The Impact of Microwaves and Ultrasound on the Hydrolysis of Banana Peels and the Growth of Fodder Yeasts
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Banana Peels—Characteristics of the Raw Material
2.2. Yeast Strains and Inoculum Preparation
2.3. Preparation of Banana Peel Hydrolysates
- (a)
- A quantity of 800 g of ground banana peel (approximately 11.75% dry weight) was weighed into a plastic container, into which 1000 mL of a 2.5% H2SO4 solution was then added. The mixture was stirred and autoclaved at 121 °C for 1 h. Afterwards, the sample was cooled to room temperature and neutralised with 30% NaOH to achieve a pH of 5.3–5.5. This hydrolysis method was used as a control and was abbreviated as PT.
- (b)
- A quantity of 800 g of ground banana peel (approximately 11.75% dry weight) was weighed into a plastic container, into which 1000 mL of a 2.5% H2SO4 solution was then measured. The mixture was stirred and autoclaved at 121 °C for 1 h. Afterwards, the sample was cooled to room temperature and neutralised with 30% NaOH to achieve a pH between 5.3 and 5.5. Then, enzymatic hydrolysis of banana peels was carried out using a mixture of commercially available preparations, Viscozyme and UltrafloMax (Novozymes A/S, Bagsværd, Denmark), which exhibit cellulase, pectinase, xylanase and invertase activities. Both enzyme preparations were dosed at 0.05 mL per gram (DM) of banana peels. The enzymatic treatment was carried out at 50 °C for 6 h. The hydrolysate obtained in this way was labelled with the abbreviation PTE.
- (c)
- A quantity of 800 g of ground banana peel (approximately 11.75% dry weight) was weighed into a plastic container, into which 1000 mL of a 2.5% H2SO4 solution was then measured. After mixing, the sample was microwaved using an LG MH 6842B microwave oven (LG Electronics, Warsaw, Poland) with a peak power output of 900 W. The sample was treated for 3 min at 600 W microwave power. Afterwards, the sample was cooled and neutralised with 30% NaOH to achieve a pH value between 5.3 and 5.5. Then, the enzymatic hydrolysis of the banana peels was carried out as described above. The hydrolysate obtained in this way was labelled with the abbreviation ME.
- (d)
- A quantity of 800 g of ground banana peel (approximately 11.75% dry weight) was weighed into a plastic container, into which 1000 mL of a 2.5% H2SO4 solution was then added. After mixing, the sample was ultrasonicated using a UP400S Ultrasonic Generator (Hielscher Ultrasonics GmbH, Teltow, Germany). The sonication parameters were as follows: operational frequency, 24 kHz; output power, 300 W/mL; and pulse mode factor, 50% per second. The sonotrode (H3) utilised had a maximum immersion depth of 90 mm, a tip diameter of 3 mm, and a peak amplitude of 210 µm. Ultrasound treatment was administered for 20 min. Afterwards, the sample was cooled and neutralised with 30% NaOH to achieve a pH value between 5.3 and 5.5. Then, the enzymatic hydrolysis of the banana peels was carried out as described above. The hydrolysate obtained after ultrasonication was labelled with the abbreviation UE.
2.4. Preparation of Culture Media and Yeast Cultivation
2.5. Sample Designations After Cultivations
2.6. Determination of Biomass Content After Cultivation
2.7. Crude Protein Assay
2.8. Dry Matter Content in the Raw Material
2.9. Cellulose, Hemicellulose, Lignin and Pectin Assay in Banana Peels
2.10. Enzymatic Activity
2.11. HPLC Analysis of Banana Peels’ Hydrolysates and Post-Cultivation Effluents
2.12. Statistical Analysis
3. Results and Discussion
3.1. Banana Peels Analysis
3.2. Activity of the Enzymatic Preparations Used in Our Experiments
3.3. Selected Sugars in Banana Peel Hydrolysates Before and After Yeast Cultivation
3.4. Selected Organic Acids in Banana Peel Hydrolysates Before and After Yeast Cultivation
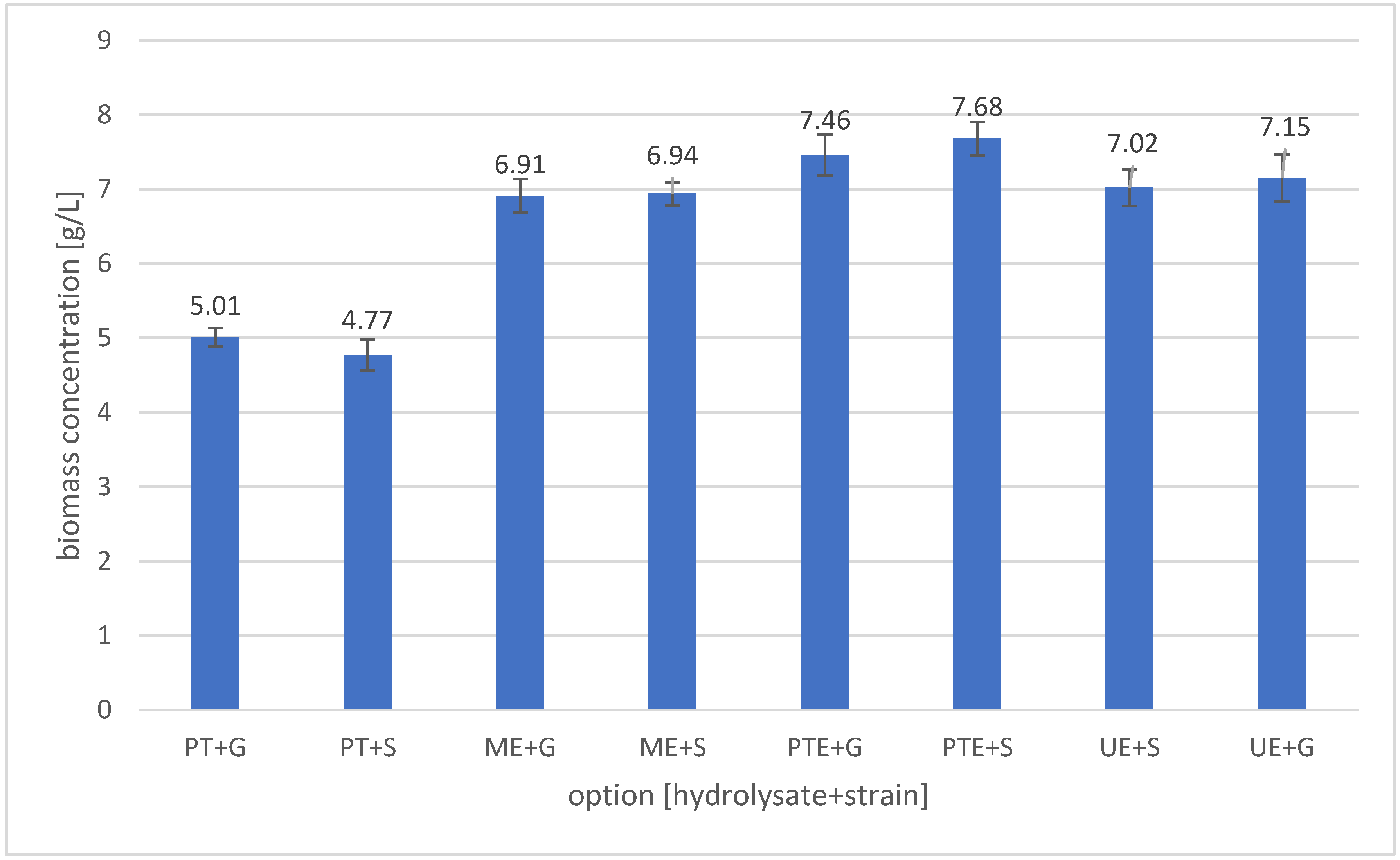
3.5. Biomass Concentration of Yeasts After Cultivation in Banana Peel Hydrolysates
3.6. Crude Protein Content in Yeast Biomass Grown on Banana Peel Hydrolysates
4. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Emaldi, U.; Trujillo, I.; García, E. Comparison of Characteristics of Bananas (Musa sp.) from the Somaclone CIEN BTA-03 and Its Parental Clone Williams. Fruits 2004, 59, 257–263. [Google Scholar] [CrossRef]
- Menezes, E.W.; Tadini, C.C.; Tribess, T.B.; Zuleta, A.; Binaghi, J.; Pak, N.; Vera, G.; Dan, M.C.T.; Bertolini, A.C.; Cordenunsi, B.R.; et al. Chemical Composition and Nutritional Value of Unripe Banana Flour (Musa acuminata, var. Nanicão). Plant Foods Hum. Nutr. 2011, 66, 231–237. [Google Scholar] [CrossRef]
- Ashokkumar, K.; Elayabalan, S.; Shobana, V.G.; Sivakumar, P.; Pandiyan, M. Nutritional Value of Cultivars of Banana (Musa spp.) and Its Future Prospects. J. Pharmacogn. Phytochem. 2018, 7, 2972–2977. [Google Scholar] [CrossRef]
- Ranjha, M.M.A.N.; Irfan, S.; Nadeem, M.; Mahmood, S. A Comprehensive Review on Nutritional Value, Medicinal Uses, and Processing of Banana. Food Rev. Int. 2022, 38, 199–225. [Google Scholar] [CrossRef]
- Huang, J.-Y.; Xu, F.; Zhou, W. Effect of LED Irradiation on the Ripening and Nutritional Quality of Postharvest Banana Fruit. J. Sci. Food Agric. 2018, 98, 5486–5493. [Google Scholar] [CrossRef] [PubMed]
- Zaini, H.B.M.; Sintang, M.D.B.; Pindi, W. The Roles of Banana Peel Powders to Alter Technological Functionality, Sensory and Nutritional Quality of Chicken Sausage. Food Sci. Nutr. 2020, 8, 5497–5507. [Google Scholar] [CrossRef]
- Bananas: Production Volume Worldwide 2023. Available online: https://www.statista.com/statistics/716037/global-banana-market-volume/ (accessed on 10 April 2025).
- Yan, L.; Fernando, W.; Brennan, M.; Brennan, C.; Jayasena, V.; Coorey, R. Effect of Extraction Method and Ripening Stage on Banana Peel Pigments. Int. J. Food Sci. Technol. 2016, 51, 1449–1456. [Google Scholar] [CrossRef]
- Carvalho, V.S.; Conti-Silva, A.C. Cereal Bars Produced with Banana Peel Flour: Evaluation of Acceptability and Sensory Profile. J. Sci. Food Agric. 2018, 98, 134–139. [Google Scholar] [CrossRef]
- Marques, O.F.C.; Sales, E.C.J.D.; Monção, F.P.; Silva, A.F.; Rigueira, J.P.S.; Pires, D.A.D.A.; Rufino, L.D.D.A.; Durães, H.F. Potential for Using Dehydrated Banana Peel as an Additive in Grass Silage. Cad. Ciênc. Agrár. 2021, 13, 1–8. [Google Scholar] [CrossRef]
- Biedunkiewicz, A. Ecophysiology of Selected Candida Species Isolated from Different Types of Utility Water under Laboratory Conditions. Appl. Ecol. Environ. Res. 2015, 13, 967–979. [Google Scholar] [CrossRef]
- Lonnie, M.; Hooker, E.; Brunstrom, J.; Corfe, B.; Green, M.; Watson, A.; Williams, E.; Stevenson, E.; Penson, S.; Johnstone, A. Protein for Life: Review of Optimal Protein Intake, Sustainable Dietary Sources and the Effect on Appetite in Ageing Adults. Nutrients 2018, 10, 360. [Google Scholar] [CrossRef] [PubMed]
- Pomeroy, J.; Jose, D.; Tyler, A.; Bloxham, P.; Culling, J. The Future of Food. Can We Meet the Needs of 9bn People? Available online: https://www.research.hsbc.com/C/1/1/320/WgCK7Wv (accessed on 20 May 2024).
- Galanakis, C.M. The Future of Food. Foods 2024, 13, 506. [Google Scholar] [CrossRef]
- Robins, S.; Iley, R.; Michell, L.; Smith, S.; Gilbert, G.; McCarthy, M.; Stein, U.; Wells, P.; Lawton, J.; Landridge, A.; et al. The Future of Food: Are Food Businesses on Track to Deliver a Sustainable Protein System by 2040? Available online: https://www.forumforthefuture.org/Handlers/Download.ashx?IDMF=f2a9339c-8a62-4462-a886-f7de0e3fd729 (accessed on 13 June 2024).
- Smith, K.; Watson, A.W.; Lonnie, M.; Peeters, W.M.; Oonincx, D.; Tsoutsoura, N.; Simon-Miquel, G.; Szepe, K.; Cochetel, N.; Pearson, A.G.; et al. Meeting the Global Protein Supply Requirements of a Growing and Ageing Population. Eur. J. Nutr. 2024, 63, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Bedsaul-Fryer, J.R.; Monroy-Gomez, J.; Van Zutphen-Küffer, K.G.; Kraemer, K. Editorial: An Introduction to Traditional and Novel Alternative Proteins for Low- and Middle-Income Countries. Curr. Dev. Nutr. 2023, 8, 102014. [Google Scholar] [CrossRef]
- Sijpestijn, G.F.; Wezel, A.; Chriki, S. Can Agroecology Help in Meeting Our 2050 Protein Requirements? Livest. Sci. 2022, 256, 104822. [Google Scholar] [CrossRef]
- Lara-Parra, A.I.; Hernández-Hernández, A.A.; Jaguey-Hernández, Y.; Jiménez-Osorio, A.S.; Castañeda-Ovando, A.; Aguilar-Arteaga, K.; Añorve-Morga, J. Exploring Alternative Sources of Protein in Food: Trends in Nutrient and Functional Features. Food Res. Int. 2025, 208, 116224. [Google Scholar] [CrossRef]
- Mensah, J.K.M.; Twumasi, P. Use of Pineapple Waste for Single Cell Protein (SCP) Production and the Effect of Substrate Concentration on the Yield. J. Food Process Eng. 2017, 40, e12478. [Google Scholar] [CrossRef]
- Prosvirnikov, D.; Tuntsev, D.; Gizzatullina, L.; Kulikova, Y.; Michaud, P.; Babich, O. Protein Production from Cellulosic Waste Using Candida utilis. Environ. Technol. Innov. 2023, 32, 103445. [Google Scholar] [CrossRef]
- Ortman, K.; Pehrson, B. Selenite and Selenium Yeast as Feed Supplements for Dairy Cows. Zentralbl. Veterinarmed. A 1997, 44, 373–380. [Google Scholar] [CrossRef]
- Baker, L.M.; Kraft, J.; Karnezos, T.P.; Greenwood, S.L. Review: The Effects of Dietary Yeast and Yeast-Derived Extracts on Rumen Microbiota and Their Function. Anim. Feed Sci. Technol. 2022, 294, 115476. [Google Scholar] [CrossRef]
- Jach, M.E.; Serefko, A.; Ziaja, M.; Kieliszek, M. Yeast Protein as an Easily Accessible Food Source. Metabolites 2022, 12, 63. [Google Scholar] [CrossRef] [PubMed]
- Dygas, D.; Kręgiel, D.; Berłowska, J. Sugar Beet Pulp as a Biorefinery Substrate for Designing Feed. Molecules 2023, 28, 2064. [Google Scholar] [CrossRef] [PubMed]
- Dygas, D.; Liszkowska, W.; Steglińska, A.; Sulyok, M.; Kręgiel, D.; Berłowska, J. Rapeseed Meal Waste Biomass as a Single-Cell Protein Substrate for Nutritionally-Enhanced Feed Components. Processes 2023, 11, 1556. [Google Scholar] [CrossRef]
- Patelski, P.; Berłowska, J.; Balcerek, M.; Dziekońska-Kubczak, U.; Pielech-Przybylska, K.; Dygas, D.; Jędrasik, J. Conversion of Potato Industry Waste into Fodder Yeast Biomass. Processes 2020, 8, 453. [Google Scholar] [CrossRef]
- Henriques, T.; Pereira, S.; Serafim, L.; Xavier, A. Two-Stage Aeration Fermentation Strategy to Improve Bioethanol Production by Scheffersomyces stipitis. Fermentation 2018, 4, 97. [Google Scholar] [CrossRef]
- Salazar-Cerezo, S.; De Vries, R.P.; Garrigues, S. Strategies for the Development of Industrial Fungal Producing Strains. J. Fungi 2023, 9, 834. [Google Scholar] [CrossRef]
- Bajić, B.; Vučurović, D.; Vasić, Đ.; Jevtić-Mučibabić, R.; Dodić, S. Biotechnological Production of Sustainable Microbial Proteins from Agro-Industrial Residues and By-Products. Foods 2022, 12, 107. [Google Scholar] [CrossRef]
- Amara, A.A.; El-Baky, N.A. Fungi as a Source of Edible Proteins and Animal Feed. J. Fungi 2023, 9, 73. [Google Scholar] [CrossRef]
- Valentino, M. Mycota of Distillery Yeast Sludge as Source of Single Cell Protein. Mycosphere 2015, 6, 241–247. [Google Scholar] [CrossRef]
- Kut, A.; Demiray, E.; Ertuğrul Karatay, S.; Dönmez, G. Second Generation Bioethanol Production from Hemicellulolytic Hydrolyzate of Apple Pomace by Pichia stipitis. Energy Sources Part Recovery Util. Environ. Eff. 2022, 44, 5574–5585. [Google Scholar] [CrossRef]
- Commission Regulation (EU) 2022/1104 of 1 July 2022 Amending Regulation (EU) No 68/2013 on the Catalogue of Feed Materials. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX%3A32022R1104 (accessed on 6 July 2024).
- Jeffries, T.W.; Van Vleet, J.R.H. Pichia stipitis Genomics, Transcriptomics, and Gene Clusters. FEMS Yeast Res. 2009, 9, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Patelski, P.; Berlowska, J.; Dziugan, P.; Pielech-Przybylska, K.; Balcerek, M.; Dziekonska, U.; Kalinowska, H. Utilisation of Sugar Beet Bagasse for the Biosynthesis of Yeast SCP. J. Food Eng. 2015, 167, 32–37. [Google Scholar] [CrossRef]
- Patelski, P.; Stanisz, M.; Antczak, A.; Balcerek, M.; Pielech-Przybylska, K.; Sapinska, E.; Dziekonska, U. Conversion of Sugar Beet Leaf Polysaccharides into Single Cell Protein. RSC Adv. 2015, 5, 20961–20965. [Google Scholar] [CrossRef]
- Papon, N.; Savini, V.; Lanoue, A.; Simkin, A.J.; Crèche, J.; Giglioli-Guivarc’h, N.; Clastre, M.; Courdavault, V.; Sibirny, A.A. Candida guilliermondii: Biotechnological Applications, Perspectives for Biological Control, Emerging Clinical Importance and Recent Advances in Genetics. Curr. Genet. 2013, 59, 73–90. [Google Scholar] [CrossRef]
- Fernandes, A.; Cruz-Lopes, L.; Esteves, B.; Evtuguin, D.V. Microwaves and Ultrasound as Emerging Techniques for Lignocellulosic Materials. Materials 2023, 16, 7351. [Google Scholar] [CrossRef] [PubMed]
- Gavrila, A.I.; Vartolomei, A.; Calinescu, I.; Vinatoru, M.; Parvulescu, O.C.; Psenovschi, G.; Chipurici, P.; Trifan, A. Ultrasound-Assisted Alkaline Pretreatment of Biomass to Enhance the Extraction Yield of Valuable Chemicals. Agronomy 2024, 14, 903. [Google Scholar] [CrossRef]
- Richel, A.; Jacquet, N. Microwave-Assisted Thermochemical and Primary Hydrolytic Conversions of Lignocellulosic Resources: A Review. Biomass Convers. Biorefinery 2015, 5, 115–124. [Google Scholar] [CrossRef]
- Ji, Q.; Yu, X.; Yagoub, A.E.A.; Chen, L.; Mustapha, A.T.; Zhou, C. Enhancement of Lignin Removal and Enzymolysis of Sugarcane Bagasse by Ultrasound-Assisted Ethanol Synergized Deep Eutectic Solvent Pretreatment. Renew. Energy 2021, 172, 304–316. [Google Scholar] [CrossRef]
- Kłosowski, G.; Mikulski, D.; Lewandowska, N. Microwave-Assisted Degradation of Biomass with the Use of Acid Catalysis. Catalysts 2020, 10, 641. [Google Scholar] [CrossRef]
- Mikulski, D.; Kłosowski, G. Delignification Efficiency of Various Types of Biomass Using Microwave-Assisted Hydrotropic Pretreatment. Sci. Rep. 2022, 12, 4561. [Google Scholar] [CrossRef]
- Pradhan, D.; Tsegaye, B.; Mathew, S.S.; Jaiswal, S.; Jaiswal, A.K. Ultrasound-Assisted Pretreatment of Lignocellulosic Biomass for Bioethanol Production. In Bioethanol Fuel Production Processes. I; CRC Press: Boca Raton, FL, USA, 2023; ISBN 978-1-003-22653-6. [Google Scholar]
- Wrolstad, R.E.; Acree, T.E.; Decker, E.A.; Penner, M.H.; Reid, D.S.; Schwartz, S.J.; Shoemaker, C.F.; Smith, D.M.; Sporns, P. Handbook of Food Analytical Chemistry: Water, Proteins, Enzymes, Lipids, and Carbohydrates; Wrolstad, R.E., Ed.; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.O.; Scarlata, C.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; NREL: Golden, CO, USA, 2012.
- Oberoi, H.S.; Sandhu, S.K.; Vadlani, P.V. Statistical Optimization of Hydrolysis Process for Banana Peels Using Cellulolytic and Pectinolytic Enzymes. Food Bioprod. Process. 2012, 90, 257–265. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Emaga, T.H.; Bindelle, J.; Agneesens, R.; Buldgen, A.; Wathelet, B.; Paquot, M. Ripening Influences Banana and Plantain Peels Composition and Energy Content. Trop. Anim. Health Prod. 2011, 43, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Mohd Jamil, N.A.; Jaffar, S.S.; Saallah, S.; Misson, M.; Siddiquee, S.; Roslan, J.; Lenggoro, W. Isolation of Cellulose Nanocrystals from Banana Peel Using One-Pot Microwave and Mild Oxidative Hydrolysis System. Nanomaterials 2022, 12, 3537. [Google Scholar] [CrossRef] [PubMed]
- Yasin, M.; Gangan, S.; Panchal, S.K. Banana Peels: A Genuine Waste or a Wonderful Opportunity? Appl. Sci. 2025, 15, 3195. [Google Scholar] [CrossRef]
- Kabenge, I.; Omulo, G.; Banadda, N.; Seay, J.; Zziwa, A.; Kiggundu, N. Characterization of Banana Peels Wastes as Potential Slow Pyrolysis Feedstock. J. Sustain. Dev. 2018, 11, 14. [Google Scholar] [CrossRef]
- Pereira, M.A.F.; Cesca, K.; Poletto, P.; de Oliveira, D. New Perspectives for Banana Peel Polysaccharides and Their Conversion to Oligosaccharides. Food Res. Int. 2021, 149, 110706. [Google Scholar] [CrossRef]
- Jamal, P.K.; Saheed, O.; Alam, Z. Bio-Valorization Potential of Banana Peels (Musa sapientum): An Overview. Asian, J. Biotechnol. 2012, 4, 1–14. [Google Scholar] [CrossRef]
- Berlowska, J.; Cieciura-Włoch, W.; Kalinowska, H.; Kregiel, D.; Borowski, S.; Pawlikowska, E.; Binczarski, M.; Witonska, I. Enzymatic Conversion of Sugar Beet Pulp: A Comparison of Simultaneous Saccharification and Fermentation and Separate Hydrolysis and Fermentation for Lactic Acid Production. Food Technol. Biotechnol. 2018, 56, 188–196. [Google Scholar] [CrossRef]
- Berłowska, J.; Pielech-Przybylska, K.; Balcerek, M.; Dziekońska-Kubczak, U.; Patelski, P.; Dziugan, P.; Kręgiel, D. Simultaneous Saccharification and Fermentation of Sugar Beet Pulp for Efficient Bioethanol Production. BioMed Res. Int. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Dziekońska-Kubczak, U.; Berłowska, J.; Dziugan, P.; Patelski, P.; Balcerek, M.; Pielech-Przybylska, K.; Robak, K. Two-Stage Pretreatment to Improve Saccharification of Oat Straw and Jerusalem Artichoke Biomass. Energies 2019, 12, 1715. [Google Scholar] [CrossRef]
- Akita, H.; Shibata, S.; Komoriya, T.; Kamei, S.; Asamoto, H.; Matsumoto, M. Simultaneous Saccharification and Fermentation for Isobutanol Production from Banana Peel. Fermentation 2024, 10, 161. [Google Scholar] [CrossRef]
- Pereira, M.A.F.; Monteiro, C.R.M.; Pereira, G.N.; Júnior, S.E.B.; Zanella, E.; Ávila, P.F.; Stambuk, B.U.; Goldbeck, R.; de Oliveira, D.; Poletto, P. Deconstruction of Banana Peel for Carbohydrate Fractionation. Bioprocess Biosyst. Eng. 2021, 44, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Palacios, S.; Ruiz, H.A.; Ramos-Gonzalez, R.; Martínez, J.; Segura, E.; Aguilar, M.; Aguilera, A.; Michelena, G.; Aguilar, C.; Ilyina, A. Comparison of Physicochemical Pretreatments of Banana Peels for Bioethanol Production. Food Sci. Biotechnol. 2017, 26, 993–1001. [Google Scholar] [CrossRef]
- NCYC 443-Meyerozyma guilliermondii|National Collection of Yeast Cultures. Available online: https://www.ncyc.co.uk/catalogue/meyerozyma-guilliermondii-443 (accessed on 11 April 2025).
- Kurtzman, C.P.; Fell, J.W. The Yeasts, A Taxonomic Study, 4th ed.; Elsevier: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Papini, M.; Nookaew, I.; Uhlén, M.; Nielsen, J. Scheffersomyces stipitis: A Comparative Systems Biology Study with the Crabtree Positive Yeast Saccharomyces cerevisiae. Microb. Cell Factories 2012, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Smith, J. Mixed β-Glucanase, Xylanase from Humicola Insolens; FAO: Rome, Italy, 2004. [Google Scholar]
- Martins, L.C.; Palma, M.; Angelov, A.; Nevoigt, E.; Liebl, W.; Sá-Correia, I. Complete Utilization of the Major Carbon Sources Present in Sugar Beet Pulp Hydrolysates by the Oleaginous Red Yeasts Rhodotorula toruloides and R. mucilaginosa. J. Fungi 2021, 7, 215. [Google Scholar] [CrossRef]
- Koivistoinen, O.M.; Arvas, M.; Headman, J.R.; Andberg, M.; Penttilä, M.; Jeffries, T.W.; Richard, P. Characterisation of the Gene Cluster for L-Rhamnose Catabolism in the Yeast Scheffersomyces (Pichia) stipitis. Gene 2012, 492, 177–185. [Google Scholar] [CrossRef]
- Fernie, A.R.; Carrari, F.; Sweetlove, L.J. Respiratory Metabolism: Glycolysis, the TCA Cycle and Mitochondrial Electron Transport. Curr. Opin. Plant Biol. 2004, 7, 254–261. [Google Scholar] [CrossRef]
- Kornberg, H.L.; Madsen, N.B. The Metabolism of C2 Compounds in Micro-Organisms. 3. Synthesis of Malate from Acetate via the Glyoxylate Cycle. Biochem. J. 1958, 68, 549–557. [Google Scholar] [CrossRef]
- Eglinton, J.M.; Heinrich, A.J.; Pollnitz, A.P.; Langridge, P.; Henschke, P.A.; de Barros Lopes, M. Decreasing Acetic Acid Accumulation by a Glycerol Overproducing Strain of Saccharomyces cerevisiae by Deleting the ALD6 Aldehyde Dehydrogenase Gene. Yeast 2002, 19, 295–301. [Google Scholar] [CrossRef]
- Remize, F.; Roustan, J.L.; Sablayrolles, J.M.; Barre, P.; Dequin, S. Glycerol Overproduction by Engineered Saccharomyces cerevisiae Wine Yeast Strains Leads to Substantial Changes in By-Product Formation and to a Stimulation of Fermentation Rate in Stationary Phase. Appl. Environ. Microbiol. 1999, 65, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, R.; Shrestha, N.; Singh, L.; Khadgi, R.K.; Chaturwedi, S.B. Production of Single Cell Protein from Banana Peel, Papaya Juice and Whey and Estimation of Protein Concentration. Tribhuvan Univ. J. Microbiol. 2022, 9(1), 28–34. [Google Scholar] [CrossRef]
- Jiru, T.M.; Melku, B. Single Cell Protein Production from Torula Yeast (Cyberlindnera Sp.) Using Banana Peel Hydrolysate. J. Adv. Microbiol. 2018, 13, 1–7. [Google Scholar] [CrossRef]
- Sharma, I.; Sharma, K. Banana peel extracts for the production of single cell protein by using Saccharomyces cerevisiae. Int. J. Adv. Res. 2017, 5, 531–535. [Google Scholar] [CrossRef]
- Azwar, A.; Mukhlishien, M.; Muslim, A.; Hadissa, P.; Ningsih, U.H.; Zanil, M.F.; Ali, J.M. Production of Single Cell Protein from Banana Peel Waste in Batch Fermentation Using Saccharomyces cerevisiae. J. Bahan Alam Terbarukan 2021, 10, 104–112. [Google Scholar] [CrossRef]
- Matos, I.T.S.R.; Cassa-Barbosa, L.A.; de Galvão, R.S.M.; Nunes-Silva, C.G.; Filho, S.A. Isolation, taxonomic identification and investigation of the biotechnological potential of wild-type Meyerozyma guilliermondii associated with amazonian termites able to ferment D-xylose. Biosci. J. 2014, 30, 260–266. [Google Scholar]
- Duarte, L.C.; Lopes, F.C.S.; Neves, I.; Girio, F.M. Yeast Biomass Production in Brewery’s Spent Grains Hemicellulosic Hydrolyzate. In Applied Biochememistry and Biotechnology, Proceedings of the Twenty-Ninth Symposium on Biotechnology for Fuels and Chemicals, Denver, CO, USA, 29 April–2 May 2007; Humana Press: Totowa, NJ, USA, 2008. [Google Scholar]
- Martini, C.; Tauk-Tornisielo, S.M.; Codato, C.B.; Bastos, R.G.; Ceccato-Antonini, S.R. A Strain of Meyerozyma guilliermondii Isolated from Sugarcane Juice Is Able to Grow and Ferment Pentoses in Synthetic and Bagasse Hydrolysate Media. World J. Microbiol. Biotechnol. 2016, 32, 80. [Google Scholar] [CrossRef]
- Reed, G.; Nagodawithana, T.W. Yeast Technology; Springer: Dordrecht, The Netherlands, 1990; ISBN 978-94-011-9773-1. [Google Scholar]
- Sheth, U.; Patel, S. Production, Economics, and Marketing of Yeast Single Cell Protein. In Food Microbiology Based Entrepreneurship: Making Money from Microbes; Amaresan, N., Dharumadurai, D., Babalola, O.O., Eds.; Springer: Singapore, 2023; pp. 133–152. ISBN 978-981-19-5041-4. [Google Scholar]

| Parameter | Value |
|---|---|
| dry matter [%] | 11.75 ± 0.57 |
| cellulose [% DM] | 10.09 ± 0.18 |
| hemicellulose [% DM] | 36.89 ± 0.95 |
| lignin [% DM] | 9.62 ± 0.4 |
| pectin [% DM] | 8.27 ± 0.2 |
| crude protein [% DM] | 7.22 ± 0.659 |
| Enzyme | Cellulase | Pectinase | Xylanase |
|---|---|---|---|
| Activity [U/mL] | |||
| Viscozyme | 23.26 ± 0.76 | 287.06 ± 8.2 | 27.41 ± 1.18 |
| UltrafloMax | 30.3 ± 0.88 | 23.94 ± 0.91 | 58.29 ± 3.15 |
| Cellobiose | Glucose | Xylose | Arabinose | |
|---|---|---|---|---|
| Sample | Concentration [g/L] | |||
| PT | 0.02 ± 0.001 | 5.23 ± 0.194 | 5.56 ± 0.298 | 0.73 ± 0.071 |
| ME | 0.32 ± 0.008 | 8.08 ± 0.439 | 8.8 ± 0.753 | 1.55 ± 0.123 |
| PTE | 0.29 ± 0.026 | 9.27 ± 0.327 | 9.24 ± 0.703 | 1.93 ± 0.154 |
| UE | 0.31 ± 0.028 | 8.55 ± 0.81 | 8.8 ± 0.373 | 1.68 ± 0.095 |
| Cellobiose | Glucose | Xylose | Arabinose | |
|---|---|---|---|---|
| Sample | Concentration [g/L] | |||
| PT + G | 0.08 ± 0.006 | 0.02 ± 0.001 | 0.36 ± 0.026 | 0.24 ± 0.012 |
| PT + S | 0.05 ± 0.005 | 0.02 ± 0.001 | 0.29 ± 0.019 | 0.27 ± 0.017 |
| ME + G | 0.05 ± 0.005 | 0.01 ± 0.001 | 0.2 ± 0.019 | 0.1 ± 0.007 |
| ME + S | 0.07 ± 0.006 | n.d. | 0.03 ± 0.002 | 0.06 ± 0.004 |
| PTE + G | 0.02 ± 0.002 | n.d. | 0.03 ± 0.001 | 0.1 ± 0.005 |
| PTE + S | 0.02 ± 0.002 | n.d. | 0.62 ± 0.056 | 0.12 ± 0.01 |
| UE + G | 0.06 ± 0.003 | n.d. | 0.12 ± 0.01 | 0.09 ± 0.007 |
| UE + S | 0.05 ± 0.005 | n.d. | 0.1 ± 0.008 | 0.09 ± 0.003 |
| Sample | Galacturonic Acid | Succinic Acid | Formic Acid | Acetic Acid |
|---|---|---|---|---|
| Concentration [g/L] | ||||
| PT | 0.64 ± 0.02 | 0.05 ± 0.003 | 0.03 ± 0.001 | 0.35 ± 0.01 |
| ME | 0.62 ± 0.025 | 0.29 ± 0.017 | 0.04 ± 0.002 | 0.39 ± 0.029 |
| PTE | 0.93 ± 0.081 | 0.26 ± 0.015 | 0.04 ± 0.001 | 0.42 ± 0.027 |
| UE | 0.73 ± 0.051 | 0.27 ± 0.01 | 0.04 ± 0.004 | 0.43 ± 0.035 |
| Sample | Galacturonic Acid | Succinic Acid | Formic Acid | Acetic Acid |
|---|---|---|---|---|
| Concentration [g/L] | ||||
| PT + G | 0.61 ± 0.023 | 0.14 ± 0.011 | 0.1 ± 0.009 | 0.39 ± 0.02 |
| PT + S | 0.61 ± 0.03 | 0.13 ± 0.007 | 0.12 ± 0.01 | 0.42 ± 0.022 |
| ME + G | 0.6 ± 0.042 | 0.26 ± 0.007 | 0.28 ± 0.011 | 0.36 ± 0.019 |
| ME + S | 0.64 ± 0.057 | 0.23 ± 0.01 | 0.36 ± 0.02 | 0.43 ± 0.013 |
| PTE + G | 0.9 ± 0.075 | 0.25 ± 0.012 | 0.17 ± 0.015 | 0.41 ± 0.013 |
| PTE + S | 0.86 ± 0.061 | 0.24 ± 0.015 | 0.23 ± 0.02 | 0.39 ± 0.021 |
| UE + G | 0.75 ± 0.074 | 0.25 ± 0.022 | 0.3 ± 0.014 | 0.47 ± 0.043 |
| UE + S | 0.74 ± 0.04 | 0.24 ± 0.008 | 0.31 ± 0.028 | 0.38 ± 0.029 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patelski, A.M.; Dziekońska-Kubczak, U.; Balcerek, M.; Pielech-Przybylska, K.; Domański, J.; Berłowska, J.; Dziugan, P. The Impact of Microwaves and Ultrasound on the Hydrolysis of Banana Peels and the Growth of Fodder Yeasts. Appl. Sci. 2025, 15, 5617. https://doi.org/10.3390/app15105617
Patelski AM, Dziekońska-Kubczak U, Balcerek M, Pielech-Przybylska K, Domański J, Berłowska J, Dziugan P. The Impact of Microwaves and Ultrasound on the Hydrolysis of Banana Peels and the Growth of Fodder Yeasts. Applied Sciences. 2025; 15(10):5617. https://doi.org/10.3390/app15105617
Chicago/Turabian StylePatelski, Andrea Maria, Urszula Dziekońska-Kubczak, Maria Balcerek, Katarzyna Pielech-Przybylska, Jarosław Domański, Joanna Berłowska, and Piotr Dziugan. 2025. "The Impact of Microwaves and Ultrasound on the Hydrolysis of Banana Peels and the Growth of Fodder Yeasts" Applied Sciences 15, no. 10: 5617. https://doi.org/10.3390/app15105617
APA StylePatelski, A. M., Dziekońska-Kubczak, U., Balcerek, M., Pielech-Przybylska, K., Domański, J., Berłowska, J., & Dziugan, P. (2025). The Impact of Microwaves and Ultrasound on the Hydrolysis of Banana Peels and the Growth of Fodder Yeasts. Applied Sciences, 15(10), 5617. https://doi.org/10.3390/app15105617










