Preparation of PS-MWNT and PETE-MWNT Antistatic Materials via In Situ Polymerization for IC Tray Applications
Abstract
1. Introduction
2. Experiments
2.1. Materials and Instrumentation
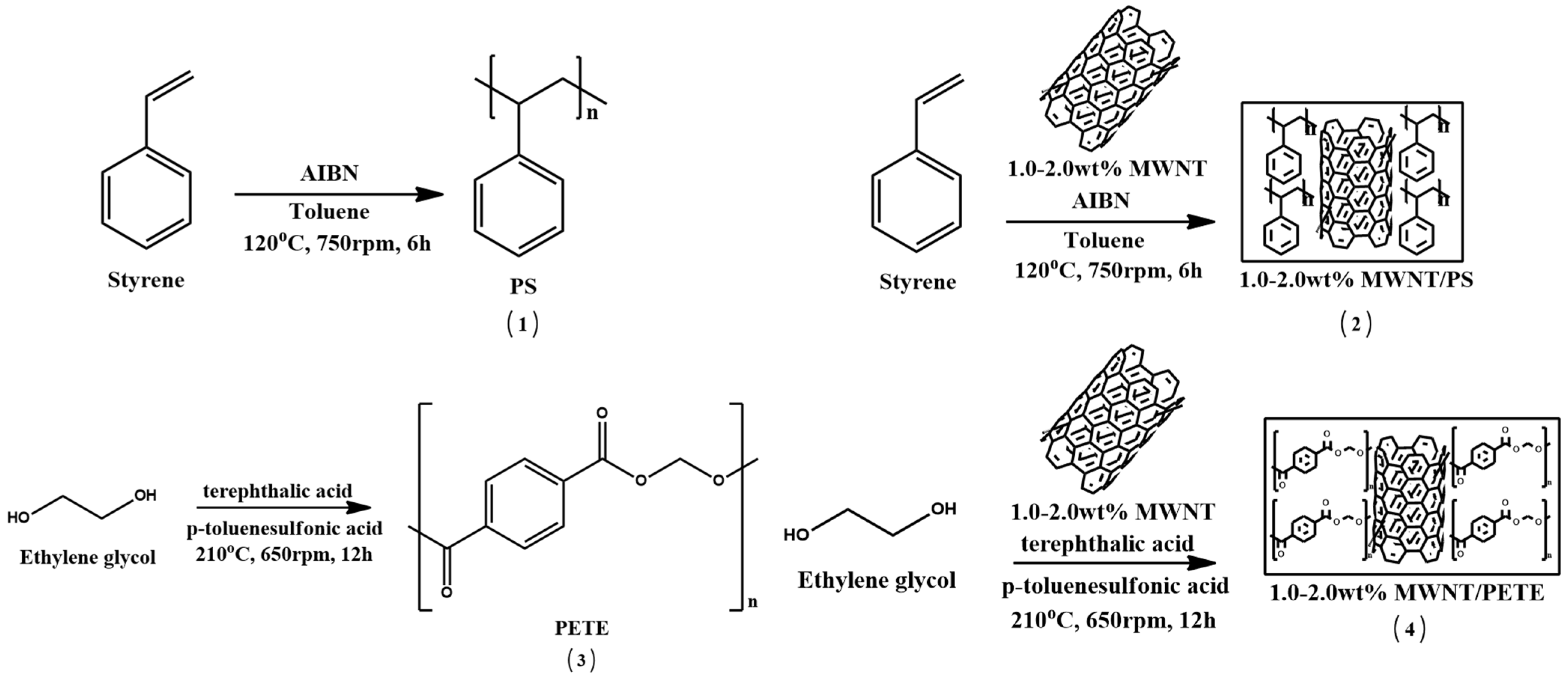
2.2. Synthesis
2.2.1. Synthesis of PS (1)
2.2.2. Synthesis of 1.0–2.0 wt% MWNT/PS Materials (2)
2.2.3. Synthesis of PETE (3)
2.2.4. Synthesis of 1.0–2.0 wt% MWNT/PETE Materials (4)
3. Results and Discussion
3.1. Synthesis and Gel Permeation Chromatography
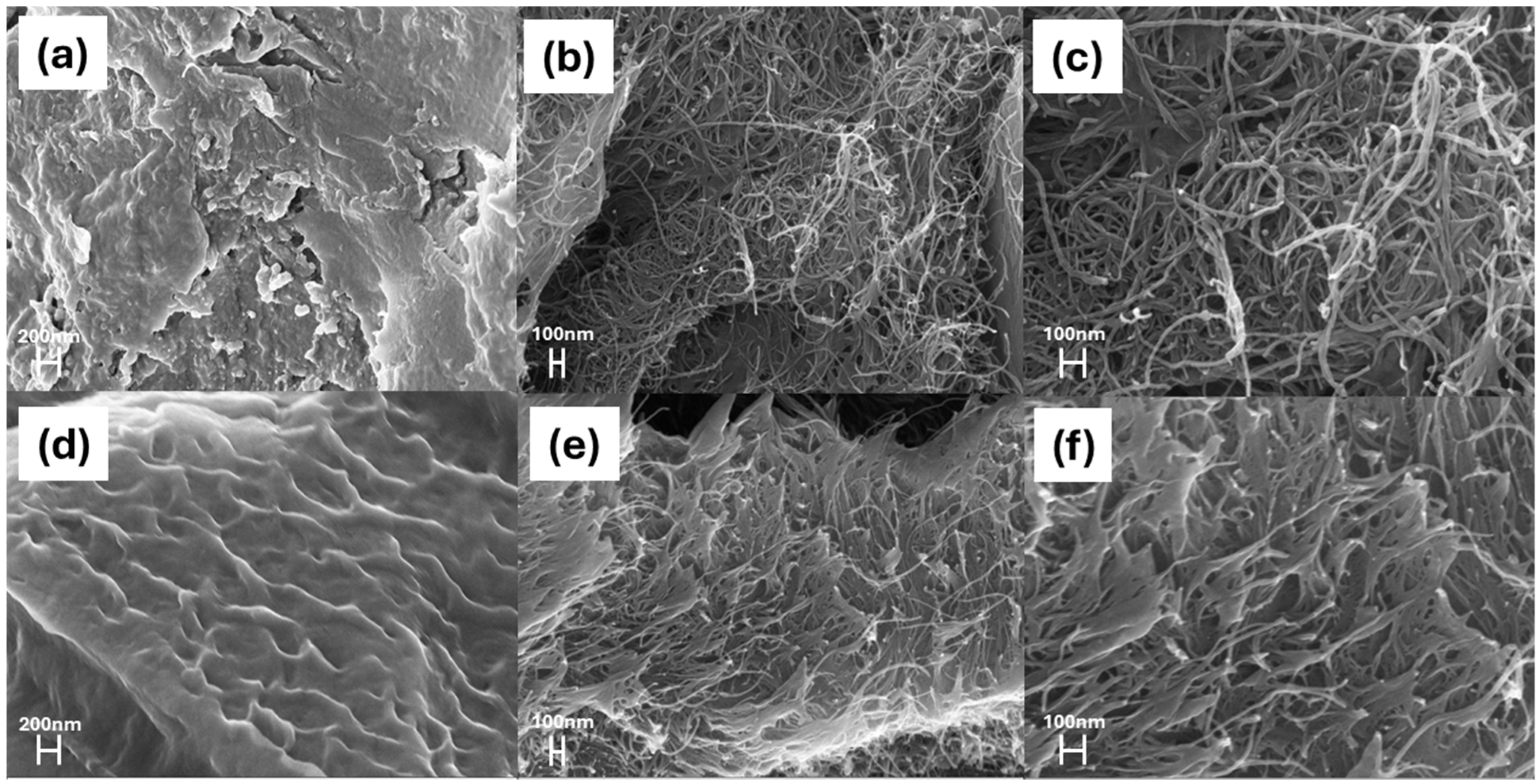
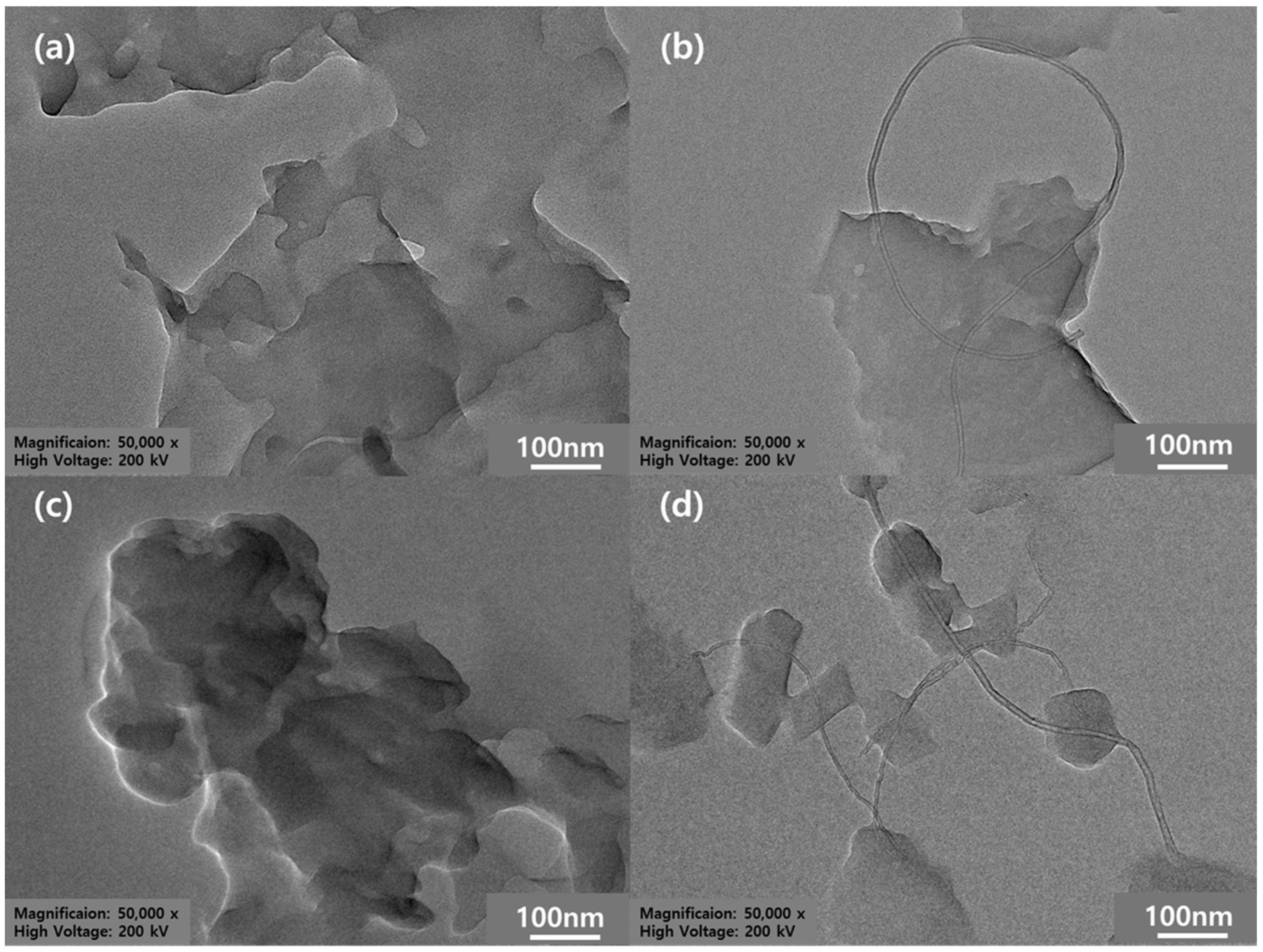
3.2. Morphology
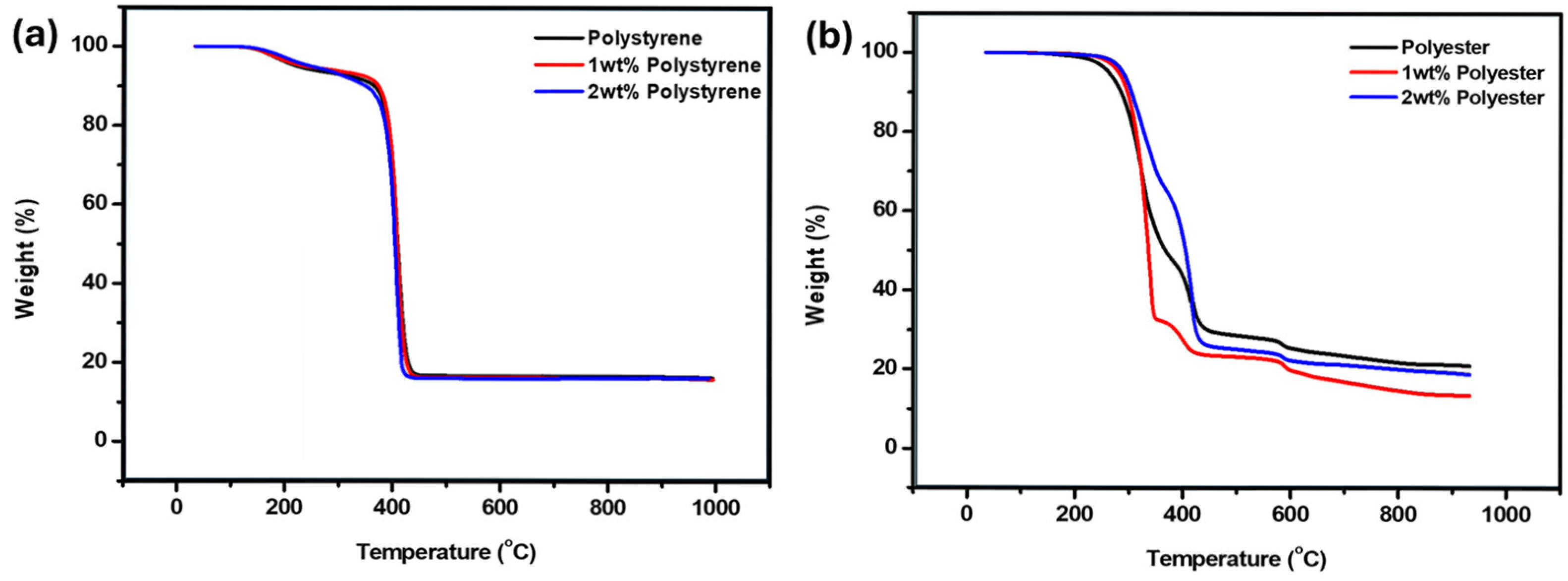
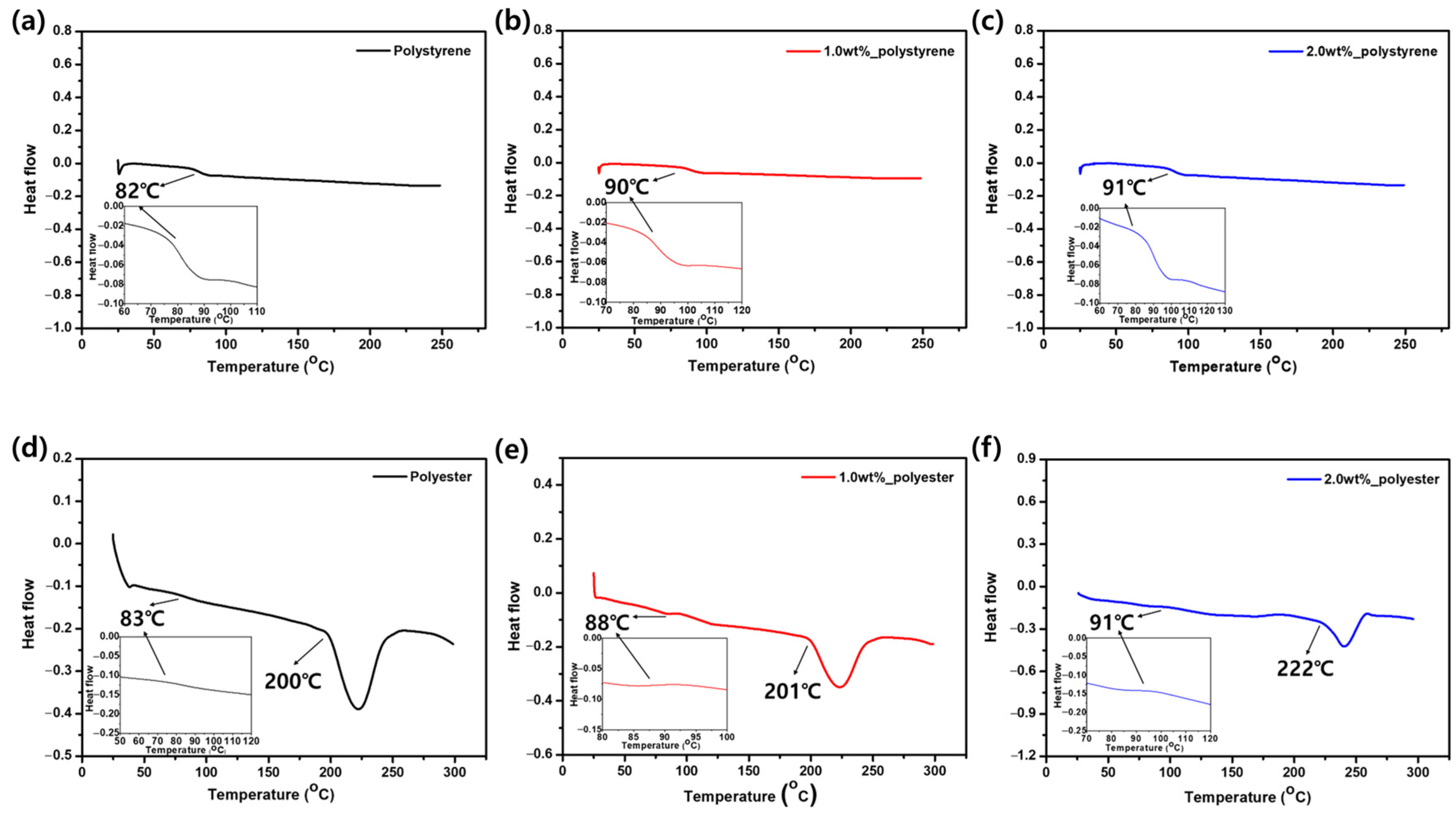
3.3. Thermal Analysis
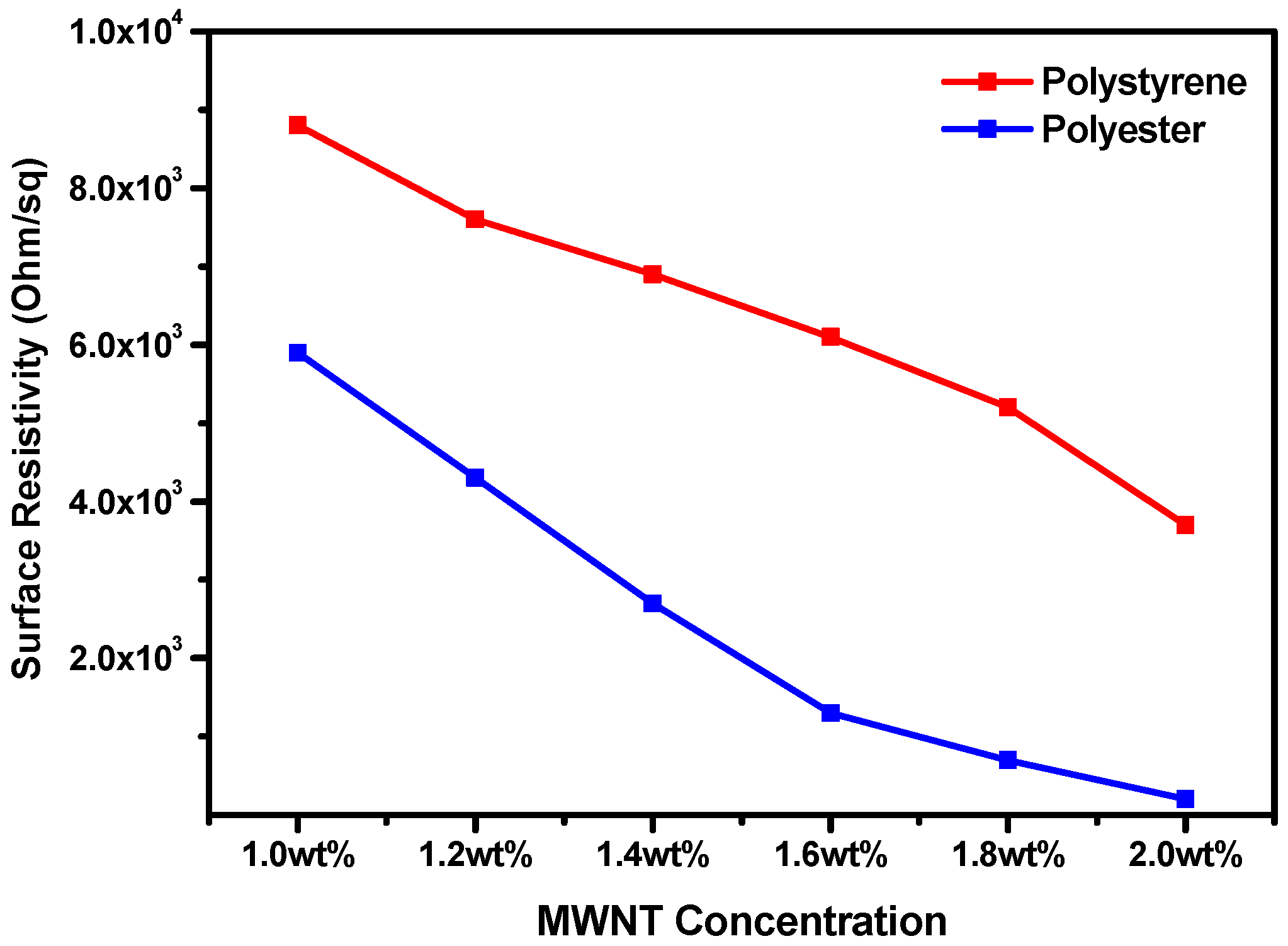
3.4. Electrical Conductivity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dahman, S.J. All Polymeric Compounds: Conductive and Dissipative Polymers in ESD Control Materials. In Proceedings of the Electrical Overstress/Electrostatic Discharge Symposium Proceedings, Las Vegas, NV, USA, 21–25 September 2003; pp. 1–7. [Google Scholar]
- Dahman, S.J.; Avlyanov, J. The Use of Conducting Polymer Composites in Thermoplastics for Tuning Surface Resistivity. In Conductive Polymers and Plastics; William Andrew Publishing: Norwich, NY, USA, 1999; pp. 225–230. [Google Scholar]
- Su, D.S. 20 years of carbon nanotubes. ChemSusChem 2011, 4, 811–813. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Dwivedy, I. International patenting activity in the field of carbon nanotubes. Curr. Appl. Phys. 2005, 5, 163–170. [Google Scholar] [CrossRef]
- Callister, W.D.; Rethwisch, D.G. Material Science and Engineering: An Introduction; John Wiley: Hoboken, NJ, USA, 2000; pp. 459–460. [Google Scholar]
- Li, J.; Ma, P.C.; Chow, W.S.; To, C.K.; Tang, B.Z.; Kim, J.K. Correlations between Percolation Threshold, Dispersion State, and Aspect Ratio of Carbon Nanotubes. Adv. Funct. Mater. 2007, 17, 3207–3215. [Google Scholar] [CrossRef]
- Tulevski, G.S.; Falk, A.L. Emergent Properties of Macroscale Assemblies of Carbon Nanotubes. Adv. Funct. Mater. 2020, 30, 1909448. [Google Scholar] [CrossRef]
- Park, S.J.; Cho, M.S.; Lim, S.T.; Choi, H.J.; Jhon, M.S. Synthesis and Dispersion Characteristics of Multi-Walled Carbon Nanotube Composites with Poly(methyl methacrylate) Prepared by In-Situ Bulk Polymerization. Macromol. Rapid Commun. 2003, 24, 1070–1073. [Google Scholar] [CrossRef]
- Xie, X.; Gao, L. Characterization of a manganese dioxide/carbon nanotube composite fabricated using an in situ coating method. Carbon 2007, 45, 2365–2373. [Google Scholar] [CrossRef]
- Mathur, R.B.; Pande, S.; Singh, B.P.; Dhami, T.L. Electrical and mechanical properties of multi-walled carbon nanotubes reinforced PMMA and PS composites. Polym. Compos. 2008, 29, 717–727. [Google Scholar] [CrossRef]
- Oliva-Avilés, A.I.; Avilés, F.; Sosa, V. Electrical and piezoresistive properties of multi-walled carbon nanotube/polymer composite films aligned by an electric field. Carbon 2011, 49, 2989–2997. [Google Scholar] [CrossRef]
- Detriche, S.; Bhakta, A.K.; N’Twali, P.; Delhalle, J.; Mekhalif, Z. Assessment of Catalyst Selectivity in Carbon-Nanotube Silylesterification. Appl. Sci. 2019, 10, 109. [Google Scholar] [CrossRef]
- Xiong, J.; Zheng, Z.; Qin, X.; Li, M.; Li, H.; Wang, X. The thermal and mechanical properties of a polyurethane/multi-walled carbon nanotube composite. Carbon 2006, 44, 2701–2707. [Google Scholar] [CrossRef]
- Uchida, T.; Kumar, S. Single wall carbon nanotube dispersion and exfoliation in polymers. J. Appl. Polym. Sci. 2005, 98, 985–989. [Google Scholar] [CrossRef]
- Datsyuk, V.; Kalyva, M.; Papagelis, K.; Parthenios, J.; Tasis, D.; Siokou, A.; Kallitsis, I.; Galiotis, C. Chemical oxidation of multiwalled carbon nanotubes. Carbon 2008, 46, 833–840. [Google Scholar] [CrossRef]
- Fujisawa, K.; Kim, H.; Go, S.; Muramatsu, H.; Hayashi, T.; Endo, M.; Hirschmann, T.; Dresselhaus, M.; Kim, Y.; Araujo, P. A Review of Double-Walled and Triple-Walled Carbon Nanotube Synthesis and Applications. Appl. Sci. 2016, 6, 109. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Y.; Shi, J.; Bai, H.; Song, B. Functionalized multi-walled carbon nanotubes improve nonisothermal crystallization of poly(ethylene terephthalate). Polym. Test. 2008, 27, 179–188. [Google Scholar] [CrossRef]
- Yuen, S.-M.; Ma, C.-C.M.; Lin, Y.-Y.; Kuan, H.-C. Preparation, morphology and properties of acid and amine modified multiwalled carbon nanotube/polyimide composite. Compos. Sci. Technol. 2007, 67, 2564–2573. [Google Scholar] [CrossRef]
- Ryszkowska, J. Quantitative image analysis of polyurethane/carbon nanotube composite micostructures. Mater. Charact. 2009, 60, 1127–1132. [Google Scholar] [CrossRef]
- Kaseem, M.; Hamad, K.; Ko, Y.G. Fabrication and materials properties of polystyrene/carbon nanotube (PS/CNT) composites: A review. Eur. Polym. J. 2016, 79, 36–62. [Google Scholar] [CrossRef]
- Faravelli, T.; Pinciroli, M.; Pisano, F.; Bozzano, G.; Dente, M.; Ranzi, E. Thermal degradation of polystyrene. J. Anal. Appl. Pyrolysis 2001, 60, 103–121. [Google Scholar] [CrossRef]
- Maafa, I.M. Pyrolysis of Polystyrene Waste: A Review. Polymers 2021, 13, 225. [Google Scholar] [CrossRef]
- Pang, K.; Kotek, R.; Tonelli, A. Review of conventional and novel polymerization processes for polyesters. Prog. Polym. Sci. 2006, 31, 1009–1037. [Google Scholar] [CrossRef]
- Samir, Z.; Boukheir, S.; Belhimria, R.; Achour, M.E.; Costa, L.C. Electrical Transport Properties of Carbon Nanotube/Polyester Polymer Composites. J. Supercond Nov. Magn. 2019, 32, 185–190. [Google Scholar] [CrossRef]
- Wang, H.; Li, H.; Lee, C.K.; Mat Nanyan, N.S.; Tay, G.S. Recent Advances in the Enzymatic Synthesis of Polyester. Polymers 2022, 14, 5059. [Google Scholar] [CrossRef] [PubMed]
- Mittal, G.; Rhee, K.Y.; Park, S.J. The Effects of Cryomilling CNTs on the Thermal and Electrical Properties of CNT/PMMA Composites. Polymers 2016, 8, 169. [Google Scholar] [CrossRef] [PubMed]
- Goto, A.; Fukuda, T. Kinetics of living radical polymerization. Prog. Polym. Sci. 2004, 29, 329–385. [Google Scholar] [CrossRef]
- Billiet, L.; Fournier, D.; Du Prez, F. Combining “click” chemistry and step-growth polymerization for the generation of highly functionalized polyesters. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 6552–6564. [Google Scholar] [CrossRef]
- Park, S.-J.; Chae, S.-W.; Rhee, J.-M.; Kang, S.-J. A Study on Electrical and Thermal Properties of Polyimide/MWNT Nanocomposites. Bull. Korean Chem. Soc. 2010, 31, 2279–2282. [Google Scholar] [CrossRef]
- Deep, N.; Mishra, P. Fabrication and characterization of thermally conductive PMMA/MWCNT nanocomposites. Mater. Today Proc. 2018, 5, 28328–28336. [Google Scholar] [CrossRef]
- Aurilia, M.; Sorrentino, L.; Iannace, S. Modelling physical properties of highly crystallized polyester reinforced with multiwalled carbon nanotubes. Eur. Polym. J. 2012, 48, 26–40. [Google Scholar] [CrossRef]
- Gao, Z.; Li, Y.; Huang, P.; Zou, R.; Li, Y.; Fu, S. Graphene nanoplatelet/cellulose acetate film with enhanced antistatic, thermal dissipative and mechanical properties for packaging. Cellulose 2023, 30, 4499–4509. [Google Scholar] [CrossRef]
| PS | PETE | |
|---|---|---|
| Mn (g/mol) | 6600 | 2640 |
| Mw (g/mol) | 16,900 | 4250 |
| D | 2.56 | 1.61 |
| Sample | Surface Resistivity (Ohm/sq) | Sample | Surface Resistivity (Ohm/sq) | ||
|---|---|---|---|---|---|
| 1 | MWNT only | 8.12 × 102 | 1 | MWNT only | 8.12 × 102 |
| 2 | PS only | >1013 | 2 | PETE only | >1013 |
| 3 | (1.0 wt%) MWNT + PS | 8.8 × 103 | 3 | (1.0 wt%) MWNT + PETE | 5.9 × 103 |
| 4 | (1.2 wt%) MWNT + PS | 7.6 × 103 | 4 | (1.2 wt%) MWNT + PETE | 4.3 × 103 |
| 5 | (1.4 wt%) MWNT + PS | 6.9 × 103 | 5 | (1.4 wt%) MWNT + PETE | 2.7 × 103 |
| 6 | (1.6 wt%) MWNT + PS | 6.1 × 103 | 6 | (1.6 wt%) MWNT + PETE | 1.3 × 103 |
| 7 | (1.8 wt%) MWNT + PS | 5.2 × 103 | 7 | (1.8 wt%) MWNT + PETE | 0.7 × 103 |
| 8 | (2.0 wt%) MWNT + PS | 3.7 × 103 | 8 | (2.0 wt%) MWNT + PETE | 0.2 × 103 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Lee, T.; Kim, S.-T.; Lee, S.; Lee, J.; Lee, H.; Park, J. Preparation of PS-MWNT and PETE-MWNT Antistatic Materials via In Situ Polymerization for IC Tray Applications. Appl. Sci. 2025, 15, 5557. https://doi.org/10.3390/app15105557
Park S, Lee T, Kim S-T, Lee S, Lee J, Lee H, Park J. Preparation of PS-MWNT and PETE-MWNT Antistatic Materials via In Situ Polymerization for IC Tray Applications. Applied Sciences. 2025; 15(10):5557. https://doi.org/10.3390/app15105557
Chicago/Turabian StylePark, Sangwook, Taegeon Lee, Sang-Tae Kim, Soonhang Lee, Jihoon Lee, Hayoon Lee, and Jongwook Park. 2025. "Preparation of PS-MWNT and PETE-MWNT Antistatic Materials via In Situ Polymerization for IC Tray Applications" Applied Sciences 15, no. 10: 5557. https://doi.org/10.3390/app15105557
APA StylePark, S., Lee, T., Kim, S.-T., Lee, S., Lee, J., Lee, H., & Park, J. (2025). Preparation of PS-MWNT and PETE-MWNT Antistatic Materials via In Situ Polymerization for IC Tray Applications. Applied Sciences, 15(10), 5557. https://doi.org/10.3390/app15105557








