SPECT and MRI Evaluation of Cerebrovascular Reactivity with CO2 Inhalation—A Preliminary Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
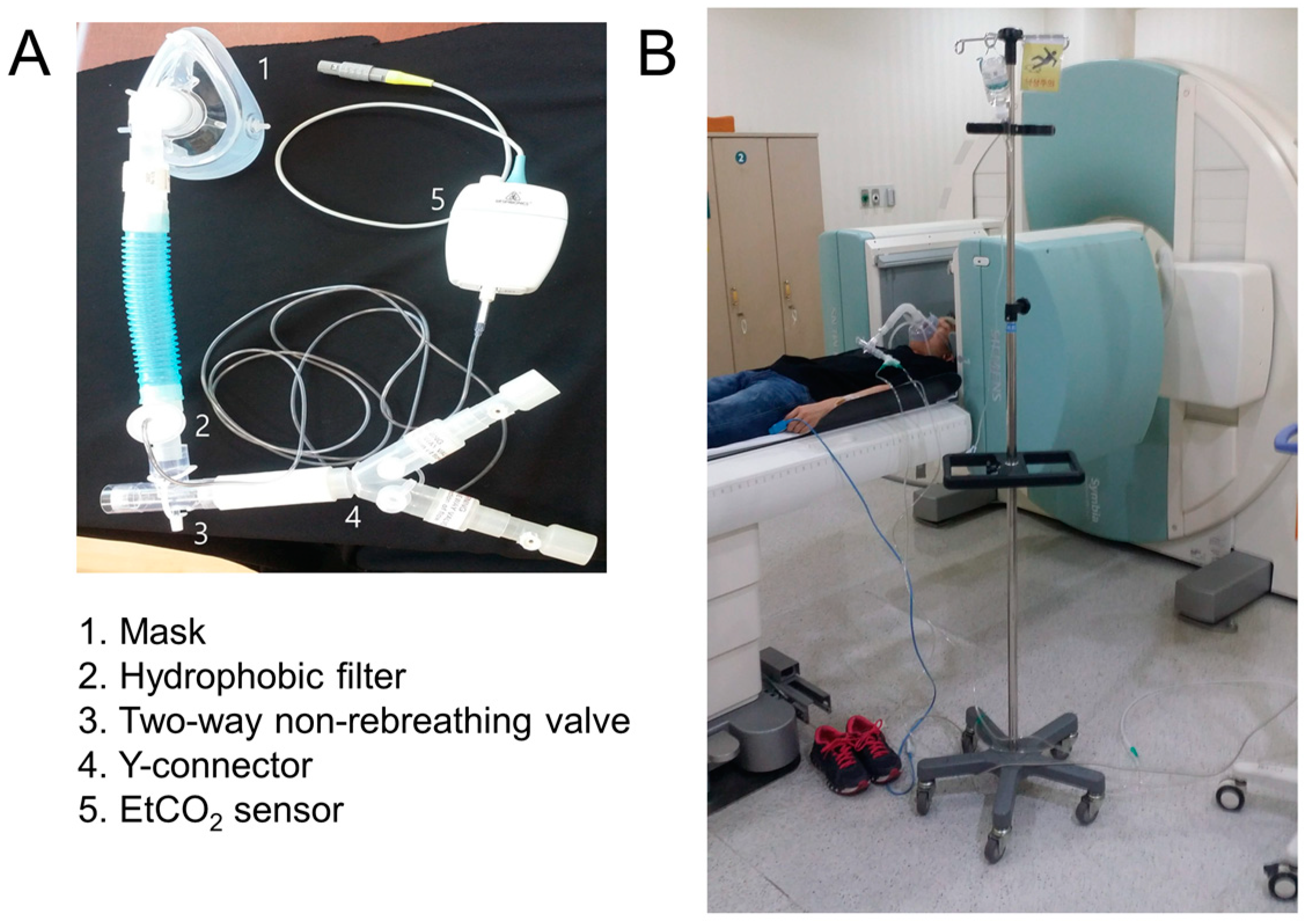
2.2. CO2 Gas Delivery System
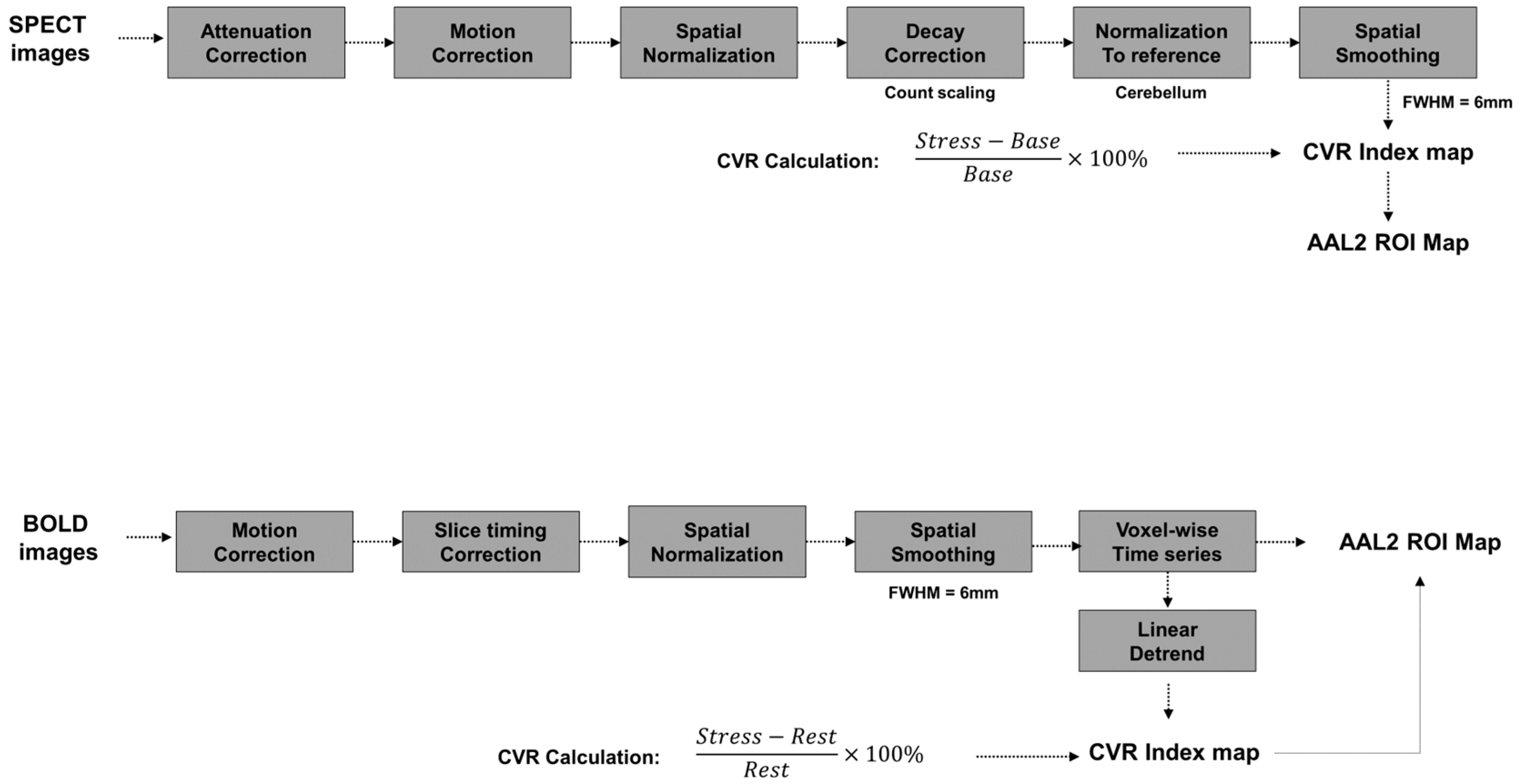
2.3. MRI Acquisition and CVR Processing
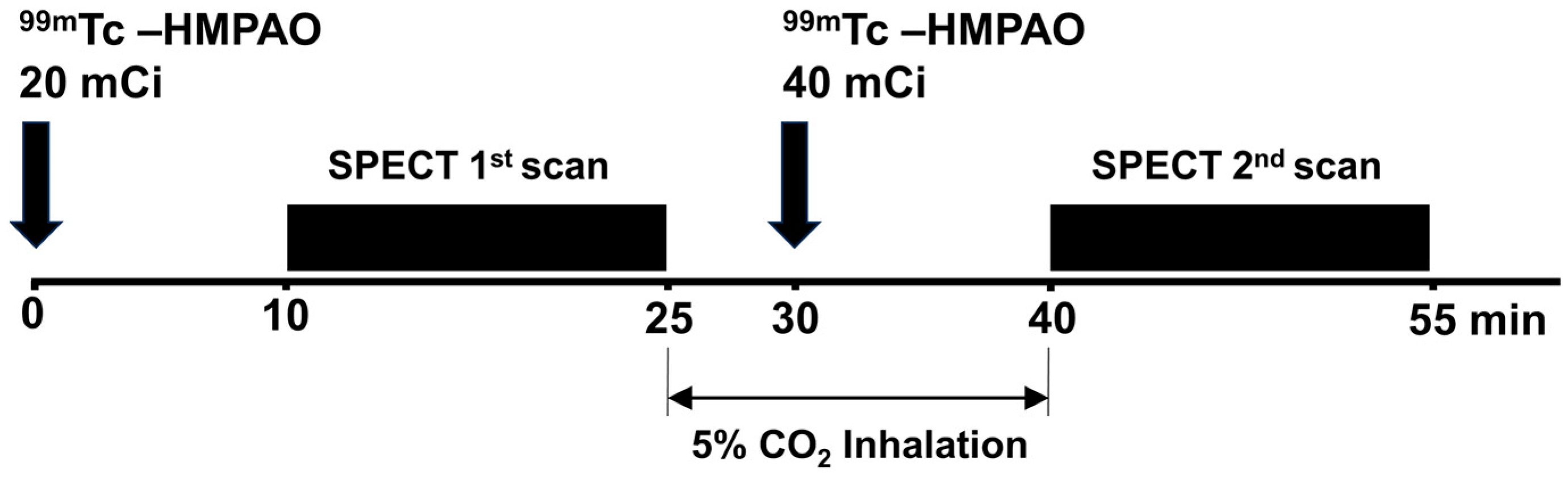
2.4. SPECT Acquisition and CVR Processing
2.5. Statistical Analysis
3. Results
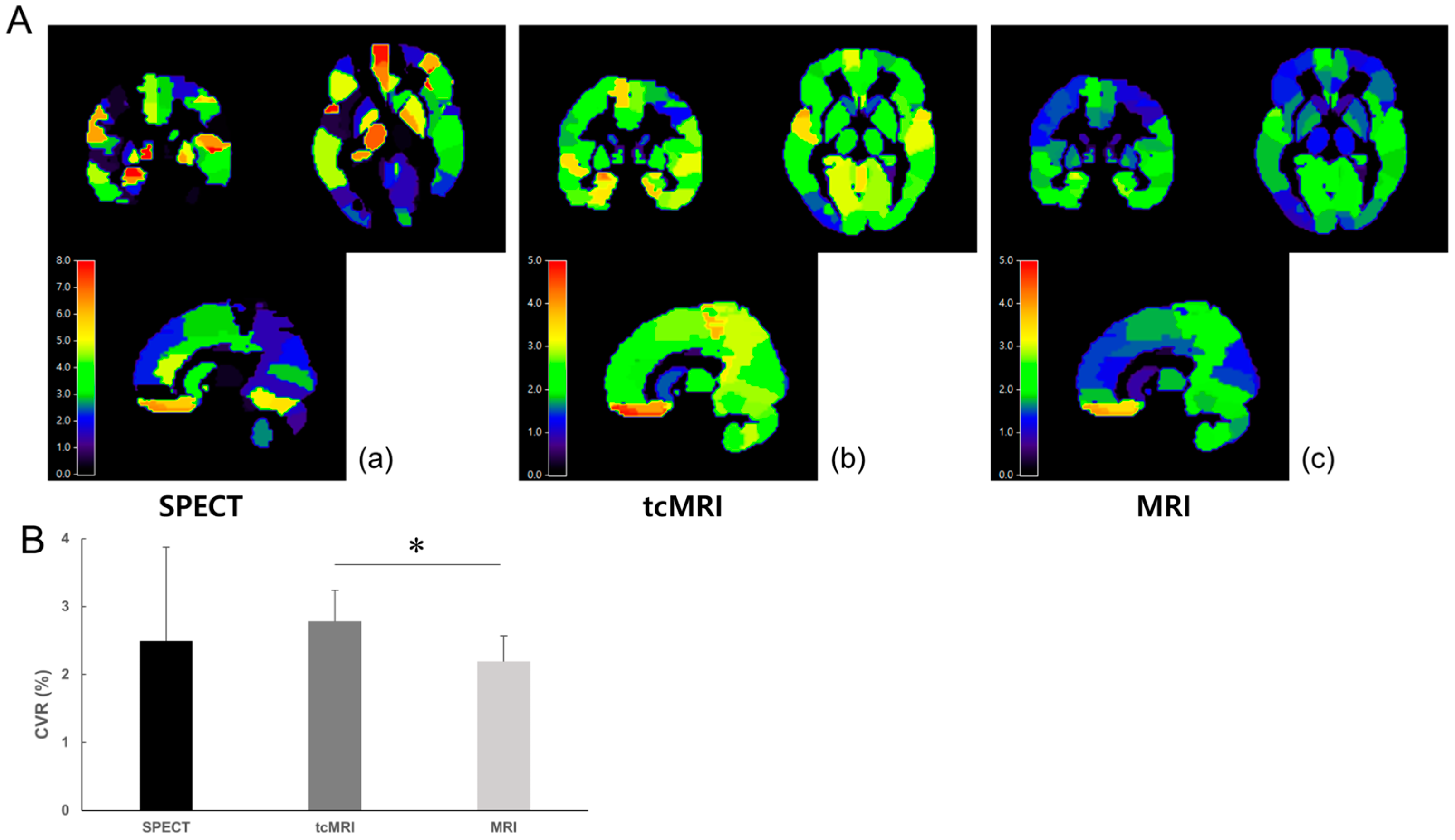
3.1. Participant Characteristics and Group-Level SPECT Results
3.2. Group-Level Comparison Between SPECT, tcMRI, and MRI
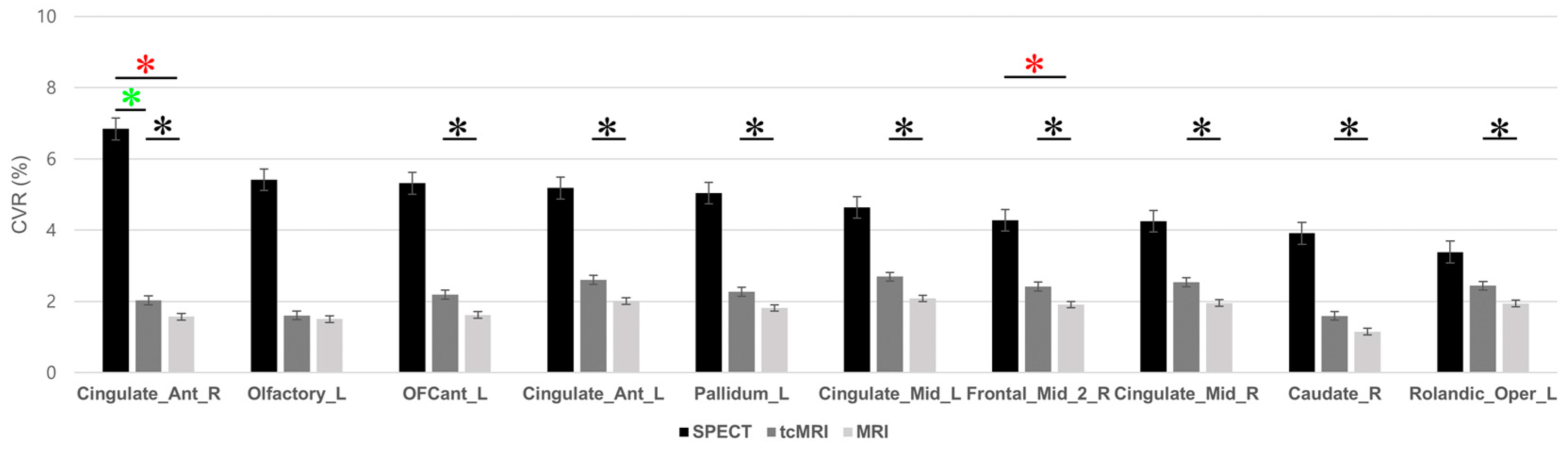
3.3. Regional-Level Comparison and ROI-Specific Differences
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| ROI Name | SPECT | tcMRI | MRI | F | p |
|---|---|---|---|---|---|
| Cingulate_Ant_R | 6.842 ± 3.580 | 2.028 ± 0.390 | 1.571 ± 0.240 | 12.35 | 0.001 |
| Vermis_1_2 | 6.357 ± 10.670 | 3.131 ± 0.480 | 2.546 ± 0.600 | 0.675 | 0.527 |
| Vermis_3 | 6.229 ± 10.290 | 3.242 ± 0.560 | 2.514 ± 0.490 | 0.666 | 0.532 |
| Cerebelum_3_R | 5.941 ± 6.560 | 2.904 ± 0.540 | 2.324 ± 0.470 | 1.514 | 0.259 |
| Olfactory_L | 5.412 ± 3.990 | 1.605 ± 1.080 | 1.504 ± 0.900 | 4.897 | 0.028 |
| OFCant_L | 5.314 ± 3.740 | 2.191 ± 1.150 | 1.617 ± 1.080 | 7.828 | 0.007 |
| Cingulate_Ant_L | 5.183 ± 2.840 | 2.604 ± 0.440 | 2.012 ± 0.280 | 6.303 | 0.013 |
| Pallidum_L | 5.041 ± 3.550 | 2.270 ± 0.260 | 1.815 ± 0.280 | 4.520 | 0.034 |
| Supp_Motor_Area_L | 4.939 ± 3.410 | 3.126 ± 0.510 | 2.433 ± 0.400 | 2.674 | 0.110 |
| Vermis_10 | 4.879 ± 5.410 | 2.633 ± 0.810 | 2.287 ± 0.860 | 1.200 | 0.335 |
| Frontal_Inf_Oper_R | 4.743 ± 3.840 | 2.412 ± 0.460 | 1.950 ± 0.400 | 2.996 | 0.088 |
| Vermis_7 | 4.723 ± 6.390 | 3.676 ± 1.110 | 2.971 ± 1.060 | 0.316 | 0.735 |
| Pallidum_R | 4.715 ± 6.450 | 2.389 ± 0.330 | 1.927 ± 0.380 | 0.928 | 0.422 |
| Cingulate_Mid_L | 4.638 ± 2.390 | 2.694 ± 0.390 | 2.083 ± 0.280 | 6.151 | 0.014 |
| Vermis_4_5 | 4.462 ± 7.790 | 3.440 ± 0.630 | 2.667 ± 0.620 | 0.249 | 0.784 |
| Rectus_R | 4.287 ± 3.630 | 1.649 ± 1.420 | 1.560 ± 1.140 | 2.712 | 0.107 |
| Frontal_Mid_2_R | 4.278 ± 1.810 | 2.418 ± 0.410 | 1.907 ± 0.470 | 9.770 | 0.003 |
| Cingulate_Mid_R | 4.251 ± 2.140 | 2.536 ± 0.460 | 1.955 ± 0.340 | 4.668 | 0.032 |
| Putamen_R | 4.212 ± 5.010 | 2.079 ± 0.290 | 1.673 ± 0.250 | 1.323 | 0.303 |
| OFCmed_R | 4.209 ± 4.060 | 2.013 ± 1.750 | 1.874 ± 1.540 | 1.384 | 0.288 |
| Heschl_R | 4.150 ± 4.340 | 3.302 ± 0.830 | 2.577 ± 0.670 | 0.499 | 0.619 |
| OFCmed_L | 4.075 ± 3.420 | 1.135 ± 1.180 | 1.161 ± 1.280 | 3.757 | 0.054 |
| Insula_R | 4.026 ± 3.810 | 2.539 ± 0.410 | 2.033 ± 0.280 | 1.528 | 0.256 |
| Vermis_8 | 3.980 ± 7.060 | 3.196 ± 0.760 | 2.618 ± 0.690 | 0.155 | 0.858 |
| Frontal_Inf_Oper_L | 3.954 ± 3.470 | 1.951 ± 0.560 | 1.581 ± 0.410 | 2.745 | 0.104 |
| Caudate_R | 3.912 ± 2.900 | 1.594 ± 0.400 | 1.149 ± 0.280 | 5.336 | 0.022 |
| OFCant_R | 3.876 ± 3.390 | 2.500 ± 1.190 | 1.883 ± 1.100 | 1.457 | 0.271 |
| Rectus_L | 3.847 ± 4.930 | 1.284 ± 0.980 | 1.158 ± 0.790 | 1.553 | 0.251 |
| Angular_R | 3.763 ± 4.280 | 2.283 ± 0.750 | 1.843 ± 0.690 | 0.941 | 0.417 |
| Caudate_L | 3.632 ± 3.210 | 1.644 ± 0.300 | 1.200 ± 0.210 | 3.136 | 0.08 |
| Parietal_Inf_L | 3.618 ± 2.770 | 2.232 ± 0.540 | 1.761 ± 0.410 | 2.075 | 0.168 |
| Precentral_R | 3.537 ± 3.340 | 2.113 ± 0.630 | 1.680 ± 0.470 | 1.459 | 0.271 |
| Paracentral_Lobule_R | 3.425 ± 3.600 | 3.300 ± 0.610 | 2.392 ± 0.490 | 0.451 | 0.647 |
| Rolandic_Oper_L | 3.384 ± 1.720 | 2.439 ± 0.420 | 1.939 ± 0.320 | 3.957 | 0.048 |
| Vermis_6 | 3.367 ± 4.960 | 3.969 ± 0.930 | 3.153 ± 0.870 | 0.154 | 0.859 |
| Supp_Motor_Area_R | 3.344 ± 2.200 | 2.821 ± 0.540 | 2.156 ± 0.420 | 1.233 | 0.326 |
References
- Liu, P.; De Vis, J.B.; Lu, H. Cerebrovascular Reactivity (CVR) MRI with CO2 Challenge: A Technical Review. NeuroImage 2019, 187, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Dogra, S.; Wang, X.; Gupta, A.; Veraart, J.; Ishida, K.; Qiu, D.; Dehkharghani, S. Acetazolamide-Augmented BOLD MRI to Assess Whole-Brain Cerebrovascular Reactivity in Chronic Steno-Occlusive Disease Using Principal Component Analysis. Radiology 2023, 307, e221473. [Google Scholar] [CrossRef] [PubMed]
- Juttukonda, M.R.; Donahue, M.J. Neuroimaging of Vascular Reserve in Patients with Cerebrovascular Diseases. NeuroImage 2019, 187, 192–208. [Google Scholar] [CrossRef]
- Fisher, J.A.; Venkatraghavan, L.; Mikulis, D.J. Magnetic Resonance Imaging–Based Cerebrovascular Reactivity and Hemodynamic Reserve. Stroke 2018, 49, 2011–2018. [Google Scholar] [CrossRef]
- Ito, H.; Kanno, I.; Kato, C.; Sasaki, T.; Ishii, K.; Ouchi, Y.; Iida, A.; Okazawa, H.; Hayashida, K.; Tsuyuguchi, N.; et al. Database of Normal Human Cerebral Blood Flow, Cerebral Blood Volume, Cerebral Oxygen Extraction Fraction and Cerebral Metabolic Rate of Oxygen Measured by Positron Emission Tomography with 15O-Labelled Carbon Dioxide or Water, Carbon Monoxide and Oxygen: A Multicentre Study in Japan. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Yezhuvath, U.S.; Lewis-Amezcua, K.; Varghese, R.; Xiao, G.; Lu, H. On the Assessment of Cerebrovascular Reactivity Using Hypercapnia BOLD MRI. NMR Biomed. 2009, 22, 779–786. [Google Scholar] [CrossRef]
- Oku, N.; Matsumoto, M.; Hashikawa, K.; Moriwaki, H.; Okazaki, Y.; Seike, Y.; Handa, N.; Uehara, T.; Kamada, T.; Nishimura, T. Carbon Dioxide Reactivity by Consecutive Technetium-99m-HMPAO SPECT in Patients with a Chronically Obstructed Major Cerebral Artery. J. Nucl. Med. 1994, 35, 32–40. [Google Scholar]
- Chen, D.-W.; Zheng, J.; Shi, J.; Yin, Y.; Song, C.; Yang, F.; Zhang, Y.-Q.; Ma, L.-N. Assessment of the Cerebral Hemodynamic Benefits of Carotid Artery Stenting for Patients with Preoperative Hemodynamic Impairment Using Cerebral Single Photon Emission Computed Tomography (SPECT) and Carbon Dioxide Inhalation. Med. Sci. Monit. 2018, 24, 5398–5404. [Google Scholar] [CrossRef]
- Ma, J.; Mehrkens, J.H.; Holtmannspoetter, M.; Linke, R.; Schmid-Elsaesser, R.; Steiger, H.-J.; Brueckmann, H.; Bruening, R. Perfusion MRI before and after Acetazolamide Administration for Assessment of Cerebrovascular Reserve Capacity in Patients with Symptomatic Internal Carotid Artery (ICA) Occlusion: Comparison with 99mTc-ECD SPECT. Neuroradiology 2007, 49, 317–326. [Google Scholar] [CrossRef]
- Wong, T.H.; Shagera, Q.A.; Ryoo, H.G.; Ha, S.; Lee, D.S. Basal and Acetazolamide Brain Perfusion SPECT in Internal Carotid Artery Stenosis. Nucl. Med. Mol. Imaging 2020, 54, 9–27. [Google Scholar] [CrossRef]
- Swenson, E.R. New Insights into Carbonic Anhydrase Inhibition, Vasodilation, and Treatment of Hypertensive-Related Diseases. Curr. Hypertens. Rep. 2014, 16, 467. [Google Scholar] [CrossRef]
- Koyama, M.; Kawashima, R.; Ito, H.; Ono, S.; Sato, K.; Goto, R.; Kinomura, S.; Yoshioka, S.; Sato, T.; Fukuda, H. SPECT Imaging of Normal Subjects with Technetium-99m-HMPAO and Technetium-99m-ECD. J. Nucl. Med. 1997, 38, 587–592. [Google Scholar]
- Catafau, A.M. Brain SPECT in Clinical Practice. Part I: Perfusion*. J. Nucl. Med. 2001, 42, 259–271. [Google Scholar] [PubMed]
- Choi, H.; Yoo, M.Y.; Cheon, G.J.; Kang, K.W.; Chung, J.-K.; Lee, D.S. Parametric Cerebrovascular Reserve Images Using Acetazolamide 99mTc-HMPAO SPECT: A Feasibility Study of Quantitative Assessment. Nucl. Med. Mol. Imaging 2013, 47, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Blockley, N.P.; Harkin, J.W.; Bulte, D.P. Rapid Cerebrovascular Reactivity Mapping: Enabling Vascular Reactivity Information to Be Routinely Acquired. NeuroImage 2017, 159, 214–223. [Google Scholar] [CrossRef]
- Leoni, R.F.; Mazzetto-Betti, K.C.; Silva, A.C.; Dos Santos, A.C.; de Araujo, D.B.; Leite, J.P.; Pontes-Neto, O.M. Assessing Cerebrovascular Reactivity in Carotid Steno-Occlusive Disease Using MRI BOLD and ASL Techniques. Radiol. Res. Pract. 2012, 2012, 268483. [Google Scholar] [CrossRef] [PubMed]
- Iutaka, T.; de Freitas, M.B.; Omar, S.S.; Scortegagna, F.A.; Nael, K.; Nunes, R.H.; Pacheco, F.T.; Maia Júnior, A.C.M.; do Amaral, L.L.F.; da Rocha, A.J. Arterial Spin Labeling: Techniques, Clinical Applications, and Interpretation. RadioGraphics 2023, 43, e220088. [Google Scholar] [CrossRef]
- Bulte, D.P.; Kelly, M.; Germuska, M.; Xie, J.; Chappell, M.A.; Okell, T.W.; Bright, M.G.; Jezzard, P. Quantitative Measurement of Cerebral Physiology Using Respiratory-Calibrated MRI. NeuroImage 2012, 60, 582–591. [Google Scholar] [CrossRef]
- Spano, V.R.; Mandell, D.M.; Poublanc, J.; Sam, K.; Battisti-Charbonney, A.; Pucci, O.; Han, J.S.; Crawley, A.P.; Fisher, J.A.; Mikulis, D.J. CO2 Blood Oxygen Level–Dependent MR Mapping of Cerebrovascular Reserve in a Clinical Population: Safety, Tolerability, and Technical Feasibility. Radiology 2013, 266, 592–598. [Google Scholar] [CrossRef]
- Blockley, N.P.; Driver, I.D.; Francis, S.T.; Fisher, J.A.; Gowland, P.A. An Improved Method for Acquiring Cerebrovascular Reactivity Maps. Magn. Reson. Med. 2011, 65, 1278–1286. [Google Scholar] [CrossRef]
- Forté, S.; Sobczyk, O.; Poublanc, J.; Duffin, J.; Hare, G.M.T.; Fisher, J.A.; Mikulis, D.; Kuo, K.H.M. Sickle Cell Cerebrovascular Reactivity to a CO2 Stimulus: Too Little, Too Slow. Front. Physiol. 2022, 13, 886807. [Google Scholar] [CrossRef] [PubMed]
- Hauser, T.-K.; Seeger, A.; Bender, B.; Klose, U.; Thurow, J.; Ernemann, U.; Tatagiba, M.; Meyer, P.T.; Khan, N.; Roder, C. Hypercapnic BOLD MRI Compared to H215O PET/CT for the Hemodynamic Evaluation of Patients with Moyamoya Disease. NeuroImage Clin. 2019, 22, 101713. [Google Scholar] [CrossRef] [PubMed]
- Blair, G.W.; Doubal, F.N.; Thrippleton, M.J.; Marshall, I.; Wardlaw, J.M. Magnetic Resonance Imaging for Assessment of Cerebrovascular Reactivity in Cerebral Small Vessel Disease: A Systematic Review. J. Cereb. Blood Flow Metab. 2016, 36, 833–841. [Google Scholar] [CrossRef]
- Pellaton, A.; Bijlenga, P.; Bouchez, L.; Cuvinciuc, V.; Barnaure, I.; Garibotto, V.; Lövblad, K.-O.; Haller, S. CO2BOLD Assessment of Moyamoya Syndrome: Validation with Single Photon Emission Computed Tomography and Positron Emission Tomography Imaging. World J. Radiol. 2016, 8, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Mandell, D.M.; Han, J.S.; Poublanc, J.; Crawley, A.P.; Stainsby, J.A.; Fisher, J.A.; Mikulis, D.J. Mapping Cerebrovascular Reactivity Using Blood Oxygen Level-Dependent MRI in Patients With Arterial Steno-Occlusive Disease. Stroke 2008, 39, 2021–2028. [Google Scholar] [CrossRef]
- Fierstra, J.; van Niftrik, C.; Warnock, G.; Wegener, S.; Piccirelli, M.; Pangalu, A.; Esposito, G.; Valavanis, A.; Buck, A.; Luft, A.; et al. Staging Hemodynamic Failure With Blood Oxygen-Level-Dependent Functional Magnetic Resonance Imaging Cerebrovascular Reactivity: A Comparison Versus Gold Standard (15O-)H2O-Positron Emission Tomography. Stroke 2018, 49, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Griswold, M.A.; Jakob, P.M.; Heidemann, R.M.; Nittka, M.; Jellus, V.; Wang, J.; Kiefer, B.; Haase, A. Generalized Autocalibrating Partially Parallel Acquisitions (GRAPPA). Magn. Reson. Med. 2002, 47, 1202–1210. [Google Scholar] [CrossRef]
- Rolls, E.T.; Joliot, M.; Tzourio-Mazoyer, N. Implementation of a New Parcellation of the Orbitofrontal Cortex in the Automated Anatomical Labeling Atlas. NeuroImage 2015, 122, 1–5. [Google Scholar] [CrossRef]
- Chang, L.-T. A Method for Attenuation Correction in Radionuclide Computed Tomography. IEEE Trans. Nucl. Sci. 1978, 25, 638–643. [Google Scholar] [CrossRef]
- Inugami, A.; Kanno, I.; Uemura, K.; Shishido, F.; Murakami, M.; Tomura, N.; Fujita, H.; Higano, S. Linearization Correction of 99mTc-Labeled Hexamethyl-Propylene Amine Oxime (HM-PAO) Image in Terms of Regional CBF Distribution: Comparison to C15O2 Inhalation Steady-State Method Measured by Positron Emission Tomography. J. Cereb. Blood Flow Metab. 1988, 8, S52–S60. [Google Scholar] [CrossRef]
- Soonawala, D.; Amin, T.; Ebmeier, K.P.; Steele, J.D.; Dougall, N.J.; Best, J.; Migneco, O.; Nobili, F.; Scheidhauer, K. Statistical Parametric Mapping of 99mTc-HMPAO-SPECT Images for the Diagnosis of Alzheimer’s Disease: Normalizing to Cerebellar Tracer Uptake. NeuroImage 2002, 17, 1193–1202. [Google Scholar] [CrossRef] [PubMed]
- Van Niftrik, C.H.B.; Piccirelli, M.; Bozinov, O.; Maldaner, N.; Strittmatter, C.; Pangalu, A.; Valavanis, A.; Regli, L.; Fierstra, J. Impact of Baseline CO2 on Blood-Oxygenation-Level-Dependent MRI Measurements of Cerebrovascular Reactivity and Task-Evoked Signal Activation. Magn. Reson. Imaging 2018, 49, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-T.; Ghosh, K.K.; Padmanabhan, P.; Langer, O.; Liu, J.; Eng, D.N.C.; Halldin, C.; Gulyás, B. PET-MR and SPECT-MR Multimodality Probes: Development and Challenges. Theranostics 2018, 8, 6210–6232. [Google Scholar] [CrossRef] [PubMed]
- Vorstrup, S.; Brun, B.; Lassen, N.A. Evaluation of the Cerebral Vasodilatory Capacity by the Acetazolamide Test before EC-IC Bypass Surgery in Patients with Occlusion of the Internal Carotid Artery. Stroke 1986, 17, 1291–1298. [Google Scholar] [CrossRef]

| Participants | Age | Gender | Hand | SPECT | fMRI | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre BP | Post BP | Gas | Count1st | Count2nd | Pre BP | Post BP | ||||
| 01 | 25 | M | Rt | 116/59 | 125/95 | CO2 | 3593 | 10,599 | 117/77 | 112/80 |
| 02 | 22 | F | Rt | 99/56 | 114/66 | CO2 | 5223 | 15,440 | 107/60 | 102/58 |
| 03 | 25 | M | Rt | 134/82 | 136/90 | CO2 | 3543 | 10,453 | 134/97 | 134/84 |
| 04 | 21 | F | Rt | 91/52 | 98/60 | CO2 | 3486 | 12,876 | 105/67 | 100/67 |
| 05 | 27 | M | Rt | 123/73 | 122/75 | CO2 | 4222 | 12,963 | 111/68 | 110/74 |
| 06 | 25 | M | Rt | 123/67 | 130/76 | CO2 | 4335 | 13,327 | 125/81 | 126/74 |
| 07 | 23 | M | Rt | 119/60 | 122/67 | CO2 | 3555 | 12,272 | 108/65 | 111/66 |
| 08 | 23 | M | Rt | 118/71 | 123/80 | AIR | 4072 | 12,220 | 125/86 | 135/91 |
| 09 | 23 | M | Rt | 124/55 | 137/70 | AIR | 4208 | 12,649 | 121/75 | 137/76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, M.-G.; Shim, J.-M.; Son, Y.-D.; Lee, Y.-B.; Kang, C.-K. SPECT and MRI Evaluation of Cerebrovascular Reactivity with CO2 Inhalation—A Preliminary Study. Appl. Sci. 2025, 15, 5352. https://doi.org/10.3390/app15105352
Song M-G, Shim J-M, Son Y-D, Lee Y-B, Kang C-K. SPECT and MRI Evaluation of Cerebrovascular Reactivity with CO2 Inhalation—A Preliminary Study. Applied Sciences. 2025; 15(10):5352. https://doi.org/10.3390/app15105352
Chicago/Turabian StyleSong, Min-Gyu, Jeong-Min Shim, Young-Don Son, Yeong-Bae Lee, and Chang-Ki Kang. 2025. "SPECT and MRI Evaluation of Cerebrovascular Reactivity with CO2 Inhalation—A Preliminary Study" Applied Sciences 15, no. 10: 5352. https://doi.org/10.3390/app15105352
APA StyleSong, M.-G., Shim, J.-M., Son, Y.-D., Lee, Y.-B., & Kang, C.-K. (2025). SPECT and MRI Evaluation of Cerebrovascular Reactivity with CO2 Inhalation—A Preliminary Study. Applied Sciences, 15(10), 5352. https://doi.org/10.3390/app15105352







