Carbon Footprint Assessment on the Viability of Utilizing Brewer’s Spent Grain to Produce Biochar
Abstract
1. Introduction
1.1. Background
1.2. Brewing Industry Waste
1.3. Biochar Production Model for BSG
1.4. Post-Production Utilization of Biochar, Syngas, and Bio-Oil
1.5. Landfilling Biomass
2. Materials and Methods
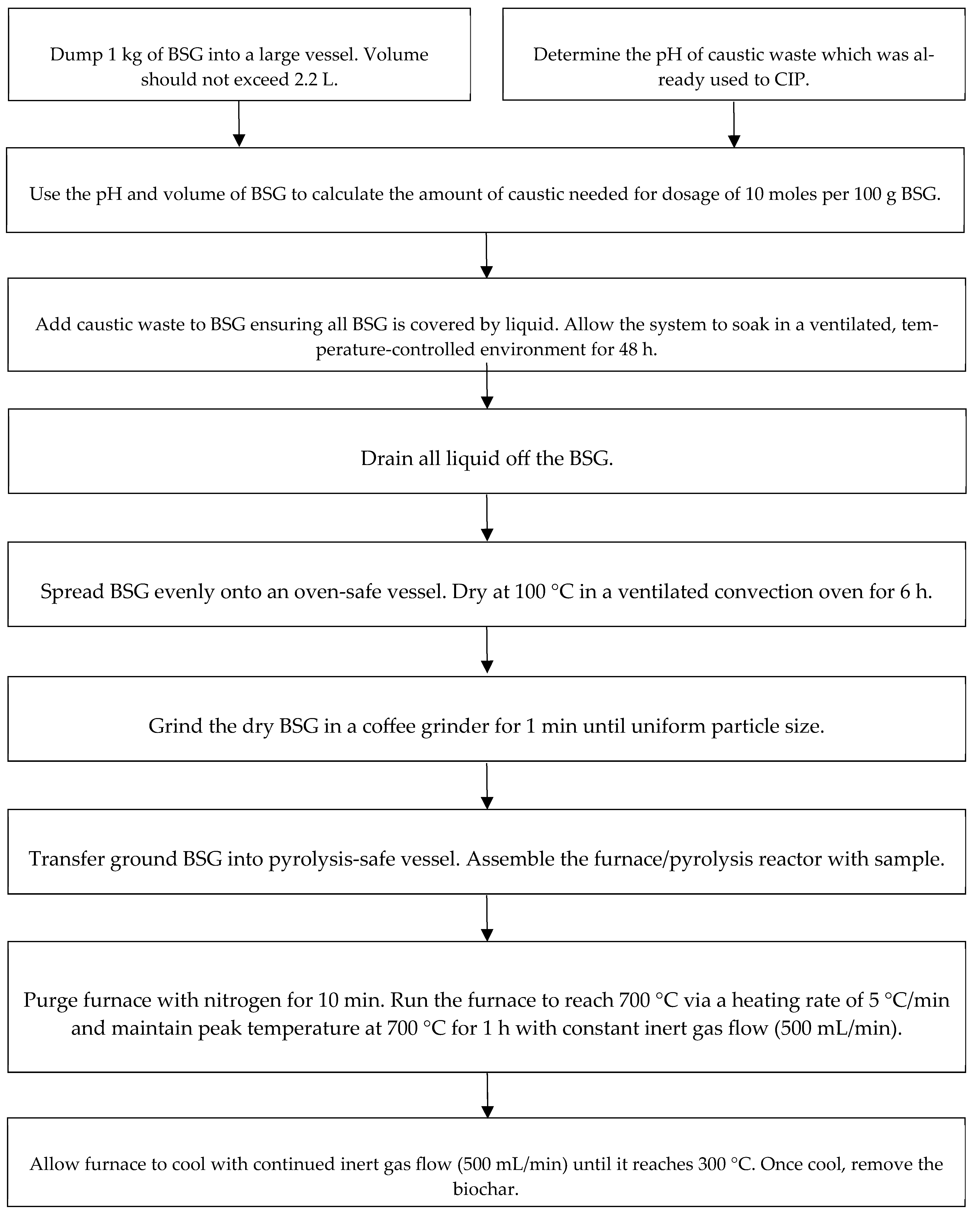
2.1. Biochar Production Method
2.2. Biochar Evaluation Methods
- M = Standardized molarity of NaOH (mol L−1);
- b = Volume of NaOH consumed (mL) by the blank;
- a = Volume of NaOH consumed (mL) by the biochar;
- = Conversion from mL to L;
- 100.09 = Molar mass of CaCO3;
- W = Mass of biochar (g);
- 2 = 1 mole of CaCO3 consumes 2 moles of H+.
2.3. Carbon Footprint Assessment (CFA) Model Assumptions and Equations
2.3.1. Inventory of BSG-to-Biochar Process Inputs and Outputs
2.3.2. Global Warming Potential (Impact Assessment) Input Phases
2.3.3. Global Warming Potential (Impact Assessment) Output Phases
2.4. Goal and Scope
2.5. System Boundary
3. Results
3.1. BSG-to-Biochar Process Inputs and Outputs
3.2. Landfilling BSG Comparison
3.3. Global Warming Potential (Impact Assessment)
3.3.1. Input Phases
3.3.2. Output Phases
4. Discussion
4.1. Comparative Models
4.2. Commercial Applicability of BSG-to-Biochar Process
4.3. Biochar End-of-Life Use
4.4. Limitations
- Collecting data on the pH of caustic waste proved challenging, and, therefore, confirmation was only obtained from a few breweries;
- In the calculated model, the oven would need to be filled to full capacity, potentially requiring drying times exceeding 6 h;
- The calculations for grinding were based on a small coffee grinder, which would not be efficient for large-scale production;
- Although the recorded average nitrogen loss was 50 L per run, the calculations indicated a requirement of 170 L for the duration of the run, suggesting an error with the flow meter. The actual flow rate for nitrogen depleted was closer to 15 mL/min, while the flow meter read 5 L/min;
- Regarding the syngas and bio-oil recovery calculations, the reference study employed an auger reactor (1 kg biomass per hour), while the present study used a smaller-scale pyrolysis furnace (30 g per run);
4.5. Future Considerations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zeko-Pivač, A.; Tišma, M.; Žnidaršič-Plazl, P.; Kulisic, B.; Sakellaris, G.; Hao, J.; Planinić, M. The potential of brewer’s spent grain in the circular bioeconomy: State of the art and future perspectives. Front. Bioeng. Biotechnol. 2022, 10, 870744. [Google Scholar] [CrossRef] [PubMed]
- Hanson, C. Guidance on Interpreting Sustainable Development Goal Target 12.3. Available online: https://champions123.org/sites/default/files/2020-09/champions-12-3-guidance-on-interpreting-sdg-target-12-3.pdf (accessed on 1 August 2024).
- Mitri, S.; Salameh, S.-J.; Khelfa, A.; Leonard, E.; Maroun, R.G.; Louka, N.; Koubaa, M. Valorization of brewers’ spent grains: Pretreatments and fermentation, a review. Fermentation 2022, 8, 50. [Google Scholar] [CrossRef]
- Cancelliere, R.; Carbone, K.; Pagano, M.; Cacciotti, I.; Micheli, L. Biochar from Brewers’ Spent Grain: A Green and Low-Cost Smart Material to Modify Screen-Printed Electrodes. Biosensors 2019, 9, 139. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Q. Sustainable mechanisms of biochar derived from brewers’ spent grain and sewage sludge for ammonia–nitrogen capture. J. Clean. Prod. 2016, 112, 3927–3934. [Google Scholar] [CrossRef]
- Xi, X.; Yan, J.; Quan, G.; Cui, L. Removal of the Pesticide Pymetrozine from Aqueous Solution by Biochar Produced from Brewer’s Spent Grain at Different Pyrolytic Temperatures. Bioresources 2014, 9, 7696–7709. [Google Scholar] [CrossRef]
- Roberts, K.G.; Gloy, B.A.; Joseph, S.; Scott, N.R.; Lehmann, J. Life cycle assessment of biochar systems: Estimating the energetic, economic, and climate change potential. Environ. Sci. Technol. 2010, 44, 827–833. [Google Scholar] [CrossRef]
- Stewart, C.E.; Zheng, J.; Botte, J.; Cotrufo, M.F. Co-generated fast pyrolysis biochar mitigates green-house gas emissions and increases carbon sequestration in temperate soils. Glob. Change Biol. Bioenergy 2013, 5, 153–164. [Google Scholar] [CrossRef]
- Matuštík, J.; Hnátková, T.; Kočí, V. Life cycle assessment of biochar-to-soil systems: A review. J. Clean. Prod. 2020, 259, 120998. [Google Scholar] [CrossRef]
- Bergman, R.D.; Gu, H.; Page-Dumroese, D.S.; Anderson, N.M. Life cycle analysis of biochar. In Biochar: A Regional Supply Chain Approach in View of Climate Change Mitigation; Bruckman, V.J., Apaydın Varol, E., Uzun, B.B., Liu, J., Eds.; Cambridge University Press: Cambridge, UK, 2016; pp. 46–69. ISBN 9781107117099. [Google Scholar]
- Miller-Robbie, L.; Ulrich, B.A.; Ramey, D.F.; Spencer, K.S.; Herzog, S.P.; Cath, T.Y.; Stokes, J.R.; Higgins, C.P. Life cycle energy and greenhouse gas assessment of the co-production of biosolids and biochar for land application. J. Clean. Prod. 2015, 91, 118–127. [Google Scholar] [CrossRef]
- Hammond, J.; Shackley, S.; Sohi, S.; Brownsort, P. Prospective life cycle carbon abatement for pyrolysis biochar systems in the UK. Energy Policy 2011, 39, 2646–2655. [Google Scholar] [CrossRef]
- Uddin, M.M.; Wright, M.M. Life cycle analysis of biochar use in water treatment plants. In Sustainable Biochar for Water and Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2022; pp. 705–735. ISBN 9780128222256. [Google Scholar]
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Brewers’ spent grain: Generation, characteristics and potential applications. J. Cereal Sci. 2006, 43, 1–14. [Google Scholar] [CrossRef]
- Wool, R.; Sun, X.S. Chapter 16: Lignin Polymers and Composites. In Bio-Based Polymers and Composites; Wool, R., Sun, X.S., Eds.; Academic Press: Amsterdam, The Netherlands, 2011; pp. 551–598. [Google Scholar]
- De, S.; Mishra, S.; Poonguzhali, E.; Rajesh, M.; Tamilarasan, K. Fractionation and characterization of lignin from waste rice straw: Biomass surface chemical composition analysis. Int. J. Biol. Macromol. 2020, 145, 795–803. [Google Scholar] [CrossRef]
- International Biochar Initiative. Standardized Product Definition and Product Testing Guidelines for Biochar That Is Used in Soil. Version 2.1. Available online: https://biochar-international.org/wp-content/uploads/2020/06/IBI_Biochar_Standards_V2.1_Final2.pdf (accessed on 1 August 2024).
- Rodriguez Correa, C.; Hehr, T.; Voglhuber-Slavinsky, A.; Rauscher, Y.; Kruse, A. Pyrolysis vs. hydrothermal carbonization: Understanding the effect of biomass structural components and inorganic compounds on the char properties. J. Anal. Appl. Pyrolysis 2019, 140, 137–147. [Google Scholar] [CrossRef]
- Surampalli, R.Y.; Zhang, T.C.; Tyagi, R.D.; Naidu, R.; Gurjar, B.R.; Ojha, C.S.P.; Yan, S.; Brar, S.K.; Ramakrishnan, A.; Kao, C.M. Carbon Capture and Storage: Physical, Chemical, and Biological Methods; American Society of Civil Engineers: Reston, VA, USA, 2015; ISBN 9780784478912. [Google Scholar]
- Yoder, J.; Galinato, S.; Granatstein, D.; Garcia-Pérez, M. Economic tradeoff between biochar and bio-oil production via pyrolysis. Biomass Bioenergy 2011, 35, 1851–1862. [Google Scholar] [CrossRef]
- Biogreen. Syngas—High Energy Pyrolysis Gas for Power Applications. Available online: https://www.biogreen-energy.com (accessed on 1 August 2024).
- Mohsen, R.A.; Abbassi, B.; Dutta, A. Assessment of greenhouse gas emissions from Ontario’s solid waste landfills: Assessment of improvement scenarios. J. Environ. Eng. 2019, 145. [Google Scholar] [CrossRef]
- Singh, B.; Wang, T.; Camps-Arbestain, M. Biochar characterization for water and wastewater treatments. In Sustainable Biochar for Water and Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2022; pp. 135–152. ISBN 9780128222256. [Google Scholar]
- Fremantle, F.E.; Finnemore, H. The Abbott versimetric charts for the conversion of metric into English measure. Br. Med. J. 1908, 2, 1384–1385. [Google Scholar] [CrossRef]
- Lawrence Berkeley National Laboratory and Earth Advantage. Laboratory Fume Hood Energy Modeler. Available online: https://fumehoodcalculator.lbl.gov (accessed on 1 August 2024).
- Brassard, P.; Godbout, S.; Pelletier, F.; Raghavan, V.; Palacios, J.H. Pyrolysis of switchgrass in an auger reactor for biochar production: A greenhouse gas and energy impacts assessment. Biomass Bioenergy 2018, 116, 99–105. [Google Scholar] [CrossRef]
- Greenhouse Gas Equivalencies Calculator-Calculations and References|US EPA. Available online: https://www.epa.gov/energy/greenhouse-gas-equivalencies-calculator-calculations-and-references (accessed on 14 March 2025).
- RTI International. Greenhouse Gas Emissions Estimation Methodologies for Biogenic Emissions from Selected Source Categories: Solid Waste Disposal Wastewater Treatment Ethanol Fermentation. Available online: https://www3.epa.gov/ttnchie1/efpac/ghg/GHG_Biogenic_Report_draft_Dec1410.pdf (accessed on 1 August 2024).
- Ran, T.; Jin, L.; Abeynayake, R.; Saleem, A.M.; Zhang, X.; Niu, D.; Chen, L.; Yang, W. Effects of brewers’ spent grain protein hydrolysates on gas production, ruminal fermentation characteristics, microbial protein synthesis and microbial community in an artificial rumen fed a high grain diet. J. Anim. Sci. Biotechnol. 2021, 12, 1. [Google Scholar] [CrossRef]
- Sanciolo, P.; Rivera, E.; Navaratna, D.; Duke, M.C. Food waste diversion from landfills: A cost–benefit analysis of existing technological solutions based on greenhouse gas emissions. Sustainability 2022, 14, 6753. [Google Scholar] [CrossRef]
- Field, J.L.; Keske, C.M.H.; Birch, G.L.; DeFoort, M.W.; Cotrufo, M.F. Distributed biochar and bioenergy coproduction: A regionally specific case study of environmental benefits and economic impacts. Glob. Change Biol. Bioenergy 2013, 5, 177–191. [Google Scholar] [CrossRef]
- Jacobs, A.A. Plant Guide for Common Barley (Hordeum vulgare L.). Available online: https://plants.usda.gov/DocumentLibrary/plantguide/pdf/pg_hovu.pdf (accessed on 1 August 2024).
- Bertholdsson, N.O. Characterization of malting barley cultivars with more or less stable grain protein content under varying environmental conditions. Eur. J. Agron. 1999, 10, 1–8. [Google Scholar] [CrossRef]
- Dang, Q.; Yu, C.; Luo, Z. Environmental life cycle assessment of bio-fuel production via fast pyrolysis of corn stover and hydroprocessing. Fuel 2014, 131, 36–42. [Google Scholar] [CrossRef]
- Jackowski, M.; Niedźwiecki, Ł.; Jagiełło, K.; Uchańska, O.; Trusek, A. Brewer’s Spent Grains-Valuable Beer Industry By-Product. Biomolecules 2020, 10, 1669. [Google Scholar] [CrossRef] [PubMed]
- Chetrariu, A.; Dabija, A. Brewer’s spent grains: Possibilities of valorization, a review. Appl. Sci. 2020, 10, 5619. [Google Scholar] [CrossRef]
- da Silva Araújo, F.P.; de Souza Cupertino, G.; de Cássia Superbi de Sousa, R.; de Castro Santana, R.; Pereira, A.F. Use of biochar produced from brewer’s spent grains as an adsorbent. Biomass Convers. Biorefinery 2024, 1–16. [Google Scholar] [CrossRef]

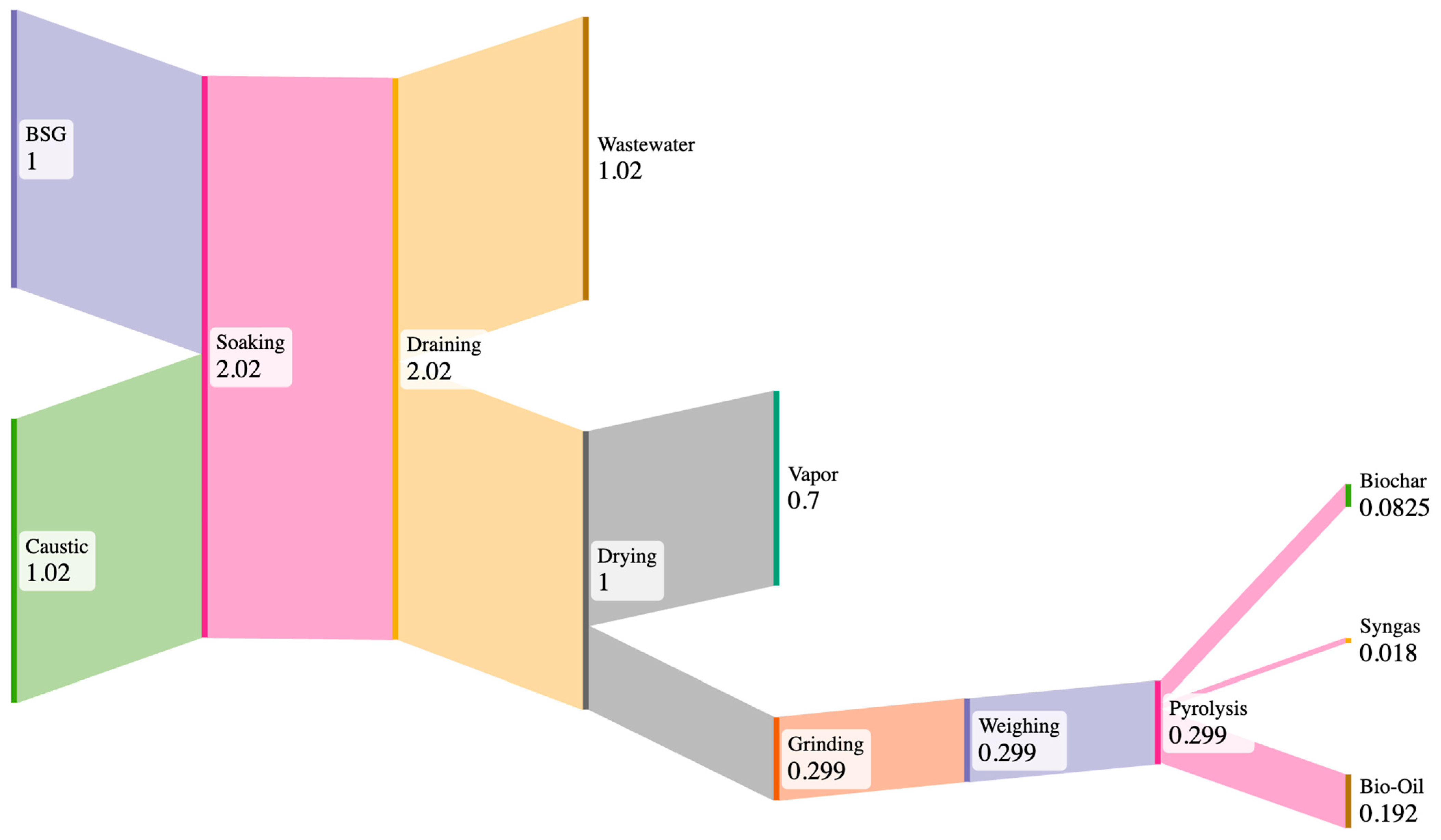

| Phase | Item | Amount per FU | Unit |
|---|---|---|---|
| Inputs | |||
| Soaking | Electricity (scale) | 0.018 | MJ |
| Electricity (ventilation) | 9.75 | MJ | |
| Drying | Electricity (oven) | 0.915 | MJ |
| Grinding | Electricity (coffee grinder) | 0.018 | MJ |
| Weighing | Electricity (scale) | 0.009 | MJ |
| Pyrolysis | Nitrogen Gas | 137 | kg |
| Electricity | 71.9 | MJ | |
| Outputs | |||
| Soaking | Wastewater (pH 5.17) | 1.02 | kg |
| Vented Fumes | 0.024 | kg | |
| Drying | Water Vapor | 0.7 | kg |
| Pyrolysis | Biochar | 0.083 | kg |
| Syngas | 0.018 | kg | |
| Bio-oil | 0.192 | kg | |
| Phase | Item | ||
|---|---|---|---|
| BSG-to-biochar Process | Input | Electricity (sum) | 9.81 |
| Nitrogen Gas | 1 × 10−4 | ||
| Output | Wastewater | 6 × 10−7 | |
| Vented Fumes | 0.074 | ||
| Biochar–Soil Application | −8.93 | ||
| Bio-Oil and Syngas Credit | −0.027 | ||
| Total | BSG-to-biochar GWP | 1.18 | |
| Landfill | Total | Landfill GWP | 3.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Newman, E.; Nitin, N.; Spang, E.; Fox, G. Carbon Footprint Assessment on the Viability of Utilizing Brewer’s Spent Grain to Produce Biochar. Appl. Sci. 2025, 15, 5525. https://doi.org/10.3390/app15105525
Newman E, Nitin N, Spang E, Fox G. Carbon Footprint Assessment on the Viability of Utilizing Brewer’s Spent Grain to Produce Biochar. Applied Sciences. 2025; 15(10):5525. https://doi.org/10.3390/app15105525
Chicago/Turabian StyleNewman, Emily, Nitin Nitin, Edward Spang, and Glen Fox. 2025. "Carbon Footprint Assessment on the Viability of Utilizing Brewer’s Spent Grain to Produce Biochar" Applied Sciences 15, no. 10: 5525. https://doi.org/10.3390/app15105525
APA StyleNewman, E., Nitin, N., Spang, E., & Fox, G. (2025). Carbon Footprint Assessment on the Viability of Utilizing Brewer’s Spent Grain to Produce Biochar. Applied Sciences, 15(10), 5525. https://doi.org/10.3390/app15105525





