Experimental Study on Multi-Cell Counting Using an Inertial Microfluidic Device
Abstract
1. Introduction
2. Materials and Methods
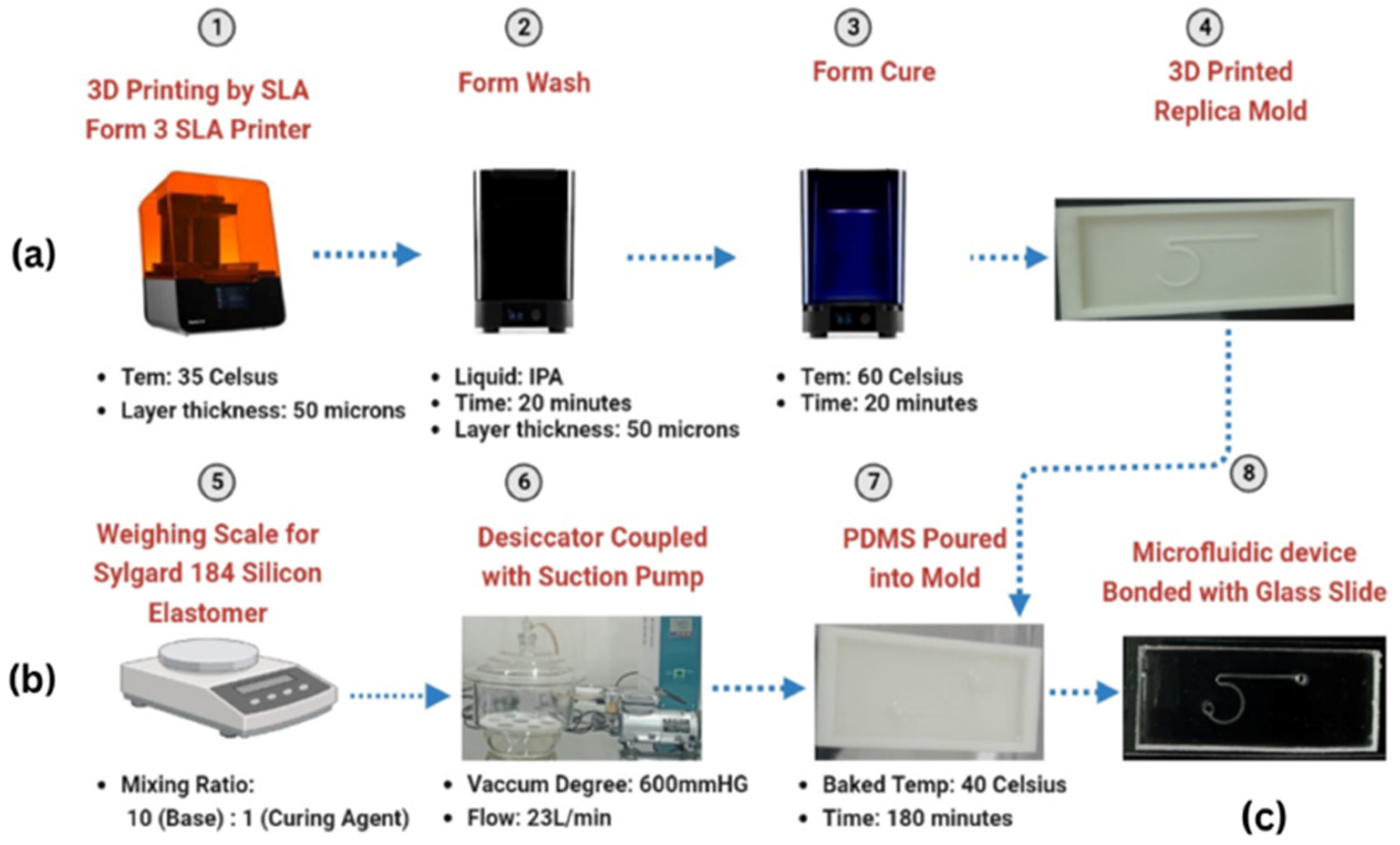
2.1. Fabrication of Microchannels
2.1.1. Mold Preparation
2.1.2. PDMS Preparation and Casting
2.2. Device Assembly
2.3. Sample Flow Preparation
2.3.1. Microparticle Selection and Ratios
2.3.2. Sample Preparation Process
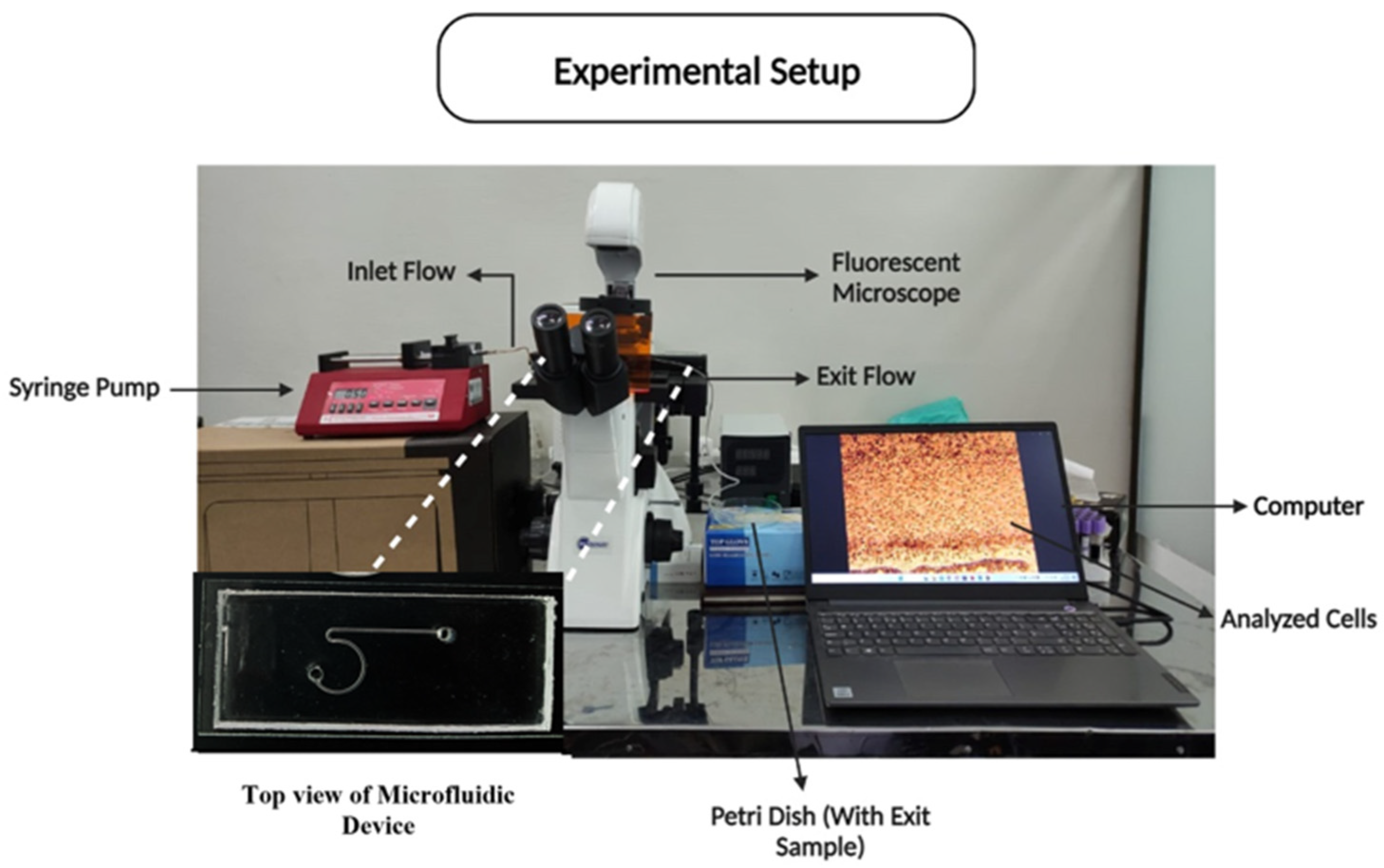
2.4. Experimental Setup and Procedure
Experimental Parameters
2.5. Experimental Variables and Setup
- Microchannel dimensions: Square channels were designed with widths and depths of 150 µm, 200 µm, and 250 µm.
- Particle ratios: Two particle ratios were used: 600:1 for normal conditions and 400:1 for diseased conditions, corresponding to the prevalence of RBC-sized and WBC-sized microparticles measuring 8 µm and 15 µm, respectively.
- Flow rates: Flow rates of 1, 5, 10, and 15 µL/min were employed to assess the impact of flow velocity on particle focusing and counting.
- Optical imaging: The sorting process and particle behavior were monitored using two microscopes: inverted and fluorescent.
2.5.1. Experimental Approach
- Channel size variation: By systematically altering the channel sizes, the study aimed to determine how microchannel dimensions influence the fluidic behavior and sorting efficiency of particles. Smaller channels are expected to have more pronounced inertial effects, which may improve the focusing and sorting of particles.
- Concentration effects: The different particle concentration ratios were selected to simulate both physiological and pathological conditions. This variation allowed for the examination of sorting efficiency under different biological conditions, offering insights into the device’s potential applications in clinical diagnostics.
- Flow rate impact: Adjusting the flow rates provided an opportunity to study the balance between the inertial lift forces and viscous drag. Lower flow rates are anticipated to produce less pronounced inertial effects, whereas higher flow rates may enhance particle migration but risk increased shear forces that could affect the sorting accuracy.
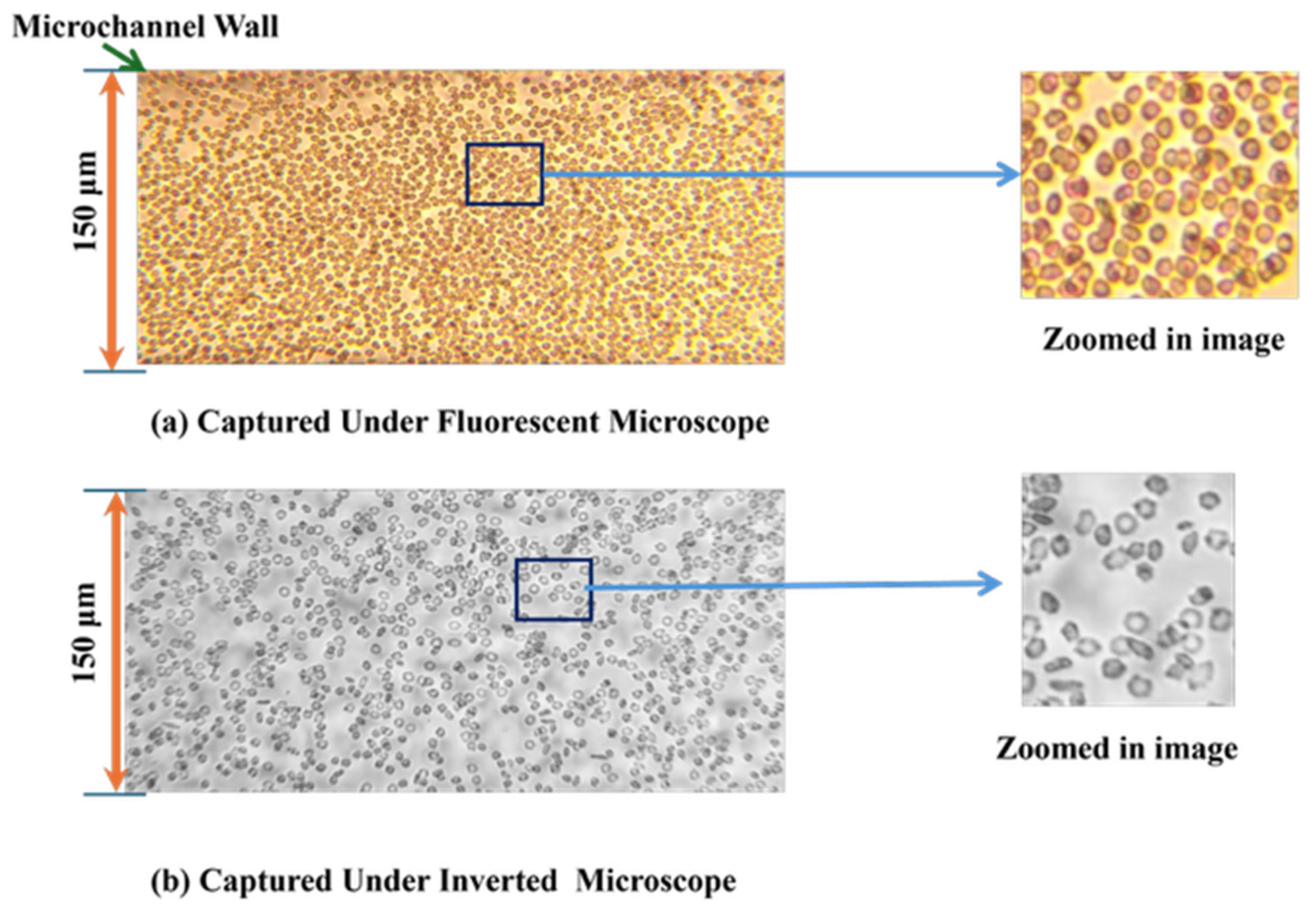
- Imaging and analysis: The use of both inverted and fluorescent microscopes enabled the capture of high-resolution images of particle movement. These data were crucial for quantifying the sorting efficiency and understanding the interaction between particles and the microchannel structure.
2.5.2. Hypothesis and Expected Outcomes
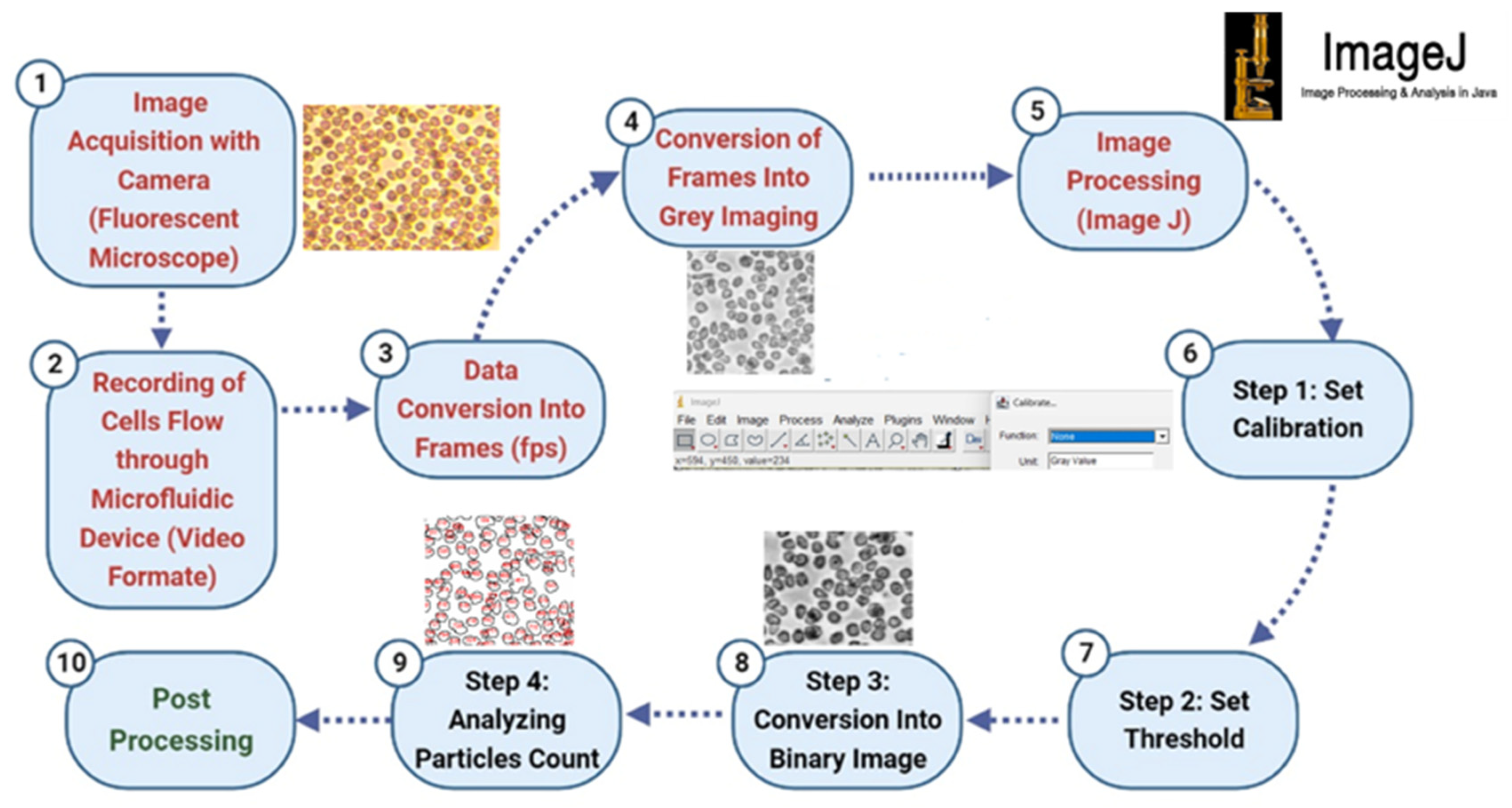
2.5.3. Counting Technique
- Step 1: Image Acquisition and Video Recording
- Step 2: Data Conversion and Frame Extraction
- Step 3: Image Preprocessing
- Step 4: Image Processing and Calibration
- Step 5: Thresholding and Binary Conversion
- Step 6: Automated Particle Counting
- Step 7: Data Compilation and Analysis
- Step 8: Validation and Post-Processing
3. Results and Discussion
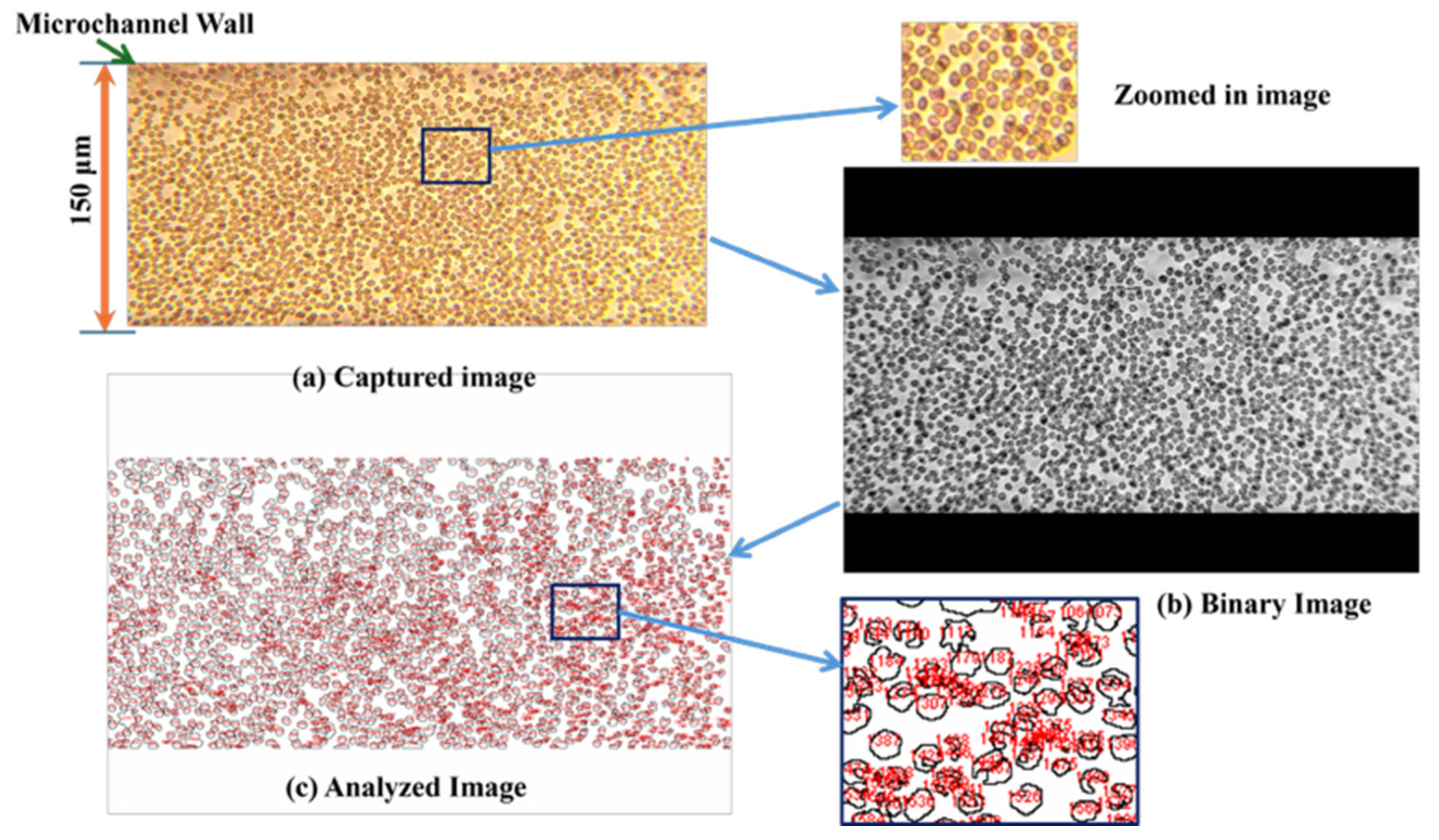
3.1. Validation of the Microfluidic Device
3.2. Analysis of 8-µm Microparticle Counting at Normal Conditions (Ratio 1)
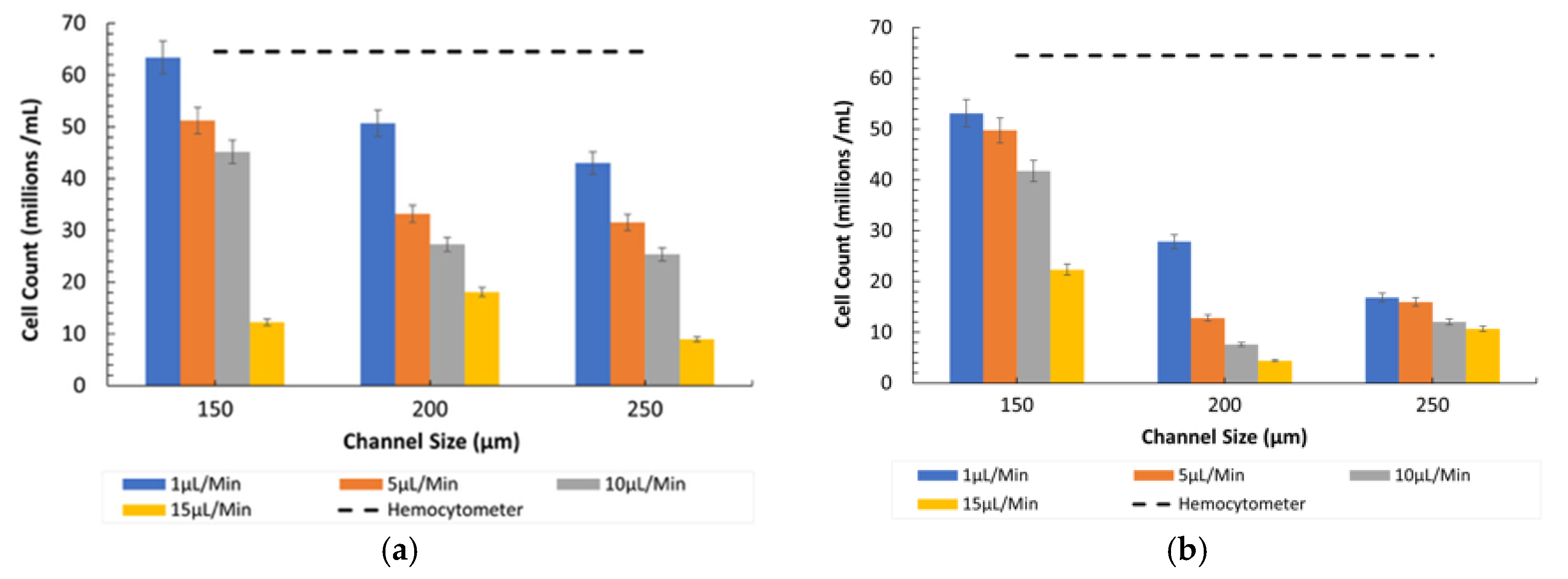
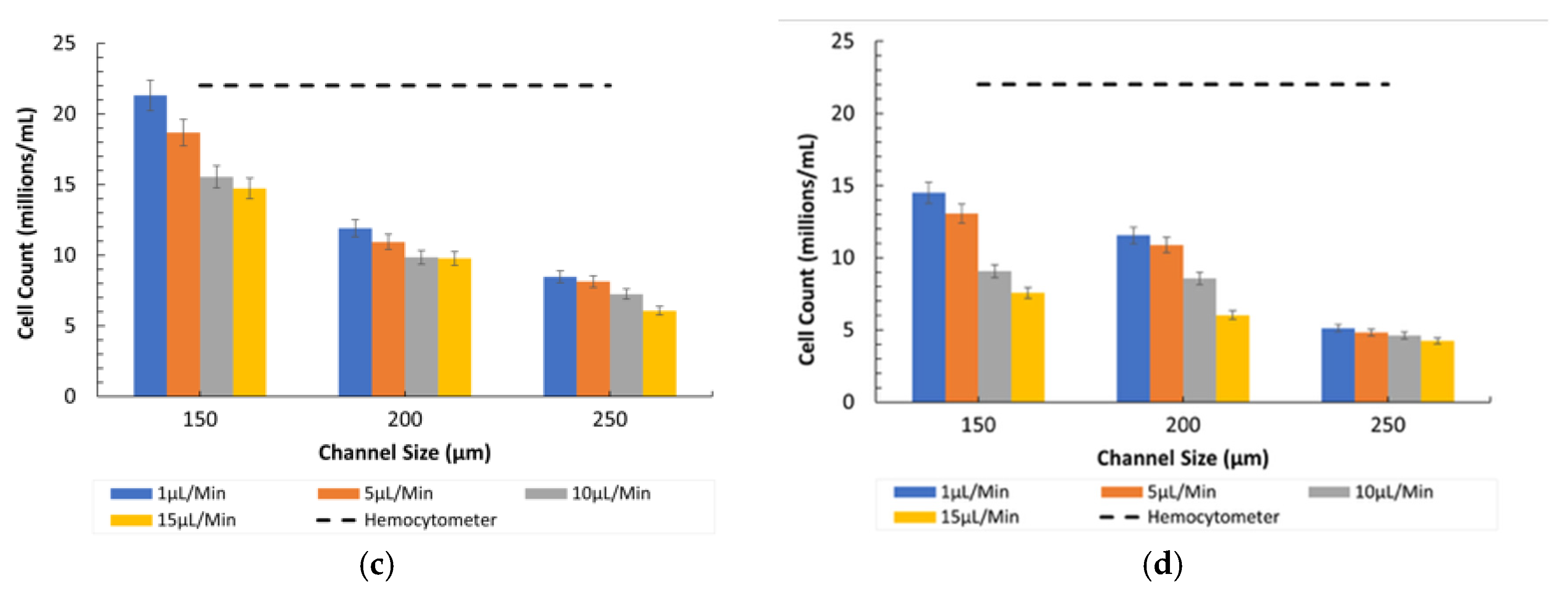
- Fluorescent vs. Inverted Microscopy: Under the Bioimager fluorescent microscope, the particle counts were slightly higher compared with the inverted microscope, likely due to enhanced visibility and contrast provided by fluorescent imaging, as shown in Figure 7. Despite this difference, both imaging methods showed a decline in counting efficiency at higher flow rates. The optimal condition for counting 8-µm particles was observed at a 150 µm channel size and a 1 µL/min flow rate under the fluorescent microscope.
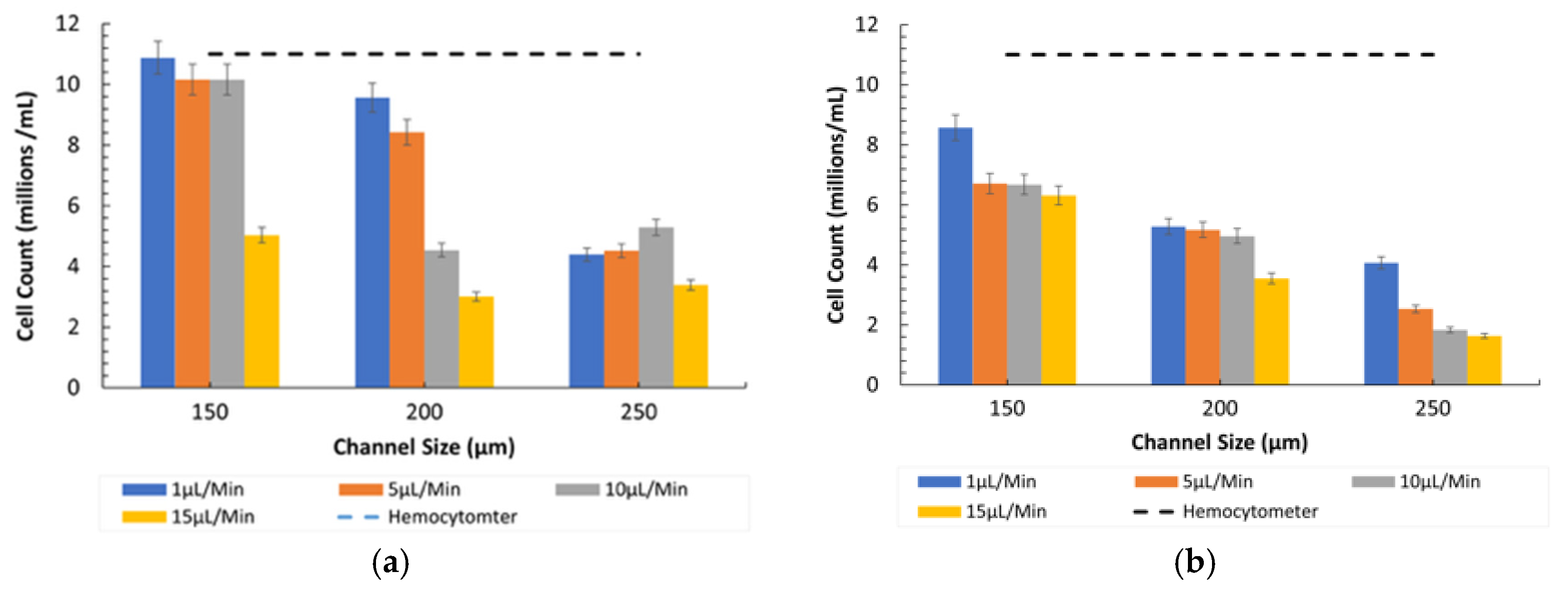
3.3. Counting of 15-µm Microparticles in Normal Conditions (Ratio 1)
- Comparison between Microscopes: As in the case of the 8-µm particles, the fluorescent microscope provided slightly higher counts due to its superior imaging capabilities, as shown in Figure 7. The inverted microscope, although slightly less efficient, remained effective at lower flow rates. For both the 8 µm and 15 µm particles, the fluorescent microscope consistently yielded slightly higher particle counts. This was due to its superior contrast and sensitivity to particles, which improved edge detection during image processing. In contrast, the inverted microscope produced slightly lower count values, resulting in fewer detected particles.
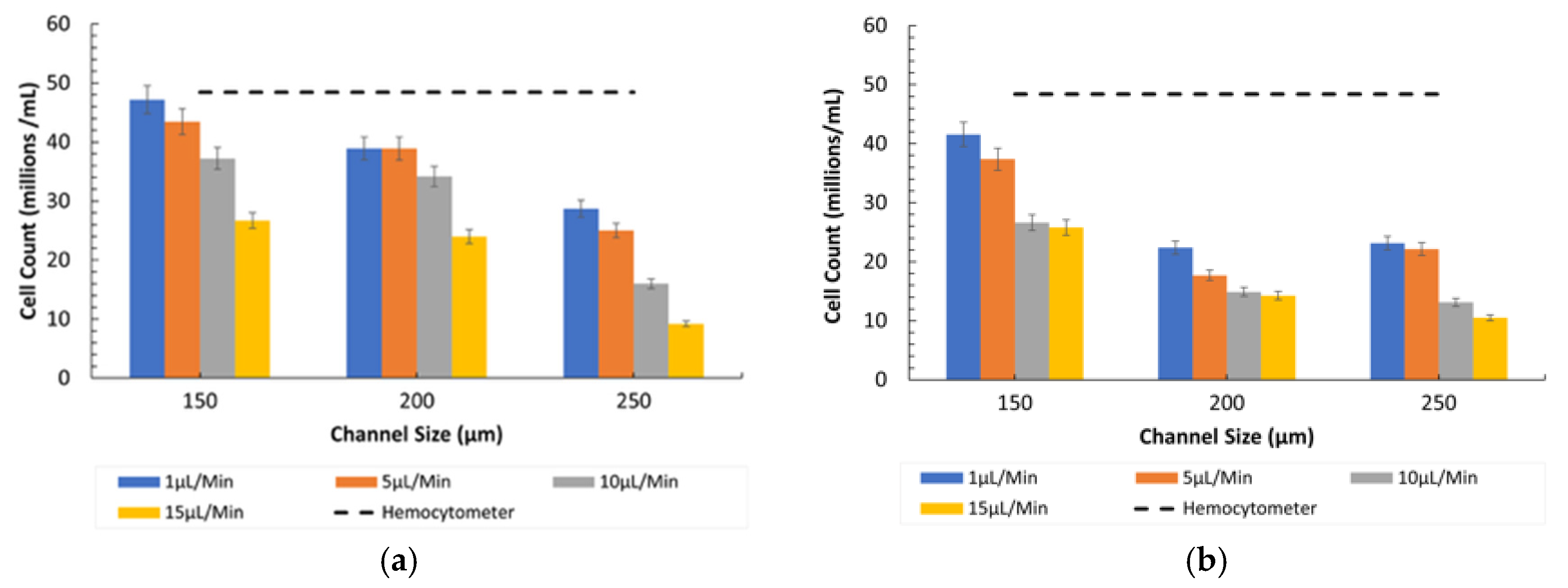
3.4. Counting Under Diseased Conditions (Ratio 2)
- Influence of Channel Size and Flow Rates: The 150 µm channel size remained the most effective in maintaining high counting efficiency at low flow rates. However, as the flow rate increased, the particles misaligned and lost their equilibrium position, resulting in a disruption to the counting efficiency, especially in wider channels (250 µm).
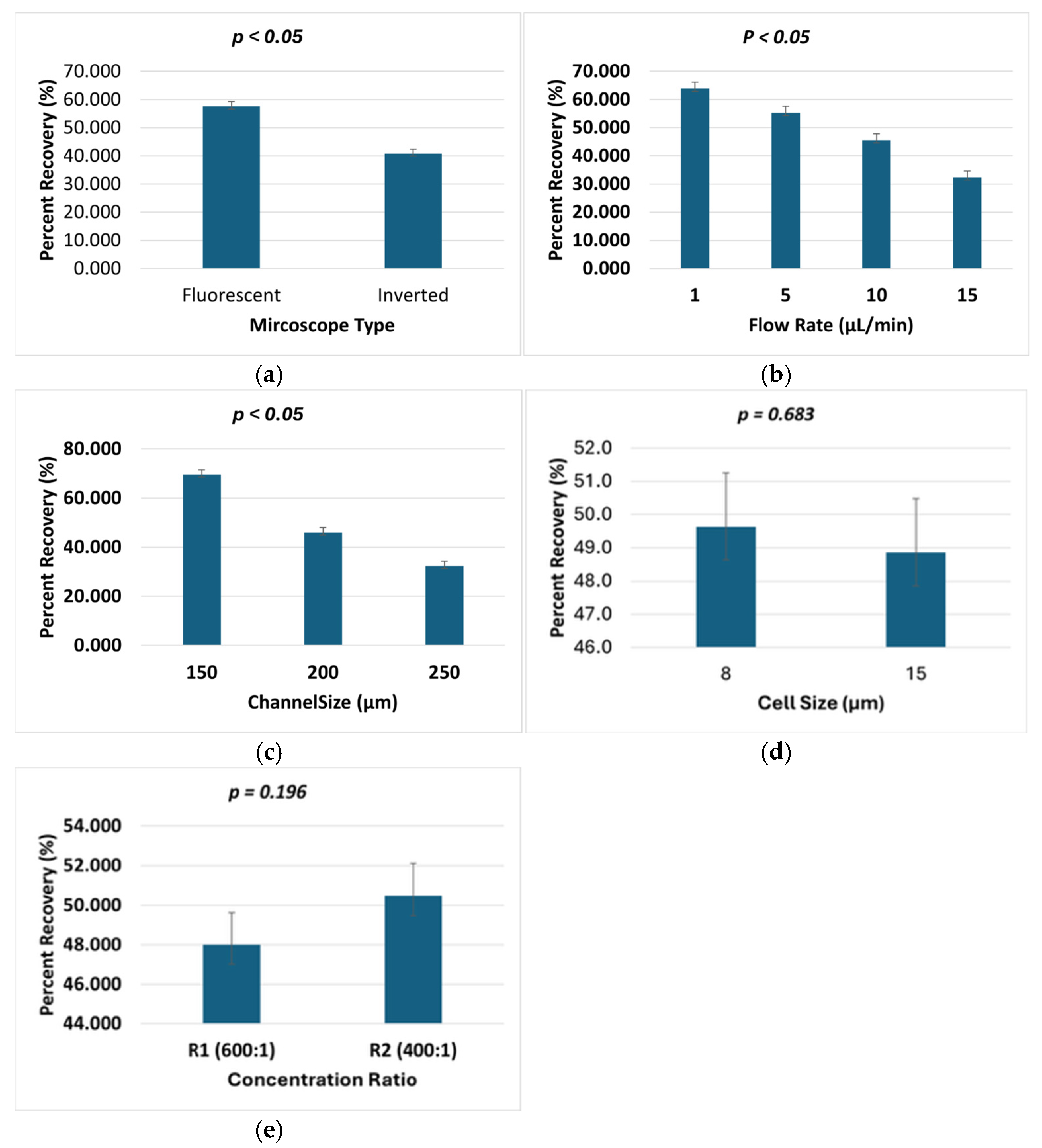
3.5. Statistical Analysis of Percent Recovery
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| a | Particle diameter |
| Dh | Hydraulic diameter of microchannel |
| v | Average fluid velocity in channel |
| ρ | Fluid density |
| μ | Dynamic viscosity of fluid |
| Re | Reynolds number (Re = ρvDh/μ) |
| C | Cell/microparticle concentration |
| R | RBC to WBC ratio |
| η | Viscosity of fluid medium |
| PDMS | Polydimethylsiloxane |
| IPA | Isopropyl alcohol |
| SLA | Stereolithography (3D printing technique) |
References
- Yang, S.M.; Lv, S.; Zhang, W.; Cui, Y. Microfluidic Point-of-Care (POC) Devices in Early Diagnosis: A Review of Opportunities and Challenges. Sensors 2022, 22, 1620. [Google Scholar] [CrossRef]
- Amaral, A.J.R.; Pasparakis, G. Cell membrane engineering with synthetic materials: Applications in cell spheroids, cellular glues and microtissue formation. Acta Biomater. 2019, 90, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Dockree, S.; Shine, B.; Pavord, S.; Impey, L.; Vatish, M. White blood cells in pregnancy: Reference intervals for before and after delivery. eBioMedicine 2021, 74, 103715. [Google Scholar] [CrossRef] [PubMed]
- Deliorman, M.; Ali, D.S.; Qasaimeh, M.A. Next-Generation Microfluidics for Biomedical Research and Healthcare Applications. Biomed. Eng. Comput. Biol. 2023, 14, 11795972231214387. [Google Scholar] [CrossRef]
- Pattanayak, P.; Singh, S.K.; Gulati, M.; Vishwas, S.; Kapoor, B.; Chellappan, D.K.; Anand, K.; Gupta, G.; Jha, N.K.; Gupta, P.K.; et al. Microfluidic chips: Recent advances, critical strategies in design, applications and future perspectives. Microfluid. Nanofluidics 2021, 25, 99. [Google Scholar] [CrossRef]
- Kumar, A.; Panda, U. Microfluidics-based devices and their role on point-of-care testing. In Biosensor Based Advanced Cancer Diagnostics: From Lab to Clinics; Academic Press: Cambridge, MA, USA, 2022; pp. 197–224. [Google Scholar] [CrossRef]
- Liberale, C.; Cojoc, G.; Bragheri, F.; Minzioni, P.; Perozziello, G.; La Rocca, R.; Ferrara, L.; Rajamanickam, V.; Di Fabrizio, E.; Cristiani, I. Integrated microfluidic device for single-cell trapping and spectroscopy. Sci. Rep. 2013, 3, 1258. [Google Scholar] [CrossRef]
- Renier, C.; Pao, E.; Che, J.; Liu, H.E.; Lemaire, C.A.; Matsumoto, M.; Triboulet, M.; Srivinas, S.; Jeffrey, S.S.; Rettig, M.; et al. Label-free isolation of prostate circulating tumor cells using Vortex microfluidic technology. npj Precis. Oncol. 2017, 1, 15. [Google Scholar] [CrossRef]
- Zhu, P.; Wang, L. Passive and active droplet generation with microfluidics: A review. Lab Chip 2016, 17, 34–75. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.Y.; Han, J.I.; Park, J.K. Inertial Microfluidics-Based Cell Sorting. BioChip J. 2018, 12, 257–267. [Google Scholar] [CrossRef]
- Ashley, B.K.; Hassan, U. Time-domain signal averaging to improve microparticles detection and enumeration accuracy in a microfluidic impedance cytometer. Biotechnol. Bioeng. 2021, 118, 4428–4440. [Google Scholar] [CrossRef]
- Seto, H.; Saiki, A.; Matsushita, R.; Mitsukami, W.; Kamba, S.; Hasegawa, M.; Miura, Y.; Hirohashi, Y.; Shinto, H. Development of microparticle counting sensor based on structural and spectroscopic properties of metal mesh device. Adv. Powder Technol. 2021, 32, 1920–1926. [Google Scholar] [CrossRef]
- Seto, H.; Kamba, S.; Kondo, T.; Ogawa, Y.; Hoshino, Y.; Miura, Y. Novel Detection Technique for Particulate Matter in Air Using Metal Mesh Device Sensors. Chem. Lett. 2014, 43, 408–410. [Google Scholar] [CrossRef]
- Huo, D.Q.; Liu, Z.; Hou, C.-J.; Yang, J.; Luo, X.-G.; Fa, H.-B.; Dong, J.-L.; Zhang, Y.-C.; Zhang, G.-P.; Li, J.-J. Recent Advances on Optical Detection Methods and Techniques for Cell-based Microfluidic Systems. Chin. J. Anal. Chem. 2010, 38, 1357–1365. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, X. Microfluidic-Based Electrical Operation and Measurement Methods in Single-Cell Analysis. Sensors 2024, 24, 6359. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, K.R.; Esene, J.E.; Woolley, A.T. Advances in multiplex electrical and optical detection of biomarkers using microfluidic devices. Anal. Bioanal. Chem. 2022, 414, 167–180. [Google Scholar] [CrossRef]
- Strohm, E.M.; Gnyawali, V.; Sebastian, J.A.; Ngunjiri, R.; Moore, M.J.; Tsai, S.S.H.; Kolios, M.C. Sizing biological cells using a microfluidic acoustic flow cytometer. Sci. Rep. 2019, 9, 4775. [Google Scholar] [CrossRef]
- Shrirao, A.B.; Fritz, Z.; Novik, E.M.; Yarmush, G.M.; Schloss, R.S.; Zahn, J.D.; Yarmush, M.L. Microfluidic flow cytometry: The role of microfabrication methodologies, performance and functional specification. Technology 2018, 6, 1–23. [Google Scholar] [CrossRef]
- Storti, F.; Bonfadini, S.; Criante, L. Simplified 3D hydrodynamic flow focusing for lab-on-chip single particle study. Sci. Rep. 2023, 13, 14671. [Google Scholar] [CrossRef]
- Ikram, K.; Hasan, S.M.; Abdullah, C.; Zulfiqar, M.; Uddin, E.; Ali, Z.; Sajid, M. Experimental Investigation of Particle Sorting in Y-Channels with Variable Bifurcation Angles for Microfluidic Applications. J. Appl. Sci. Eng. 2025, 28, 1679–1687. [Google Scholar] [CrossRef]
- Scott, S.M.; Ali, Z. Fabrication methods for microfluidic devices: An overview. Micromachines 2021, 12, 319. [Google Scholar] [CrossRef]
- Leuthner, M.; Helou, M.; Reisbeck, M.; Hayden, O. Quantitative Magnetic Flow Cytometry in High Hematocrit Conditions for Point-of-Care Testing. bioRxiv 2024. [Google Scholar] [CrossRef]
- Chen, Y.W.; Chiang, P.J. An automated approach for hemocytometer cell counting based on image-processing method. Measurement 2024, 234, 114894. [Google Scholar] [CrossRef]
- Ashkani, A.; Jafari, A.; Ghomsheh, M.J.; Dumas, N.; Funfschilling, D. Enhancing particle focusing: A comparative experimental study of modified square wave and square wave microchannels in lift and Dean vortex regimes. Dent. Sci. Rep. 2024, 14, 2679. [Google Scholar] [CrossRef]
- Tanriverdi, S.; Cruz, J.; Habibi, S.; Amini, K.; Costa, M.; Lundell, F.; Mårtensson, G.; Brandt, L.; Tammisola, O.; Russom, A. Elasto-inertial focusing and particle migration in high aspect ratio microchannels for high-throughput separation. Microsyst. Nanoeng. 2024, 10, 87. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Yuan, D.; Zhang, J.; Li, W. A Review of Secondary Flow in Inertial Microfluidics. Micromachines 2020, 11, 461. [Google Scholar] [CrossRef]
- Ramachandraiah, H.; Ardabili, S.; Faridi, A.M.; Gantelius, J.; Kowalewski, J.M.; Mårtensson, G.; Russom, A. Dean flow-coupled inertial focusing in curved channels. Biomicrofluidics 2014, 8, 034117. [Google Scholar] [CrossRef] [PubMed]
- Waheed, S.; Cabot, J.M.; Macdonald, N.P.; Lewis, T.; Guijt, R.M.; Paull, B.; Breadmore, M.C. 3D printed microfluidic devices: Enablers and barriers. Lab Chip 2016, 16, 1993–2013. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Liu, X.; Hou, K.; Vyas, C.; Bartolo, P. Stereolithography 3D printing of microgroove master moulds for topography-induced nerve guidance conduits. Int. J. Bioprint. 2024, 10, 489–507. [Google Scholar] [CrossRef]
- Lai, A.; Altemose, N.; White, J.A.; Streets, A.M. On-ratio PDMS bonding for multilayer microfluidic device fabrication. J. Micromech. Microeng. 2019, 29, 107001. [Google Scholar] [CrossRef]
- Sun, D. Micro-Channel Surface Treatment Method. 2020. Available online: https://typeset.io/papers/micro-channel-surface-treatment-method-2h0vsv4eaa (accessed on 21 January 2025).
- Hassan, U.; Watkins, N.N.; Reddy, B.; Damhorst, G.; Bashir, R. Microfluidic differential immunocapture biochip for specific leukocyte counting. Nat. Protoc. 2016, 11, 714. [Google Scholar] [CrossRef]
- Zeniou, A.; Kefallinou, D.; Dimitrakellis, P.; Xenogiannopoulou, E.; Grigoriou, M.; Dimoulas, A.; Boumpas, D.T.; Tserepi, A.; Gogolides, E. Atmospheric Pressure Plasma Functionalization of Sealed PDMS Microfluidics: Application to Capillary Pumping and Enhanced Cell Growth. Chempluschem 2024, 89, e202400290. [Google Scholar] [CrossRef] [PubMed]
- Perrira, N.; Shuib, A.S.; Phang, S.W.; Muda, A.S. Experimental Investigation of Blood Mimicking Fluid Viscosity for Application in 3D-Printed Medical Simulator. J. Phys. Conf. Ser. 2022, 2222, 012016. [Google Scholar] [CrossRef]
- Diez-Silva, M.; Dao, M.; Han, J.; Lim, C.T.; Suresh, S. Shape and Biomechanical Characteristics of Human Red Blood Cells in Health and Disease. MRS Bull./Mater. Res. Soc. 2010, 35, 382. [Google Scholar] [CrossRef] [PubMed]
- Components of Blood—Blood Disorders—Merck Manual Consumer Version. Available online: https://www.merckmanuals.com/home/blood-disorders/biology-of-blood/components-of-blood (accessed on 24 September 2024).
- Complete Blood Count—Health Encyclopedia—University of Rochester Medical Center. Available online: https://www.urmc.rochester.edu/encyclopedia/content.aspx?contenttypeid=167&contentid=complete_blood_count (accessed on 24 September 2024).
- Pahwa, R.; Goyal, A.; Jialal, I. Chronic Inflammation. In Pathobiology of Human Disease: A Dynamic Encyclopedia of Disease Mechanisms; Elsevier Inc.: Amsterdam, The Netherlands, 2023; pp. 300–314. [Google Scholar] [CrossRef]
- Hansen, J.T.; Netter, F.H.; Machado, C.A.G.; Craig, J.A.; Perkins, J.A.; Marzejon, K.W.; DaVanzo, T.S. Netter’s Clinical Anatomy. 2022, p. 554. Available online: https://geekymedics.com/blood-components/ (accessed on 24 September 2024).
- Zhang, N.; Wu, J.; Wang, Q.; Liang, Y.; Li, X.; Chen, G.; Ma, L.; Liu, X.; Zhou, F. Global burden of hematologic malignancies and evolution patterns over the past 30 years. Blood Cancer J. 2023, 13, 82. [Google Scholar] [CrossRef]
- Sanganwar, G.P.; Gupta, R.B. Nano-mixing of dipyridamole drug and excipient nanoparticles by sonication in liquid CO2. Powder Technol. 2009, 196, 36–49. [Google Scholar] [CrossRef]
- Optimizing Sorting of Micro-Sized Bio-Cells in Symmetric Serpentine Microchannel using Machine Learning. arXiv 2023, arXiv:2308.01701. [CrossRef]
- Stone, L.R.; Gray, D.R.; Remple, K.; Beaudet, M.P. Accuracy and precision comparison of the hemocytometer and automated cell counting methods. FASEB J. 2009, 23, 827.2. [Google Scholar] [CrossRef]
- Teng, J.; Chu, J.-C.; Liu, C.; Xu, T.; Lien, Y.-F.; Cheng, J.-H.; Huang, S.; Jin, S.; Dang, T.; Zhang, C.; et al. Fluid Dynamics in Microchannels. In Fluid Dynamics, Computational Modeling and Applications; InTech Open: Rijeka, Croatia, 2012. [Google Scholar] [CrossRef][Green Version]

| RBC Ratio | Condition/Disease | Explanation |
|---|---|---|
| 600:1 | Normal [36] | This is the typical ratio in healthy human blood, reflecting a normal immune response. |
| 400:1 | Inflammatory disease, infection [37] | A decrease in the RBC ratio due to an increase in WBCs indicates the body is fighting an infection or dealing with inflammation. |
| 250–300:1 | Severe inflammation, chronic infection [38] | A significantly lower ratio may indicate severe infections or chronic inflammatory diseases. |
| 100–200:1 | Leukemia, severe sepsis [39] | A very low ratio suggests serious conditions like leukemia, where WBC production is abnormally high, or severe sepsis. |
| 50–100:1 | Acute leukemia [36] | In cases of acute leukemia, the WBC count can become so high that it drastically reduces the RBC ratio. |
| <50:1 | Advanced leukemia, other hematologic malignancies [40] | In very advanced cases, the WBCs can almost outnumber RBCs, indicative of severe hematologic malignancies. |
| Sr No. | Test Parameters | Description |
|---|---|---|
| 1 | Squared channel size (µm) | 150, 200, 250 |
| 2 | Particle concentration (8 and 15 µm, respectively) | R1 = 600:1, R2 = 400:1 |
| 3 | Flow rates (μL/min) | 1, 5, 10, 15 |
| 4 | Optical equipment | Inverted microscope, Bioimager fluorescent microscope |
| Ratio | Particle Size | Microfluidic Device Count (cells/mL) | Hemocytometer Count (cells/mL) | Percentage Error (%) |
|---|---|---|---|---|
| 600:1 | 8 µm | 6.45 × 107 | 6.57 × 107 | 1.8% |
| 600:1 | 15 µm | 1.10 × 107 | 1.11 × 107 | 1.1% |
| 400:1 | 8 µm | 4.50 × 107 | 4.60 × 107 | 2.2% |
| 400:1 | 15 µm | 2.16 × 107 | 2.20 × 107 | 1.9% |
| Factor | F | Sig. (p-Value) |
|---|---|---|
| Microscope type | 79.453 | 0.000 |
| Flow rate | 51.007 | 0.000 |
| Channel size | 131.357 | 0.000 |
| Cell size | 0.169 | 0.683 |
| Concentration ratio | 1.712 | 0.196 |
| Cell size × concentration ratio | 19.010 | 0.000 |
| Microscope × flow rate | 3.322 | 0.025 |
| Cell size × flow rate | 2.667 | 0.055 |
| Flow rate × channel size | 1.884 | 0.097 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zulfiqar, M.; Manshad, D.; Uddin, E.; Mubashar, A.; Ali, Z.; Irfan, M.; Ullah, S.; Li, J. Experimental Study on Multi-Cell Counting Using an Inertial Microfluidic Device. Appl. Sci. 2025, 15, 5701. https://doi.org/10.3390/app15105701
Zulfiqar M, Manshad D, Uddin E, Mubashar A, Ali Z, Irfan M, Ullah S, Li J. Experimental Study on Multi-Cell Counting Using an Inertial Microfluidic Device. Applied Sciences. 2025; 15(10):5701. https://doi.org/10.3390/app15105701
Chicago/Turabian StyleZulfiqar, Muhammad, Danish Manshad, Emad Uddin, Aamir Mubashar, Zaib Ali, Muhammad Irfan, Sibghat Ullah, and Jingmin Li. 2025. "Experimental Study on Multi-Cell Counting Using an Inertial Microfluidic Device" Applied Sciences 15, no. 10: 5701. https://doi.org/10.3390/app15105701
APA StyleZulfiqar, M., Manshad, D., Uddin, E., Mubashar, A., Ali, Z., Irfan, M., Ullah, S., & Li, J. (2025). Experimental Study on Multi-Cell Counting Using an Inertial Microfluidic Device. Applied Sciences, 15(10), 5701. https://doi.org/10.3390/app15105701





