans Houtt. Ann. Dunarea Jos Univ. Galati Fascicle IV—Food Technol. 2023, 47, 95–109. [Google Scholar] [CrossRef]
- Arifin, W.N.; Zahiruddin, W.M. Sample Size Calculation in Animal Studies Using Resource Equation Approach. Malays. J. Med. Sci. 2017, 24, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Poovitha, S.; Parani, M. In Vitro and in Vivo α-Amylase and α-Glucosidase Inhibiting Activities of the Protein Extracts from Two Varieties of Bitter Gourd (Momordica charantia L.). BMC Complement. Altern. Med. 2016, 16, 185. [Google Scholar] [CrossRef]
- Ali, R.B.; Atangwho, I.J.; Kuar, N.; Ahmad, M.; Mahmud, R.; Asmawi, M.Z. In Vitro and in Vivo Effects of Standardized Extract and Fractions of Phaleria Macrocarpa Fruits Pericarp on Lead Carbohydrate Digesting Enzymes. BMC Complement. Altern. Med. 2013, 13, 39. [Google Scholar] [CrossRef]
- Hossain, M.A.; Mostofa, M.; Awal, M.A.; Chowdhury, E.H.; Sikder, M.H. Histomorphological and Morphometric Studies of the Pancreatic Islet Cells of Diabetic Rats Treated with Aqueous Extracts of Momordica charantia (Karela) Fruits. Asian Pac. J. Trop. Dis. 2014, 4, S698–S704. [Google Scholar] [CrossRef]
- Schober, P.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Khadayat, K.; Marasini, B.P.; Gautam, H.; Ghaju, S.; Parajuli, N. Evaluation of the Alpha-Amylase Inhibitory Activity of Nepalese Medicinal Plants Used in the Treatment of Diabetes Mellitus. Clin. Phytoscience 2020, 6, 34. [Google Scholar] [CrossRef]
- Peungvicha, P.; Vallisuta, O.; Mangmool, S.; Sirithamwanich, T.; Sirithamwanich, R. Anti-Hyperglycemic Effect and Subchronic Toxicity of the Combined Extract from Sattagavata—Mathurameha—Tubpikarn Anti-Diabetic Herbal Formulae. Thai J. Pharm. Sci. 2018, 42, 6–13. [Google Scholar] [CrossRef]
- Florence, N.T.; Benoit, M.Z.; Jonas, K.; Alexandra, T.; Désiré, D.D.P.; Pierre, K.; Théophile, D. Antidiabetic and Antioxidant Effects of Annona Muricata (Annonaceae), Aqueous Extract on Streptozotocin-Induced Diabetic Rats. J. Ethnopharmacol. 2014, 151, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Alipin, K.; Sari, E.M.A.P.; Setiawati, T.I.A. Kidney Histology in Streptozotocin-Induced Diabetic Male Wistar Rats Treated with Combined Extract of Temulawak Rhizome and Belimbing Wuluh Fruit. Nusant. Biosci. 2017, 9, 312–317. [Google Scholar] [CrossRef]
- Atkinson, M.A. Organisation of the Human Pancreas in Health and in Diabetes. Diabetologia 2020, 63, 1966–1973. [Google Scholar] [CrossRef]
- Garcia, T.S.; Rech, T.H.; Bauermann, C. Pancreatic Size and Fat Content in Diabetes: A Systematic Review and Meta-Analysis of Imaging Studies. PLoS ONE 2017, 12, e0180911. [Google Scholar] [CrossRef] [PubMed]
- Virostko, J.; Williams, J.; Hilmes, M.; Bowman, C.; Wright, J.J.; Du, L.; Kang, H.; Russell, W.E.; Powers, A.C.; Moore, D.J. Pancreas Volume Declines During the First Year After Diagnosis of Type 1 Diabetes and Exhibits Altered Diffusion at Disease Onset. Diabetes Care 2018, 42, 248–257. [Google Scholar] [CrossRef]
- Saisho, Y.; Butler, A.E.; Meier, J.J.; Monchamp, T.; Rizza, R.A.; Butler, P.C. Pancreas Volumes in Humans from Birth to Age One Hundred Taking Into Account Sex, Obesity, and Presence of Type-2 Diabetes. Clin. Anat. 2007, 20, 933–942. [Google Scholar] [CrossRef]
- Al-Mrabeh, A.; Hollingsworth, K.G.; Steven, S.; Taylor, R. Morphology of the Pancreas in Type 2 Diabetes: Effect of Weight Loss with or without Normalisation of Insulin Secretory Capacity. Diabetologia 2016, 59, 1753–1759. [Google Scholar] [CrossRef]
- Yagihashi, S. Diabetes and Pancreas Size, Does It Matter? J. Diabetes Investig. 2016, 8, 413. [Google Scholar] [CrossRef]
- Zafar, M.; Naqvi, S.N.-H. Effects of STZ-Induced Diabetes on the Relative Weights of Kidney, Liver and Pancreas in Albino Rats: A Comparative Study. Int. J. Morphol. 2010, 28, 135–142. [Google Scholar] [CrossRef]
- Alabi, T.D.; Brooks, N.L.; Oguntibeju, O.O. Leaf Extracts of Anchomanes Difformis Ameliorated Kidney and Pancreatic Damage in Type 2 Diabetes. Plants 2021, 10, 300. [Google Scholar] [CrossRef]
- Vallon, V.; Thomson, S.C. Renal Function in Diabetic Disease Models: The Tubular System in the Pathophysiology of the Diabetic Kidney. Annu. Rev. Physiol. 2013, 74, 351–375. [Google Scholar] [CrossRef]
- Malini, D.M.; Fitriani, N.; Laila, A.; Ratningsih, N.; Setiawati, T. Morphological and Histological Kidney Structure in Diabetic Rats Model Treated with Ethanol Extracts of Jengkol Fruit Peel (Archidendron Pauciflorum). J. Biol. Udayana 2021, 25, 208–217. [Google Scholar] [CrossRef]
- Eleazu, C.O.; Iroaganachi, M.; Okafor, P.N.; Ijeh, I.I.; Eleazu, K.C. Ameliorative Potentials of Ginger (Z. Officinale Roscoe) on Relative Organ Weights in Streptozotocin Induced Diabetic Rats. Int. J. Biomed. Sci. 2013, 9, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Asuk, A.A.; Atangwho, I.J.; Ugwu, M.N.; Unimke, B. Jatropha Curcas Leaf Extract and Fractions Attenuate Hyperglycemia, Tissue Oxidation, and Kidney Dysfunction in Diabetic Rats. J. Biochem. Techol. 2021, 12, 84–90. [Google Scholar] [CrossRef]
- Mohamed, J.; Nafizah, N.; Zariyantey; Budin, S.B. Mechanisms of Diabetes-Induced Liver Damage: The Role of Oxidative Stress and Inflammation. Sultan Qaboos Univ. Med. J. 2016, 16, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Lestari, K.; Hwang, J.; Hartini Kariadi, S.; Wijaya, A.; Ahmad, T.; Subarnas, A.; Supriyatna; Muchtaridi, M. Screening for PPAR γ Agonist from Myristica Fragrans Houtt Seeds for the Treatment of Type 2 Diabetes by in Vitro and in Vivo. Med. Health Sci. J. 2012, 12, 7–15. [Google Scholar] [CrossRef]
- Pashapoor, A.; Mashhadyrafie, S.; Mortazavi, P. Ameliorative Effect of Myristica Fragrans (Nutmeg) Extract on Oxidative Status and Histology of Pancreas in Alloxan Induced Diabetic Rats. Folia Morphol. 2020, 79, 113–119. [Google Scholar] [CrossRef]
- Somani, R.S.; Singhai, A.K. Hypoglycaemic and Antidiabetic Activities of Seeds of Myristica Fragrans in Normoglycaemic and Alloxan-Induced Diabetic Rats. Asian J. Exp. Sci. 2008, 22, 95–102. [Google Scholar]
- Rajamani, S.; Suganthi, R.; Ravichandrana, M.K.; Anuradha, C.V. Food Seasoning Spices Mixture Improves Glucose Metabolism and Lipid Profile in Fructose-Fed Hyperinsulinemic Rats. J. Med. Food 2005, 8, 502–507. [Google Scholar] [CrossRef]
- Zhao, W.; Song, F.; Hu, D.; Chen, H.; Zhai, Q.; Lu, W.; Zhao, J.; Zhang, H.; Chen, W.; Gu, Z.; et al. The Protective Effect of Myristica Fragrans Houtt. Extracts against Obesity and Inflammation by Regulating Free Fatty Acids Metabolism in Nonalcoholic Fatty Liver Disease. Nutrients 2020, 12, 2507. [Google Scholar] [CrossRef]
- Lestari, K.; Diantini, A.; Barliana, M.I.; Achmad, T.H.; Subarnas, A.; Abdulah, R.; Lesmana, R.; Hwang, J.K. Potential Natural Dual Agonist PPAR α/γ -Induced Antidiabetic and Anti- Dyslipidemic Properties of Safrole-Free Nutmeg Seed (Myristica Fragrans Houtt) Extract. Nat. Prod. J. 2019, 9, 248–253. [Google Scholar] [CrossRef]
- Saputra, N.T.; Suartha, I.N.; Dharmayudha, A.A.G.O. Diabetagonic Agent Streptozotocin to Make White Rats Male Diabetes Mellitus. Bul. Vet. Udayana 2018, 10, 116–121. [Google Scholar] [CrossRef][Green Version]
- Munjiati, N.E.; Sulistiyowati, R. Kurniawan Pengaruh Pemberian Streptozotocin Dosis Tunggal Terhadap Kadar Glukosa Wistar. Meditory 2021, 9, 62–67. [Google Scholar] [CrossRef]
- Estoppey, P.; Clair, C.; Auderset, D.; Puder, J.J. Sex differences in type 2 diabetes. Cardiovasc. Med. 2023, 26, 96–99. [Google Scholar] [CrossRef]
- Kellett, G.L.; Brot-laroche, E.; Mace, O.J.; Leturque, A. Sugar Absorption in the Intestine: The Role of GLUT2. Annu. Rev. Nutr. 2008, 28, 35–54. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.M.; Loo, D.D.F.; Hirayama, B.A.; Human, C. Biology of Human Sodium Glucose Transporters. Physiol. Rev. 2011, 91, 733–794. [Google Scholar] [CrossRef]
- Koepsell, H. Glucose Transporters in the Small Intestine in Health and Disease. Pflügers Arch. Eur. J. Physiol. 2020, 472, 1207–1248. [Google Scholar] [CrossRef]
- Gromova, L.V.; Fetissov, S.O.; Gruzdkov, A.A. Mechanisms of Glucose Absorption in the Small Intestine in Health and Metabolic Diseases and Their Role in Appetite Regulation. Nutrients 2021, 13, 2474. [Google Scholar] [CrossRef]
- Maria, C.; Cefalo, A.; Cinti, F.; Moffa, S.; Impronta, F.; Sorice, G.P.; Mezza, T.; Pontecorvi, A.; Giaccari, A. Sotagliflozin, the First Dual SGLT Inhibitor: Current Outlook and Perspectives. Cardiovasc. Diabetol. 2019, 18, 20. [Google Scholar] [CrossRef]
- Kareem, M.A.; Krushna, G.S.; Hussain, S.A.; Devi, K.L. Effect of Aqueous Extract of Nutmeg on Hyperglycaemia, Hyperlipidaemia and Cardiac Histology Associated with Isoproterenol-Induced Myocardial Infarction in Rats. Trop. J. Pharm. Res. 2009, 8, 337–344. [Google Scholar] [CrossRef]
- Maidadi, B.; Ntchapda, F.; Miaffo, D.; Mahamad, A.T. Journal of Traditional and Complementary Medicine Diabetes Mellitus: Preventive and Curative Therapies with Aqueous Extract of Rytigynia Senegalensis Blume (Rubiaceae) in Wistar Rats. J. Tradit. Complement. Med. 2023, 13, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Grover, J.K.; Yadav, S.; Vats, V. Medicinal Plants of India with Anti-Diabetic Potential. J. Ethnopharmacol. 2002, 81, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Sathish, G.; Jayanthi, M.; Muthukumaran, J.; Muruganathan, U.; Ramachandran, V. Ameliorating Effect of Eugenol on Hyperglycemia by Attenuating the Key Enzymes of Glucose Metabolism in Streptozotocin-Induced Diabetic Rats. Mol. Cell. Biochem. 2014, 385, 159–168. [Google Scholar] [CrossRef]
- Thomas, H.Y.; Versypt, A.N.F. Pathophysiology of Mesangial Expansion in Diabetic Nephropathy: Mesangial Structure, Glomerular Biomechanics, and Biochemical Signaling and Regulation. J. Biol. Eng. 2022, 16, 19. [Google Scholar] [CrossRef]
- Bhatt, H.B.; Smith, R.J. Fatty Liver Disease in Diabetes Mellitus. HepatoBiliary Surg. Nutr. 2015, 4, 101–108. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Merino, J.; Sun, Q.; Fitó, M.; Salas-Salvadó, J. Dietary Polyphenols, Mediterranean Diet, Prediabetes, and Type 2 Diabetes: A Narrative Review of the Evidence. Oxidative Med. Cell. Longev. 2017, 2017, 6723931. [Google Scholar] [CrossRef]

| Results | % Difference | Effect * |
|---|---|---|
| Acute effects | ||
| AUC-OSTT < negative control | −12.56 | + |
| AUC-OSTT > positive control (10 mg acarbose/kg BW) | 4.50 | 0 |
| AUC-OGTT < negative control | −8.50 | + |
| AUC-OGTT > positive control (5 mg glibenclamide/kg BW) | 2.67 | 0 |
| Long-term effects (28 days) | ||
| FBG > normal control | 392.27 | 0 |
| FBG < negative control | −14.29 | + |
| FBG < positive control (10 mg acarbose/kg BW) | −8.29 | + |
| FBG < initial FBG (week 0) | −12.61 | + |
| FBG > normal control + 1.84 mg total phenolics from ME/kg BW | 385.84 | 0 |
| HbA1c > normal control | 104.75 | 0 |
| HbA1c < negative control | −11.50 | + |
| HbA1c < positive control (10 mg acarbose/kg BW) | −2.97 | + |
| HbA1c > normal control + 1.84 mg total phenolics from ME/kg BW | 97.25 | 0 |
| Group | Relative Weight of Organs (%) | |||
|---|---|---|---|---|
| Pancreas | Right Kidney | Left Kidney | Liver | |
| Normal rats + aquadest (normal control) | 0.36 ± 0.07 ab | 0.37 ± 0.02 a | 0.37 ± 0.03 a | 3.73 ± 0.29 a |
| Hyperglycemic rats + aquadest (negative control) | 0.29 ± 0.04 a | 0.50 ± 0.03 b | 0.49 ± 0.05 b | 4.67 ± 0.56 b |
| Hyperglycemic rats + 10 mg acarbose/kg BW (positive control) | 0.31 ± 0.03 ab | 0.50 ± 0.03 b | 0.47 ± 0.04 b | 4.73 ± 0.44 b |
| Hyperglycemic rats + 1.84 mg total phenolics from ME/kg BW | 0.35 ± 0.15 ab | 0.46 ± 0.04 b | 0.46 ± 0.03 b | 4.49 ± 0.66 b |
| Normal rats + 1.84 mg total phenolics from ME/kg BW | 0.44 ± 0.07 b | 0.38 ± 0.02 a | 0.39 ± 0.02 a | 3.33 ± 0.13 a |
| vs. | FBG | HbA1c | BW | RW of Liver | RW of Right Kidney | RW of Left Kidney | RW of Pancreas |
|---|---|---|---|---|---|---|---|
| FBG | 1 | 0.948 | −0.871 | 0.762 | 0.906 | 0.858 | −0.461 |
| HbA1c | 1 | −0.893 | 0.794 | 0.902 | 0.830 | −0.427 | |
| BW | 1 | −0.815 | −0.874 | −0.838 | 0.329 | ||
| RW of liver | 1 | 0.725 | 0.687 | −0.589 | |||
| RW of right kidney | 1 | 0.912 | −0.241 | ||||
| RW of left kidney | 1 | −0.199 | |||||
| RW of pancreas | 1 |
| Experimental Parameters | Hyperglycemic Rats + ME | Hyperglycemic Rats (Negative Control) | Effects |
|---|---|---|---|
| Glycemic parameters | |||
| AUC-OSTT | 330.83 | 378.33 | ↓12.56% |
| AUC-OGTT | 330.50 | 361.21 | ↓8.50% |
| FBG (mg/dL) | 446.00 | 520.33 | ↓14.29% |
| HbA1c (%) | 10.77 | 12.17 | ↓11.50% |
| Body weight loss (g) | 44.33 | 45.00 | ↓0.26% |
| Daily water intake (mL) | 116.73 | 129.88 | ↓10.13% |
| Relative weight of organs | |||
| Pancreas (%) | 0.35 | 0.29 | ↑20.69% |
| Right kidney (%) | 0.46 | 0.50 | ↓8.00% |
| Left kidney (%) | 0.46 | 0.49 | ↓6.12% |
| Liver (%) | 4.49 | 4.67 | ↓3.85% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasbullah; Dewi, F.N.A.; Faridah, D.N.; Indrasti, D.; Andarwulan, N.; Średnicka-Tober, D. Antihyperglycemic Potential of Mace Water Extract from Myristica fragrans Houtt. Appl. Sci. 2025, 15, 5706. https://doi.org/10.3390/app15105706
Hasbullah, Dewi FNA, Faridah DN, Indrasti D, Andarwulan N, Średnicka-Tober D. Antihyperglycemic Potential of Mace Water Extract from Myristica fragrans Houtt. Applied Sciences. 2025; 15(10):5706. https://doi.org/10.3390/app15105706
Chicago/Turabian StyleHasbullah, Fitriya Nur Annisa Dewi, Didah Nur Faridah, Dias Indrasti, Nuri Andarwulan, and Dominika Średnicka-Tober. 2025. "Antihyperglycemic Potential of Mace Water Extract from Myristica fragrans Houtt" Applied Sciences 15, no. 10: 5706. https://doi.org/10.3390/app15105706
APA StyleHasbullah, Dewi, F. N. A., Faridah, D. N., Indrasti, D., Andarwulan, N., & Średnicka-Tober, D. (2025). Antihyperglycemic Potential of Mace Water Extract from Myristica fragrans Houtt. Applied Sciences, 15(10), 5706. https://doi.org/10.3390/app15105706













