Thermography as a Method to Evaluate Temperature Changes in the Acropodial Region of a Warmblood Horse Following the Application of an Ice Boot Pack: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal
2.2. Thermal Imaging and Data Recording
2.3. Statistical Analysis
3. Results
3.1. Thermography Before Applying Ice Boot Pack
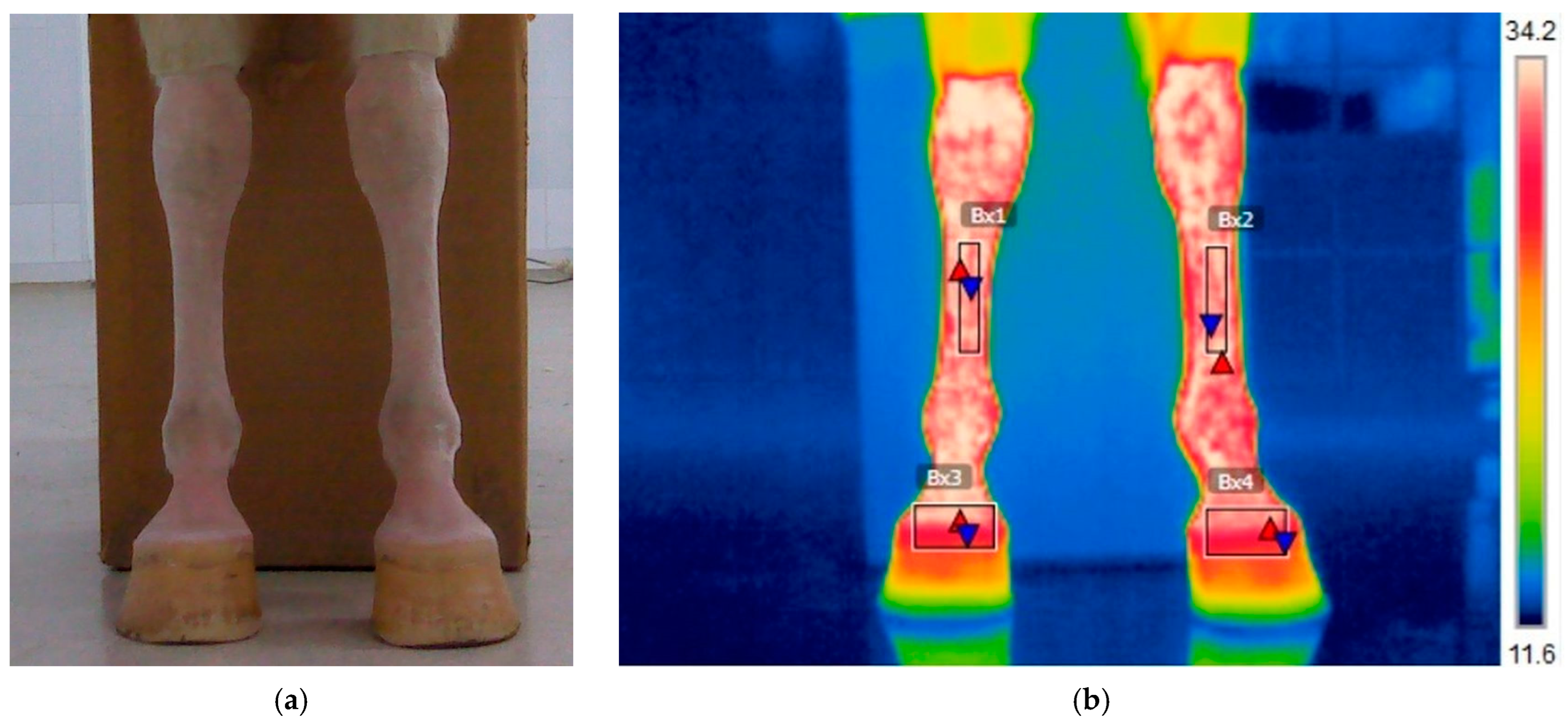
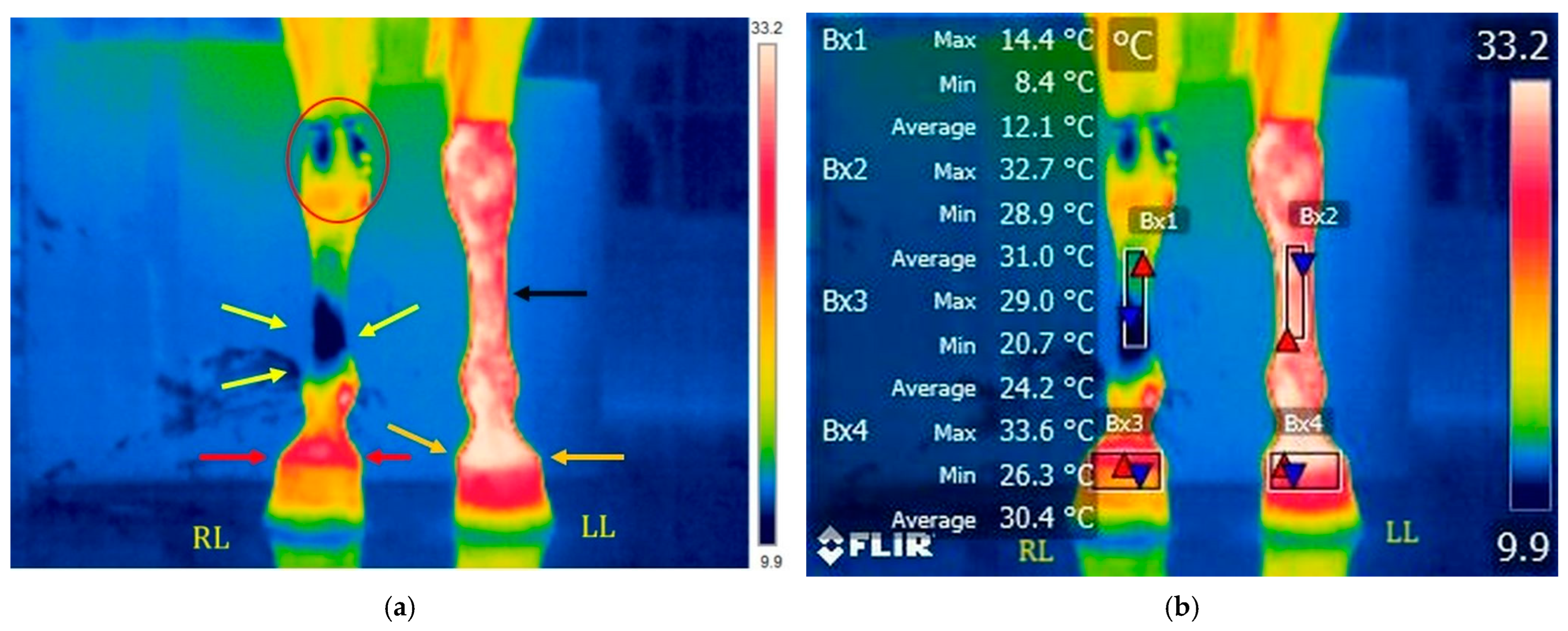
3.1.1. T1: Before Training on the Treadmill
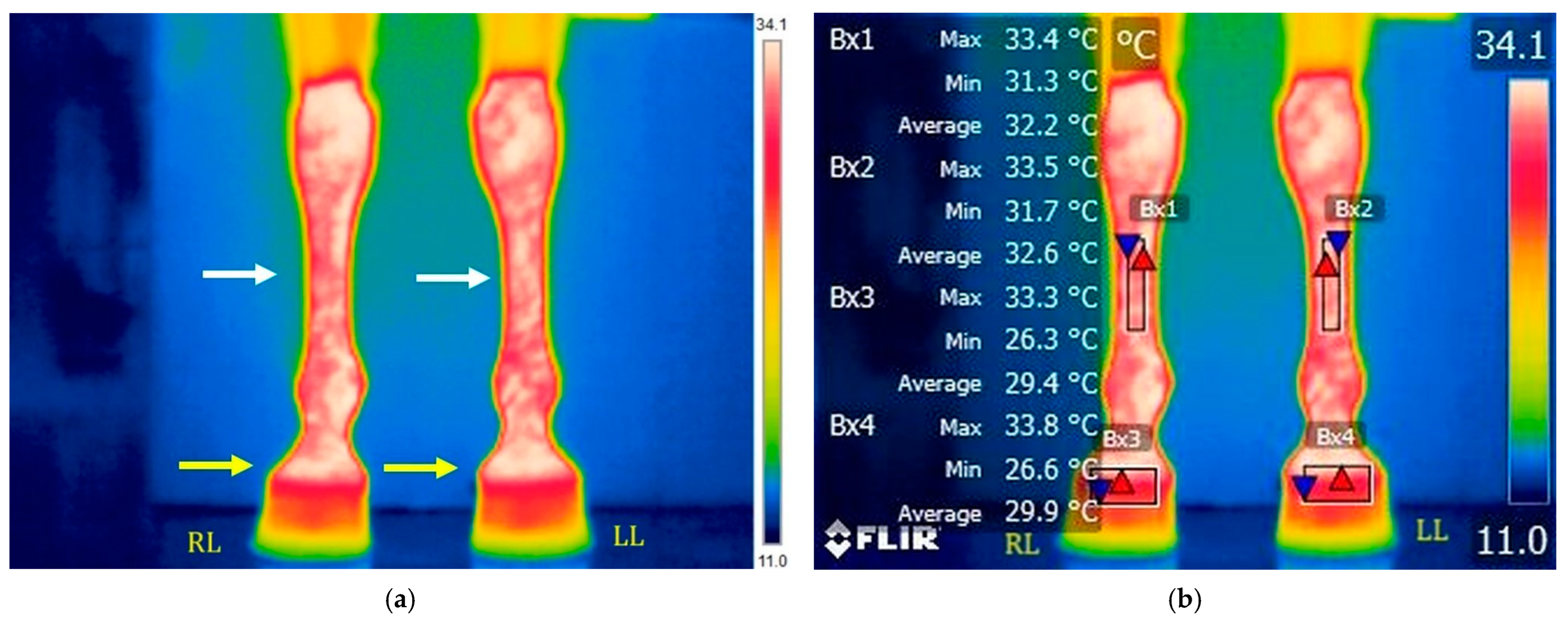
3.1.2. T2: After Training on the Treadmill
3.2. Thermography After Applying Ice Boot Pack
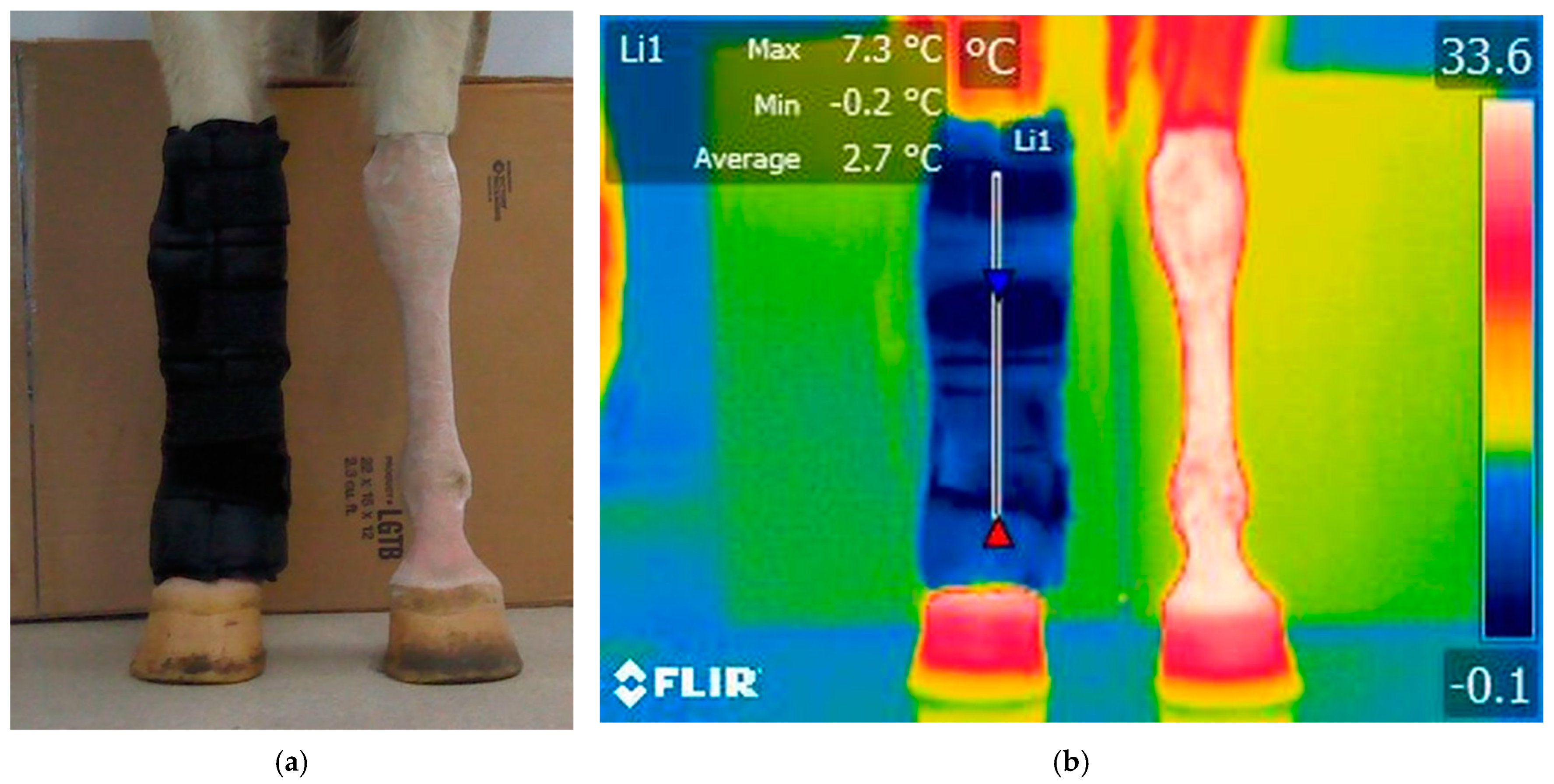
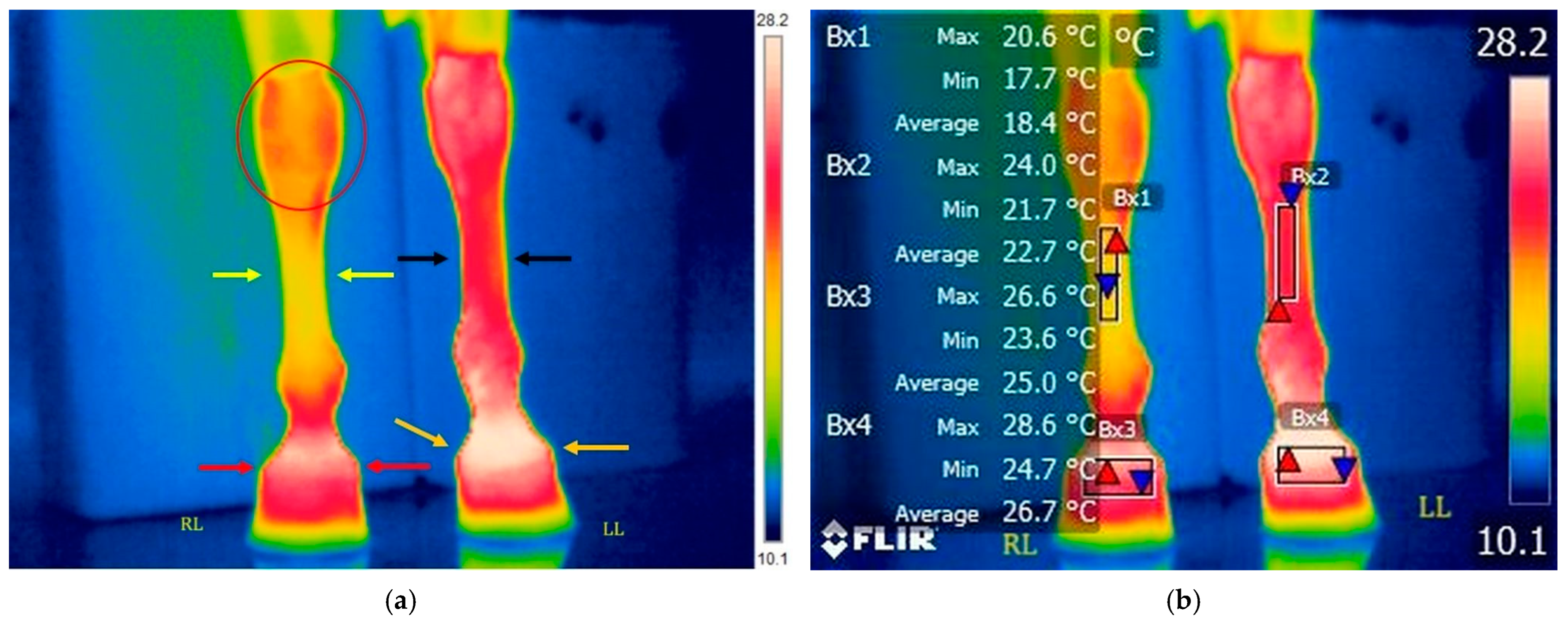
3.2.1. T3: Immediately After Ice Boot Treatment (Right Limb) and 20 Min After Treadmill Exercise (Left Limb)
3.2.2. T4: 10 Min After Ice Boot Pack Treatment (Right Limb) and 30 Min After Treadmill Exercise (Left Limb)
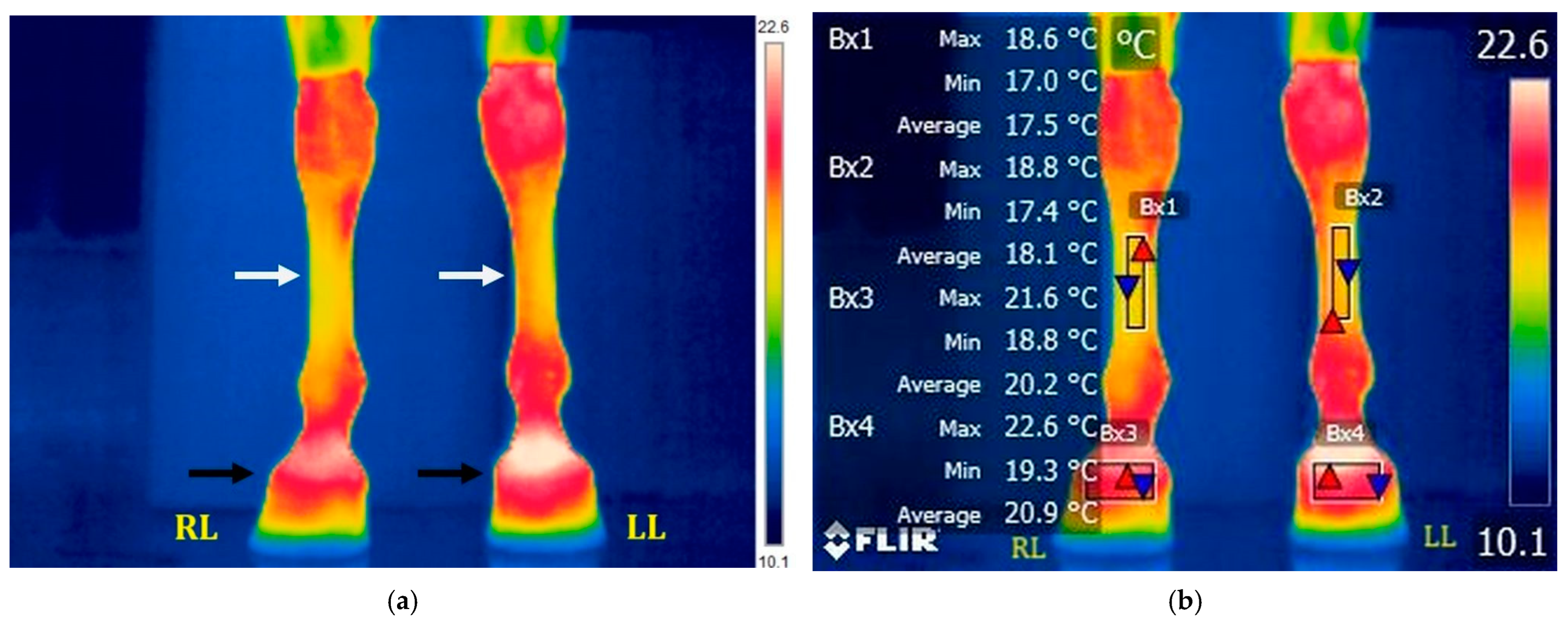
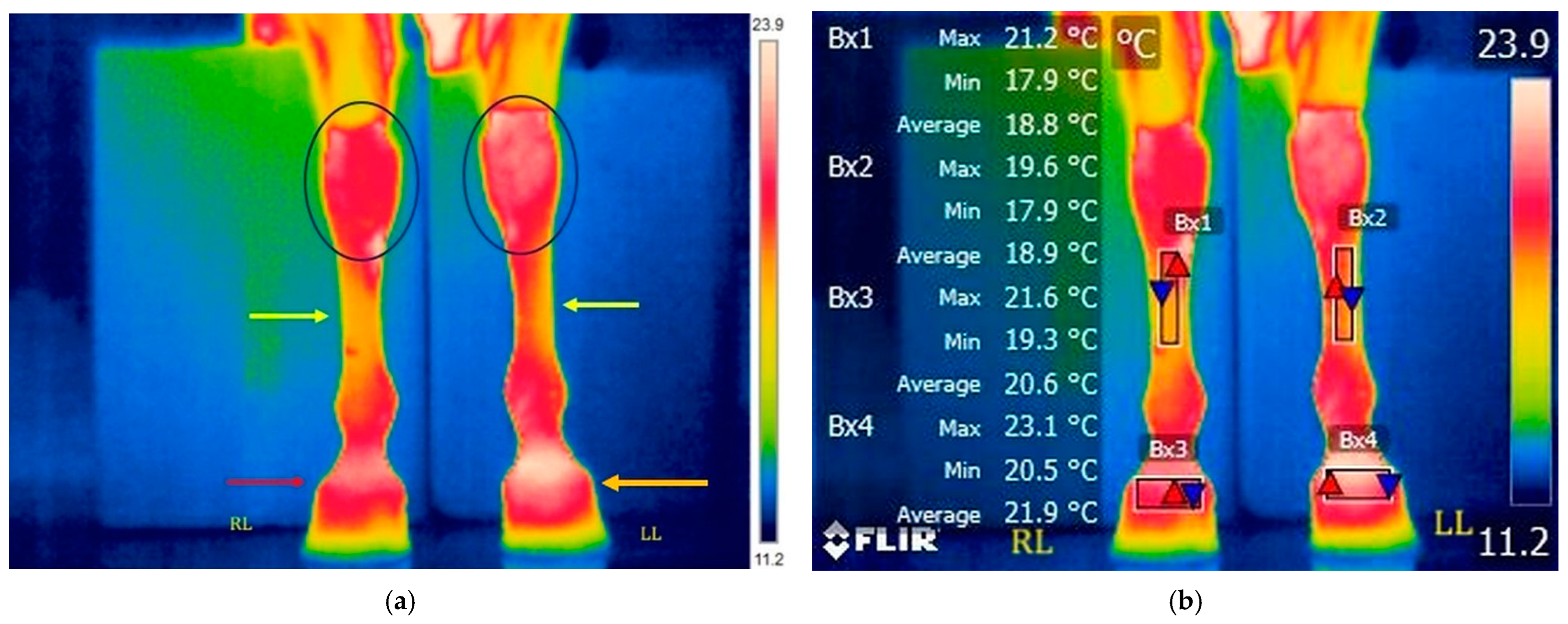
3.2.3. T5: 20 Min After Ice Boot Pack Treatment (Right Limb) and 40 Min After Treadmill Exercise (Left Limb)
3.2.4. T6: 30 Min After Ice Boot Pack Treatment (Right Limb) and 50 Min After Treadmill Exercise (Left Limb)
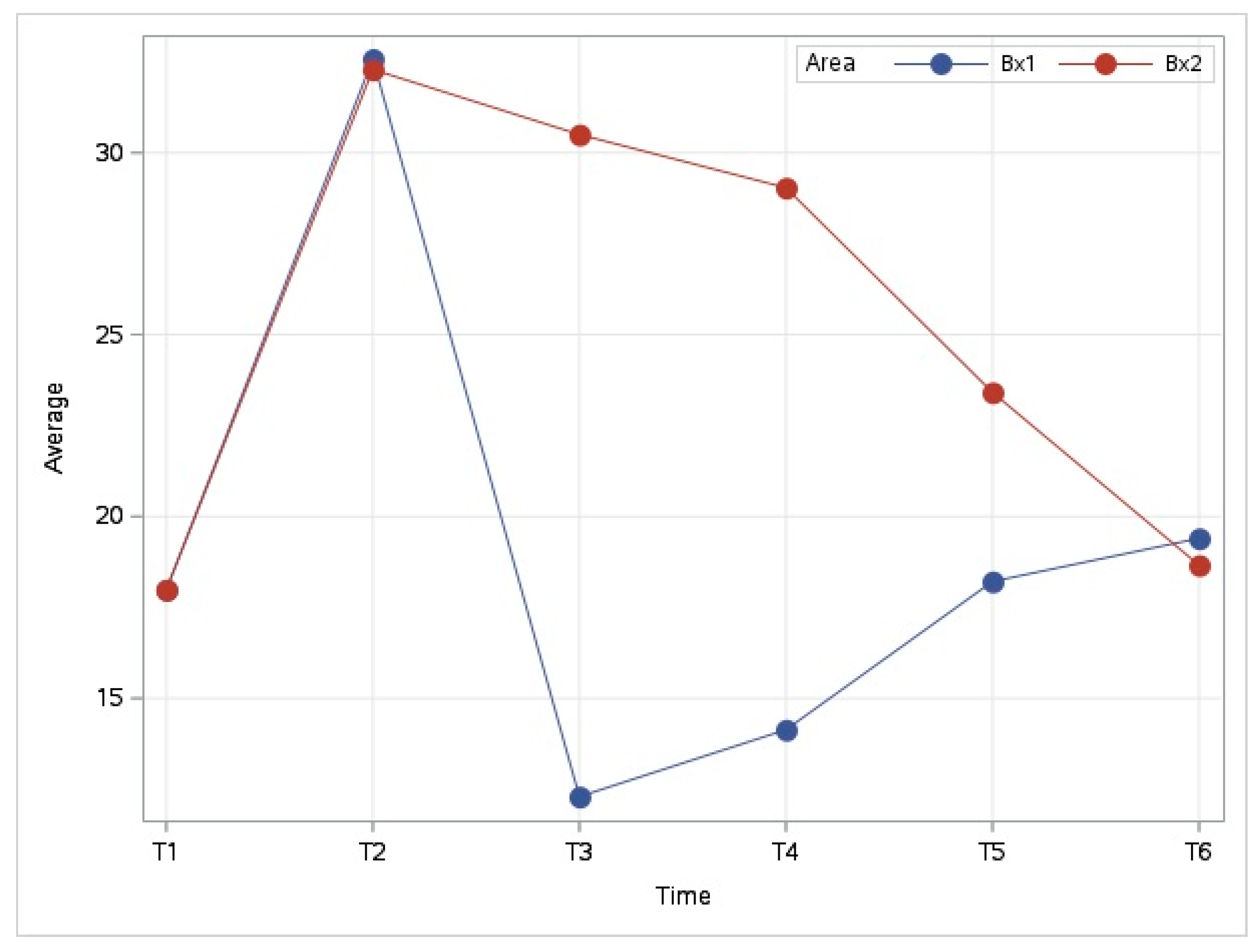
3.3. Temperature Evolution at Diferent Time Periods (T1–T6)
3.3.1. Evolution of Temperature in the Middle of the Metacarpal Area (Bx1–Bx2)
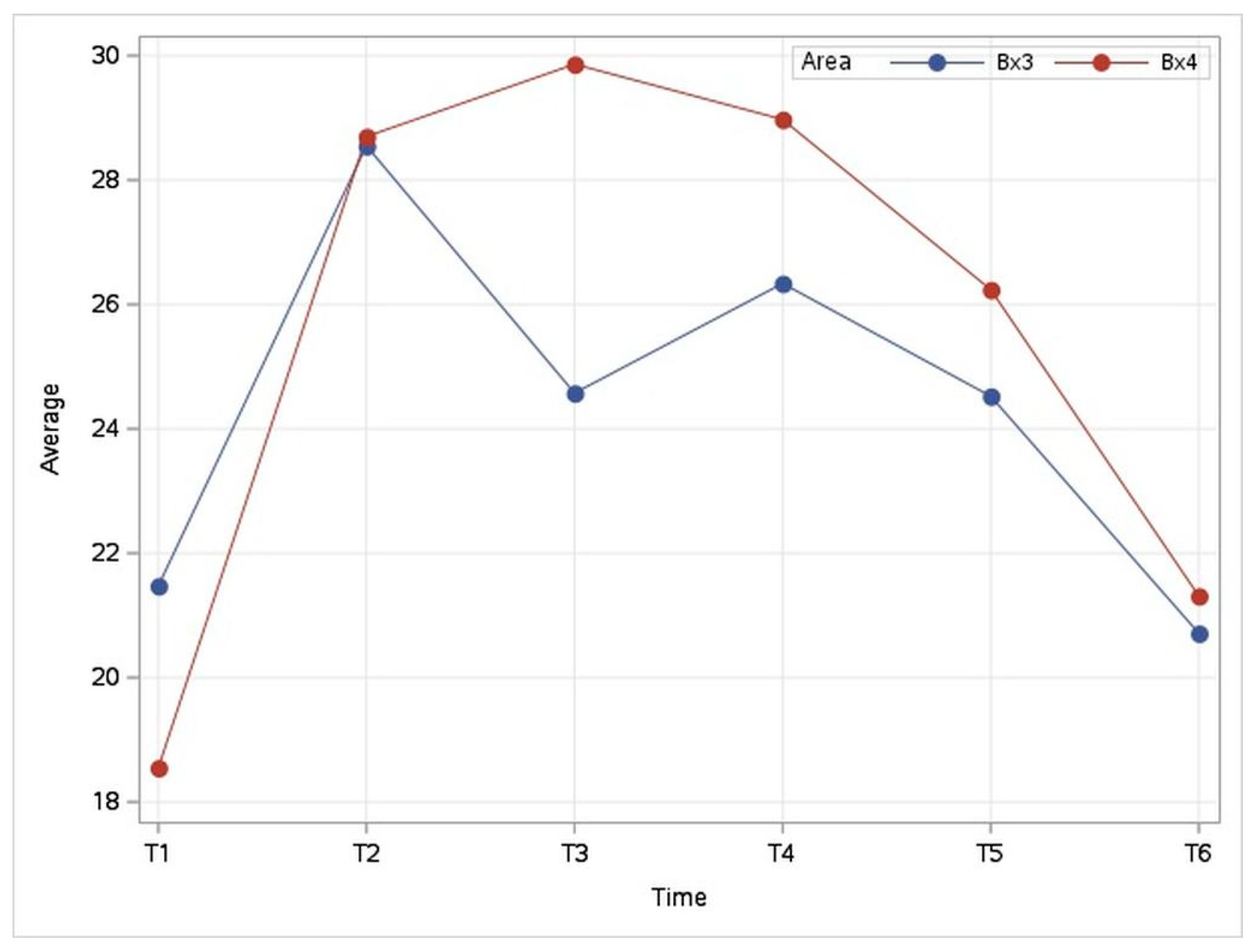
3.3.2. Evolution of Temperature in the Coronary Band Area (Bx3–Bx4)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soroko, M.; Howell, K. Infrared Thermography: Current Applications in Equine Medicine. J. Equine Vet. Sci. 2016, 60, 90–96.e2. [Google Scholar] [CrossRef]
- Casas-Alvarado, A.; Martinez-Burnez, J.; Mora-Medina, P.; Hernandez-Avalos, I.; Dominguez-Olivia, A.; Lezama-Garcia, K.; Gomez-Prado, J.; Mota-Rojas, D. Thermal and Circulatory Changes in Diverse Body Regions in Dogs and Cats Evaluated by Infrared Thermography. Animals 2022, 12, 789. [Google Scholar] [CrossRef]
- Soroko-Dubrovina, M.; Śniegucka, M.; Dobrowolski1, M.; Dudek, K. Application of thermography in the assessment of physical effort on body surface temperature distribution in racehorses. Pol. J. Vet. Sci. 2024, 27, 221–227. [Google Scholar] [CrossRef]
- Zaha, C.; Schuszler, L.; Dascalu, R.; Nistor, P.; Florea, T.; Rujescu, C.; Sicoe, B.; Igna, C. Thermographic Image of the Hoof Print in Leisure and Cross-Country Warmblood Horses: A Pilot Study. Vet. Sci. 2023, 10, 470. [Google Scholar] [CrossRef]
- Repac, J.; Alvarez, L.X.; Lamb, K.; Gillette, R.L. Evaluation of Thermographic Imaging in Canine Hindlimb Muscles After 6 min of Walking—A Pilot Study. Front. Vet. Sci. 2020, 7, 224. [Google Scholar] [CrossRef]
- Casas-Alvarado, A.; Mota-Rojas, D.; Hernandez-Avaloz, I.; Mora-Medina, P.; Olmos-Hernandez, A.; Verduzco-Mendoza, A.; Reyes-Sotelo, B.; Martinez-Brunes, J. Advances in infrared thermography: Surgical aspects, vascular changes, and pain monitoring in veterinary medicine. J. Term. Biol. 2020, 92, 102664. [Google Scholar] [CrossRef]
- Soroko, M.; Howell, K.; Dudek, K.; Henklewski, R.; Ska, P.Z.N. The influence of breed, age, gender, training level and ambient temperature on forelimb and back temperature in racehorses. Anim. Sci. J. 2015, 88, 347–355. [Google Scholar] [CrossRef]
- Zaha, C.; Schuszler, L.; Dascalu, R.; Nistor, P.; Florea, T.; Imre, K.; Rujescu, C.; Sicoe, B.; Igna, C. Evaluation of thermal changes of the sole surface in horses with Palmar Foot Pain: A pilot Study. Biology 2023, 12, 423. [Google Scholar] [CrossRef]
- Masko, M.; Domino, M.; Lewczuk, D.; Jasinski, T. Horse Behavior, Physiology and Emotions during. Animals 2020, 10, 921. [Google Scholar] [CrossRef]
- Kaneps, A.J. Tissue Temperature Response to Hot and Cold Therapy in the Metacarpal Region of a Horse. Proc. Annu. Conv. AAEP Am. Assoc. Equine Pract. 2000, 46, 208–213. [Google Scholar]
- Guitart, A.S. The Use of Percutaneous Thermal Sensing Microchips for Body Temperature Measurements in Horses Prior to, during and after Treadmill Exercise. Animals 2020, 10, 2274. [Google Scholar] [CrossRef] [PubMed]
- Van Eps, A.W.; Pollitt, C.C. Equine laminitis: Cryotherapy reduces the severity of the acute lesion. Equine Vet. J. 2004, 36, 255–260. [Google Scholar] [CrossRef]
- Kang, H.; Zsoldos, R.R.; Skinner, J.E.; Gaughan, J.B.; Mellor, V.A.; Sole-Guitart, A. The Use of Percutaneous Thermal Sensing Microchips to Measure Body Temperature in Horses during and after Exercise Using Three Different Cool-Down Methods. Animals 2022, 12, 1267. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.D. Endotoxemia in Horses A Review of Cellular and Humoral Mediators Involved in its Pathogenesis. J. Vet. Intern. Med. 1999, 5, 167–181. [Google Scholar] [CrossRef]
- Parsons, C.S.; Orsini, J.A.; Krafty, R.; Capewell, L.; Boston, L. Risk factors for development of acute laminitis in horses during hospitalization: 73 cases (1997–2004). J. Am. Vet. Med. Assoc. 2007, 230, 885–889. [Google Scholar] [CrossRef]
- Gomez Alvares, C.B.; Rhodin, M.; Bystrom, A.; Back, W.; Rene van Weeren, P. Back kinematics of healthy trotting horses during treadmill versus over ground locomotion. Equine Vet. J. 2009, 41, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Nankervis, K.J.; Launder, E.J.; Murray, R.C. The use of Treadmills within the Rehabilitation of Horses. J. Equine Vet. Sci. 2017, 53, 108–115. [Google Scholar] [CrossRef]
- King, C.M.; Evans, D.L.; Roset, R.J. Acclimation to treadmill exercise. Equine Vet. J. 2006, 18, 453–456. [Google Scholar] [CrossRef]
- Schamhardt, H.C.; Merkens, H.W. Kinematics of treadmill versus overground locomotion in horses. Equine Vet. J. 2016, 27, 453–456. [Google Scholar] [CrossRef]
- Fredricson, I.; Drevemo, S.; Dalin, G.; Hjerten, G.; Bjorne, K.; Rynge, R.; Franzen, G. Treadmill for equine locomotion analysis. Equine Vet. J. 1983, 15, 111–115. [Google Scholar] [CrossRef]
- Buchner, H.H.F.; Savelberg, H.H.C.M.; Schamhardt, H.C.; Merkens, H.W.; Barneveld, A. Habituation of horses to treadmill locomotion. Equine Vet. J. 1994, 26, 13–15. [Google Scholar] [CrossRef]
- Verdegaal, E.J.M.M.; Howarth, G.S.; Mcwhorter, T.J.; Delesalle, C.J.G. Thermoregulation during Field Exercise in Horses Using Skin Temperature Monitoring. Animals 2023, 14, 136. [Google Scholar] [CrossRef]
- Quintanar, M.N.; Pu, T.; Burd, M.A. Thermodynamic effects of commercially available ice boots. Open Vet. J. 2018, 8, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Van Eps, A.W.; Walters, L.J.; Baldwin, G.I.; McGarry, M.; Pollit, C.C. Distal Limb Cryotherapy for the Prevention of Acute Laminitis. Clin. Tech. Equine Pract. 2004, 3, 64–70. [Google Scholar] [CrossRef]
- van Eps, A.W.; Pollitt, C.C. Equine laminitis model: Cryotherapy reduces the severity of lesions evaluated seven days after induction with oligofructose. Equine Vet. J. 2009, 41, 741–746. [Google Scholar] [CrossRef]
- Van Eps, A.W. Therapeutic Hypothermia to Prevent and Treat Acute Laminitis. Vet. Clin. North Am. Equine Pract. 2010, 26, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Ziemann, E.A.; Olek, R.A.; Kujach, S.; Grzywacz, T.; Garsztka, T. Five-Day Whole-Body Cryostimulation, Blood Inflammatory Markers, and Performance in High- Ranking Professional Tennis Players. J. Athl. Train. 2012, 47, 664–672. [Google Scholar] [CrossRef]
- Kullmann, A.; Holcombe, S.J.; Hurcombe, S.D.; Roessner, H.A.; Hauptman, J.C.; Geor, R.J. Prophylactic digital cryotherapy is associated with decreased incidence of laminitis in horses diagnosed with colitis. Equine Vet. J. 2014, 46, 554–559. [Google Scholar] [CrossRef]
- Morgan, J.; Stefanovski, D.; Lenfest, M.; Chatterjee, S. Novel dry cryotherapy system for cooling the equine digit. Vet. Rec. Open 2018, 5, e000244. [Google Scholar] [CrossRef]
- Proctor-Brown, L.; Hicks, R.; Colmer, S.; Guilfoyle, D.; Dallap-Schaer, B.; Johnson, A.J.; Tomlinson, J. Research in Veterinary Science Distal limb pathologic conditions in horses treated with sleeve-style digital cryotherapy (285 cases). Res. Vet. Sci. 2018, 121, 12–17. [Google Scholar] [CrossRef]
- Taylor, P.; Worster, A.A.; Gaughan, E.M.; Hoskinson, J.J.; Sargeant, J.; Erb, J.H. Effects of external thermal manipulation on laminar temperature and perfusion scintigraphy of the equine digit Effects of external thermal manipulation on laminar temperature and perfusion scintigraphy of the equine digit. N. Z. Vet. J. 2014, 48, 37–41. [Google Scholar] [CrossRef]
- Roszkowska, K.; Witkowska-Pilaszewicz, O.; Przewozny, M.; Cywinska, A. Whole body and partial body cryotherapies—Lessons from human practice and possible application for horses. BMC Vet. Res. 2018, 14, 394. [Google Scholar] [CrossRef]
- Algafly, A.A.; George, K.P. The effect of cryotherapy on nerve conduction velocity, pain threshold and pain tolerance. Br. J. Sports Med. 2007, 41, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Wiecek, M.; Szymura, J.; Sproull, J.; Szygula, Z. Whole-Body Cryotherapy Is an Effective Method of Reducing Abdominal Obesity in Menopausal Women with Metabolic Syndrome. J. Clin. Med. 2020, 9, 2797. [Google Scholar] [CrossRef] [PubMed]
- Mawhinney, C.; Low, D.A.; Jones, H.; Green, D.J.; Costello, J.T.; Gregson, W. Cold Water Mediates Greater Reductions in Limb Blood Flow than Whole Body Cryotherapy. Med. Sci. Sports Exerc. 2017, 49, 1252–1260. [Google Scholar] [CrossRef]
- Takahashi, T.; Matsui, A.; Mukai, K.; Ohmura, H.; Hiraga, A.; Aida, H. The Effects of Inclination (Up and Down) of the Treadmill on the Electromyogram Activities of the Forelimb and Hind limb Muscles at a Walk and a Trot in Thoroughbred Horses. JEVS 2014, 25, 73–77. [Google Scholar] [CrossRef]
- Weishaupt, M.A.; Wiestner, T.; Hogg, H.P.; Jordan, P.; Auer, J.A. Compensatory load redistribution of horses with induced weightbearing hindlimb lameness trotting on a treadmill. Equine Vet. J. 2004, 36, 727–733. [Google Scholar] [CrossRef]
- Simon, E.L.; Gaughan, E.M.; Epp, T.; Spire, M. Influence of exercise on thermographically determined surface temperatures of thoracic and pelvic limbs in horses. J. Am. Vet. Assoc. 2006, 229, 1940. [Google Scholar] [CrossRef]
- Soroko, M.; Górniak, W.; Godlewska, M.; Howell, K. The effect of training on infrared thermographic images of the forelimb and hindlimb joints of healthy racehorses. Pol. J. Vet. Sci. 2022, 25, 83–92. [Google Scholar] [CrossRef]
- Yarnell, K.; Fleming, J.; Stratton, T.D.; Brassington, R. Monitoring changes in skin temperature associated with exercise in horses on a water treadmill by use of infrared thermography. J. Therm. Biol. 2014, 45, 110–116. [Google Scholar] [CrossRef]
- Burke, M.J.; Tomlinson, J.E.; Blikslager, A.T.; Johnson, A.L.; Dallap-Schaer, B.L. Evaluation of digital cryotherapy using a commercially available sleeve style ice boot in healthy horses and horses receiving i.v. endotoxin. Equine Vet. J. 2018, 50, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Redaelli, V.; Bergero, D.; Zucca, E.; Ferrucci, F.; Costa, L.N.; Crosta, L.; Das, F.L. Journal of Equine Veterinary Science Use of Thermography Techniques in Equines: Principles and Applications. J. Equine Vet. Sci. 2014, 34, 345–350. [Google Scholar] [CrossRef]
- Hodgson, D.R.; Davis, R.E.; McConaghy, F.F. Thermoregulation in the horse in response to exercise. Br. Vet. J. 1994, 150, 219–235. [Google Scholar] [CrossRef]
- McKeever, K.H.; Eaton, T.L.; Kearns, C.F.; Lehnhard, R.A. Age related decreased in thermoregulation and cardiovascular function in horses. Equine Vet. J. 2010, 42, 220–227. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Titto, C.G.; Orihuela, A.; Martínez-Burnes, J.; Gómez-Prado, J.; Torres-Bernal, F.; Flores-Padilla, K.; Carvajal-de la Fuente, V.; Wang, D. Physiological and Behavioral Mechanisms of Thermoregulation in Mammals. Animals 2021, 11, 1733. [Google Scholar] [CrossRef] [PubMed]
- Periard, J.D.; Eijsvogels, T.M.H.; Daanen, H.A.M. Exercises under heat stress: Thermoregulation, hydration, performance implications and mitigation strategies. Physiol. Rev. 2021, 101, 1873–1979. [Google Scholar] [CrossRef]
- Marlin, D.J.; Scott, C.M.; Schroter, R.C.; Harris, R.C.; Harris, P.A.; Roberts, C.A.; Millis, P.A. Physiological responses of horses to a treadmill simulated speed and endurance test in high heat and humidity before and after humid heat acclimation. Equine Vet. J. 1999, 31, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Crook, T.K.; Wilson, A. The effect of treadmill speed and gradient on equine hindlimb muscle activity. Equine Vet. J. 2010, 42, 412–416. [Google Scholar] [CrossRef]
- Pollitt, C.C.; Van Eps, A.W. Prolonged, continuous distal limb cryotherapy in the horse. Equine Vet. J. 2004, 36, 216–220. [Google Scholar] [CrossRef]
- Turner, T.A. Thermography as an Aid to the Clinical Lameness Evaluation. Vet. Clin. North Am. Equine Pract. 1991, 7, 311–338. [Google Scholar] [CrossRef]
- Mccarthy, R.N.; Jeffcott, L.B.; Clinical, E. Effects of treadmill exercise on cortical bone in the third metacarpus of young horses. Res. Vet. Sci. 1992, 52, 28–37. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaha, C.; Schuszler, L.; Ciresan, A.; Căsălean, T.; Spataru, I.; Torda, I.; Cocioba, V.; Hutu, I.; Degi, J.; Rujescu, C.; et al. Thermography as a Method to Evaluate Temperature Changes in the Acropodial Region of a Warmblood Horse Following the Application of an Ice Boot Pack: A Pilot Study. Appl. Sci. 2025, 15, 5524. https://doi.org/10.3390/app15105524
Zaha C, Schuszler L, Ciresan A, Căsălean T, Spataru I, Torda I, Cocioba V, Hutu I, Degi J, Rujescu C, et al. Thermography as a Method to Evaluate Temperature Changes in the Acropodial Region of a Warmblood Horse Following the Application of an Ice Boot Pack: A Pilot Study. Applied Sciences. 2025; 15(10):5524. https://doi.org/10.3390/app15105524
Chicago/Turabian StyleZaha, Cristian, Larisa Schuszler, Alexandru Ciresan, Tudor Căsălean, Irina Spataru, Iuliu Torda, Vlad Cocioba, Ioan Hutu, Janos Degi, Ciprian Rujescu, and et al. 2025. "Thermography as a Method to Evaluate Temperature Changes in the Acropodial Region of a Warmblood Horse Following the Application of an Ice Boot Pack: A Pilot Study" Applied Sciences 15, no. 10: 5524. https://doi.org/10.3390/app15105524
APA StyleZaha, C., Schuszler, L., Ciresan, A., Căsălean, T., Spataru, I., Torda, I., Cocioba, V., Hutu, I., Degi, J., Rujescu, C., & Dascălu, R. (2025). Thermography as a Method to Evaluate Temperature Changes in the Acropodial Region of a Warmblood Horse Following the Application of an Ice Boot Pack: A Pilot Study. Applied Sciences, 15(10), 5524. https://doi.org/10.3390/app15105524








