Effects of Varying Flooding Durations on the Soil Reinforcement Capacity of Dominant Riparian Plants in the Yangtze River Basin
Abstract
1. Introduction
2. Materials and Methods
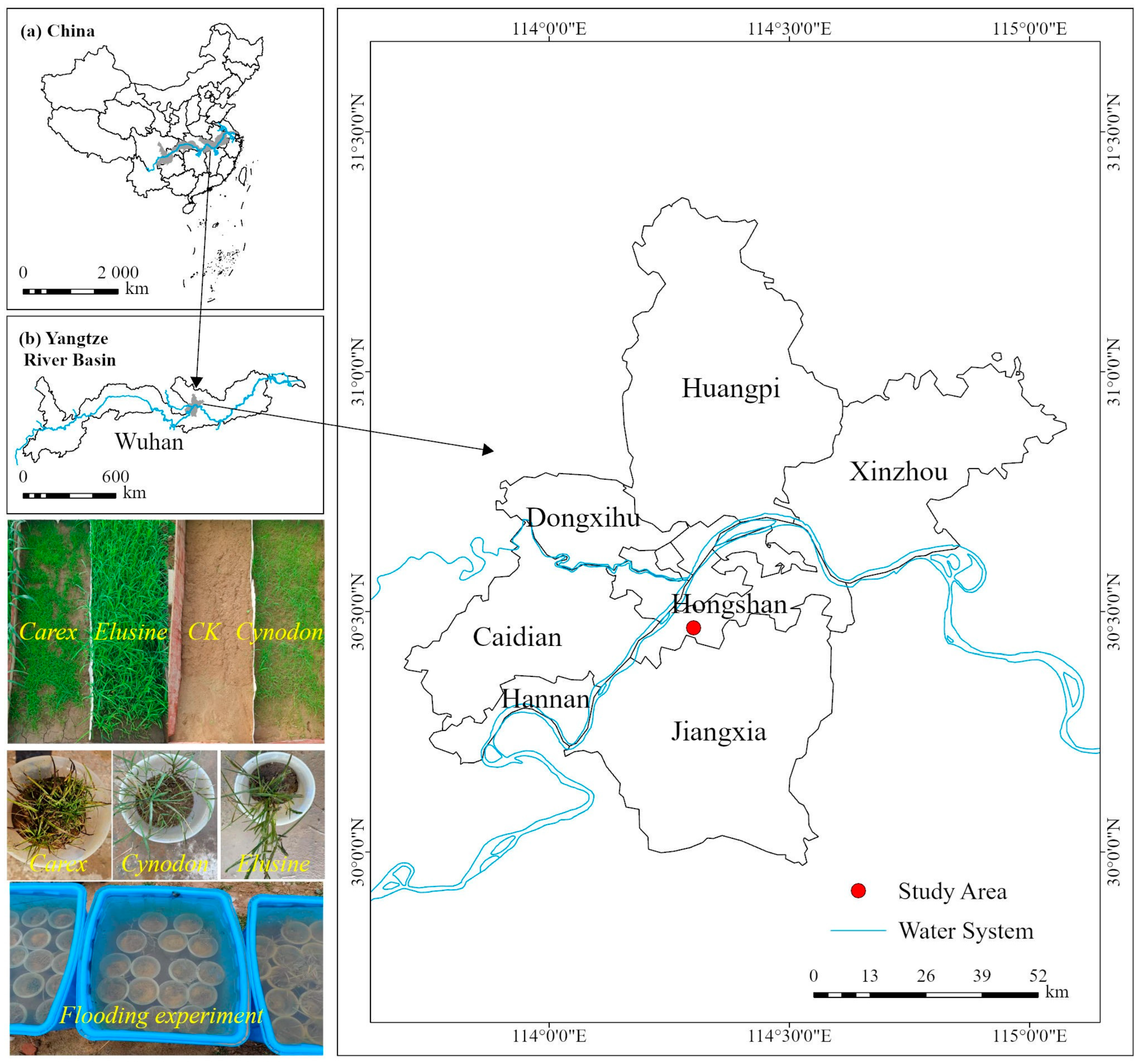
2.1. Study Area
2.2. Plant Materials
2.3. Experimental Method
2.4. Single-Root Stretching Test for Plants
2.5. Root–Soil Strength
2.6. Statistical Analysis
3. Results
3.1. Response of Plant Root Growth to Varying Flooding Durations
3.1.1. Root Morphology
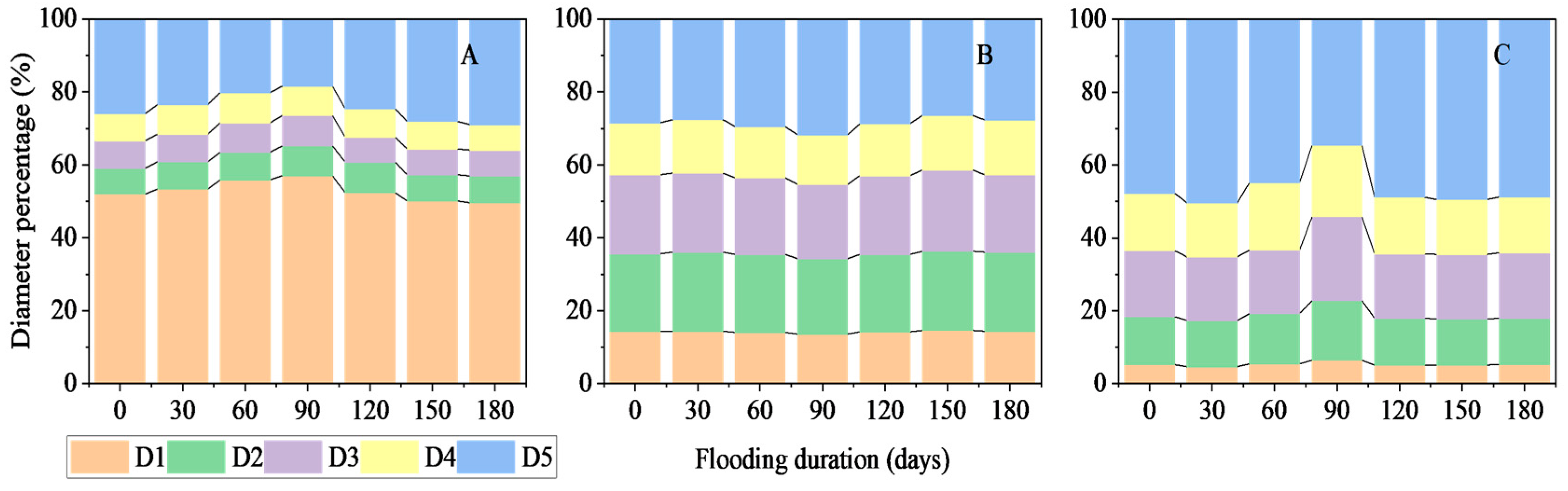
3.1.2. Root Diameter Percentages
3.2. Response of Various Plant Root Mechanical Properties to Different Flooding Durations
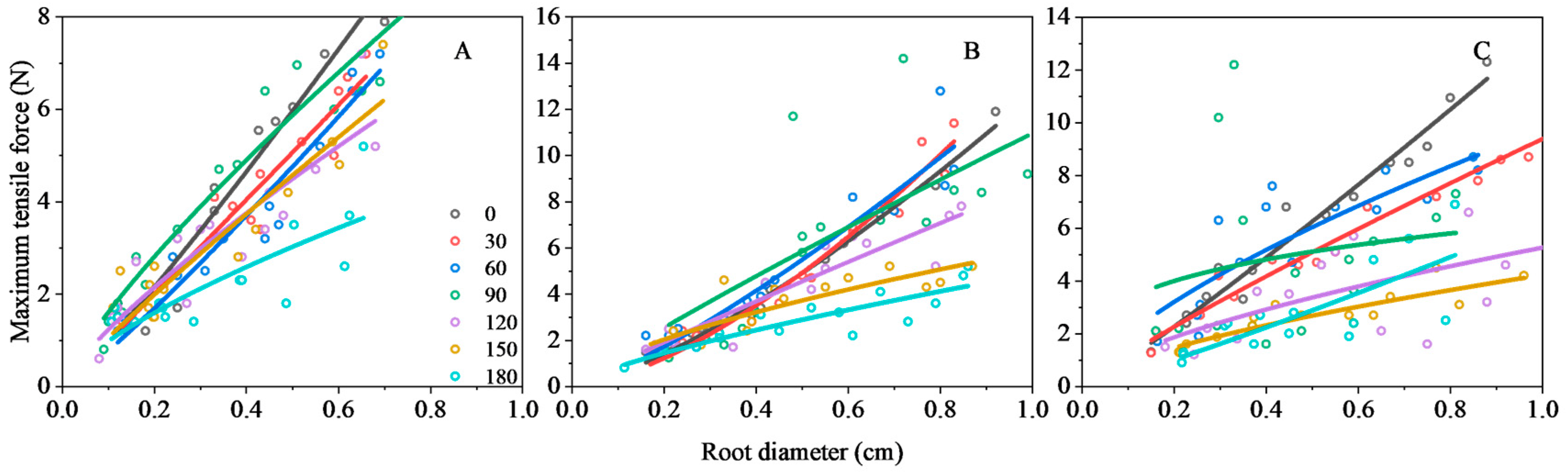
3.2.1. Maximum Tensile Force of the Root
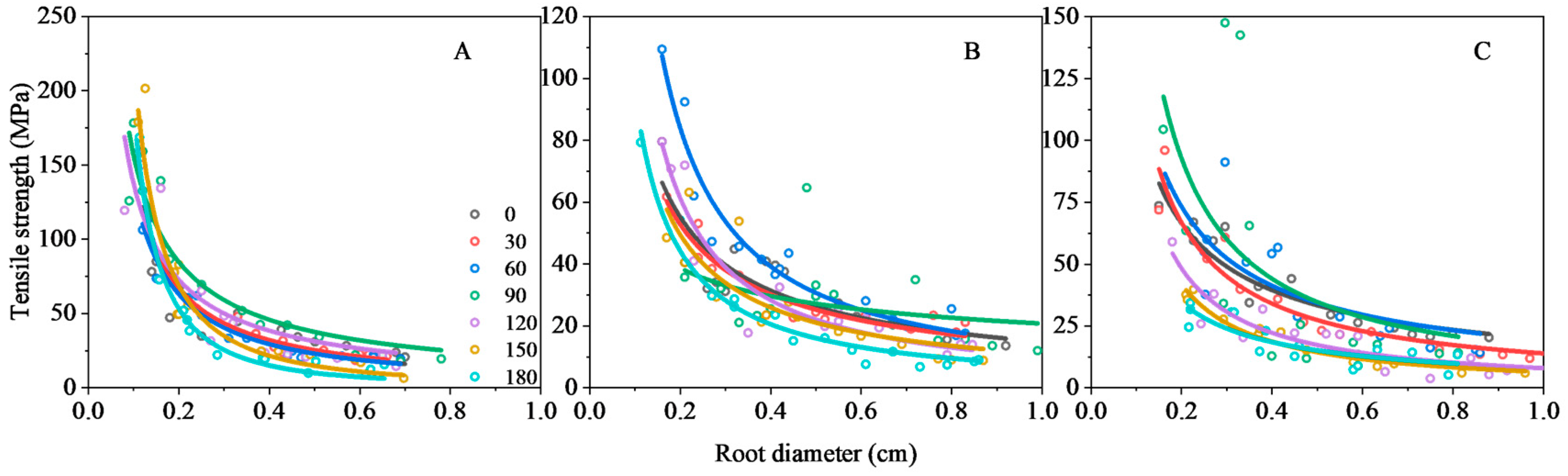
3.2.2. Tensile Strength of the Root
3.2.3. Elastic Modulus of the Root
3.3. Root–Soil Strength Test
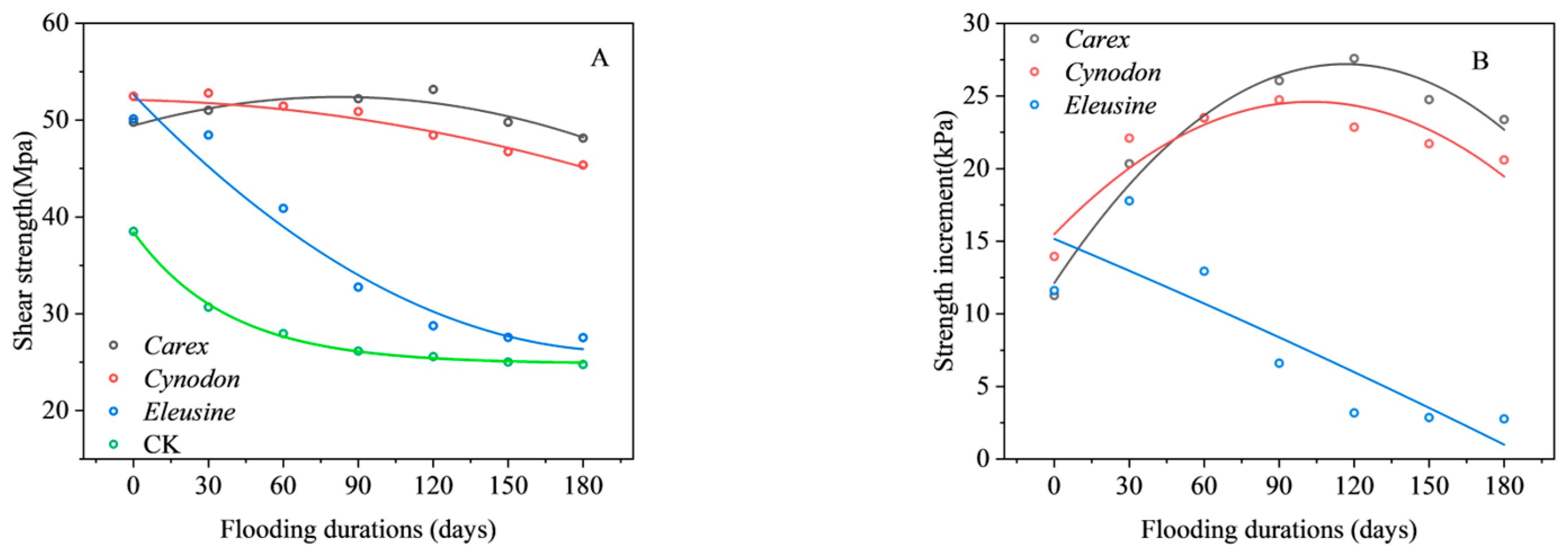
3.3.1. Shear Strength and Increment
3.3.2. Friction Angle and Cohesion
4. Discussion
4.1. Varying Flooding Durations Affect Root Morphology
4.2. Root Diameter and Varying Flooding Durations Affect Root Strength
4.3. Root Strength Affects Root–Soil Strength
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wei, S.; Zhang, K.; Liu, C.; Cen, Y.; Xia, J. Effects of different vegetation components on soil erosion and response to rainfall intensity under simulated rainfall. Catena 2024, 235, 107652. [Google Scholar] [CrossRef]
- Weifeng, S.; Lihua, C.; Xiuping, L. Review of theories of soil reinforcement by root system in forest. Bull. Soil Water Conserv. 2008, 28, 180–186. [Google Scholar] [CrossRef]
- Garssen, A.G.; Baattrup-Pedersen, A.; Voesenek, L.A.; Verhoeven, J.T.; Soons, M.B. Riparian plant community responses to increased flooding: A meta-analysis. Glob. Chang. Biol. 2015, 21, 2881–2890. [Google Scholar] [CrossRef] [PubMed]
- Colmer, T.D.; Voesenek, L. Flooding tolerance: Suites of plant traits in variable environments. Funct. Plant Biol. 2009, 36, 665–681. [Google Scholar] [CrossRef] [PubMed]
- Deng, T.; Du, Q.; Zhu, Y.; Queenborough, S.A. Environmental drivers of herbaceous plant diversity in the understory community of a warm-temperate forest. Plant Divers. 2025, 47, 282–290. [Google Scholar] [CrossRef]
- Löbmann, M.T.; Geitner, C.; Wellstein, C.; Zerbe, S. The influence of herbaceous vegetation on slope stability–a review. Earth Sci. Rev. 2020, 209, 103328. [Google Scholar] [CrossRef]
- Zhang, Z.; Wan, C.; Zheng, Z.; Hu, L.; Feng, K.; Chang, J. Plant community characteristics and their responses to environmental factors in the water level fluctuation zone of the three gorges reservoir in China. Environ. Sci. Pollut. Res. 2013, 20, 7080–7091. [Google Scholar] [CrossRef]
- Li, X.; He, D.; Chen, G.; Yang, J.; Yang, Z.; Guo, X.J.; Wang, C.; Zhu, S.; Huang, Y.; Chen, H.; et al. Responses of leaf functional traits to different hydrological regimes and leaf economics spectrum in the dominant riparian of Three Gorges Reservoir, China. Front. Plant Sci. 2022, 13, 939452. [Google Scholar] [CrossRef]
- Deng, H.; Zhang, X.; Zhang, C.; Wang, Y.; Wang, D.-Y. Release Characteristics of Mercury from Submersed Typical Herbaceous Plants in the Water-Level Fluctuation Zone of the Three Gorges Reservoir Area. Environ. Sci. 2017, 38, 987–992. (In Chinese) [Google Scholar] [CrossRef]
- Xian, X.D.; Feng, Y.L.; Willison, J.M.; Ai, L.J.; Wang, P.; Wu, Z.N. Restoring ecosystem services to littoral zones of rivers in the urban core of Chongqing, China. Environ. Sci. Pollut. Res. 2015, 22, 12576–12584. [Google Scholar] [CrossRef]
- Wantzen, K.M.; Rothhaupt, K.O.; Mörtl, M.; Cantonati, M.; Tóth, L.G.; Fischer, P. Ecological effects of water-level fluctuations in lakes: An urgent issue. Hydrobiologia 2008, 613, 1–4. [Google Scholar] [CrossRef]
- Yuan, X.Z.; Zhang, Y.W.; Liu, H.; Xiong, S.; Li, B.; Deng, W. The littoral zone in the Three Gorges Reservoir, China: Challenges and opportunities. Environ. Sci. Pollut. Res. 2013, 20, 7092–7102. [Google Scholar] [CrossRef]
- Fan, D.Y.; Xiong, G.M.; Zhang, A.Y.; Liu, X. Effect of water-lever regulation on species selection for ecological restoration practice in the water-level fluctuation zone of Three Gorges Reservoir. Chin. Plant Ecol. 2015, 39, 416–432. [Google Scholar] [CrossRef]
- Gonzalez-Ollauri, A.; Mickovski, S.B. Plant-soil reinforcement response under different soil hydrological regimes. Geoderma 2017, 285, 141–150. [Google Scholar] [CrossRef]
- Kim, J.H.; Fourcaud, T.; Jourdan, C.; Maeght, J.L.; Mao, Z.; Metayer, J.; Meylan, L.; Pierret, A.; Rapidel, B.; Roupsard, O.; et al. Vegetation as a driver of temporal variations in slope stability: The impact of hydrological processes. Geophys. Res. Lett. 2017, 44, 4897–4907. [Google Scholar] [CrossRef]
- Zhou, C.; Lu, Y.Z.; Huang, Y.H. Oedometer expansion and direct shear tests on vetiver root-reinforced expansive soil with different water contents. Chin. Geotech. Eng. 2016, 38, 30–35. [Google Scholar] [CrossRef]
- Zhao, D.H.; Ji, X.D.; Zhang, X. Friction performance of root-soil interface of Betula platyphylla in Northwestern Hebei Province, China. Trans. SAE 2021, 37, 124–131. [Google Scholar] [CrossRef]
- Zeng, H.; Wu, M.; Zhou, C.; Chen, Q.; Gong, Z. Experimental study on reinforcement mechanism of vegetated slopes with root system and vertical geotextile belts. Chin. Geotech. Eng 2020, 42 (Suppl. S2), 151–156. [Google Scholar] [CrossRef]
- Kong, G.Q.; Wen, L.; Liu, H.L.; Wang, C.-Q. Strength properties of root compound soil and morphological observation of plant root. Rock Soil Mech. 2019, 40, 3717–3723. [Google Scholar] [CrossRef]
- Marzini, L.; Ciofini, D.; Agresti, J.; Ciaccheri, L.; D’Addario, E.; Disperati, L.; Siano, S.; Osticioli, I. Exploring the Potential of Portable Spectroscopic Techniques for the Biochemical Characterization of Roots in Shallow Landslides. Forests 2023, 14, 825. [Google Scholar] [CrossRef]
- Wu, T.H. Root reinforcement of soil: Review of analytical models, test results, and applications to design (Review). Can. Geotech. J. 2013, 50, 259–274. [Google Scholar] [CrossRef]
- Waldrom, L. The shear resistance of root permeated Ho-mo-geneous and stratified. Soil Sci. Soc. Am. Proc. 1977, 41, 843–849. [Google Scholar] [CrossRef]
- Zhu, J.; Mao, Z.; Wang, Y.; Wang, Y.; Li, T.; Wang, K.; Langendoen, E.J.; Zheng, B. Soil moisture and hysteresis affect both magnitude and efficiency of root reinforcement. Catena 2022, 219, 106574. [Google Scholar] [CrossRef]
- Shen, J.-H.; Zeng, B.; Lei, S.-T.; Su, X.-L.; Huang, W.-J. Seed submergence tolerance of four annual species growing in the water-level-fluctuation zone of Three Gorges Reservoir, China, and effects of long-term submergence on their seed germination. Chin. Plant Ecol. 2011, 35, 237. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Voesenek, L. Flooding stress: Acclimations and genetic diversity. Annu. Rev. Plant Biol. 2008, 59, 313–339. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Ling, L.I.U.; Xinyue, H.U. Effects of flooding stress on morphological structure and physiological and biochemical characteristics of Conyza canadensis. Ecol. Sci. 2020, 39, 134–141. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, J.; Cheng, P. Response of Carex cinerascens to waterlogging stress. Hydroecology 2017, 38, 24–29. [Google Scholar] [CrossRef]
- Huang, S.; Jiang, S.; Liang, J.; Chen, M.; Shi, Y. Current knowledge of bermudagrass responses to abiotic stresses. Breed. Sci. 2019, 69, 215–226. [Google Scholar] [CrossRef]
- Yuan, Z.; Ni, X.; Chen, C.; Zhang, S.; Chen, X.; Yang, Z. Effects of different water conditions on the biomass, root morphology and aerenchyma formation in bermudagrass (Cynodon dactylon (L.) Pers). BMC Plant Biol. 2022, 22, 266. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, Q.; Werner, A.D.; Li, Y.; Jiang, S.; Tan, Z. Root-induced changes of soil hydraulic properties–A review. Hydrol 2020, 589, 125203. [Google Scholar] [CrossRef]
- Boldrin, D.; Leung, A.K.; Bengough, A.G. Effects of root dehydration on biomechanical properties of woody roots of Ulex europaeus. Plant Soil 2018, 431, 347–369. [Google Scholar] [CrossRef]
- Xue, Y.; Zhao, Y.; Wang, K.; Duan, X.; Du, Y. Tensile Mechanical Properties and Influencing Factors of Typical Shrub Roots in Mopan Mountain. Southwest For. Univ. 2019, 39, 99–107. [Google Scholar] [CrossRef]
- Hu, W.; Hui, D.; Huangfu, C. Soil water status dominates growth and nitrogen acquisition strategy of Carex thunbergii in response to nitrogen and water additions. Soils Sediments 2024, 24, 2623–2637. [Google Scholar] [CrossRef]
- Wang, X.; Huang, P.; Ma, M.; Shan, K.; Wu, S. Effects of riparian pioneer plants on soil aggregate stability: Roles of root traits and rhizosphere microorganisms. Sci. Total Environ. 2024, 940, 173584. [Google Scholar] [CrossRef]
- Genet, M.; Stokes, A.; Salin, F.; Mickovski, S.B.; Fourcaud, T.; Dumail, J.-F.; van Beek, R. The influence of cellulose content on tensile strength in tree roots. Plant Soil 2005, 278, 1–9. [Google Scholar] [CrossRef]
- Hao, Y.-Z.; Zhao, J.-Y.; Lu, M.; Wang, Q.; Peng, W.-Q.; Chen, Z. Effect of plant roots on river bank stabilization after composite vegetation planting. Hydroecol 2020, 41, 42–50. [Google Scholar] [CrossRef]
- Liu, Y.-B.; Hu, X.-S.; Yu, D.-M.; Zhu, H.-L.; Li, G.-R. Influence of the roots of mixed-planting species on the shear strength of saline loess soil. Mt. Sci. 2021, 18, 806–818. [Google Scholar] [CrossRef]
- Jie, S.; Fan, D.; Xie, Z.; Zhang, X.; Xiong, G. Feature of leaf photosynthetic and leaf nutrient traits in reservoir riparian region of Three Gorges Reservoir, China. Acta Ecol. Sin. 2012, 32, 172. [Google Scholar] [CrossRef][Green Version]
- Chun-tao, Z.; Xiao-lou, Z.H.U.; Kai-feng, C.A.I.; Yu, Y.-G. Evaluation of shade tolerance of Carex species available for garden-environment planting. Beijing For. Univ. 2010, 32, 207–212. [Google Scholar] [CrossRef]
- Zhu, K.-W.; Chen, Y.-C.; Zhang, S.; Lei, B.; Yang, Z.-M.; Huang, L. Vegetation of the water-level fluctuation zone in the Three Gorges Reservoir at the initial impoundment stage. Glob. Ecol. Conserv. 2020, 21, e00866. [Google Scholar] [CrossRef]
- Liu, L.-Y.; Fan, X.-F.; Teng, K.; Yue, Y.-S.; Chang, Z.-H.; Wu, J.-Y. Advances in Development of Genetic Diversity of Carex L. Acta Agrestia Sin. 2021, 29, 1148. [Google Scholar] [CrossRef]
- Ai-Ying, Z.; Da-Yong, F.; Liang, M.; Dan, Y.; Gao-Ming, X.; Zong-Qiang, X. Intra-annual variations in leaf traits of Cynodon dactylon (L.) Pers. during exposure period in riparian zone of Three Gorges Reservoir Area. Plant Sci. J. 2022, 40, 453–461. [Google Scholar] [CrossRef]
- Liu, S.-L.; Chen, J.-X.; Yang, Y.; Chen, Z.-W.; Lu, L.-L.; Mu, Y.-H. Effects of milkvetch (Astragalus sinicus) decomposition leachates on germination and seedling growth of Eleusine (Eleusine indica). Acta Pratacult. Sin. 2022, 31, 209. [Google Scholar] [CrossRef]
- Jiang, W.; Xiao, N.; Xiao, H. Niche and interspecific associations of dominant plants in the water-level-fluctuating zones of the reservoirs in the Jinshajiang River watershed. Lake Sci. 2023, 35, 236–246. [Google Scholar]
- Chen, J.; Zeng, C.; Wei, H.; Liu, Y.; Wang, Z.; Jia, Z. Effects of mixed intercropping of Cynodon dactylon and Hemarthria altissima on their biomass under different flooding conditions. Acta Ecol. Sin. 2017, 37, 54–61. [Google Scholar] [CrossRef][Green Version]
- Shi, Y.; Feng, Y.; Wang, J.; Bai, Z.; Feng, X.; Chen, B. Optimal allocation of technical reclamation and ecological restoration for a cost-effective solution in Pingshuo Opencast Coal Mine area of China. Environ. Manag. 2025, 373, 123951. [Google Scholar] [CrossRef]



| Species | Parameters | Equation | R2 | Significance |
|---|---|---|---|---|
| Carex | Diameter | y = 0.62 + 4.45 × 10−4x − 7.5 × 10−7x2 | 0.74 | <0.05 |
| Length | y = 686.79 + 8.28x − 4.02 × 10−2x2 | 0.83 | <0.01 | |
| Surface area | y = 140.99 + 1.69x − 8.17 × 10−3x2 | 0.94 | <0.01 | |
| Volume | y = 2.17 + 2.76 × 10−2x − 1.16 × 10−4x2 | 0.80 | <0.01 | |
| Cynodon | Diameter | y = 0.64 + 1.90 × 10−4x − 2.51 × 10−7x2 | 0.58 | >0.05 |
| Length | y = 735.79 + 3.55x − 1.88 × 10−2x2 | 0.82 | <0.01 | |
| Surface area | y = 149.00 + 0.47x − 2.45 × 10−3x2 | 0.77 | <0.01 | |
| Volume | y = 2.42 + 4.38 × 10−3x − 1.67 × 10−5x2 | 0.95 | <0.01 | |
| Eleusine | Diameter | y = 0.67 + 4.37 × 10−4x − 4.97 × 10−7x2 | 0.90 | <0.01 |
| Length | y = 394.49 − 0.16x − 1.93 × 10−3x2 | 0.84 | <0.01 | |
| Surface area | y = 68.78 + 0.40x − 2.03 × 10−3x2 | 0.89 | <0.01 | |
| Volume | y = 1.14 + 1.44 × 10−3x − 1.20 × 10−5x2 | 0.95 | <0.01 |
| Flooding Durations (Days) | Carex | Cynodon | Eleusine | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Equation | R2 | Significance | Equation | R2 | Significance | Equation | R2 | Significance | |
| 0 | y = 12.87x1.11 | 0.96 | <0.01 | y = 13.23x1.40 | 0.97 | <0.01 | y = 9.75x0.99 | 0.97 | <0.01 |
| 30 | y = 12.22x1.01 | 0.93 | <0.01 | y = 15.95x1.54 | 0.93 | <0.01 | y = 9.39x0.88 | 0.96 | <0.01 |
| 60 | y = 10.38x1.12 | 0.93 | <0.01 | y = 13.14x1.26 | 0.89 | <0.01 | y = 8.74x0.69 | 0.70 | <0.01 |
| 90 | y = 10.25x0.81 | 0.93 | <0.01 | y = 10.97x0.90 | 0.47 | >0.05 | y = 6.17x0.27 | 0.04 | >0.05 |
| 120 | y = 7.84x0.80 | 0.25 | >0.05 | y = 8.73x0.90 | 0.86 | <0.01 | y = 5.27x0.64 | 0.35 | >0.05 |
| 150 | y = 8.58x0.91 | 0.86 | <0.05 | y = 5.88x0.66 | 0.70 | <0.01 | y = 4.23x0.65 | 0.77 | <0.01 |
| 180 | y = 4.9x0.70 | 0.61 | <0.05 | y = 4.88x0.75 | 0.75 | <0.01 | y = 6.32x1.13 | 0.54 | <0.05 |
| Flooding Durations (Days) | Carex | Cynodon | Eleusine | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Equation | R2 | Significance | Equation | R2 | Significance | Equation | R2 | Significance | |
| 0 | y = 10.76x−1.14 | 0.8 | <0.01 | y = 12.97x−0.72 | 0.81 | <0.01 | y = 12.42x−1.02 | 0.91 | <0.01 |
| 30 | y = 12.29x−1.05 | 0.98 | <0.01 | y = 19.99x−0.49 | 0.88 | <0.01 | y = 13.42x−0.97 | 0.91 | <0.01 |
| 60 | y = 10.79x−1.1 | 0.96 | <0.01 | y = 14.33x−1.1 | 0.94 | <0.01 | y = 19.37x−0.83 | 0.63 | <0.05 |
| 90 | y = 20.37x−0.89 | 0.87 | <0.01 | y = 15.73x−0.54 | 0.55 | >0.05 | y = 16.52x−1.07 | 0.32 | >0.05 |
| 120 | y = 16.57x−0.92 | 0.99 | <0.01 | y = 10.17x−1.12 | 0.90 | <0.01 | y = 7.98x−1.12 | 0.81 | <0.01 |
| 150 | y = 4.68x−1.67 | 0.9 | <0.01 | y = 11.04x−0.93 | 0.70 | <0.01 | y = 6.44x−1.62 | 0.95 | <0.01 |
| 180 | y = 2.87x−1.81 | 0.98 | <0.01 | y = 7.47x−1.1 | 0.96 | <0.01 | y = 8.1x−0.9 | 0.71 | <0.01 |
| Flooding Durations (Days) | Carex | Cynodon | Eleusine | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Equation | R2 | Significance | Equation | R2 | Significance | Equation | R2 | Significance | |
| 0 | y = 252.5x−0.69 | 0.62 | <0.05 | y = 348.3x−0.41 | 0.71 | <0.01 | y = 506.7x−0.33 | 0.84 | <0.01 |
| 30 | y = 219.6x−0.94 | 0.95 | <0.01 | y = 282.7x−0.34 | 0.60 | <0.05 | y = 276.3x−0.61 | 0.76 | <0.01 |
| 60 | y = 197x−1.03 | 0.93 | <0.01 | y = 270.15x−0.99 | 0.76 | <0.01 | y = 205.5x−0.68 | 0.45 | >0.05 |
| 90 | y = 239.9x−1.06 | 0.89 | <0.01 | y = 554.9x−0.99 | 0.24 | >0.05 | y = 331.4x−1.22 | 0.68 | <0.01 |
| 120 | y = 434.6x−0.63 | 0.64 | <0.05 | y = 241.8x−0.99 | 0.47 | >0.05 | y = 177.4x−1.65 | 0.92 | <0.01 |
| 150 | y = 489.1x−1.88 | 0.98 | <0.01 | y = 415.8x−0.59 | 0.43 | >0.05 | y = 289.6x−1.22 | 0.77 | <0.01 |
| 180 | y = 149.9x−1.41 | 0.72 | <0.01 | y = 382.5x−0.83 | 0.71 | <0.01 | y = 217.6x−1.21 | 0.81 | <0.01 |
| Species | Parameters | Equation | R2 | Significance |
|---|---|---|---|---|
| Carex | Shear strength | y = 49.43 + 7.2 × 10−2x − 4.38 × 10−4x2 | 0.82 | <0.05 |
| Strength increment | y = 27.84 + 2.86 × 10−3x − 2.09 × 10−5x2 | 0.97 | <0.01 | |
| Cynodon | Shear strength | y = 52.80 − 5.01 × 10−3x − 1.87 × 10−4x2 | 0.98 | <0.01 |
| Strength increment | y = 15.49 + 0.18x − 8.63 × 10−4x2 | 0.84 | <0.01 | |
| Eleusine | Shear strength | y = 52.50 − 0.27x + 6.79 × 10−4x2 | 0.96 | <0.01 |
| Strength increment | y = 15.16 − 0.07x − 3.78 × 10−5x2 | 0.74 | <0.05 | |
| CK | Shear strength | y = 37.31 − 0.19 × 10−2x + 6.78 × 10−4x2 | 0.96 | <0.01 |
| Species | Parameters | Equation | R2 | Significance |
|---|---|---|---|---|
| Carex | Friction angle | y = 27.84 + 2.86 × 10−3x − 2.09 × 10−5x2 | 0.74 | <0.05 |
| Cohesion | y = 23.08 + 6.92 × 10−2x − 42 × 10−4x2 | 0.83 | <0.01 | |
| Cynodon | Friction angle | y = 23.54 − 7.77 × 10−3x + 1.28 × 10−5x2 | 0.92 | <0.01 |
| Cohesion | y = 31.02 − 1.73 × 10−3x − 2 × 10−4x2 | 0.97 | <0.01 | |
| Eleusine | Friction angle | y = 22.39 − 2.22 × 10−2x + 7.87 × 10−5x2 | 0.96 | <0.01 |
| Cohesion | y = 32.04 + 0.25x + 5.99 × 10−4x2 | 0.94 | <0.01 | |
| CK | Friction angle | y = 20.36 − 1.22 × 10−2x + 1.92 × 10−5 x2 | 0.97 | <0.01 |
| Cohesion | y = 18.76 − 0.18x + 6.57 × 10−4x2 | 0.95 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Xing, Z.; Xiao, H.; Tao, G. Effects of Varying Flooding Durations on the Soil Reinforcement Capacity of Dominant Riparian Plants in the Yangtze River Basin. Appl. Sci. 2025, 15, 5376. https://doi.org/10.3390/app15105376
Wang P, Xing Z, Xiao H, Tao G. Effects of Varying Flooding Durations on the Soil Reinforcement Capacity of Dominant Riparian Plants in the Yangtze River Basin. Applied Sciences. 2025; 15(10):5376. https://doi.org/10.3390/app15105376
Chicago/Turabian StyleWang, Pengcheng, Zifa Xing, Henglin Xiao, and Gaoliang Tao. 2025. "Effects of Varying Flooding Durations on the Soil Reinforcement Capacity of Dominant Riparian Plants in the Yangtze River Basin" Applied Sciences 15, no. 10: 5376. https://doi.org/10.3390/app15105376
APA StyleWang, P., Xing, Z., Xiao, H., & Tao, G. (2025). Effects of Varying Flooding Durations on the Soil Reinforcement Capacity of Dominant Riparian Plants in the Yangtze River Basin. Applied Sciences, 15(10), 5376. https://doi.org/10.3390/app15105376






