Customized Pediatric Hand EXoskeleton for Activities of Daily Living (PHEX): Design, Development, and Characterization of an Innovative Finger Module
Abstract
1. Introduction
2. Materials and Methods
2.1. Analysis of Requirements
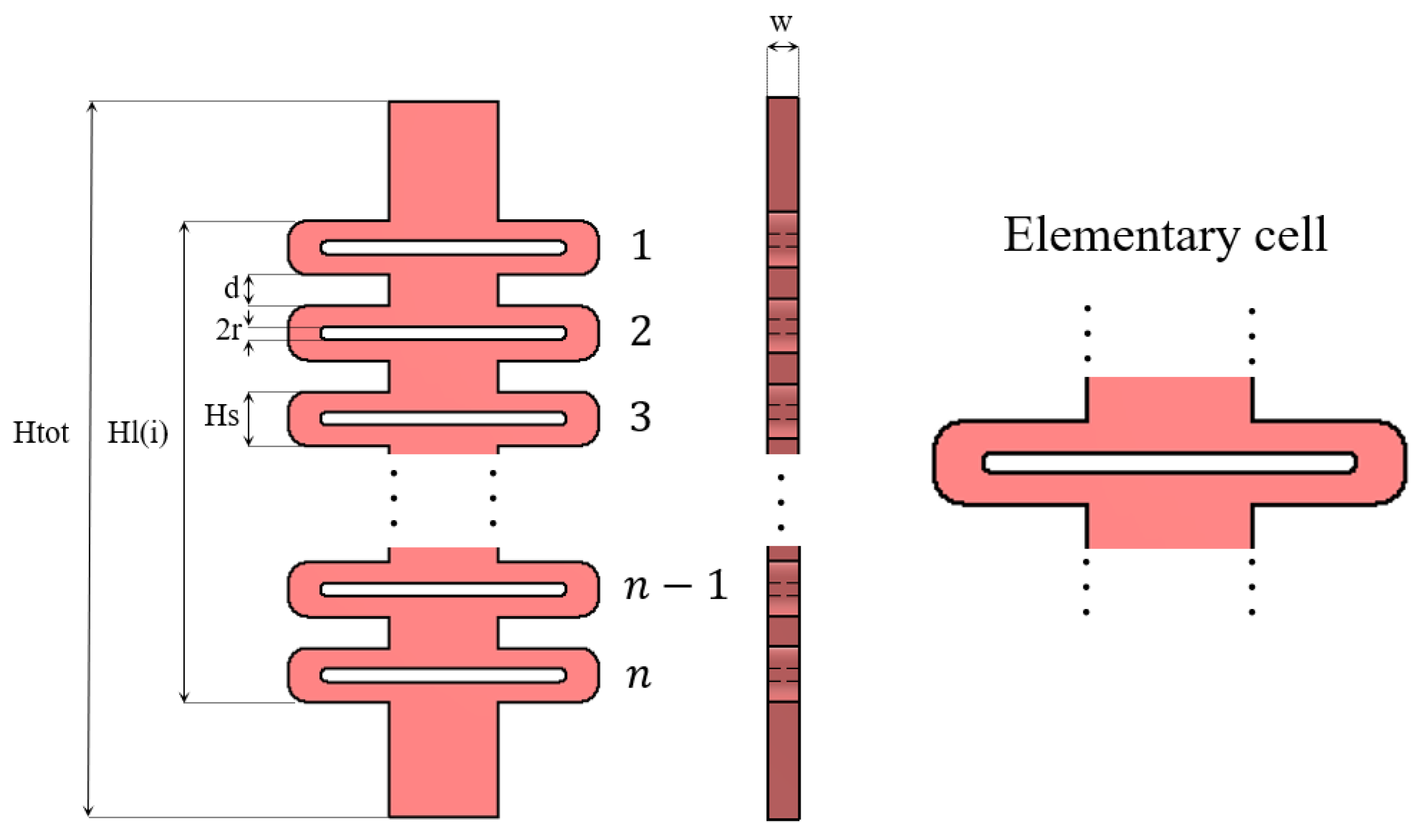
2.2. Cable Routing Modeling
- is the distance from joint 1 to the anchor point
- is the length of the phalanx
- is the position of the ring as defined in Figure 1
- is the diameter of the i-th phalanx
- is the height of the anchor point
- and are defined as:
2.3. Design of Compliant Structures
2.4. Exoskeleton Prototyping
2.5. Characterization of Mechanical Components
3. Results
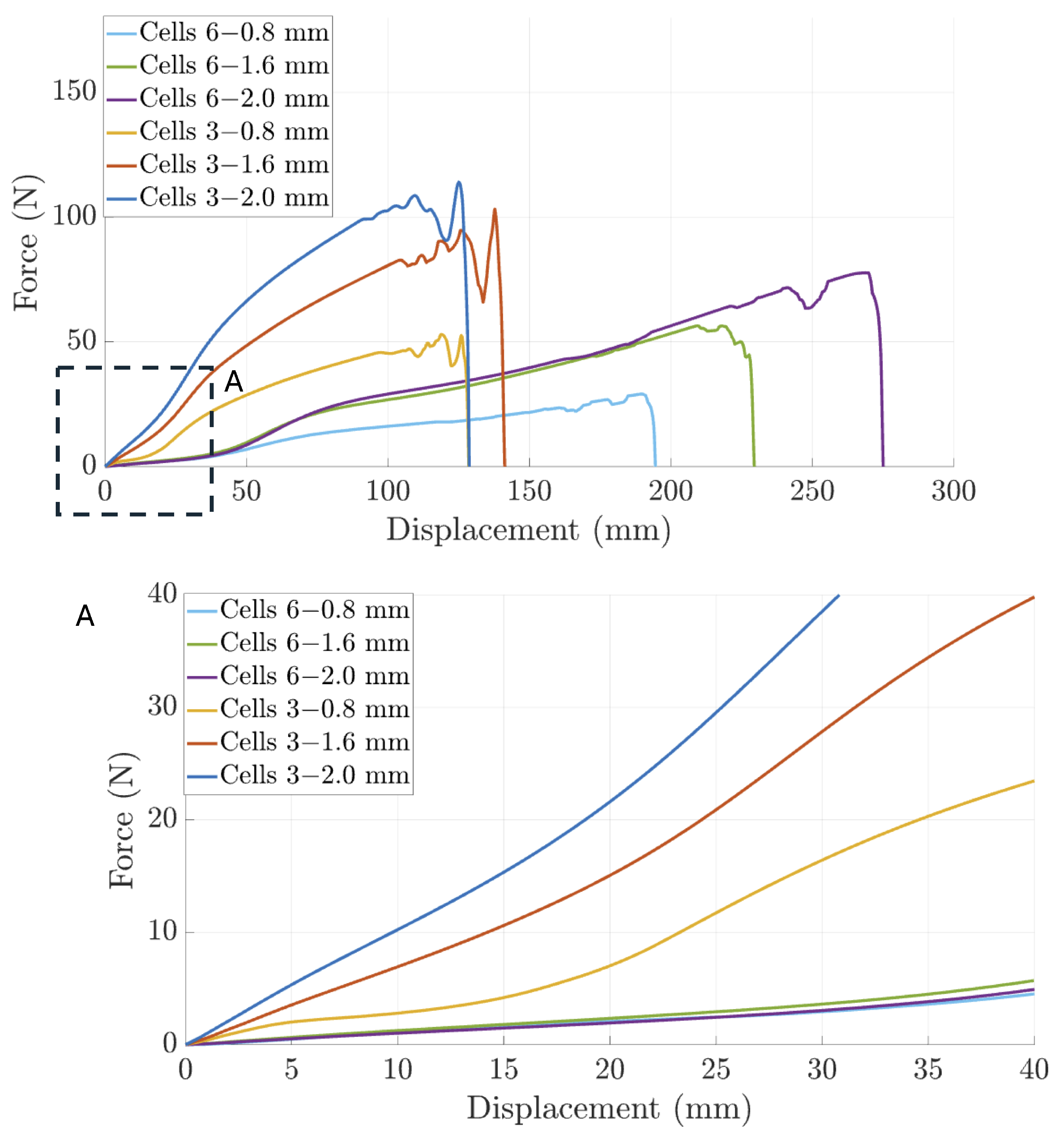
3.1. Characterization of Compliant Dorsal Links
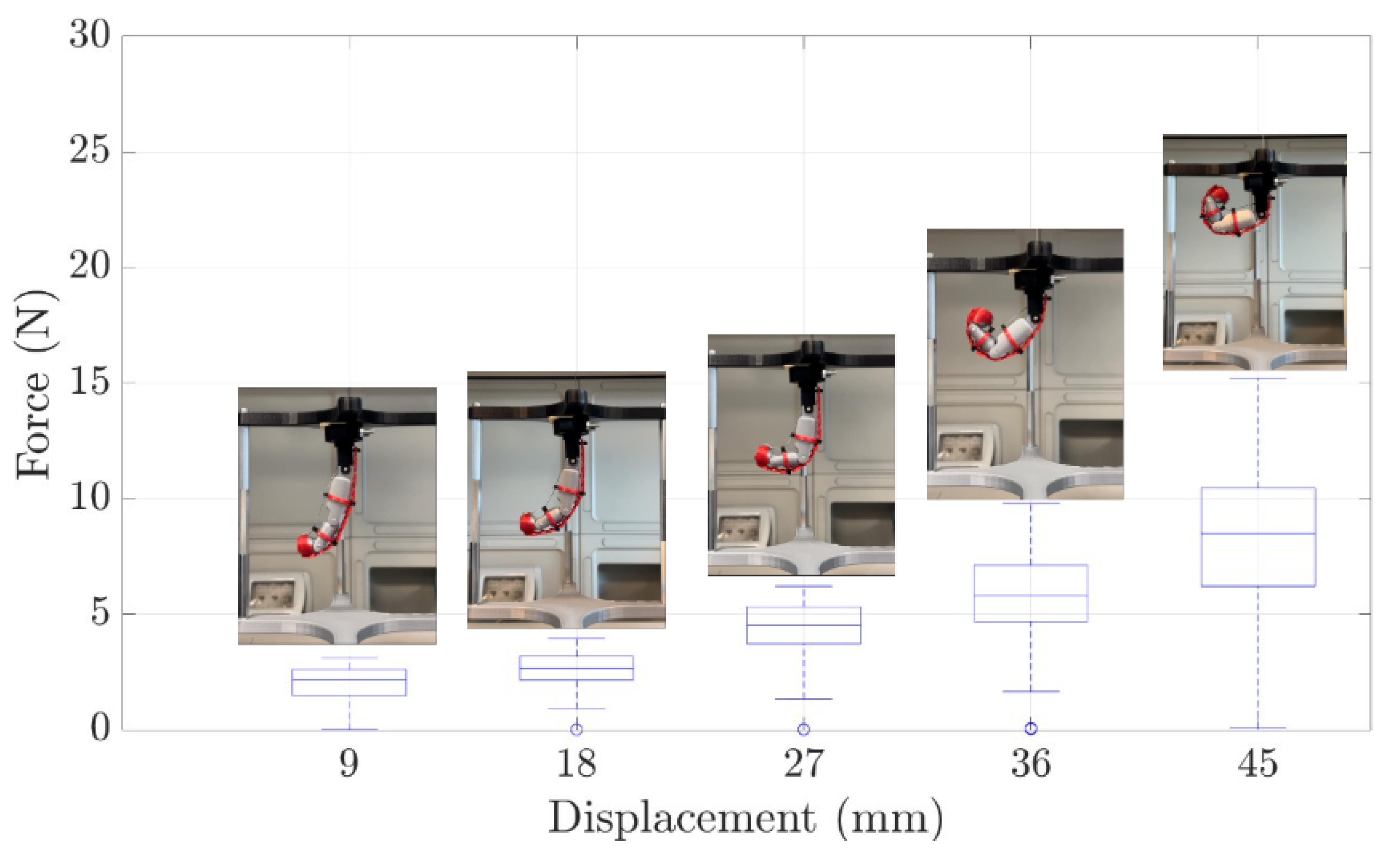
3.2. Experimental Evaluation of the Finger Module Prototype
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADL | Activities of daily living |
| ASD | Autism disorder spectrum |
| BPI | Brachial plexus injury. |
| CMC | Carpometacarpal joint |
| CP | Cerebral palsy |
| DIP | Distal interphalangeal joint |
| DMD | Duchenne muscular distrophy |
| DOF | Degree of freedom |
| IP | Interphalangeal joint |
| MAS | Modified Ashworth Scale |
| MCP | Metacarpophalangeal joint |
| PIP | Proximal interphalangeal joint |
| TBI | Traumatic brain injury |
| TRL | Technology Readiness Levels |
Appendix A. Set Up Parameters of Ultimaker Factor 4
| Material | Infill Density [%] | Infill Pattern | Printing Temperature [°] | Build Plate Temperature [°] | Print Speed [mm/s] | Fan Speed [%] | Wall thikness [mm] |
|---|---|---|---|---|---|---|---|
| TPU | 50 | Zig Zag | 225 | 40 | 35 | 50 | 0.8 |
| Nylon | 20 | Triangles | 250 | 40 | 80 | 75 | 1.2 |
Appendix B. Complete Behavior of Compliant Dorsal Links
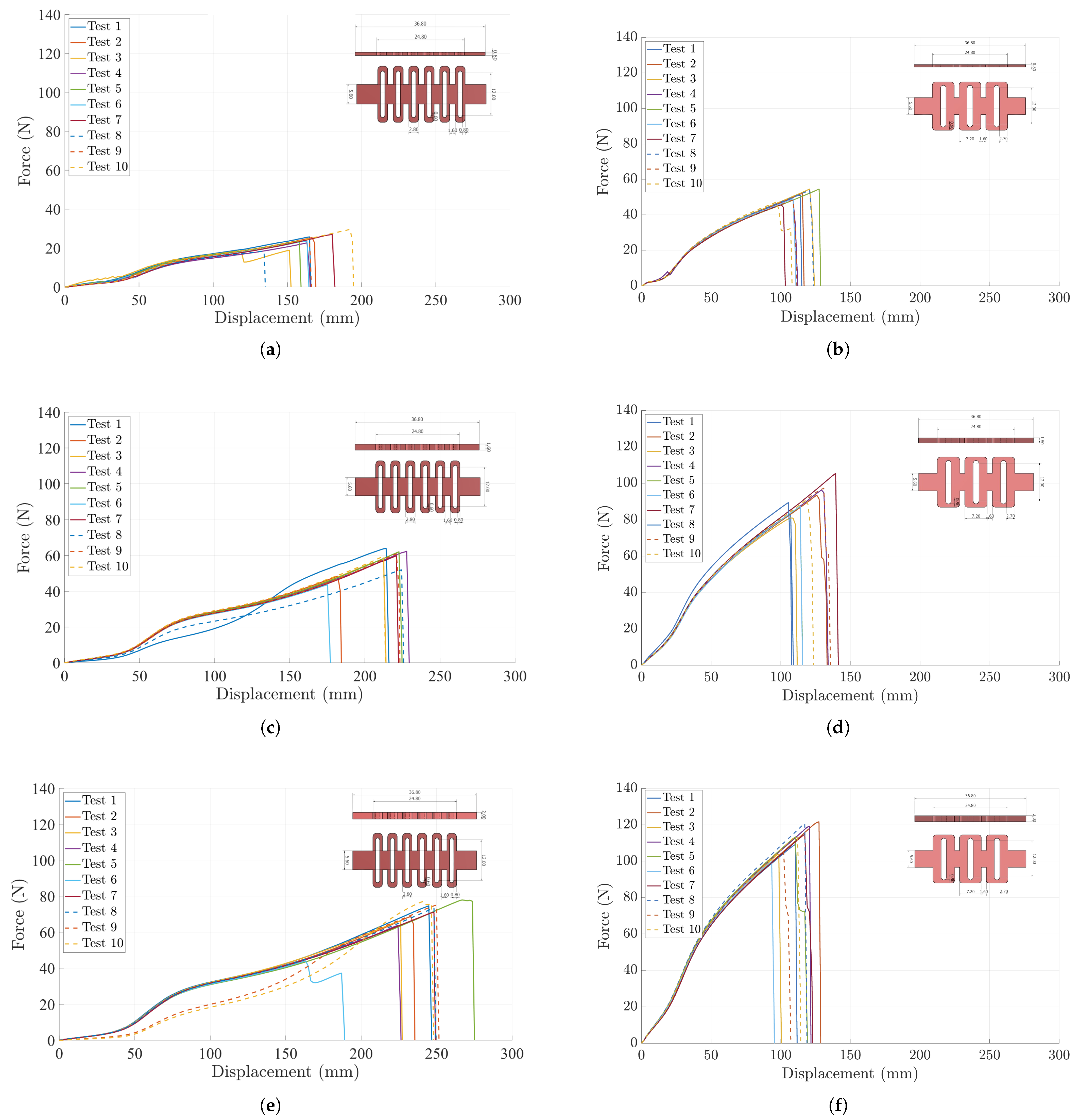
References
- Focaroli, V.; Taffoni, F.; Velardi, A.; Caravale, B.; Keller, F. Object Exploration and Manipulation in Infants at Typical vs. Elevated Likelihood for ASD: A Review. Children 2024, 11, 825. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.L.; Lobo, M.A. Design and development of the first exoskeletal garment to enhance arm mobility for children with movement impairments. Assist. Technol. 2018, 30, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Garcia, L.; Kilby, J.; McNair, P. Robotic devices for paediatric rehabilitation: A review of design features. Biomed. Eng. Online 2021, 20, 1–33. [Google Scholar] [CrossRef]
- Randazzo, L.; Iturrate, I.; Perdikis, S.; Millán, J.D.R. mano: A wearable hand exoskeleton for activities of daily living and neurorehabilitation. IEEE Robot. Autom. Lett. 2017, 3, 500–507. [Google Scholar] [CrossRef]
- Bützer, T.; Dittli, J.; Lieber, J.; van Hedel, H.J.; Meyer-Heim, A.; Lambercy, O.; Gassert, R. PEXO-A pediatric whole hand exoskeleton for grasping assistance in task-oriented training. In Proceedings of the 2019 IEEE 16th International Conference on Rehabilitation Robotics (ICORR), Toronto, ON, Canada, 24–28 June 2019; Volume 16, pp. 108–114. [Google Scholar] [CrossRef]
- Søraa, R.; Fosch-Villaronga, E. Exoskeletons for all: The interplay between exoskeletons, inclusion, gender, and intersectionality. Paladyn J. Behav. Robot. 2020, 11, 217–227. [Google Scholar] [CrossRef]
- Fosch-Villaronga, E.; Čartolovni, A.; Pierce, R.L. Promoting inclusiveness in exoskeleton robotics: Addressing challenges for pediatric access. Paladyn J. Behav. Robot. 2020, 11, 327–339. [Google Scholar] [CrossRef]
- Du Plessis, T.; Djouani, K.; Oosthuizen, C. A review of active hand exoskeletons for rehabilitation and assistance. Robotics 2021, 10, 40. [Google Scholar] [CrossRef]
- Bunch, P.M.; Altes, T.A.; McIlhenny, J.; Patrie, J.; Gaskin, C.M. Skeletal development of the hand and wrist: Digital bone age companion—A suitable alternative to the Greulich and Pyle atlas for bone age assessment? In Skeletal Radiology; Springer: New York, NY, USA, 2017; Volume 46, pp. 785–793. [Google Scholar] [CrossRef]
- Snyder, R.; Schneider, L.; Owings, C.; Reynolds, H. Anthropometry of Infoants, Children, and Youths to Age 18 for Product Safety Design. 1977. Available online: https://trid.trb.org/View/57257 (accessed on 19 June 2024).
- Aubin, P.; Petersen, K.; Sallum, H.; Walsh, C.; Correia, A.; Stirling, L. A pediatric robotic thumb exoskeleton for at-home rehabilitation: The Isolated Orthosis for Thumb Actuation (IOTA). Int. J. Intell. Comput. Cybern. 2014, 7, 233–252. [Google Scholar] [CrossRef]
- Haarman, C.J.W. Development of a Functional Hand Orthosis for Boys with Duchenne Muscular Dystrophy. Master’s Thesis, University of Twente, Enschede, The Netherlands, 30 September 2016. Available online: https://ris.utwente.nl/ws/portalfiles/portal/60773794/CONFIDENTIAL_PDEng_Thesis_Claudia_Haarman.pdf (accessed on 13 September 2024).
- Refour, E.; Sebastian, B.; Ben-Tzvi, P. Design and implementation of an exoskeleton glove for infant medical rehabilitation. Int. Des. Eng. Tech. Conf. Comput. Inf. Eng. Conf. 2018, 51807, V05AT07A062. [Google Scholar] [CrossRef]
- Siyi. 2017. Syrebo. Available online: https://it.syrebo.com/rehabilitationequipment-for-home/soft-robotic-glove-children.html (accessed on 20 July 2024).
- Syrebo Care. Newest Model: Syrebo C12 Stroke Hand Rehabilitation Gloves with 6 Training Modes, 9 Strength Levels. 2023. Available online: https://www.youtube.com/watch?v=Scd-Nf-W3tM (accessed on 23 July 2024).
- Bianchi, M.; Secciani, N.; Ridolfi, A.; Vannetti, F.; Pasquini, G. Kinematics-based strategy for the design of a pediatric hand exoskeleton prototype. In Advances in Italian Mechanism Science: Proceedings of the Second International Conference of IFToMM Italy, Cassino, Italy, 29–30 November 2018; Springer: Berlin/Heidelberg, Germany, 2019; pp. 501–508. [Google Scholar] [CrossRef]
- Dittli, J.; Goikoetxea-Sotelo, G.; Lieber, J.; Gassert, R.; Meyer-Heim, A.; Van Hedel, H.J.; Lambercy, O. A Tailorable Robotic Hand Orthosis to Support Children with Neurological Hand Impairments: A Case Study in a Child’s Home. In Proceedings of the 2023 International Conference on Rehabilitation Robotics (ICORR), Singapore, 24–28 September 2023; pp. 1–6. [Google Scholar] [CrossRef]
- Lieber, J.; Dittli, J.; Lambercy, O.; Gassert, R.; Meyer-Heim, A.; van Hedel, H.J. Clinical utility of a pediatric hand exoskeleton: Identifying users, practicability, and acceptance, and recommendations for design improvement. J. Neuroeng. Rehabil. 2022, 19, 17. [Google Scholar] [CrossRef]
- Dittli, J.; Vasileiou, C.; Asanovski, H.; Lieber, J.; Lin, J.B.; Meyer-Heim, A.; Lambercy, O. Design of a compliant, stabilizing wrist mechanism for a pediatric hand exoskeleton. In Proceedings of the 2022 International Conference on Rehabilitation Robotics (ICORR), Rotterdam, The Netherlands, 25–29 July 2022; pp. 1–6. [Google Scholar] [CrossRef]
- Srivastava, R.; Singh, M.; Gomes, G.D.; Murray, N.; Devine, D. SM-EXO: Shape Memory alloy-based Hand EXOskeleton for Cobotic Application. In Proceedings of the 2022 31st IEEE International Conference on Robot and Human Interactive Communication (RO-MAN), Napoli, Italy, 29 August–2 September 2022; pp. 1277–1284. [Google Scholar] [CrossRef]
- Tran, P.; Jeong, S.; Lyu, F.; Herrin, K.; Bhatia, S.; Elliott, D. FLEXotendon glove-III: Voice-controlled soft robotic hand exoskeleton with novel fabrication method and admittance grasping control. IEEE/ASME Trans. Mechatron. 2022, 27, 3920–3931. [Google Scholar] [CrossRef]
- Tran, P.; Elliott, D.; Herrin, K.; Desai, J.P. Towards comprehensive evaluation of the FLEXotendon glove-III: A case series evaluation in pediatric clinical cases and able-bodied adults. Biomed. Eng. Lett. 2023, 13, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Noronha, B.; Accoto, D. Exoskeletal devices for hand assistance and rehabilitation: A comprehensive analysis of state-of-the-art technologies. IEEE Trans. Med. Robot. Bionics 2021, 3, 525–538. [Google Scholar] [CrossRef]
- Khatik, V.; Saxena, A. On Optimal Tendon Routing Based Design of Biologically Inspired Underactuated Hand Exoskeleton for Gross Grasping. IEEE Trans. Med. Robot. Bionics 2024, 6, 600–617. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, F.; Yang, L.; Fu, Y. Design and modeling of a hybrid soft-rigid hand exoskeleton for poststroke rehabilitation. Int. J. Mech. Sci. 2021, 212, 106831. [Google Scholar] [CrossRef]
- Salazar, G.; Russi-Vigoya, M.N. Technology readiness level as the foundation of human readiness level. Ergon. Des. 2021, 29, 25–29. [Google Scholar] [CrossRef]
- Kuo, F.-L.; Lee, H.-C.; Hsiao, H.-Y.; Lin, J.-C. Robotic-assisted hand therapy for improvement of hand function in children with cerebral palsy: A case series study. Eur. J. Phys. Rehabil. Med. 2020, 56, 237–242. [Google Scholar] [CrossRef]
- Bressi, F.; Santacaterina, F.; Cricenti, L.; Campagnola, B.; Nasto, F.; Assenza, C.; Morelli, D.; Cordella, F.; Lapresa, M.; Zollo, L.; et al. Robotic-assisted hand therapy with Gloreha Sinfonia for the improvement of hand function after pediatric stroke: A case report. Appl. Sci. 2022, 12, 4206. [Google Scholar] [CrossRef]
- Battraw, M.A.; Young, P.R.; Welner, M.E.; Joiner, W.M.; Schofield, J.S. Characterizing Pediatric Hand Grasps During Activities of Daily Living to Inform Robotic Rehabilitation and Assistive Technologies. In Proceedings of the 2022 International Conference on Rehabilitation Robotics (ICORR), Rotterdam, The Netherlands, 25–29 July 2022; pp. 1–6. [Google Scholar] [CrossRef]
- Buchholz, B.; Armstrong, T.J.; Goldstein, S.A. Anthropometric data for describing the kinematics of the human hand. Ergonomics 1992, 35, 261–273. [Google Scholar] [CrossRef]
- Gerez, L.; Chen, J.; Liarokapis, M. On the development of adaptive, tendon-driven, wearable exo-gloves for grasping capabilities enhancement. IEEE Robot. Autom. Lett. 2019, 4, 422–429. [Google Scholar] [CrossRef]
- Lapresa, M.; Ceccarelli, A.; Taffoni, F.; Tagliamonte, N.L.; Zollo, L.; Cordella, F. Analysis of hand intra-finger couplings during flexion movements in the free space. IEEE Access 2023, 11, 90084–90093. [Google Scholar] [CrossRef]
- Sun, Y.; Ye, W.; Chen, Y.; Fan, W.; Feng, J.; Sareh, P. Geometric design classification of kirigami-inspired metastructures and metamaterials. Structures 2021, 33, 3633–3643. [Google Scholar] [CrossRef]
- Lei, Q.E.; Shu, J.; Wang, J.; Cheung, H.Y.; Cheung, J.P.; Wong, W.F.; Tong, R.K. Design and characterize of kirigami-inspired springs and the application in vertebrae exoskeleton for adolescent idiopathic scoliosis brace treatment. Front. Mech. Eng. 2023, 9, 1152930. [Google Scholar] [CrossRef]
- Cuellar, J.S.; Smit, G.; Zadpoor, A.A.; Breedveld, P. Ten guidelines for the design of non-assembly mechanisms: The case of 3D-printed prosthetic hands. Proc. Inst. Mech. Eng. Part H 2018, 232, 962–971. [Google Scholar] [CrossRef]
- Nini, L.; Tontini, L.; Tagliamonte, N.; Zollo, L.; Taffoni, F. Design of compliant components in linkage-driven prosthetic fingers: A Multi-Material printing based method. IEEE Access 2024, 12, 190959–190971. [Google Scholar] [CrossRef]
- Bullock, I.M.; Borràs, J.; Dollar, A.M. Assessing assumptions in kinematic hand models: A review. In Proceedings of the 2012 4th IEEE RAS & EMBS International Conference on Biomedical Robotics and Biomechatronics (BioRob), Rome, Italy, 24–27 June 2012; pp. 139–146. [Google Scholar] [CrossRef]






| Reference | Func | Disease | Stage of Development | Age [y] | Actuation | Type | Tested Grasps | Weight [g] | DoA | Sensors | Motion Intention | Assisted District | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Motor | Transm. | Hand | Total | Fingers | Joint | ||||||||||
| IOTA (2014) [11] | R 1 | CP 3 | Prototype | 7–12 | Servo | Ind. DOF | Rigid | Opposition | 230 | – | 2 | Encoders, bend | Explicit | 1 | , |
| Haarman et al. (2016) [12] | A 2 | DMD 4 | Prototype | 20–23 | DC | Constr. slide | Soft-rigid | Pinch, power | <75 | 225 | 2 | – | – | 2–3 | , , |
| Refour et al. (2018) [13] | R | CP | Clinical trial | 1–3 | DC | Coupl. DOF | Rigid | Pinch | – | – | 2 | Encoders, FSR | Explicit | 1–2 | , , , IP |
| SYREBO (2018) [14,15] | R | Stroke | Commercial | 5–12 | Pneum. | Bladder | Soft | Pinch, power, opposition | <150 | <2000 | 5 | – | Explicit | 1–5 | , , , IP |
| Bianchi et al. (2019) [16] | A | CP | Prototype | – | Servo | Coupl. DOF | Rigid | – | – | – | 1 | – | – | 2–5 | , , |
| PEXO (2019) [5,17,18,19] | A | CP | Clinical trial | 6–12 | DC | Constr. slide | Soft-rigid | Pinch, power, opposition | 84–101 | 492 | 3 | TTL trigger | Implicit/Explicit | 1–5 | CMC, , |
| SM-EXO (2022) [20] | R | ASD 5 | Prototype | – | – | Cable on glove | Soft | – | 50 | – | – | KPTs | Implicit | 1–3 | , , , IP |
| FLEXotendon Glove-III (2023) [21,22] | A | TBI 6, BPI 7 | Clinical trial | 12 | DC | Cable on glove | Soft | Pinch, power, opposition | 131 | 5100 | 5 | Tension sensors | Explicit | 1–3 | , , , IP |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Angelo, E.; Latini, G.; Ceccarelli, A.; Nini, L.; Tagliamonte, N.L.; Zollo, L.; Taffoni, F. Customized Pediatric Hand EXoskeleton for Activities of Daily Living (PHEX): Design, Development, and Characterization of an Innovative Finger Module. Appl. Sci. 2025, 15, 5694. https://doi.org/10.3390/app15105694
D’Angelo E, Latini G, Ceccarelli A, Nini L, Tagliamonte NL, Zollo L, Taffoni F. Customized Pediatric Hand EXoskeleton for Activities of Daily Living (PHEX): Design, Development, and Characterization of an Innovative Finger Module. Applied Sciences. 2025; 15(10):5694. https://doi.org/10.3390/app15105694
Chicago/Turabian StyleD’Angelo, Elisa, Gianmarco Latini, Alessandro Ceccarelli, Ludovica Nini, Nevio Luigi Tagliamonte, Loredana Zollo, and Fabrizio Taffoni. 2025. "Customized Pediatric Hand EXoskeleton for Activities of Daily Living (PHEX): Design, Development, and Characterization of an Innovative Finger Module" Applied Sciences 15, no. 10: 5694. https://doi.org/10.3390/app15105694
APA StyleD’Angelo, E., Latini, G., Ceccarelli, A., Nini, L., Tagliamonte, N. L., Zollo, L., & Taffoni, F. (2025). Customized Pediatric Hand EXoskeleton for Activities of Daily Living (PHEX): Design, Development, and Characterization of an Innovative Finger Module. Applied Sciences, 15(10), 5694. https://doi.org/10.3390/app15105694









