Texture Analysis of Near-Infrared Vein Images During Reactive Hyperemia in Healthy Subjects
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Technologies
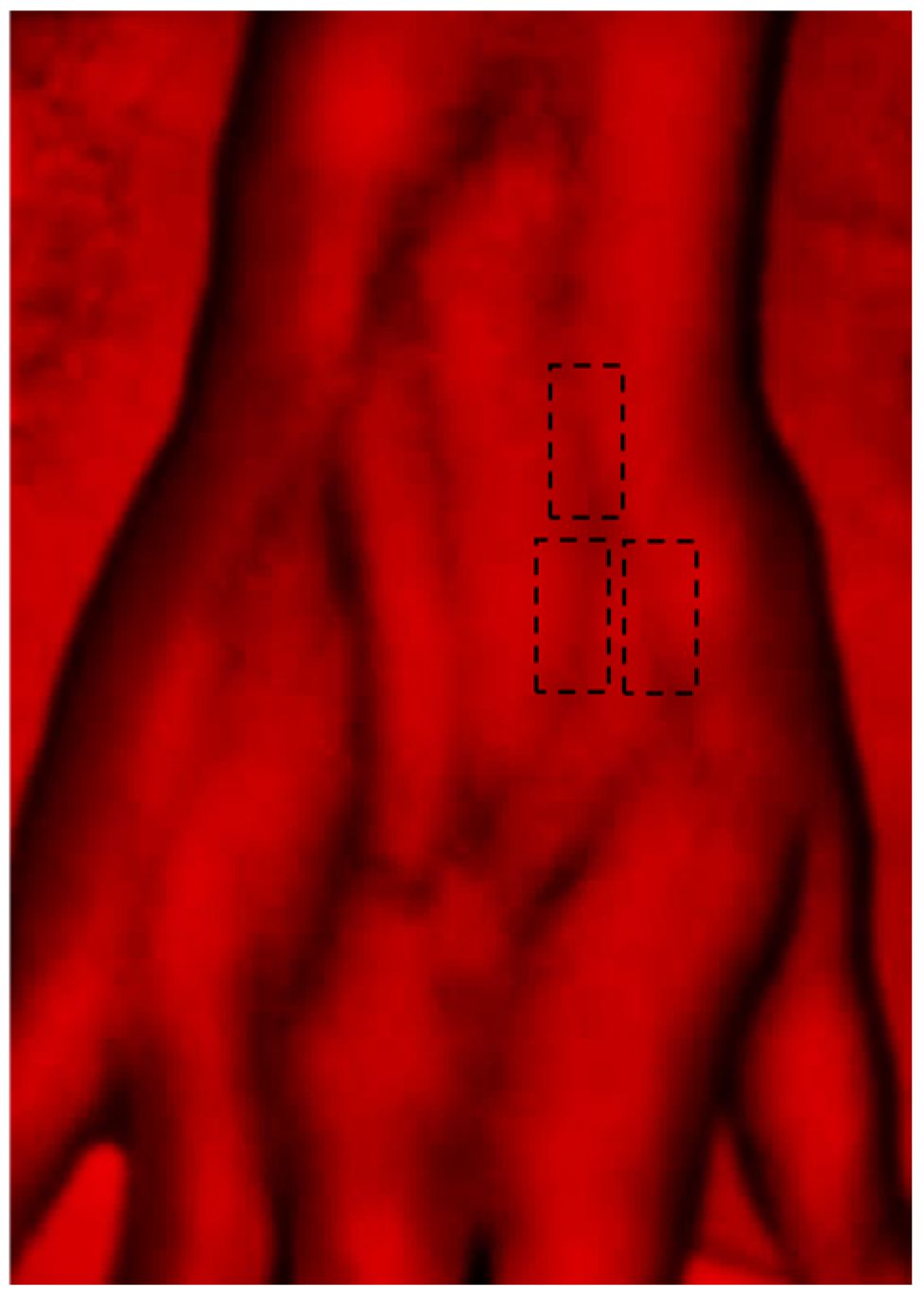
2.3. Methodology
2.4. Analytics
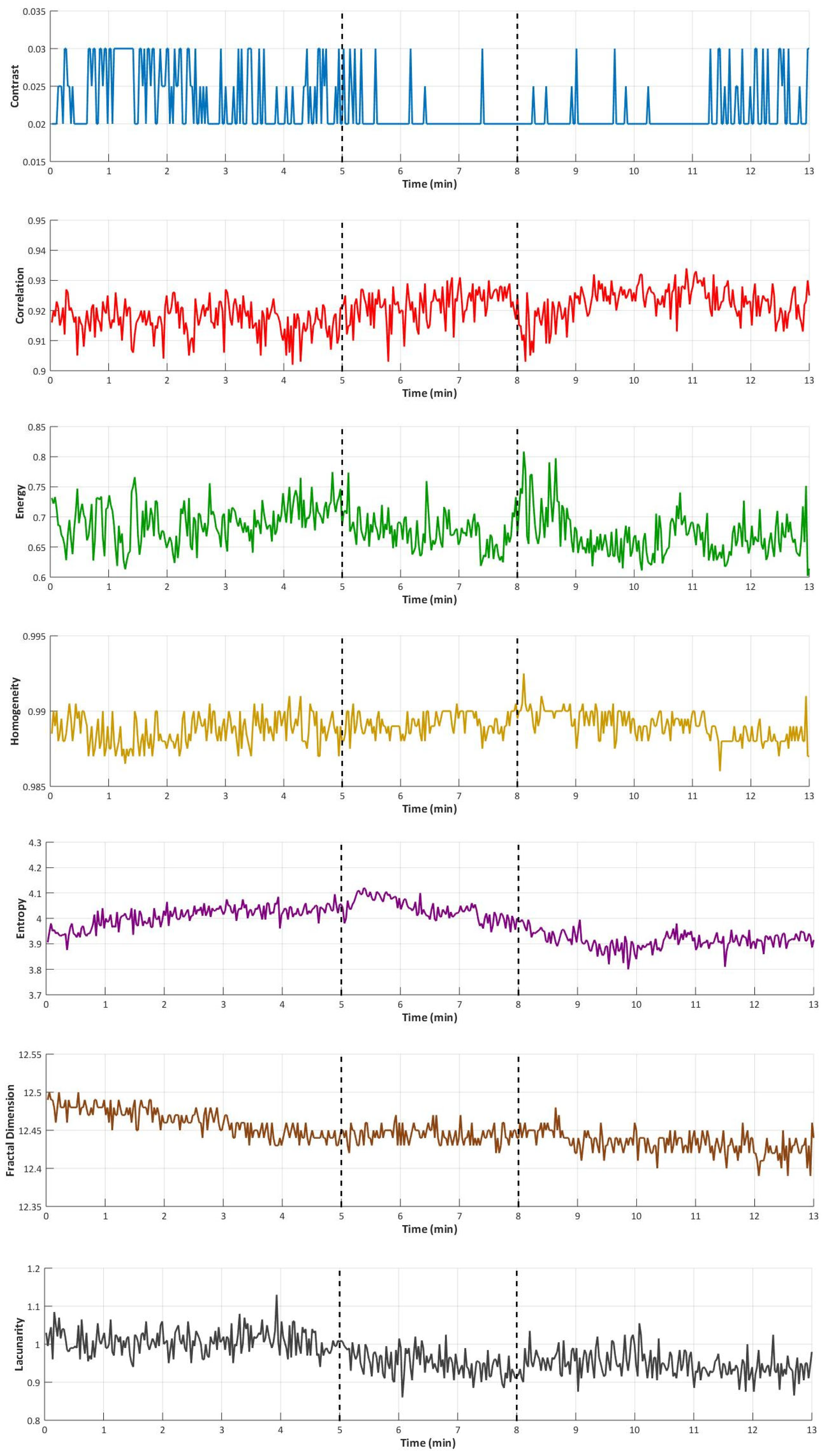
3. Results
4. Discussion
4.1. Interpretation of Classical Texture Parameters
4.2. Divergence Between Morphology and Texture
4.3. Composite Parameters and Their Physiological Meaning
4.4. Possible Relation with Movement Artifacts
4.5. Study Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cleman, J.; Xia, K.; Haider, M.; Nikooie, R.; Scierka, L.; Romain, G.; Attaran, R.R.; Grimshaw, A.; Mena-Hurtado, C.; Smolderen, K.G. A state-of-the-art review of quality-of-life assessment in venous disease. J. Vasc. Surg. Venous Lymphat. Disord. 2024, 12, 101725. [Google Scholar] [CrossRef]
- Skoog, J.; Nelzén, O.; Zachrisson, H. Venous Compliance in Great Saphenous Vein Incompetence: Pre- and Post-interventional Changes. EJVES Vasc. Forum 2020, 47, 78–82. [Google Scholar] [CrossRef]
- Spiridon, M.; Corduneanu, D. Chronic Venous Insufficiency: A Frequently Underdiagnosed and Undertreated Pathology. Maedica 2017, 12, 59–61. [Google Scholar]
- Zhang, F.; Song, H.-X.; He, Z.-P.; Zheng, L.-H.; Han, Y.-R.; Wang, B.-Y.; Liu, P. Analysis of computed tomography venography for the diagnosis and endovascular treatment of iliac venous compression syndrome with venous leg ulcers: A retrospective study. Sci. Rep. 2024, 14, 22314. [Google Scholar] [CrossRef]
- Zhu, L.-L.; Wang, Y.-H.; Zhou, Q. Progress in Research on the Mechanisms and Interventions of Phlebitis from the Perspective of Vascular Endothelial Cell and Signaling Pathway. J. Inflamm. Res. 2023, 16, 6469–6481. [Google Scholar] [CrossRef]
- Schulman, S.; Makatsariya, A.; Khizroeva, J.; Bitsadze, V.; Kapanadze, D. The Basic Principles of Pathophysiology of Venous Thrombosis. Int. J. Mol. Sci. 2024, 25, 11447. [Google Scholar] [CrossRef]
- Kassaï, B.; Boissel, J.-P.; Cucherat, M.; Sonie, S.; Shah, N.R.; Leizorovicz, A. A systematic review of the accuracy of ultrasound in the diagnosis of deep venous thrombosis in asymptomatic patients. Thromb. Haemost. 2004, 91, 655–666. [Google Scholar]
- Burnside, P.R.; Green, E.; Kline, J.A. Indirect computed tomography venography: A report of vascular opacification. Emerg. Radiol. 2010, 17, 195–201. [Google Scholar] [CrossRef]
- Arnoldussen, C.W.K.P.; De Graaf, R.; Wittens, C.H.A.; De Haan, M.W. Value of magnetic resonance venography and computed tomographic venography in lower extremity chronic venous disease. Phlebol. J. Venous Dis. 2013, 28, 169–175. [Google Scholar] [CrossRef]
- Kistner, R.L. Diagnosis of chronic venous insufficiency. J. Vasc. Surg. 1986, 3, 185–188. [Google Scholar] [CrossRef][Green Version]
- Aulagnier, J.; Hoc, C.; Mathieu, E.; Dreyfus, J.F.; Fischler, M.; Le Guen, M. Efficacy of Accuvein to facilitate peripheral intravenous placement in adults presenting to an emergency department: A randomized clinical trial. Acad. Emerg. Med. 2014, 21, 858–863. [Google Scholar] [CrossRef]
- Pan, C.-T.; Francisco, M.D.; Yen, C.-K.; Wang, S.-Y.; Shiue, Y.-L. Vein pattern locating technology for cannulation: A review of the low-cost vein finder prototypes utilizing near infrared (NIR) light to improve peripheral subcutaneous vein selection for phlebotomy. Sensors 2019, 19, 3573. [Google Scholar] [CrossRef]
- Francisco, M.D.; Chen, W.-F.; Pan, C.-T.; Lin, M.-C.; Wen, Z.-H.; Liao, C.-F.; Shiue, Y.-L. Competitive real-time near infrared (NIR) vein finder imaging device to improve peripheral subcutaneous vein selection in venipuncture for clinical laboratory testing. Micromachines 2021, 12, 373. [Google Scholar] [CrossRef]
- Vyas, V.; Sharma, A.; Goyal, S.; Kothari, N. Infrared vein visualization devices for ease of intravenous access in children: Hope versus hype. Anaesthesiol. Intensiv. Ther. 2021, 53, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, X.; Zhang, L.; Lou, X.; Su, X.; Wang, X.; Sun, F.; He, X. Infrared Vein Imaging for Insertion of Peripheral Intravenous Catheter for Patients Requiring Isolation for Severe Acute Respiratory Syndrome Coronavirus 2 Infection: A Nonrandomized Clinical Trial. J. Emerg. Nurs. 2022, 48, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Renno, I.; Horch, R.E.; Ludolph, I.; Cai, A.; Arkudas, A. Vein Visualization With a Near Infrared Imaging Device and Its Impact on Students’ and Nurses’ Skills in an Academic Teaching University Hospital. J. Infus. Nurs. 2024, 47, 249–254. [Google Scholar] [CrossRef]
- Fraifeld, A.D.; Thompson, J.A. Incorporating Near Infrared Light Vein Visualization Technology Into Peripheral Intravenous Access Protocols. J. Infus. Nurs. 2023, 46, 313–319. [Google Scholar] [CrossRef]
- Gupta, S.; Mehta, N.; Bajpai, M.; Gaurav, V. Near-infrared vein finder for assessment of superficial venous malformations. Indian Dermatol. Online J. 2023, 14, 448–449. [Google Scholar] [CrossRef]
- Goldschmidt, E.; Faraji, A.H.; Jankowitz, B.T.; Gardner, P.; Friedlander, R.M. Use of a near-infrared vein finder to define cortical veins and dural sinuses prior to dural opening. J. Neurosurg. 2020, 133, 1202–1209. [Google Scholar] [CrossRef]
- Castellano, G.; Bonilha, L.; Li, L.M.; Cendes, F. Texture analysis of medical images. Clin. Radiol. 2004, 59, 1061–1069. [Google Scholar] [CrossRef]
- Li, Z.; Yu, L.; Wang, X.; Yu, H.; Gao, Y.; Ren, Y.; Wang, G.; Zhou, X. Diagnostic Performance of Mammographic Texture Analysis in the Differential Diagnosis of Benign and Malignant Breast Tumors. Clin. Breast Cancer 2018, 18, e621–e627. [Google Scholar] [CrossRef]
- Jan, Y.-K.; Hung, I.Y.-J.; Cheung, W.C. Texture Analysis in Musculoskeletal Ultrasonography: A Systematic Review. Diagnostics 2025, 15, 524. [Google Scholar] [CrossRef]
- Lubner, M.G.; Smith, A.D.; Sandrasegaran, K.; Sahani, D.V.; Pickhardt, P.J. CT texture analysis: Definitions, applications, biologic correlates, and challenges. Radiographics 2017, 37, 1483–1503. [Google Scholar] [CrossRef]
- Kunimatsu, A.; Yasaka, K.; Akai, H.; Sugawara, H.; Kunimatsu, N.; Abe, O. Texture Analysis in Brain Tumor MR Imaging. Magn. Reson. Med. Sci. 2022, 21, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Sun, W.; Wu, D.; Pang, P.; Guo, X.; Yu, C.; Lu, W.; Tang, G. Value of texture analysis based on dynamic contrast-enhanced magnetic resonance imaging in preoperative assessment of extramural venous invasion in rectal cancer. Insights Into Imaging 2022, 13, 1–11. [Google Scholar] [CrossRef]
- Canellas, R.; Mehrkhani, F.; Patino, M.; Kambadakone, A.; Sahani, D. Characterization of portal vein thrombosis (neoplastic versus bland) on CT images using software-based texture analysis and thrombus density (hounsfeld units). Am. J. Roentgenol. 2016, 207, W81–W87. [Google Scholar] [CrossRef]
- Ito, K.; Muraoka, H.; Hirahara, N.; Sawada, E.; Tokunaga, S.; Kaneda, T. Texture analysis of low-flow vascular malformations in the oral and maxillofacial region: Venous malformation vs. lymphatic malformation. Pol. J. Radiol. 2022, 87, 494–499. [Google Scholar] [CrossRef]
- Rosenberry, R.; Nelson, M.D. Reactive hyperemia: A review of methods, mechanisms, and considerations. Am. J. Physiol. Integr. Comp. Physiol. 2020, 318, R605–R618. [Google Scholar] [CrossRef]
- Marathe. A Novel Wireless Vein Finder. In International Conference on Circuits, Communication, Control and Computing; IEEE: Piscataway, NJ, USA, 2014. [Google Scholar]
- Shourav, M.K.; Choi, J.; Kim, J.K. Visualization of superficial vein dynamics in dorsal hand by near-infrared imaging in response to elevated local temperature. J. Biomed. Opt. 2021, 26, 026003. [Google Scholar] [CrossRef]
- Gonzalez, R.C.; Woods, R.E.; Eddins, S.L. Digital Image Processing Using MATLAB; Pearson/Prentice Hall: Upper Saddle River, NJ, USA, 2004; 609p. [Google Scholar]
- Gould, D.J.; Vadakkan, T.J.; Poché, R.A.; Dickinson, M.E. Multifractal and Lacunarity Analysis of Microvascular Morphology and Remodeling. Microcirculation 2011, 18, 136–151. [Google Scholar] [CrossRef]
- Myint, S.W.; Lam, N. A study of lacunarity-based texture analysis approaches to improve urban image classification. Comput. Environ. Urban Syst. 2005, 29, 501–523. [Google Scholar] [CrossRef]
- Kara, M.; Pradel, C.; Phan, C.; Miquel, A.; Arrivé, L. CT features of vertebral venous congestion simulating sclerotic metastases in nine patients with thrombosis of the superior vena cava. Am. J. Roentgenol. 2016, 207, 80–86. [Google Scholar] [CrossRef]
- Asciutto, G.; Mumme, A.; Marpe, B.; Köster, O.; Asciutto, K.; Geier, B. MR Venography in the Detection of Pelvic Venous Congestion. Eur. J. Vasc. Endovasc. Surg. 2008, 36, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Steenbeek, M.P.; van der Vleuten, C.J.M.; Schultze Kool, L.J.; Nieboer, T.E. Noninvasive diagnostic tools for pelvic congestion syndrome: A systematic review. Acta Obstet. Et. Gynecol. Scand. 2018, 97, 776–786. [Google Scholar] [CrossRef] [PubMed]
- Galindo, P.; Gasca, C.; Argaiz, E.R.; Koratala, A. Point of care venous Doppler ultrasound: Exploring the missing piece of bedside hemodynamic assessment. World J. Crit. Care Med. 2021, 10, 310–322. [Google Scholar] [CrossRef]
- Darvall, K.; Sam, R.; Bate, G.; Adam, D.; Silverman, S.; Bradbury, A. Photoplethysmographic venous refilling times following ultrasound guided foam sclerotherapy for symptomatic superficial venous reflux: Relationship with clinical outcomes. Eur. J. Vasc. Endovasc. Surg. 2010, 40, 267–272. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guven, H. Plethysmography: A game changer for chronic venous insufficiency diagnosis, treatment, and follow-up. Vascular 2024, 33, 192–199. [Google Scholar] [CrossRef]
- Kim, D.; Kim, Y.; Yoon, S.; Lee, D. Preliminary study for designing a novel vein-visualizing device. Sensors 2017, 17, 304. [Google Scholar] [CrossRef]
- Okazaki, K.; Fu, Q.; Martini, E.R.; Shook, R.; Conner, C.; Zhang, R.; Crandall, C.G.; Levine, B.D. Vasoconstriction during venous congestion: Effects of venoarteriolar response, myogenic reflexes, and hemodynamics of changing perfusion pressure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, 1354–1359. [Google Scholar] [CrossRef]
- Fujii, N.; McGarr, G.W.; McNeely, B.D.; Ichinose, M.; Nishiyasu, T.; Kenny, G.P. KCa and KV channels modulate the venoarteriolar reflex in non-glabrous human skin with no roles of KATP channels, NOS, and COX. Eur. J. Pharmacol. 2020, 866, 172828. [Google Scholar] [CrossRef]
- Vironicka, A.S.; Sathiaseelan, J.G.R. Texture feature extraction with medical image using glcm and machine learning techniques. Ictact J. Image Video Process. 2022, 4. [Google Scholar] [CrossRef]
- Indra, Z.; Jusman, Y. Performance of GLCM Algorithm for Extracting Features to Differentiate Normal and Abnormal Brain Images. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1082, 012011. [Google Scholar] [CrossRef]
- Soh, L.-K.; Tsatsoulis, C. Texture analysis of SAR sea ice imagery using gray level co-occurrence matrices. IEEE Trans. Geosci. Remote Sens. 1999, 37, 780–795. [Google Scholar] [CrossRef]
- Depeursinge, A.; Foncubierta-Rodriguez, A.; Van De Ville, D.; Müller, H. Three-dimensional solid texture analysis in biomedical imaging: Review and opportunities. Med. Image Anal. 2014, 18, 176–196. [Google Scholar] [CrossRef]
- Materka, A. Texture analysis methodologies for magnetic resonance imaging. Dialog-Clin. Neurosci. 2004, 6, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jain, R.; Khalil, K.; Griffith, B.; Bosca, R.; Rao, G.; Rao, A. Texture feature ratios from relative CBV maps of perfusion MRI are associated with patient survival in glioblastoma. Am. J. Neuroradiol. 2016, 37, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Persichini, R.; Lai, C.; Teboul, J.-L.; Adda, I.; Guérin, L.; Monnet, X. Venous return and mean systemic filling pressure: Physiology and clinical applications. Crit. Care 2022, 26, 150. [Google Scholar] [CrossRef]
- Silva, H.; Rezendes, C. Revisiting the Venoarteriolar Reflex–Further Insights from Upper Limb Dependency in Healthy Subjects. Biology 2024, 13, 715. [Google Scholar] [CrossRef]
- Rantalainen, T.; Chivers, P.; Beck, B.R.; Robertson, S.; Hart, N.H.; Nimphius, S.; Weeks, B.K.; McIntyre, F.; Hands, B.; Siafarikas, A. Please Don’t Move-Evaluating Motion Artifact From Peripheral Quantitative Computed Tomography Scans Using Textural Features. J. Clin. Densitom. 2018, 21, 260–268. [Google Scholar] [CrossRef]
- Alizadeh, M.; Conklin, C.J.; Middleton, D.M.; Shah, P.; Saksena, S.; Krisa, L.; Finsterbusch, J.; Faro, S.H.; Mulcahey, M.; Mohamed, F.B. Identification of ghost artifact using texture analysis in pediatric spinal cord diffusion tensor images. Magn. Reson. Imaging 2018, 47, 7–15. [Google Scholar] [CrossRef]

| Total | Females | Males | |
|---|---|---|---|
| N | 14 | 8 | 6 |
| Age | 21.5 ± 4.2 | 21.5 ± 1.2 | 21.0 ± 6.4 |
| Height (m) | 1.73 ± 0.1 | 1.69 ± 0.1 | 1.75 ± 0.1 |
| Body mass (kg) | 63.0 ± 8.5 | 61.0 ± 6.0 | 65.0 ± 10.5 |
| Body mass index (kg/m2) | 23.1 ± 2.3 | 23.2 ± 2.1 | 21.0 ± 3.0 |
| Systolic blood pressure (mmHg) | 111.0 ± 8.5 | 107.5 ± 8.7 | 114.0 ± 6.8 |
| Diastolic blood pressure (mmHg) | 74.0 ± 6.7 | 76.0 ± 7.4 | 74.0 ± 5.7 |
| Menstrual cycle duration (days) | - | 28 ± 5 | - |
| Menstrual cycle day | - | 8 ± 15 | - |
| Parameter | Description | Equation |
|---|---|---|
| Contrast | A measure of the intensity difference between neighboring pixels; indicates how much texture varies locally. | |
| Correlation | A measure of how strongly the intensity of one pixel is related to its neighbor; reflects linear patterns in texture. | |
| Energy | A measure of image uniformity; calculated as the sum of squared values in the co-occurrence matrix. | |
| Homogeneity | A measure of the similarity between neighboring pixel intensities; higher values indicate smoother and more uniform textures. | |
| Entropy | A measure of randomness or complexity in the distribution of co-occurring gray levels within the GLCM; higher values indicate greater texture irregularity and disorder. | |
| Fractal dimension | A non-integer exponent that describes how detail in a pattern changes with scale. Calculated with the box-counting method, where ε represents the size (side length) of the box used to cover the structure and N(ε) is the number of boxes of size ε that are needed to completely cover the object | |
| Lacunarity | A scalar value that characterizes the distribution of gaps or voids in a binary structure. It is calculated as the ratio between the square of the variance (σ) and the square of the mean (µ) of the pixel intensities. | |
| Entropy-to-width ratio | A measure of the amount of texture detail present in relation to the visible size of the vein. | Entropy/Width |
| Correlation-to-width ratio | A measure of the consistency of the texture pattern relative to the vein’s width. | Correlation/Width |
| Total entropic content | A measure of the overall texture variation and visual complexity across the entire vein image. | Entropy × Width |
| Correlation-to-entropy ratio | A measure of the amount of order in the texture in relation to its level of complexity. | Correlation/Entropy |
| Parameters | Baseline (Phase I) | Occlusion (Phase II) | Recovery (Phase III) | p-Value (O vs. B) | p-Value (R vs. B) |
|---|---|---|---|---|---|
| Contrast | 0.021 (0.015; 0.025) | 0.020 (0.015; 0.024) | 0.021 (0.017; 0.025) | 0.397 | 0.737 |
| Correlation | 0.902 (0.882; 0.922) | 0.913 (0.900; 0.929) | 0.920 (0.914; 0.946) | 0.023 * | 0.074 |
| Energy | 0.699 (0.628; 0.786) | 0.661 (0.540; 0.716) | 0.696 (0.631; 0.795) | 0.600 | 0.157 |
| Homogeneity | 0.989 (0.987; 0.992) | 0.990 (0.988; 0.992) | 0.989 (0.987; 0.991) | 0.512 | 0.912 |
| Entropy | 4.25 (4.15; 4.42) | 4.16 (4.00; 4.34) | 4.18 (4.11; 4.42) | 0.198 | 0.083 |
| Fractal dimension | 12.462 (12.439; 12.480) | 12.463 (12.429; 12.63) | 12.463 (12.423; 12.485) | 0.060 | 0.319 |
| Lacunarity | 1.06 (0.95; 1.15) | 0.98 (0.72; 1.05) | 0.97 (0.79; 1.05) | 0.136 | 0.024 * |
| Vein width (mm) | 2.19 (2.12; 2.28) | 2.55 (2.34; 2.54) | 2.28 (2.14; 2.33) | <0.001 * | 0.020 * |
| Entropy-to-width ratio | 1.87 (1.69; 2.01) | 1.67 (1.57; 1.79) | 1.80 (1.67; 1.91) | <0.001 * | 0.228 |
| Correlation-to-width ratio | 0.403 (0.375; 0.420) | 0.368 (0.350; 0.388) | 0.404 (0.379; 0.412) | <0.001 * | 0.641 |
| Total entropic content | 9.03 (8.50; 9.48) | 10.09 (9.37; 10.68) | 8.99 (8.26; 9.95) | <0.001 * | 0.858 |
| Correlation-to-entropy ratio | 0.213 (0.205; 0.223) | 0.217 (0.207; 0.229) | 0.221 (0.210; 0.230) | 0.540 | 0.026 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, H.; Rezendes, C. Texture Analysis of Near-Infrared Vein Images During Reactive Hyperemia in Healthy Subjects. Appl. Sci. 2025, 15, 5702. https://doi.org/10.3390/app15105702
Silva H, Rezendes C. Texture Analysis of Near-Infrared Vein Images During Reactive Hyperemia in Healthy Subjects. Applied Sciences. 2025; 15(10):5702. https://doi.org/10.3390/app15105702
Chicago/Turabian StyleSilva, Henrique, and Carlota Rezendes. 2025. "Texture Analysis of Near-Infrared Vein Images During Reactive Hyperemia in Healthy Subjects" Applied Sciences 15, no. 10: 5702. https://doi.org/10.3390/app15105702
APA StyleSilva, H., & Rezendes, C. (2025). Texture Analysis of Near-Infrared Vein Images During Reactive Hyperemia in Healthy Subjects. Applied Sciences, 15(10), 5702. https://doi.org/10.3390/app15105702





